Abstract
Background
A broad capacity for deliberate self-regulation plays a key role in emotion regulation. This longitudinal investigation from infancy to preschool age examines genotype by environment (G × E) interaction in the development of self-regulation, using molecular measures of children’s genotypes and observed measures of the quality of early mother-child relationship, as reflected in attachment organization in infancy.
Methods
In 89 children, we assessed the polymorphism in the serotonin transporter gene (5-HTTLPR, ss/sl vs. ll allele status), security of attachment to mothers at 15 months in the Strange Situation, and children’s ability for self-regulation at 25, 38, and 52 months, using behavioral batteries of tasks that called for deliberately suppressing a dominant response and performing instead a sub-dominant response.
Results
There was a robust G × E interaction between genetic risk and the quality of early relationship. Among children who carried a short 5-HTTLPR allele (ss/sl), those who were insecurely attached developed poor regulatory capacities, but those who were securely attached developed as good regulatory capacities as children who were homozygotic for the long allele (ll). There was no effect of security for ll homozygotes.
Conclusions
Those findings, consistent with diathesis-stress model, bridge research on self-regulation in typically developing children with research on non-human primates and research on psychopathology. They also indicate that a secure attachment relationship can serve as a protective factor in the presence of risk conferred by a genotype.
Keywords: ATTACHMENT, EMOTION REGULATION, GENETICS, G × E interactions, EFFORTFUL CONTROL, 5-HTTLPR polymorphism, PARENT-child RELATIONSHIPS
Interplay of Genes and Early Mother-Child Relationship in the Development of Self-Regulation from Toddler to Preschool Age Children’s capacity for deliberate self-control, often studied as self-regulation, inhibitory control, or effortful control, has been inherently linked to emotion regulation. Fox and Calkins (2003) listed effortful control as an intrinsic temperament factor that underpins self-control of emotion. Sometimes, emotion regulation is seen as a subset of broader self-regulatory processes, and sometimes, emotion regulation and self-regulation are seen as having consonant qualities (e.g., Kopp, 1989). Self-regulation, the capacity to control deliberately one’s affect and behavior, accounts for children’s growing capacity to voluntarily uncouple their behavioral response from the immediate emotional impulse (Eisenberg & Spinrad, 2004; Kopp, 1982). For example, children gradually become able to suppress or delay touching prohibited objects or engaging in a strongly desired but prohibited action when so instructed, to suppress an aggressive or angry act when such an act is unacceptable, and to sustain an aversive activity when requested to do so by a caregiver. Such deliberate effortful control and the control of emotion share a key requirement – a suppression of an impulsive response and instead, carrying out an opposite act. Consequently, effortful control and modulated expression of both positive and negative affect have been often conceptually and empirically linked (Carlson & Wang, 2007; Eisenberg, Fabes, Guthrie, & Reiser, 2000; Eisenberg & Spinrad, 2004; Kieras, Tobin, Graziano, & Rothbart, 2005; Kochanska, Murray, & Harlan, 2000; Kochanska, Aksan, Penney, & Doobay, 2007; Posner & Rothbart, 2000; Rothbart & Bates, 2006).
Because of its critical importance for socialization, the fundamental capacity for self-regulation has been extensively studied (Kopp, 1982; Kochanska et al., 2000). The ability to suppress a predominant response –often one immediately desired by the child --and to perform instead a subdominant response, often unappealing, but required by caregivers and other social agents begins to develop in the second year (Kochanska et al., 2001; Kochanska, et al., 2000; Posner & Rothbart, 2000; Rothbart & Bates, 2006). Individual differences in the capacity for self-regulation have been strongly implicated in many aspects of adaptive development and psychopathology (Maccoby, 2007).
A modern approach to development must involve the integration of constructs across multiple levels, “from neurons to neighborhoods” (Shonkoff & Phillips, 2000). Consequently, those constructs include both biological and environmental factors that interface in complex ways (Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000; Fox & Calkins, 2003). “Genotype × Environment” (G × E) interactions are among most often studied forms of that interface (Rutter, Moffit, & Caspi, 2006). A G × E interaction occurs when environmental experience moderates the effect of a person’s genotype on physical or mental health outcomes, or when a genotype moderates an environmental effect (Moffit, Caspi, & Rutter, 2005).
In developmental psychology, studies of such interactions have often focused on children’s temperament as a moderator of socialization (Bates, Pettit, Dodge, & Ridge, 1998; Belsky, 1997; Belsky, Hsieh, & Crnic, 1998; Kochanska, Aksan, & Joy, 2007; Rothbart & Bates, 2006). In those studies, temperament has been considered a proxy for genotype, even though, with a few exceptions (Fowles & Kochanska, 2000), it has been measured behaviorally. Acknowledging genetic contributions to temperament, other scholars have adopted behavior genetic designs, such as twin and adoption studies (Goldsmith, 2003; Goldsmith, Lemery, Buss, & Campos, 1999).
Recently, a surge in inter-disciplinary collaborations has brought exciting advances in the study of G × E interactions. In a landmark article, Caspi and colleagues (Caspi et al., 2003) demonstrated that a history of stressors moderates the effect of genetic risk on depression. In developmental science, research on non-human primates that allows for systematic manipulation of early environment has led to groundbreaking insights into G × E interactions in early social-emotional development (Suomi, 2004, 2005, 2006; Champoux, Bennett, Shannon, Higley, Lesch, & Suomi, 2002).
Multiple studies of G × E interactions have focused on a polymorphism in the serotonin (5-HT) transporter gene regulatory region (5-HTTLPR). Serotonin is an inhibitory neurotransmitter in the central nervous system. Dysfunctions in the serotonergic system have been strongly implicated in broadly ranging regulation of mood, attention, and psychopathology (Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001; Champoux et al., 2002; Lucki, 1998; Sourbrie, 1986; van Goozen, Fairchild, Snoek, & Harold, 2007). The 5-HTT gene has also been linked to neural areas that are parts of the executive attention network involved in self-regulation (Posner, Rothbart, & Sheese, 2007).
The 5-HTTLPR polymorphism has two common alleles, the short (s) and the long (l). The short allele (s) has been linked to reduced 5-HTT transcription, lower 5-HTT protein levels, and diminished serotonin re-uptake compared to individuals with the long (l) allele. Individuals who are either homozygous for the short allele (ss) or heterozygous (sl) have been found to be at risk for a range of emotional and behavioral maladaptive outcomes. Those outcomes include under-regulated, impulsive, excessively and inappropriately aggressive, risk-taking behavior, alcohol use, as well as depressive or anxious psychopathology (Barr et al., 2004; Lesch et al., 1996; Propper & Moore, 2006; Suomi, 2005).
Even more importantly, however, both human and non-human studies have increasingly documented substantial G × E interactions between the genetic risk associated with 5-HTTLPR polymorphism (having a short allele) and environmental or experiential factors. Generally, those studies have shown that environmental factors moderate the link between the genetic risk and maladaptive outcomes: Individuals who carry a short allele develop a host of problems when they also experience sub-optimal or stressful environmental conditions. Suomi and colleagues have repeatedly demonstrated that monkeys with ss or sl genotype show a range of significant self-regulatory problems (impulsivity, inappropriate aggression, orienting problems, risk taking), but only if they had been separated from their mothers and raised in a peer nursery. For monkeys raised in natural, supportive mother-infant relationships, there was no effect of genotype (Champoux et al., 2002; Suomi, 2004, 2005, 2006).
Emerging non-experimental evidence with human children has consistently dovetailed with those findings. The “high-risk genotype” (having a short allele, ss or sl) has been found to confer a risk for depression, but only in children who have been exposed to maltreatment, abuse, or neglect (Kaufman et al. 2004, 2006), and for fearfulness, but only in children who have grown up in a family with poor social support (Fox et al., 2005).
To our knowledge, however, no study with human children has addressed G × E interactions in the development of self-regulation, using a multi-trait multi-method longitudinal design, molecular measures of the 5-HTTLPR genotype, repeated assessments of self-regulation from toddler to preschool age, and observational measures of the early rearing environment, specifically, attachment security in infancy. We present such data in the current article.
A secure early mother-child relationship has been repeatedly implicated as important for the development of emotional regulatory abilities, ranging from regulating one’s own emotional arousal to complex executive capacities (Cole, Martin, & Dennis, 2004; Hofer, 1994; Schore, 2001; Sroufe, 1996, 2005). Biological factors, however, including genes, also play an important role, as demonstrated by molecular (Diamond, Briand, Fossella, & Gehlbach, 2004; Posner, 2005; Suomi, 2005) and behavioral genetic studies (Goldsmith, 2003; Goldsmith et al., 1999). Most pertinent to the current study is the following hypothesis, based on research with primates (Suomi, 2006, p. 52): “Secure attachment relationships somehow confer resiliency to individuals who carry alleles that may otherwise increase their risk for adverse developmental outcomes (“maternal buffering”)”.
To date, however, few studies of G × E interactions operationalized early environmental risk specifically as insecure attachment. Often, beneficial or harmful early parent-child relationships have been approximated using other measures. In his work with primates, Suomi (2004) compared peer and maternal rearing. Kaufman et al. (2004) identified the history of child maltreatment using legal and social agencies’ records. Fox et al. (2005) used mothers’ reports of perceived social support.
To examine the hypothesis that early security may be an important environmental factor that moderates the effect of genotype on children’s self-regulation, we assessed children’s attachment security with their mothers at the end of the first year, using the established Strange Situation paradigm. Children’s genotype, 5-HTTLPR polymorphism, was assessed as ss/sl or ll allele status.
We targeted a major aspect of self-regulation: children’s effortful control, or the capacity to deliberately suppress a dominant behavior and to perform instead a sub-dominant behavior (Rothbart & Bates, 2006). We assessed it behaviorally at 25, 38, and 52 months, using a multi-task battery at each age.
Based on the extant non-human and human research, we expected security to moderate the impact of the genotype on children’s capacity for self-regulation. In children with high-risk genotypes, who have a short allele, ss or sl, we expected to find the effect of their early attachment security, such that those who had been insecure would develop poorer self-regulatory capacities than those who had been secure. We did not expect to find such effect in children homozygous for the long allele, ll.
In this context, we also examined whether the G × E effect would conform to the differential succeptibility hypothesis or to the genetic vulnerability hypothesis, a subject of a recent debate (Belsky, 2005; Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2007). The former hypothesis predicts that children with certain vulnerable traits --highly reactive, highly negative, carriers of the short 5-HTT allele --respond more strongly to both negative and positive variations in the environment, and thus, may end up with worse or better outcomes than children without those dispositions. In contrast, the latter hypothesis, while also predicting that children with those vulnerabilities do worse than others when exposed to harmful environments, it does not predict that they would do better than their low-risk peers when exposed to beneficial environments. In other words, beneficial environments can merely buffer children from risks conferred by genotypes.
Method
Participants and Design
Participants responded to ads in local community venues. The two-parent families represented a broad range of education, from high school (24% of mothers, 30% of fathers) to post-college (21% of mothers, 20% of fathers), and of income, from under $20,000 (8%) to over $70,000 (34%). Among mothers, 91% were White, 3% Hispanic, 1% each African American, Asian, Pacific Islander, and 3% “other” non-White. Among fathers, 83% were White, 8% Hispanic, 3% African American, 3% Asian, and 3% “other” or missing. In 20% of families, one or both parents were non-White. This research complied with all ethical principles, including informed consent, and it has been annually reviewed by the authors’ IRB.
The longitudinal study included multiple assessments during lengthy home and laboratory sessions: at Time 1, when children were 7 months (N = 102, 51 girls); at Time 2, 15 months (N = 101, 51 girls), at Time 3, 25 months (N = 100, 50 girls), at Time 4, 38 months (N = 100, 50 girls); at Time 5, 52 months (N = 99, 49 girls). Only mother-child data are presented here.
The sessions were videotaped for later behavioral coding. Independent teams coded different measures. Typically, 15%-20% of cases were used for reliability, with more used for rare codes. The coders realigned periodically to prevent drift. The measures were aggregated at multiple levels to assure their robustness (Rushton, Brainerd, & Pressley, 1983).
Assessment of Children’s Attachment Security, 15 Months
Paradigm
The standard Strange Situation was conducted at the beginning of the session. It was coded by professional attachment coders at another university, blind to all information regarding the children.
Coding
Reliability, kappa, based on 20 randomly selected cases, was .78 for the four attachment categories (secure, avoidant, resistant, disorganized/unclassifiable) and .85 for the coding of secure versus insecure attachment. All cases coded with low confidence by one coder and all disorganized/unclassifiable cases were double-coded and adjudicated.
Genotyping: Assessment of 5-HTTLPR Status, 52 Months
Mothers of 89 children agreed to participate in this assessment. DNA was obtained using buccal swabs and genotype at the 5HTTLPR was determined for each sample (Barry, Kochanska, & Philibert, in press; Bradley, Dodelzon, Sandhu, & Philibert, 2005; Philibert et al., 2002). Eighty eight of 89 samples were successfully genotyped. There were 13 ss homozygotes (3 girls, 10 boys), 47 sl heterozygotes (23 girls, 24 boys), and 28 ll homozygotes (18 girls, 10 boys). Hardy Weinberg equilibrium testing was non-significant (p < 0.66). The difference in gender distribution across different genotypes, ss/sl vs. ll was not significant, χ2 = 3.35, df = 1, p < .10.
There were 48 secure and 40 insecure children (12 avoidant, 16 resistant, and 12 disorganized/unclassifiable). There were no significant differences in the distribution of security vs. insecurity across gender, χ2 = 2.22, df = 1, ns. The difference in the distribution of security vs. insecurity across ss/sl and ll genotypes, however, was significant, χ2 = 6.93, df = 1, p < .01; among insecure children, 33 had ss/sl genotype, and 7 were ll homozygotes, whereas among secure children, 27 had ss/sl genotype, and 21 were ll homozygotes.
Assessment of Children’s Self-Regulation, 25, 38, and 52 Months
Batteries of tasks, most of which were multi-trial to yield robust scores, were administered. They were interspersed with other contexts during the laboratory sessions. These batteries, developed in our laboratory, have been described in other articles (e.g., Kochanska, Coy, & Murray, 2001; Kochanska et al., 2000, 2007), and are widely used in the field. Thus, the description here will be brief (information is available upon request).
The tasks capture the following five inter-related core aspects of the child’s capacity to deliberately suppress a dominant response and to perform instead a sub-dominant response: delaying, slowing down gross and fine motor activity, suppressing/initiating activity to signal, lowering voice, and effortful attention. Not all functions were assessed at all ages; some tasks were repeated, and new tasks were added as permitted by the child’s increasing maturity. Every task was presented as a game rather than a prohibition or request, and the child was praised regardless of performance.
Battery at 25 months
There were five tasks. Four captured delaying (two tasks that required waiting to reach for an M&M placed under a cup, and in two tasks that required waiting to unwrap a gift) and one captured suppressing/initiating activity to signal (taking turns while building a block tower).
Battery at 38 months
There were nine tasks. Three delaying tasks involved waiting to reaching for M&M, deliberately choosing a prize from a box filled with small toys, and waiting to unwrap a gift). Two slowing-down tasks called for slowing motor activity (walking a 6-ft line; and guiding a toy turtle slowly along a curved path to the barn). One task called for suppressing or inhibiting a response to one type of signal and producing or initiating a response to another (a turn-taking game). Lowering voice was tapped in a whispering task. Two effortful attention (Stroop-like) tasks required ignoring a dominant perceptual feature of a stimulus for the sake of a subdominant feature, Day-Night and Snow-Grass (Carlson and Moses, 2001).
Battery at 52 months
There were 14 tasks. Five delaying tasks again involved waiting to reach for an M&M, waiting to chew an M&M placed on the child’s tongue, deliberately choosing a prize from a box filled with toys, and waiting to unwrap two gifts. Three slowing-down tasks called for slowing fine and gross motor activity (drawing lines, walking a line, and moving a toy toward a play barn). Three suppressing/initiating activity to signal tasks involved a go-no go response (to red and green signs, and to commands given by a toy bird and toy dragon), and turn-taking while building a block tower. Lowering voice was assessed in a whispering task. Two effortful attention (Stroop-like) tasks were Day-Night and Snow-Grass.
Coding and reliability
The codes were strongly behaviorally based and required little inference. For each trial, higher score reflected better capacity for self-regulation (coding manuals are available from the first author). The scores were then averaged across trials, where applicable. Reliability of coding was extremely high across all three ages and across many teams. Reliabilities, kappas, ranged from .71 to 1.00, and alphas ranged from .81 to 1.00.
Data aggregation
The scores were averaged across trials, where applicable. The individual task scores were then standardized and aggregated into self-regulation composites at 25, 38, and 52 months (Cronbach’s alphas .71, .67, and .72; means and standard deviations, −.01 and .66; −.01 and .52; −.01 and .53, respectively). Those composites were longitudinally stable, r‘s ranging from .37 to .57, all p‘s < .001, average r = .49, alpha = .73, and thus they were aggregated across 25, 38, and 52 months into an overall composite of self-regulation, M = −.01, SD = .46.
Results
Multiple regression analysis predicting self-regulation
First, we conducted a hierarchical multiple regression, where the overall composite of self-regulation across 25, 38, and 52 months was the dependent variable, attachment security (insecure vs. secure), genotype (5-HTTLPR status, homo-or heterozygotic for the short allele, ss/sl, versus homozygotic for the long allele, ll), and their interaction, attachment security × genotype, were the predictors, and child gender was the covariate. Table 1 presents the results.
Table 1.
Children’s attachment security to mothers at 15 months, 5-HTTLPR status, and their interaction as predictors of children’s self-regulation from 25 to 52 months
| Predictor(s) | Outcome: children’s overall self-regulation compositea |
|||||
|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | ||||
| F | Beta | F | Beta | F | Beta | |
| Child genderb | 13.97*** | −.37 | 9.83** | −.32 | 9.86** | −.31 |
| R2 = .14, F(1,86) = 13.97*** | ||||||
| Attachment securityc | 1.29 | .12 | 4.13* | .24 | ||
| 5-HTTLPR statusd | 3.48+ | .19 | 7.60** | .48 | ||
| R2 = .20, F(3,84) = 6.97*** | ||||||
| Attachment security ×5-HTTLPR status | 4.14* | −.40 | ||||
| R2 = .24, F(4,83) = 6.46*** | ||||||
Composite of self-regulation at 25, 38, and 52 months.
0 = Girls, 1 = Boys.
0 = Insecure, 1 = Secure.
0 = ss/sl, 1 = ll
p < .10.
p < .05.
p <.01.
p < .001.
In the final equation, girls had higher scores on the self-regulation composite; girls, M = .13, SD = .38, boys, M = −.15, SD = .50, consistent with many published studies (Bjorklund & Kipp, 1996). The effects of security and genotype were significant, but they were qualified by the significant security × genotype interaction, and thus should not be interpreted.
Follow-up tests of the interaction
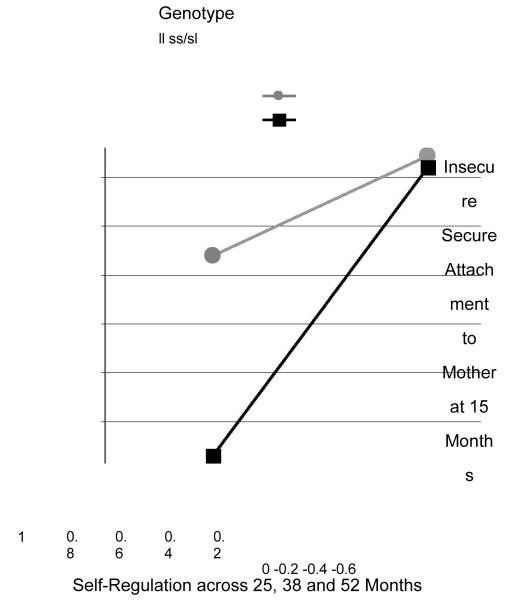
To probe the interaction effect, we performed tests of the simple slopes (Aiken & West, 1991). The results indicated that the effect of security on children’s self-regulation was significant for children with high-risk genotypes, ss/sl, b=1.18,SE= .50, p < .02, but not for children with low-risk genotypes, ll, b = .41, SE = 1.56, ns. As depicted in Figure 1, among children who carried a short 5HTTLPR allele (ss/sl), those who were insecurely attached developed poor regulatory capacities, but those who were securely attached developed as good regulatory capacities as children who were homozygotic for the long allele (ll). There was no effect of security for ll homozygotes.
Figure 1.
Security of infants’ attachment to mother at 15 months moderates the effect of infants’ 5-HTTLPR genotypes on the overall self-regulation composite across 25, 38 and 52 months (simple slopes of attachment security on ss/sl and ll children’s self-regulation).
Discussion
This study bridges a molecular genetic approach with the science of relationships within a longitudinal design using extensive observational measures. The straightforward findings dovetail with the extant human and non-human research, inform the debate on G × E interactions in human development, and have implications for research on risk and resilience. The expected significant G × E interaction was supported. Children’s attachment security moderated the effect of their genotypes: 5-HTTLPR polymorphism (specifically, having a short allele, ss or sl) was associated with a diminished self-regulatory capacity from age 2 to 4 ½. That risk, however, was significant only for children who had been insecurely attached to their mothers at the end of the first year, and it was absent for children who had been securely attached. As Suomi (2006) proposed, secure attachment indeed served as a buffer against risk otherwise conferred by the child’s genotype.
In addition to the analyses for the overall composite of self-regulation across 25, 38, and 52 months, we also examined parallel equations predicting self-regulation scores at each of those assessments. The standardized regression coefficients for the G × E interaction terms were, respectively, −.29, −.28, and −.40. Although, as often happens when composite measures are disaggregated (Rushton et al., 1983), only the last of the three reached significance, p < .05, nevertheless those separate data suggest that a similar process operated across the studied age range. Consequently, given also the considerable developmental stability of the self-regulation construct, we presented the most robust analyses for the trait-like self-regulation composite from 25 to 52 months. Future research on G × E interactions regarding other developmental constructs should not, however, preclude a possibility that those effects may appear in only some, and not all, developmental periods.
Our findings appear more consistent with the genetic vulnerability hypothesis than with the differential susceptibility hypothesis (Belsky, 2005; Belsky et al., 2007). Children with high-risk genotypes who had formed secure attachment with their mothers had as good --but not better --selfregulatory capacity as children with low-risk genotypes. Security seemed to function as a buffer against the risk conferred by having the short 5-HTT allele.
Future studies need to address the causal mechanisms that account for those effects. One such potential mechanism involves a possibility that security is particularly consequential for children with high-risk genotypes, because it can enhance (or hinder) effective emotional arousal modulation in individuals who are genetically less well equipped to handle this task (Herrmann et al., 2007; Hofer, 1987). In contrast, children with low-risk genotypes possess more effective physiological regulation capacities, and for them, relationship-based supports are less important. This may be a promising future direction of research.
Limitations of this study include a relatively small sample, and the findings should be replicated in a larger group of families. Furthermore, the contemporary developmental psychopathology approach calls for the study of risk and protective factors in both typically developing and atypical populations, whereas in this study, the children were normally developing and the families were generally well functioning. Most measures likely fell mostly within the adaptive range and their variability was relatively constrained, although the sample did include sufficient numbers of secure and insecure children to permit the analyses, consistent with the distribution of security reported in the review by van IJzendoorn, Schuengel, and Bakermans–Kranenburg (1999) for “normal US samples, age < 24 months” (p. 230). Yet even so, the findings were significant. The studied effects are likely to be amplified in children and families at a higher risk, for example, families where early parent-child relationships are grossly sub-optimal (Kaufman et al., 2004).
Conclusion
The findings inform both basic and translational research on emotional development. They support diathesis-stress or dual-risk models by demonstrating an interaction between genotype and environment in children’s development of effective self-regulation, a critical skill implicated in modulating emotion expression and exercising restraint over immediate desires. The results support attachment scholars’ views that early security provides a foundation and scaffolding for the child’s self-regulation (Hofer, 1994; Schore, 2001; Sroufe, 1996, 2005). They additionally reveal that the role of security is particularly significant for children whose genotypes may put them at risk for self-control deficiencies. Early security can be seen as a critical protective factor that can offset or buffer developmental risks conferred by genetics, consistent with several bodies of animal and adult human research.
Research that bridges molecular genetics and social relationships to explain adaptive and maladaptive developmental pathways is only beginning to blossom. That research undoubtedly holds promise for a fuller understanding of the complex nature of adaptive and maladaptive pathways in emotional and social development.
Growing evidence documents genotype by environment (G × E) interactions for adaptive and maladaptive developmental outcomes.
Polymorphism in the serotonin transporter gene, 5-HTTLPR (ss/sl vs. ll allele status), specifically, having a short allele (ss/sl), has been linked to deficits of self-regulation and other forms of psychopathology. Environment has been shown to moderate that link, such that individuals who carry a short allele develop poor outcomes when they also experience sub-optimal or stressful environmental conditions.
This longitudinal multi-method study found that among ss/sl children, those who as infants had been insecurely attached to their mothers developed poor regulatory capacities in toddler and preschool years, but those who had been securely attached developed as good regulatory capacities as children homozygotic for the long allele (ll). There was no effect of security for ll homozygotes.
The findings, consistent with diathesis-stress model, indicate that secure attachment can serve as a protective factor in the presence of risk conferred by a genotype.
ACKNOWLEDGMENTS
This research has been funded by the grants from NIMH, RO1 MH63096 and KO2 MH01446 to Grazyna Kochanska, who was also supported by the Stuit Professorship, and by RO1 DA015789 to Robert A. Philibert. We thank many students and staff, including Nazan Aksan, Lea Boldt, Amanda Hollatz, Sara Penney, Theresa Prisco, Sarah Stellern, and Jarilyn Woodard for help with data collection and coding, Dianna Edwards for help with genotyping, and the participants in Family Study for their enthusiastic commitment to this research.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury, CA: 1991. [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promotor gene (5-HTTLPR) with temperament in 12-month-old infants. The Journal of Child Psychology and Psychiatry. 2001;6:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Archives of General Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barry RA, Kochanska G, Philibert RA. G × E interactions in the organization of attachment: Mothers’ responsiveness as a moderator of children’s genotypes. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2008.01935.x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JE, Pettit GS, Dodge KA, Ridge B. Interaction of temperamental resistance to control and restrictive parenting in the development of externalizing behavior. Developmental Psychology. 1998;34:982–995. doi: 10.1037//0012-1649.34.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Theory testing, effect-size evaluation, and differential susceptibility to rearing influence: The case of mothering and attachment. Child Development. 1997;68:598–600. [PubMed] [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3: Differential susceptibility to rearing influence? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Bjorklund DF, Kipp K. Parental investment theory and gender Differences in the evolution of inhibition mechanisms. Psychological Bulletin. 1996;120:163–188. doi: 10.1037/0033-2909.120.2.163. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. The relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136B:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cognitive Development. 2007;22:489–510. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. Journal of Personality & Social Psychology. 2000;78:136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: Sharpening the definition. Child Development. 2004;75:334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Kochanska G. Temperament as a moderator of pathways to conscience in children: The contribution of electrodermal activity. Psychophysiology. 2000;37:788–795. [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The Development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Genetics of emotional development. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. Oxford University Press; New York, NY: 2003. pp. 295–408. [Google Scholar]
- Goldsmith HH, Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Developmental Psychology. 1999;35:972–985. [PubMed] [Google Scholar]
- Herrmann MJ, Huter T, Muller F, Muhlberger A, Pauli P, Reif A, Renner T, Canli T, Fallgatter AJ, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cerebral Cortex. 2007;17:1160–1163. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: a psychobiologist’s view. Child Development. 1987;58:633–647. [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators in attachment, separation, and loss. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. 2-3. Vol. 59. 1994. pp. 192–207. (Monographs of the Society for Research in Child Development). Serial No. 240. [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor–5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kieras JE, Tobin RM, Graziano WG, Rothbart MK. You can’t always get what you want: Effortful control and children’s responses to undesirable gifts. Psychological Science. 2005;16:391–396. doi: 10.1111/j.0956-7976.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N, Joy ME. Children’s fearfulness as a moderator of parenting in early socialization: Two longitudinal studies. Developmental Psychology. 2007;43:222–237. doi: 10.1037/0012-1649.43.1.222. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N, Penney SJ, Doobay AF. Early positive emotionality as a heterogeneous trait: Implications for children’s self-regulation. Journal of Personality and Social Psychology. 2007;93:1054–1066. doi: 10.1037/0022-3514.93.6.1054. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Development. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25:343–354. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maccoby EE. Historical overview of socialization research and theory. In: Grusec JE, Hastings PD, editors. Handbook of socialization: Theory and research. Guilford Press; New York: 2007. pp. 13–41. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Philibert R, Caspers K, Langbehn D, Troughton EP, Yucuis R, Sandhu HK, Cadoret RJ. The association of a HOPA polymorphism with major depression and phobia. Comprehensive Psychiatry. 2002;43:404–410. doi: 10.1053/comp.2002.33489. [DOI] [PubMed] [Google Scholar]
- Posner MI. Genes and experience shape brain networks of conscious Control. In: Laureys S, editor. The Boundaries of consciousness: Neurobiology and neuropathology progress in brain research. Elsevier; Boston, MA: 2005. pp. 173–183. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Developmental Science. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Thomas KM, Levy KN, Fossella J, et al. An approach to the psychobiology of personality disorders. Development and Psychopathology. 2003;15:1093–1106. doi: 10.1017/s0954579403000506. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner RM, Eisenberg N, editors. Handbook of Child Psychology. Wiley; New York: 2006. pp. 99–166. Social, emotional, and personality development. [Google Scholar]
- Rushton JP, Brainerd CJ, Pressley M. Behavioral development and construct validity: The principle of aggregation. Psychological Bulletin. 1983;94:18–38. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schore AN. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal. 2001;22:7–66. [Google Scholar]
- Shonkoff JP, Phillips DA. From neurons to neighborhoods: The science of early childhood development. National Academy Press; Washington, DC, US: 2000. [PubMed] [Google Scholar]
- Sroufe LA. Emotional development: The organization of emotional life in the early years. Cambridge University Press; New York: 1996. [Google Scholar]
- Sroufe LA. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attachment & Human Development. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. How gene-environment interactions shape biobehavioral development: Lessons from studies with rhesus monkeys. Research in Human Development. 2004;1:205–222. [Google Scholar]
- Suomi SJ. Genetic and environmental factors influencing the expression of impulsive aggression and serotonergic functioning in rhesus monkeys. In: Tremblay RE, Hartup WW, Archer J, editors. Developmental origins of aggression. Guilford Press; New York: 2005. pp. 63–82. [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Sourbrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986;9:319–335. [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: Meta-analysis of precursors, concomitants, and sequelae. Development and Psychopathology. 1999;11:225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]