Abstract
Cardiac allograft vasculopathy is thought to be triggered by an alloreactive response to the donor coronary vasculature, resulting in smooth muscle cell proliferation and ultimate occlusion of the donor coronary arteries. To determine whether allogeneic lymphocytes are capable of regulating endothelial-derived smooth muscle cell (SMC) growth factors, human aortic endothelial cells (HAECs) were exposed to allogeneic lymphocytes. The HAEC-lymphocyte co-cultures were assessed for (a) lymphocyte proliferation in response to the allogeneic HAECs; (b) release of soluble factors that stimulate human aortic SMC proliferation; and (c) alteration of HAEC mRNA levels for a panel of known SMC growth factors. Co-culture conditioned medium increased SMC proliferation, compared to medium conditioned by HAECs alone. HAECs exposed to allogeneic lymphocytes increased their expression of mRNA for basic fibroblast growth factor, transforming growth factors alpha and beta, and platelet derived growth factor A and B chains. These results demonstrate that allogeneic lymphocytes are capable of inducing HAECs to increase mRNA levels for several mesenchymal growth factors and to release bioactive products capable of stimulating SMC cell proliferation in vitro. Additionally, the data support the hypothesis that alloreactive lymphocytes can stimulate allogeneic donor endothelial cells to produce growth factors that may contribute to the intimal thickening seen in cardiac allograft vasculopathy.
Full text
PDF


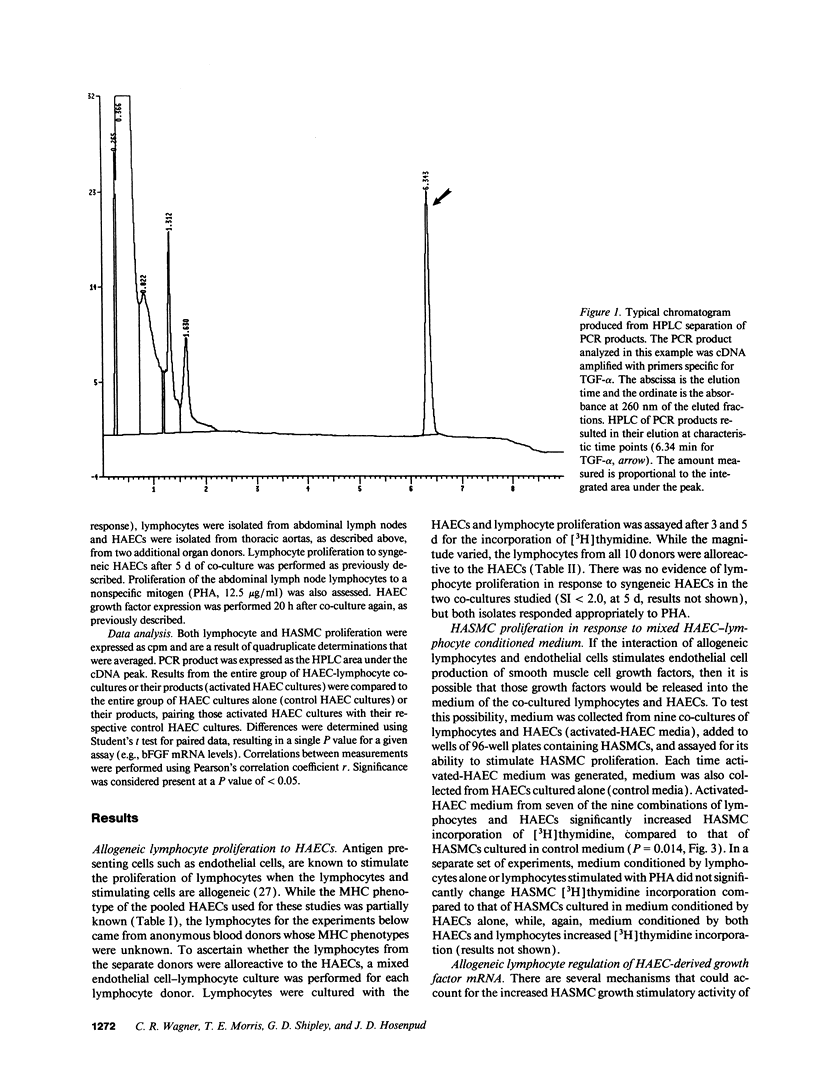
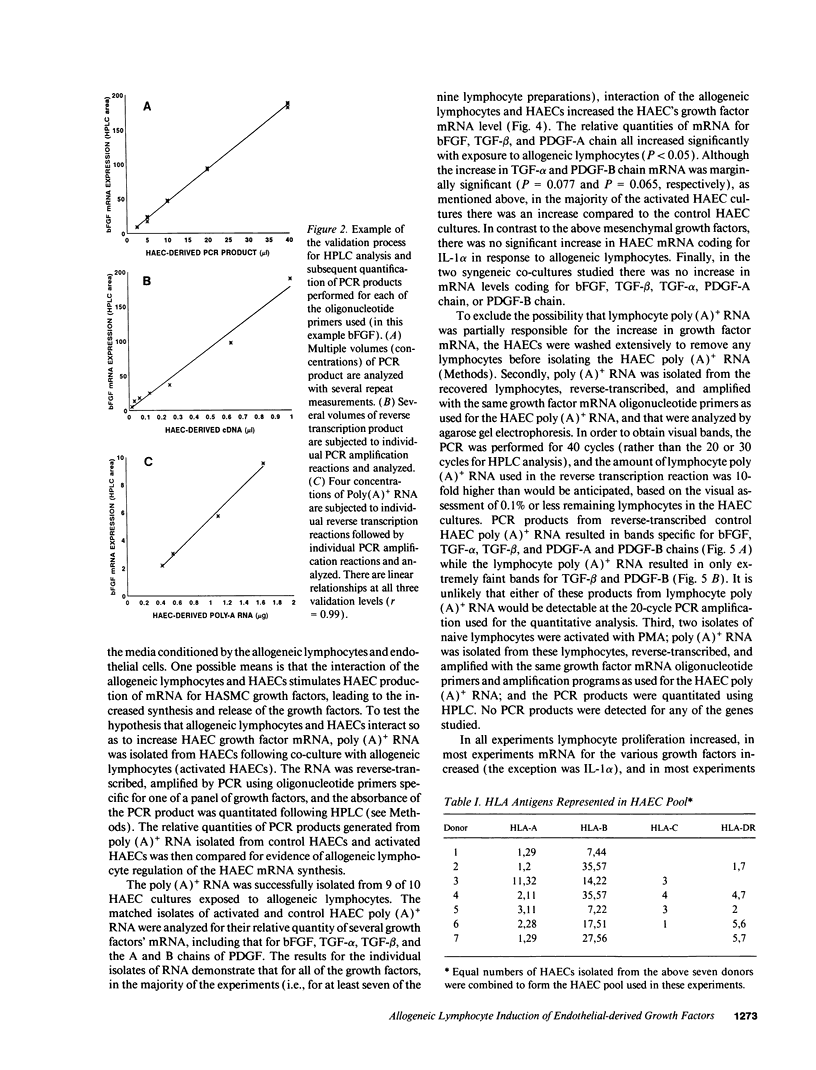
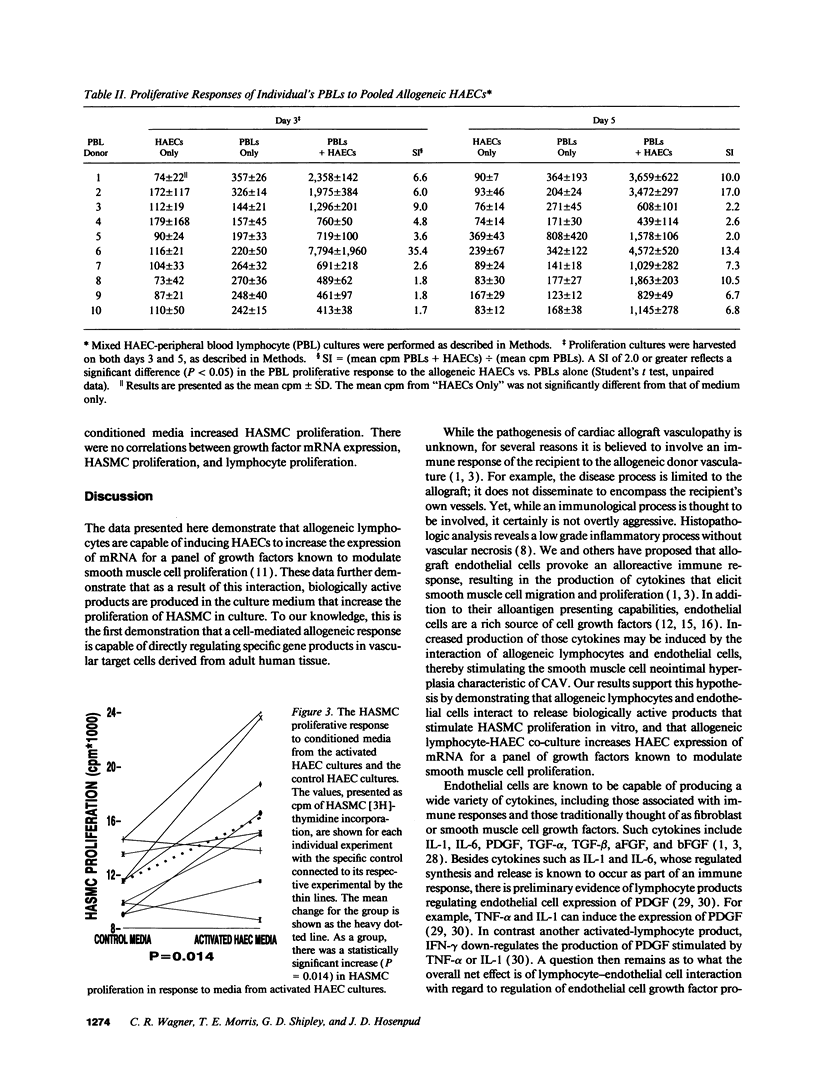


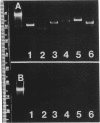
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Fukui T., Ohue M., Nomura H., Yamada M., Nakamura S. Complete nucleotide sequence of the gene for human interleukin 1 alpha. Nucleic Acids Res. 1986 Apr 25;14(8):3167–3179. doi: 10.1093/nar/14.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Hajjar K. A., Hajjar D. P., Silverstein R. L., Nachman R. L. Tumor necrosis factor-mediated release of platelet-derived growth factor from cultured endothelial cells. J Exp Med. 1987 Jul 1;166(1):235–245. doi: 10.1084/jem.166.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Evensen S. A., Henriksen T., Thorsby E. The human mixed lymphocyte-endothelium culture interaction. Transplantation. 1975 Jun;19(6):495–504. doi: 10.1097/00007890-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Hosenpud J. D., Chou S. W., Wagner C. R. Cytomegalovirus-induced regulation of major histocompatibility complex class I antigen expression in human aortic smooth muscle cells. Transplantation. 1991 Nov;52(5):896–903. doi: 10.1097/00007890-199111000-00027. [DOI] [PubMed] [Google Scholar]
- Hosenpud J. D., Shipley G. D., Wagner C. R. Cardiac allograft vasculopathy: current concepts, recent developments, and future directions. J Heart Lung Transplant. 1992 Jan-Feb;11(1 Pt 1):9–23. [PubMed] [Google Scholar]
- Hoshi H., Kan M., Chen J. K., Mckeehan W. L. Comparative endocrinology-paracrinology-autocrinology of human adult large vessel endothelial and smooth muscle cells. In Vitro Cell Dev Biol. 1988 Apr;24(4):309–320. doi: 10.1007/BF02628833. [DOI] [PubMed] [Google Scholar]
- Ikeda U., Ikeda M., Oohara T., Oguchi A., Kamitani T., Tsuruya Y., Kano S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am J Physiol. 1991 May;260(5 Pt 2):H1713–H1717. doi: 10.1152/ajpheart.1991.260.5.H1713. [DOI] [PubMed] [Google Scholar]
- Katz E. D., Dong M. W. Rapid analysis and purification of polymerase chain reaction products by high-performance liquid chromatography. Biotechniques. 1990 May;8(5):546–555. [PubMed] [Google Scholar]
- Laden A. M., Sinclair R. A. Thickening of arterial intima in rat cardiac allografts. A light and electron microscopic study. Am J Pathol. 1971 Apr;63(1):69–84. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Salomon R. N., Payne D. D., Schoen F. J., Pober J. S. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989 Aug;21(4):3677–3684. [PubMed] [Google Scholar]
- McDonald K., Rector T. S., Braulin E. A., Kubo S. H., Olivari M. T. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989 Aug 1;64(5):359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- Oni A. A., Ray J., Hosenpud J. D. Coronary venous intimal thickening in explanted cardiac allografts. Evidence demonstrating that transplant coronary artery disease is a manifestation of a diffuse allograft vasculopathy. Transplantation. 1992 Jun;53(6):1247–1251. doi: 10.1097/00007890-199206000-00015. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E. A., Smith C. R., Petrossian G. A., Barr M. L., Reemtsma K. Humoral immune responses after cardiac transplantation: correlation with fatal rejection and graft atherosclerosis. Surgery. 1989 Aug;106(2):203–208. [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Salomon R. N., Hughes C. C., Schoen F. J., Payne D. D., Pober J. S., Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991 Apr;138(4):791–798. [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Shaddy R. E., Hansen J. C., Cowley C. G. Effects of T cells on platelet-derived growth factor-like protein secretion from endothelial cells. J Heart Lung Transplant. 1992 Jan-Feb;11(1 Pt 1):48–57. [PubMed] [Google Scholar]
- Shipley G. D., Keeble W. W., Hendrickson J. E., Coffey R. J., Jr, Pittelkow M. R. Growth of normal human keratinocytes and fibroblasts in serum-free medium is stimulated by acidic and basic fibroblast growth factor. J Cell Physiol. 1989 Mar;138(3):511–518. doi: 10.1002/jcp.1041380310. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Shibano K., Okane M., Kono I., Matsui Y., Yamane K., Kashiwagi H. Interferon-gamma modulates messenger RNA levels of c-sis (PDGF-B chain), PDGF-A chain, and IL-1 beta genes in human vascular endothelial cells. Am J Pathol. 1989 Jan;134(1):35–43. [PMC free article] [PubMed] [Google Scholar]
- Thieme T. R., Hefeneider S. H., Wagner C. R., Burger D. R. Recombinant murine and human IL 1 alpha bind to human endothelial cells with an equal affinity, but have an unequal ability to induce endothelial cell adherence of lymphocytes. J Immunol. 1987 Aug 15;139(4):1173–1178. [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]