Abstract
Background
Type I diabetes (T1D) in both humans and BioBreeding (BB) rats is an autoimmune disease that results in complete destruction of islets and insulin dependency for life. Glucagon-like peptide 1 (GLP-1) promotes β cell proliferation and neogenesis and has a potent insulinotropic effect. We hypothesized that expression of GLP-1 before disease onset would increase islet mass, delay diabetes and prolong survival of BB rats.
Methods
Vascular smooth muscle cells retrovirally transduced to secrete GLP-1 were seeded into TheraCyte™ encapsulation devices, implanted subcutaneously (SQ) and rats monitored for diabetes.
Results
In untreated control rats plasma GLP-1 levels were 34.5 to 39.5 pg/ml whereas in treated rats plasma levels were elevated ranging from 90 pg/ml to 250.4 pg/ml. Hypoglycemia was not detected and this was anticipated from the glucose regulated action of GLP-1. Diabetes onset in untreated rats occurred at 56.5±0.6 SEM (n=6) days of age and in GLP-1 treated rats was delayed until 76.4±3.3 SEM (n=5) days of age (p<0.001). After disease onset untreated control rats showed rapid weight loss and elevated blood glucose (>650 mg/dl) and did not survive beyond 11 days. At 5 days after diabetes onset insulin secreting islets were absent in untreated rats. In contrast, treated rats maintained weight for up to 143 days of age and showed insulin secreting beta cells.
Conclusions
Sustained GLP-1 expression delivered by encapsulated cells before diabetes onset in BB rats showed improved clinical outcome suggesting the potential for treating patients using long lasting GLP-1 analogues.
Keywords: Diabetes, GLP-1, BB rats, bioisolator, β cells
Introduction
Type I diabetes (T1D), also known as Juvenile diabetes, is an immune mediated disease that is associated with a near complete loss of the insulin secretory function of pancreatic β cells that results in insulin-dependence for life[1]. T1D is among the most common chronic childhood illnesses, affecting 18 to 20 per 100,000 children a year with an increasing incidence of 3-4% annually, currently afflicting 1.4 million Americans [2]. Tight blood glucose control is required to delay or prevent the onset of late complications that are debilitating and associated with five-fold increased mortality [3-6]. While environmental factors play a significant role, there is increasing data regarding predisposing genetic factors involved in the pathophysiology of T1D. The association between HLA, the major histocompatibility complex, and type 1 diabetes is well established in humans and spontaneously diabetic BB DR.lyp/lyp rats [7]. T1D results from autoimmune destruction of the insulin-producing β cells. This process occurs in genetically susceptible subjects, is probably triggered by one or more environmental agents, and usually progresses over many months or years during which the subject is asymptomatic and euglycemic. This long latent period is a reflection of the large number of functioning β cells that must be lost before hyperglycemia occurs.
T1D in both patients and BB DR.lyp/lyp rats is an autoimmune disease that is characterized by lymphocytic infiltration in the islet of Langerhans (insulitis), followed by T-cell mediated destruction of β cells [8]. The primary difference between human T1D and BB DR.lyp/lyp rats is a lymphopenia defect present at birth, which results in a dramatic decrease in the number of CD4+ and CD8+ T lymphocytes. Similarities between the BB DR.lyp/lyp rats and T1D patients in terms of clinical presentation and disease progression include hyperglycemia, polyuria, polydipsia, ketoacidosis, and insulin dependency for life [9, 10]. In BB DR.lyp/lyp rats the peak incidence occurs during the time of sexual maturation and at maximum weight gain. The rats die from severe hyperglycemia and ketoacidosis within 1-2 weeks of onset unless insulin is administered. There is no effect of gender in the BB DR.lyp/lyp rat. All BB DR.lyp/lyp animals are lymphopenic and 100% of animals develop T1D during adolescence (50-65 days of age). BB DR+/+ and BB DR lyp/+ rats are neither lymphopenic nor spontaneously diabetic. The lyp/lyp genotype is associated with an islet inflammatory infiltration which is detectable about 3-5 days before the onset of hyperglycemia [11]. The recessive mode of inheritance[12] is explained by a deletion of a base-pair resulting in the truncation of the lyp protein, now recognized as a novel immune-associated nucleotide related gene (Gimap5) [13]. Recently, a large case control study in Sweden showed the GIMAP5 gene is associated with islet autoimmunity in T1D patients, perhaps making the BB DR.lyp/lyp rat a more relevant animal model for the disease [14]. In our BB DR.lyp/lyp rat colony the disease is irreversible after onset, rats never recover any islet function and will only survive if treated with exogenous insulin. The predictability of disease onset in our BB DR.lyp/lyp rats is a result of many years of breeding [12].
Glucagon-like peptide 1 (GLP-1), a gut released hormone, promotes β cell proliferation and neogenesis and has a potent insulinotropic effect [15-17]. GLP-1 exerts glucoregulatory actions via slowing of gastric emptying, glucose-dependent inhibition of glucagon secretion and acts on the brain to induce a satiety signal [15-17]. GLP-1 is produced by posttranslational processing of proglucagon in intestinal L cells and secreted in two biologically active forms, GLP-1 (7-36) amide and GLP-1 (7-37). These active forms have extremely short (< 5 minutes) circulating plasma half lives [15, 18]. The enzyme initially responsible for degrading active circulating GLP-1 is dipeptidyl peptidase 4 (DPP-4) that cleaves an N-terminal dipeptide from active GLP-1 rendering it non-functional [15]. Recent therapies are based on delivery of DPP-4 inhibitors to enhance bioavailability of GLP-1 [15, 19-21]. Also, the extremely rapid inactivation of GLP-1 has led to the use of exendin-4, a protease resistant reptilian GLP-1 analogue, as treatment for T2D [19, 20, 22]. In both humans and rodents infusion of GLP-1 has been shown to normalize blood glucose and to reduce post-prandial blood glucose excursions [15-17, 23].
Current GLP-1 gene transfer approaches to treat diabetes in rodents include GLP-1 delivery by plasmids [24-26] and adenovirus [27, 28]. Adenovirus delivery of a vector encoding a secretable form of GLP-1 to diabetic Zucker rats showed significant decreases in blood glucose and increased islet mass and function [28]. We hypothesized that continuous expression of GLP-1 before disease onset of T1D would increase islet mass, delay diabetes and prolong survival of BB DR.lyp/lyp rats. To achieve sustained GLP-1 delivery we implanted encapsulated vascular smooth muscle cells (VSMC) retrovirally transduced to secrete hormone constitutively. Because GLP-1 only increases β cell insulin secretion in the presence of elevated blood glucose, the risks of developing hypoglycemia from sustained delivery are low, providing a significant safety feature for this therapy [15-17]. Transduced VSMC were implanted in TheraCyte™ immunoisolation devices that possess an inner immunoisolation membrane and an outer vascularization membrane. These devices can be easily implanted subcutaneously and provide a potentially important approach to gene therapy as they permit the use of transduced allogeneic cells for gene delivery without the need for immune suppression. We have shown that TheraCyte™ encapsulation devices seeded with allogeneic VSMC transduced to express rat EPO provided sustained elevations of HCT for at least 15 months [29]. Islets encapsulated in TheraCyte™ devices have successfully treated rat models of T1D [30].
Materials and Methods
Animals
BB DR.lyp/lyp rats were generated in Seattle from an established breeding colony [13]. Male and female BB DR.lyp/lyp rats were studied. The standard cross-intercross breeding was used to generate BB DR.lyp/lyp rats which all develop diabetes between 50-65 days of age. Animals were genotyped and phenotyped between 25-30 days of age [31]. In brief, two drops of tail vein blood were obtained for blood lymphocyte phenotyping of R73, CD4 and CD8 antibody positive cells. The fraction of fluorescent R73 positive T cells among mononuclear cells was determined on a BD Facscan (Beckton Dickenson, San Jose, CA). Animals were kept under specific pathogen free (SPF) condition with a standard light/dark 12 hours cycle and received regular diet and water ad lib. To obtain pancreatic tissue for histology untreated control BB DR.lyp/lyp rats received daily exogenous insulin starting at 40 days of age, before diabetes onset, to prevent hyperglycemia and severe weight loss. All animal studies have been approved by the University of Washington Animal Care Committee.
Transduction of rat vascular smooth muscle cells (VSMC)
We constructed a retrovirus encoding a furin cleavable GLP-1 peptide that is based on a published GLP-1 secreting vector [24, 32]. The vector is designated pLhIL-GLP-1-SN. It encodes the human insulin leader sequence (hIL) 5′ to the first residue of 7-37 GLP-1 separated by a furin cleavable sequence (RGRR). The vector encodes selectable neo gene under the control of SV40 promoter. Rat VSMC were prepared by enzymatic digestion of aorta from male Fisher 344 and Wistar rats. Cells were characterized by positive staining for muscle cell-specific actins with HHF35 antibody [33] while staining negative for von Willebrand factor,[33] an endothelial cell specific marker. Ecotropic PE501 and amphotropic PA317 retrovirus packaging cell lines,[34, 35] NIH 3T3 thymidine kinase negative cells,[34] and primary cultures of rat smooth muscle cells were grown in Dulbecco/Vogt-modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in humidified 5% CO2 at 37°C. Early passage smooth muscle cells were exposed to 16-hr virus harvests from PA317-LhIL-GLP-1-SN amphotropic virus-producing cell lines for a period of 24 hr in the presence of polybrene (4μg/ml). pLhIL-GLP-1-SN virus with a titer of 1.5×106 IU/ml was used to infect primary VSMC cells. Infected cells were selected in medium containing G418 at 1 mg/ml and were plated at limiting dilution in 96 well plates in regular tissue culture medium (DMEM) and conditioned medium from 48 wells was screened for GLP-1 (Linco). As a control vector we used LASN, a virus encoding human adenosine deaminase regulated by LTR promoter and neo gene under the control of SV40 promoter [36].
Subcutaneous implantation of bioisolator devices
Bioisolator devices obtained from TheraCyte™ Inc. Irvine, CA were loaded with transduced VSMC and implanted subcutaneously in anesthetized animals. BB DR.lyp/lyp rats and a control rat between 39-43 days of age weighing 190-230 gm were anesthetized with an IP injection of ketamine (44mg/kg) plus Xylazine (6mg/kg) and acepromazine (0.75mg/kg). Skin was shaved from the abdomen, swabbed with betadine and a midline incision made in the skin and a small pocket created by blunt dissection with a hemostat. A device seeded with VSMC is placed into the pocket and the incisions closed with sutures.
Diagnosis of diabetes, glucose and weight monitoring
Starting at 35 days of age, all rats were weighed daily. Blood glucose was monitored daily using a blood glucose meter (Ascencia Contour, Bayer, Leverkusen, Germany) starting at 37 days of age. Diabetes was diagnosed when blood glucose levels exceeded 200mg/dL on two consecutive days. Normal control and GLP-1 treated BB DR.lyp/lyp rats were monitored and did not receive exogenous insulin. Untreated control BB DR.lyp/lyp rats received insulin daily from 40 days of age to achieve normoglycemia and preserve health to permit harvest of pancreas. Blood glucose values of >650mg/dL were above the glucometer sensitivity range and were read as high.
Insulin and GLP-1 assays
For insulin assays 0.2 ml of blood was collected into EDTA containing tubes, plasma was separated and stored at −20°C. For GLP-1 levels, 0.2 ml of blood was collected into EDTA containing tubes with the addition of 5μl of DPP-4 inhibitor (Linco Research Inc., Missouri) and plasma was separated and stored at −20°C. ELISA was used to measure insulin (Crystal Chem Inc, Illinois) and GLP-1 (Linco Research Inc, Missouri).
Pancreas Histology
Pancreatic tissue was subjected to staining using insulin and glucagon specific antibodies for the identification of islet β and α cells respectively. Pancreata were fixed in 10% paraformaldehyde/PBS and embedded in paraffin. Sections were stained with hematoxylin and eosin and immunostained using guinea pig anti-insulin (Dako), or a murine monoclonal anti rat glucagon antibody (Sigma), followed by biotinylated secondary antibody, ABC-Elite (Vector Laboratories, Burlingame CA) and 3,3′-diaminobenzidine [37] by the Department of Pathology Histology Core, University of Washington.
Results
VSMC transduction and treatment of BB DR.lyp/lyp rats before diabetes onset
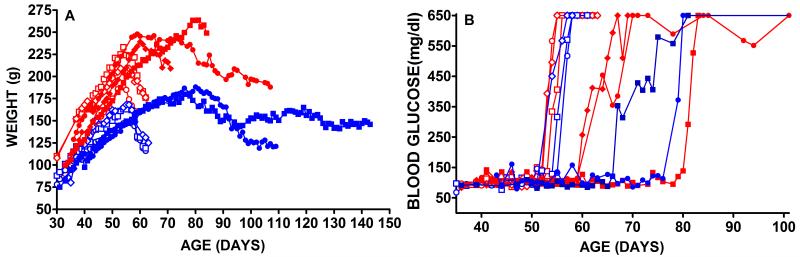
We generated amphotropic pLhIL-GLP-1-SN retrovirus using PA317 packaging cells and transduced, selected and cloned Wistar rat vascular smooth muscle cells. We monitored bioactive GLP-1 secretion from 48 cloned cell lines and the highest hormone expression was 294.4 pg GLP-1 per 106 cells per 24hrs and this clone was used in all implantation experiments. We seeded 40μl (4.5cm × 1cm × 2mm) TheraCyte™ devices with 107 transduced cells and implanted a single device subcutaneously into male or female BB DR.lyp/lyp rats. Control rats received mock surgery or implants seeded with 107 LASN transduced cells. Mock surgery consisted of anesthesia, SQ incision, pocket dissection and wound closure without implantation of a bioisolator device. All rats received implants between 39 and 43 days of age before diabetes onset and loss of β cells by autoimmune destruction. Control and treated rats were monitored daily for weight (Figure 1A) and blood glucose (Figure 1B). In three male and three female BB DR.lyp/lyp control rats that received no treatment diabetes onset was between 55 and 58 days of age and was observed as rapid weight loss (Figure 1A open symbols) and as elevated blood glucose (Figure 1B open symbols). In the 6 untreated male and female control rats blood glucose levels >650 mg/dl were reached between 55 and 58 days of age (56.5±0.6 SEM n=6). In BB DR.lyp/lyp rats the onset of hyperglycemia is rapid, within 3 days there is a change from normoglycemia to blood glucose levels >650mg/dl (Figure 1B). In all treated rats there was a delay in diabetes onset defined as two consecutive blood glucose levels >200mg/dl (Figure 1B). In the 5 treated male and female rats elevated blood glucose levels >650 mg/dl were reached between 67 and 84 days of age (76.4±3.3 SEM, n=5) and this delay in age of diabetes onset is significantly different from mean age of onset of 56.5±0.6 SEM in untreated control rats (n=6) (p<0.001). Untreated control male and female BB DR.lyp/lyp rats showed severe weight loss following onset of elevated blood glucose (Figure 1A). In these untreated animals weight loss preceded the occurrence of blood glucose values >650mg/dl by 1 to 3 days. The observation of weight loss preceding diabetes onset has been previously reported in BB DR.lyp/lyp rats [11]. These control diabetic rats did not receive exogenous insulin and were euthanized within 9 to 11 days of onset because of ill health or excessive weight loss >20% (Figure 1A). All male and female BB DR.lyp/lyp rats exposed to sustained GLP-1 delivery showed increases in weight beyond the ages when weight loss was observed in untreated diabetic rats (Figure 1A). Both female and one treated male rat showed weight gain to about 80 days of age that was then followed by weight loss. One female rat (Figure 1A, blue square symbol) maintained a weight of around 150g until 143 days of age when she was euthanized for tissue harvest. This rat showedsteady weight control for at least 70 days longer than untreated control rats (Figure 1A). In untreated diabetic BB rats serum insulin levels were undetectable and in treated BB rats serum insulin levels were within the normal range of 1-5 ng/ml. In untreated control rats plasma GLP-1 levels were 34.5 to 39.5 pg/ml, within the normal range for rats, [24-26] whereas in GLP-1 treated rats plasma levels were elevated and ranged from 90 pg/ml to 250.4 pg/ml. The GLP-1 levels were not correlated with diabetes onset. Hypoglycemia was not detected in any of the treated rats and this was anticipated from the known glucose regulated action of GLP-1 [15-17].
Figure 1. Male and Female BB DR.lyp/lyp rats untreated or receiving GLP-1 secreting encapsulated cells before diabetes onset.
Serial weights of males and females (panel A) and blood glucose (panel B). Two female (solid blue symbols) and three male (solid red symbols) BB DR.lyp/lyp rats were treated at 40-45 days of age, before onset of diabetes, with encapsulated VSMC transduced to secrete GLP-1. Untreated control BB DR.lyp/lyp rats are shown as open symbols; females (blue) and males (red). All rats received 107 encapsulated transduced cells.
Pancreas Histology
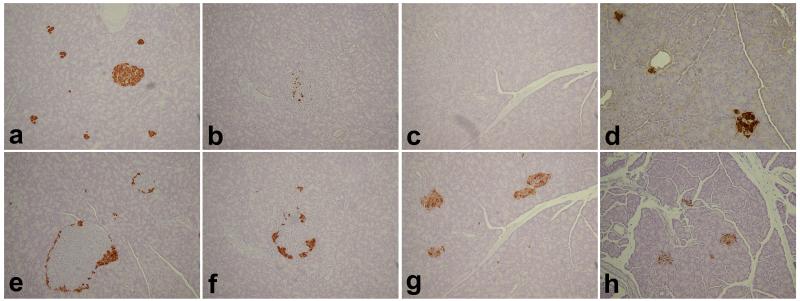
Pancreatic sections from BB DR.+/+ wild type control and GLP-1 treated and untreated BB DR.lyp/lyp rats were stained for insulin and glucagon (Figure 2). The untreated BB DR.+/+ control rat (panel a) showed islets with insulin staining β cells and, as is typical of rodents, glucagon staining α cells around the periphery (panel e) [38, 39]. In pancreas from an untreated BB DR.lyp/lyp rat at diabetes onset limited numbers of insulin staining β cells were present (panel b). However, pancreatic sections showed glucagon staining α cells around the periphery of the islets (panel f) similar to that observed in a control rat (panel e). In contrast, an untreated BB DR.lyp/lyp rat at 5 days post diabetes onset did not posses any insulin secreting β cells (panel c) and glucagon staining α cells were in loose aggregates (panel g). In contrast, a female BB DR.lyp/lyp treated with GLP-1 secreting VSMC that was sacrificed at 143 days of age (Figure 1A, 1B, blue squares) showed clusters of insulin secreting β cells in pancreatic islets (panel d). In this animal glucagon staining of pancreas showed aggregates of α cells (panel h) and not adiscrete peripheral distribution that was observed for the normal rat (panel e) or the GLP-1 untreated BB DR.lyp/lyp rat sacrificed at diabetes onset (panel f).
Figure 2. Pancreas tissue from BB DR+/+wild type, GLP-1 treated and untreated BB DR.lyp/lyp rats.
Pancreas sections were stained for insulin (a-d) and glucagon (e-h). Untreated BB DR+/+ wild type control rat (panels a/e); untreated BB DR.lyp/lyp rat at diabetes onset (panels b/f) and at 5 days post diabetes onset (panels c/g) and GLP-1 treated female BB DR.lyp/lyp rat at 143 days of age (panels d/h). Untreated BB DR.lyp/lyp control rats received daily insulin from 40 days of age to prevent hyperglycemia, severe weight loss and preserve health. Magnification 20x.
Discussion
We showed that the strategy of using a leader sequence adjacent to a furin cleavage site 5′ to bioactive GLP-1 peptide was successful in permitting GLP-1 secretion from transduced VSMC. This approach has been used in previous studies [24, 28]. The levels of plasma GLP-1 generated from encapsulated VSMC ranged from 90 pg/ml to 250.4 pg/ml and were sufficient to delay diabetes onset and control weight loss in treated BB DR.lyp/lyp diabetic rats. Also, these levels were achieved without administration of DPP-4 inhibitors to reduce GLP-1 degradation [15, 19-21]. If necessary increased levels of GLP-1 secretion from transduced VSMC could be achieved by increasing copy number. We have shown that amphotropic virus produced from PA317 packaging cells generates VSMC transductants with copy numbers of ≤ 2 [40].
In our breeding colony all BB DR.lyp/lyp rats develop spontaneous T1D during adolescence at 50 to 65 days of age and at 5 days post diabetes onset insulin secreting β cells are absent as these cells have been destroyed by the autoimmune process. Untreated rats do not survive for more than 11 days after diabetes onset and as this occurs between 50 - 65 days of age untreated lifespan is usually <76 days of age. GLP-1 treated rats survived for up to 143 days of age showing that sustained delivery of this hormone significantly delayed diabetes onset and induced prolonged weight control. Diabetes onset was significantly delayed in treated rats where elevated blood glucose levels >650 mg/dl were reached at a mean age of 76.4±3.3 SEM days in contrast to untreated rats that showed a mean age of onset of 56.5±0.6 SEM (p<0.001).
Histological examination of pancreas showed that wild type islets from BB DR+/+ rats are clearly demarcated with central insulin producing β cells surrounded by a ring of glucagon producing α cells in the islet mantle, as has been reported in rodents [38, 39, 41]. This distribution pattern of α cells was observed in pancreas of BB DR.lyp/lyp rat at diabetes onset but not in pancreas from a rat 5 days post diabetes onset. The change in distribution of α cells from a distinct ring to aggregates was also observed in GLP-1 treated BB DR.lyp/lyp rats and probably results from the specific immune mediated destruction of β cells interrupting normal islet architecture. [8]. In contrast to normal control rats, insulin secreting pancreatic β cells from a treated BB DR.lyp/lyp rat were in small clusters and were adjacent to a common ductal area indicative of islet neogenesis that is known to take place at pancreatic ductal cells [15-17]. In this GLP-1 treated animal glucagon staining α cells were present in aggregates and did not show the ring distribution observed in normal control rats or diabetic rats at disease onset.
The finding that insulin secreting pancreatic islets were present at up to 143 days of age in a BB DR.lyp/lyp rat treated with GLP-1 is significant. Although normoglycemia was not sustained, the presence of insulin secreting islets may be clinically important as residual insulin secretion in T1D protects against severe ketoacidosis [5, 42]. These data suggest that sustained elevated GLP-1 blood levels improved the balance between β cell regeneration/proliferation and immune mediated β cell destruction. These results are encouraging clinically. Treated rats had significantly longer life, were able to maintain health and exhibited reasonable weight control whereas untreated BB DR.lyp/lyp rats die within 11 days post onset with no survivors. Even at high doses and blood levels hypoglycemia is not produced by administration of GLP-1. The strict glucose dependence of GLP-1 action on insulin secretion in islets is a safeguard against provoking hypoglycemia in cases where GLP-1 blood levels are elevated. The SQ implantation of Theracyte™ devices is a very safe and easy procedure and employs a well characterized allogeneic cell line that was administered to all rats. The improved clinical outcome in treated T1D rats suggests the potential for treatment of humans using long lasting GLP-1 analogues or novel GLP-1 formulations.
Acknowledgements
This work was supported by a University of Washington Bridge Fund (WO), Junior Faculty Award (1-05-JF-32) (D.H.M) and a Mentor Based Postdoctoral Fellowship Award (Å.L.) from the American Diabetes Association, the National Institutes of Health (AI42380), the Virus Molecular Biology and Cell Core (W.O.) of the University of Washington Diabetes and Endocrinology Research Center (DK17047) as well as by the Robert H. Williams Endowment at the University of Washington. We gratefully thank Kelly Lee Hudkins, Department of Pathology, Histology Core, University of Washington.
Footnotes
The authors have no conflicts of interest.
References
- 1.Falorni A, Kockum I, Sanjeevi CB, et al. Pathogenesis of insulin-dependent diabetes mellitus. Bailliere’s Clin Endocrinol Met. 1995;9:25–46. doi: 10.1016/s0950-351x(95)80803-5. [DOI] [PubMed] [Google Scholar]
- 2.Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RR, Peyrot M. Implications of the DCCT. Looking beyond tight control. Diabetes Care. 1994;17:235–236. doi: 10.2337/diacare.17.3.235. [DOI] [PubMed] [Google Scholar]
- 4.Crofford OB. Diabetes control and complications. Ann Rev Med. 1995;46:267–279. doi: 10.1146/annurev.med.46.1.267. [DOI] [PubMed] [Google Scholar]
- 5.DCCT The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Annals of Internal Medicine. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Borch-Johnsen K, Kreiner S, Deckert T. Mortality of Type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2930 Danish Type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia. 1986;29:767–772. doi: 10.1007/BF00873214. [DOI] [PubMed] [Google Scholar]
- 7.Mathis D, Vence L, Benoist C. Beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 8.Kukreja A, Maclaren NK. Autoimmunity and Diabetes. J Clin Endocrinol Metab. 1999;84:4371–4378. doi: 10.1210/jcem.84.12.6212. DOI: 10.1210/jc.84.12.4371. [DOI] [PubMed] [Google Scholar]
- 9.Scott J. The spontaneously diabetic BB rat: sites of the defects leading to autoimmunity and diabetes mellitus. A review. Curr Top Microbiol Immunol. 1990;156:1–14. doi: 10.1007/978-3-642-75239-1_1. [DOI] [PubMed] [Google Scholar]
- 10.Mordes JP, Desemone J, Rossini AA. The BB rat. Diabetes Metab Rev. 1987;3:725–750. doi: 10.1002/dmr.5610030307. [DOI] [PubMed] [Google Scholar]
- 11.Bieg S, Simonson WT, Elefson K, et al. Rel B is an early marker of autoimmune islet inflammation in the biobreeding (BB) rat. Pancreas. 2000;20:47–54. doi: 10.1097/00006676-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bieg S, Koike G, Jiang J, et al. Genetic isolation of the Lyp region on chromosome 4 in the diabetes prone BioBreeding (BB) rat. Mammalian Genome. 1998;9:324–326. doi: 10.1007/s003359900759. [DOI] [PubMed] [Google Scholar]
- 13.MacMurray AJ, Moralejo DH, Kwitek AE, et al. Lymphopenia in the BB Rat Model of Type 1 Diabetes is Due to a Mutation in a Novel Immune-Associated Nucleotide (Ian)-Related Gene. Genome Res. 2002;12:1029–1039. doi: 10.1101/gr.412702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin JH, Janer M, McNeney B, et al. IA-2 autoantibodies in incident type I diabetes patients are associated with a polyadenylation signal polymorphism in GIMAP5. Genes Immun. 2007;8:185–192. doi: 10.1038/sj.gene.6364413. [DOI] [PubMed] [Google Scholar]
- 15.Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes, Obesity and Metabolism. 2007;9:23–31. doi: 10.1111/j.1463-1326.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 17.Amori RE, Lau J, Pittas AG. Efficacy and Safety of Incretin Therapy in Type 2 Diabetes: Systematic Review and Meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Fehmann HC, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 19.Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective. Biochem Biophys Res Commun. 2002;294:1–4. doi: 10.1016/S0006-291X(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 20.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 21.Holst J. Treatment of Type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin Emerg Drugs. 2004;9:155–166. doi: 10.1517/eoed.9.1.155.32952. [DOI] [PubMed] [Google Scholar]
- 22.Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 23.Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;8:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 24.Oh S, Lee M, Ko KS, et al. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7:478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 25.Soltani N, Kumar M, Glinka Y, et al. In vivo expression of GLP-1/IgG-Fc fusion protein enhances beta-cell mass and protects against streptozotocin-induced diabetes. Gene Ther. 2007;14:981–988. doi: 10.1038/sj.gt.3302944. [DOI] [PubMed] [Google Scholar]
- 26.Prud’homme GJ, Draghia-Akli R, Wang Q. Plasmid-based gene therapy of diabetes mellitus. Gene Ther. 2007;14:553–564. doi: 10.1038/sj.gt.3302907. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y-S, Shin S, Shigihara T, et al. Glucagon-Like Peptide-1 Gene Therapy in Obese Diabetic Mice Results in Long-Term Cure of Diabetes by Improving Insulin Sensitivity and Reducing Hepatic Gluconeogenesis. Diabetes. 2007;56:1671–1679. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 28.Parsons GB, Souza DW, Wu H, et al. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
- 29.Yanay O, Flint LY, Brzezinski M, et al. Long term erythropoietin gene expression from transduced cells in bioisolator devices. Hum Gene Ther. 2003;14:1587–1593. doi: 10.1089/104303403322542239. [DOI] [PubMed] [Google Scholar]
- 30.Sweet IR, Yanay O, Waldron L, et al. Treatment of Diabetic Rats with Encapsulated Islets. J Cell Mol Med. 2008;12:2644–2650. doi: 10.1111/j.1582-4934.2008.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller J, Kwitek AE, Hawkins T, et al. Introgression of F344 rat genomic DNA on BB rat chromosome 4 generates diabetes-resistant lymphopenic BB rats. Diabetes. 2006;55:3351–3357. doi: 10.2337/db06-0715. [DOI] [PubMed] [Google Scholar]
- 32.Hui H, Yu R, Bousquet C, et al. Transfection of pancreatic-derived beta-cells with a minigene encoding human glucagon-like peptide-1 regulates glucose-dependent insulin synthesis and secretion. Endocrinology. 2002;143:3529–3539. doi: 10.1210/en.2001-210979. [DOI] [PubMed] [Google Scholar]
- 33.Geary RL, Clowes AW, Lau S, et al. Gene transfer in baboons using prosthetic vascular grafts seeded with retrovirally-transduced smooth muscle cells: A model for local and systemic gene therapy. Hum Gene Ther. 1994;5:1213–1218. doi: 10.1089/hum.1994.5.10-1211. [DOI] [PubMed] [Google Scholar]
- 34.Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch CM, Clowes MM, Osborne WRA, et al. Long-term expression of human adenosine deaminase in vascular smooth muscle cells of rats: A model for gene therapy. Proc Natl Acad Sci USA. 1992;89:1138–1142. doi: 10.1073/pnas.89.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taneda S, Segerer S, Hudkins KL, et al. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol. 2001;159:2355–2369. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn Y, Xu G, Marselli L, et al. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia. 2007;50:334–342. doi: 10.1007/s00125-006-0536-5. [DOI] [PubMed] [Google Scholar]
- 39.Moralejo DH, Hansen CT, Treuting P, et al. Differential effects of leptin receptor mutation on male and female BBDR.Gimap5-/Gimap5- spontaneously diabetic rats. Physiological Genomics. 2010;41:9–20. doi: 10.1152/physiolgenomics.00186.2009. DOI: 10.1152/physiolgenomics.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seppen J, Barry S, Lam GM, et al. Retroviral preparations derived from PA317 packaging cells contain inhibitors that co-purify with viral particles and are devoid of viral vector RNA. Hum Gene Ther. 2000;11:771–775. doi: 10.1089/10430340050015662. [DOI] [PubMed] [Google Scholar]
- 41.Martinic MM, von Herrath MG. Real-time imaging of the pancreas during development of diabetes. Immunological Reviews. 2008;221:200–213. doi: 10.1111/j.1600-065X.2008.00581.x. [DOI] [PubMed] [Google Scholar]
- 42.Madsbad S, Alberti KG, Binder C, et al. Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J. 1979;2:1257–1259. doi: 10.1136/bmj.2.6200.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]