Abstract
Astrocytes play a critical role in maintaining cerebral homeostasis and their dysregulation is thought to contribute to the pathogenesis of several diseases, including brain cancer and metastasis. Similar to the human disease, we found that lung and melanoma metastases in the mouse brain are accompanied by a reactive gliosis. To begin to study the biology of astrocytes and examine how these cells might contribute to metastasis formation and progression in the brain, we generated a conditionally immortal astrocyte cell line from H-2Kb-tsA58 mice. Astrocytes grown in culture expressed glial fibrillary acid protein (GFAP), glutamate receptor 1, and the N-methyl-d-aspartate (NMDA) receptor. Astrocytes also expressed glial-specific transporters excitatory amino acid transporter 1 (EAAT1) and EAAT2. Astrocytes grown under permissive conditions (33°C) expressed SV40 large T antigen and had a doubling time of 36 hours, whereas expression of SV40 large T antigen was negligible in astrocytes grown at 37°C for 72 hours, which coincided with a plateau in cell division. In a co-culture assay with human lung adenocarcinoma cells (PC14-PE6), astrocytes activated programs in the tumor cells that signal for cell division and survival. Hence, the immortalized cell line will be useful for studying the role of astrocytes in disease processes in the brain, such as metastasis.
Keywords: brain metastasis, astrocyte, MAPK, macrophage, GFAP
Introduction
Astrocytes contribute to cerebral homeostasis by lending support to the blood-brain barrier (1,2), regulating the central nervous system (CNS) response to inflammation (3), and participating in synaptic transmission (4). In addition, recent studies have shown that the signaling that takes place between astrocytes and neurons plays an important role in modulating local blood flow in the brain (5,6). Emerging evidence suggests that astrocytes may also be involved in the initiation and propagation of several neurological disorders. Indeed, to date, astrocytes have been implicated in the pathogenesis of multiple sclerosis (7), amyotrophic lateral sclerosis (8,9), epilepsy (10), clinical pain syndromes (11), and other human CNS diseases (12). Given their important regulatory role in both health and disease processes, it is not surprising that astrocytes have been the focus of considerable investigative effort.
Much of our understanding of astrocyte biology is derived from in vitro examinations performed on human (13) and rodent (14) astrocytes. Studies of astrocytes in cell culture have provided investigators with an opportunity to predict astrocyte gene expression in the brain (15), to identify factors that regulate astrocytic expression of cell adhesion molecules (16) and chemokines (17), and to establish in vitro models of the blood-brain barrier that can be used for CNS drug discovery and development (18). The concept of the blood-brain barrier has evolved over recent years to include several different cell types, such as neurons, pericytes, astrocytes, and microvascular endothelial cells, that collaborate to form a “neurovascular unit” (19). When modeling the blood-brain barrier in vitro, investigator’s frequently rely on cerebral microvascular endothelial cells that have been transfected with plasmids coding for the simian virus 40 (SV40) large T antigen (20,21). Perhaps two of the greatest advantages of using immortalized cell lines are that they greatly reduce the frequency with which stock cultures need to be replenished, and they may also minimize some of the phenotypic drift that tends to occur in cells that are maintained in cell culture for extended periods of time.
Recently, our laboratory generated a number of conditionally immortal microvascular endothelial cell lines from transgenic mice that harbor a temperature-sensitive SV40 large T antigen (H-2Kb-tsA58 mice) (22). This mouse line appears to provide an improved source of immortalized cells inasmuch as it overcomes many of the limitations associated with the in vitro transfection process (e.g., initial requirement for large number of cells, different sites of gene integration, multiple copy number) (22,23). The presence of the thermolabile large T antigen in these cells also provides the investigator with a means with which to control the level of cell differentiation (22). Moreover, the immortalized cell lines generated in our laboratory have significantly contributed to our studies of the tumor blood supply by providing us with a resource to define the molecular basis for different anti-angiogenic therapies (24,25), identify new factors that encourage the vascularization of tumors (26), and to precisely model the tumor-associated blood vasculature in vitro (27).
In the present report, we wanted to establish a conditionally immortal astrocyte cell line from H-2Kb-tsA58 mice that could be utilized in conjunction with the existing brain endothelial cell line and provide us with a working model of the blood-brain barrier. The generation of an astrocyte cell line could also be used to study any reciprocal signaling that takes place between astrocytes and tumor cells, and these investigations could potentially lead to the identification of new targets for anti-cancer therapy. The methodology utilized to generate immortal astrocyte cells from H-2Kb-tsA58 mice is described below.
Materials and Methods
Reagents
The following antibodies were titrated and used in this study: anti-GFAP from Biocare Medical (Concord, CA); anti-EAATI (H-50), anti-EAAT2 (H-85), anti-glutamate receptor-1 (sc-28799), and N-methyl-D-aspartic acid (NMDA) receptor epsilon 1 (sc-9056) from Santa Cruz Biotechnology (Santa Cruz, CA); SV40 large T antigen (Oncogene Research, San Diego, CA); phospho-Akt (Ser-473), Akt, phospho-ERK1/2 (Thr-202/Tyr-204), P44/42 (Cell Signaling Technology, Beverley, MA); β-actin (Sigma, St. Louis, MO); CD68 from Serotech (Raleigh, North Carolina). Secondary reagents included a peroxidase-conjugated goat anti-rabbit IgG F(abV)2, Cy3 conjugated goat anti-rabbit IgG, Cy5 conjugated goat anti-rat IgG from Jackson ImmunoResearch Laboratories (West Grove, PA) and streptavidin-conjugated Alexa 594 fluorescent IgG from Molecular Probes (Eugene, OR). A tyramide signal amplification reagent (NEN Life Science Products, Boston, MA) was also used in the study according to the manufacturer’s instructions.
Animals
Neonatal mice homozygous for a temperature-sensitive SV40 large T antigen (H-2KbtsA58 mice: CBA/ca × C57Bl/10 hybrid; Charles River Laboratories, Wilmington, MA), were used to isolate astrocytes. Male athymic nude mice (NCI-nu) and C3H mice were purchased from the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). All mice were housed and maintained in pathogen-free conditions in accordance with institutional guidelines and the current regulations of the National Institutes of Health, the United States Department of Health and Human Services, and the United States Department of Agriculture.
Tumor Cell Lines and Culture Conditions
PC14-PE6 human lung adenocarcinoma cells (28) and K-1735 murine melanoma cells (29) were maintained in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), non-essential amino acids, l-glutamine, a two-fold vitamin solution (all from Life Technologies, Grand Island, NY), and a penicillin/streptomycin mixture (Flow Laboratories, Rockville, MD). Cultures were maintained at 37°C in 5% CO2 and 95% air and determined to be free of Mycoplasma and pathogenic murine viruses (assayed by Scientific Applications International Co., Frederick, MD).
Experimental Brain Metastasis Model
Examinations performed on human brain tissue revealed that reactive gliosis is a hallmark of brain metastasis (30). To determine if the same phenomenon is a characteristic of brain metastases in mice, we injected 10 nude mice with PC14-PE6 lung adenocarcinoma cells and 10 C3H mice with K-1735 melanoma cells via the common carotid artery. In brief, mice were anesthetized with methoxyflurane and the skin was prepared for surgery in standard fashion. The mice were placed in the supine position and viewed under a dissecting microscope. The carotid artery was prepared for injection distal to the point of division into the internal and external carotid arteries. A ligature of 4-0 silk suture was placed in the distal part of the common carotid artery. A second ligature was placed and loosely tied proximal to the injection site. A sterile cotton tip applicator was inserted under the artery just distal to the injection site to elevate the carotid artery and to prevent any bleeding from the carotid artery that may occur as a result of retrograde flow. A small incision was made in the artery and a 30-gauge glass cannula was inserted into the lumen. In both PC14-PE6 and K-1735 tumor models, 2.5 × 105 cancer cells in 100-µl of PBS was injected slowly into the common carotid artery and at this time the second ligature was tightened and the skin closed with sutures. 7 Three mice from each tumor group were killed 7 days after tumor cell injection, three were killed 14 days after injection, and four mice were killed 28 days after tumor cell injection. Brain tissue was collected and placed into OCT compound (Miles Laboratories, Elkhart, IN) to be snap-frozen in liquid nitrogen. The presence of visible brain metastases was confirmed by histology.
Astrocyte Isolation
Neonatal mice were euthanised in a carbon dioxide chamber and the skin was scrubbed with betadyne solution. Sterile microforceps (Roboz Surgical Instrument Co., Gaithersburg, MD) were used to remove the skin from the skull and microscissors were used to create a circular posterior incision from the opening of the left ear to the opening of the right ear. Another incision was made along the brain midline and the skull was divided to allow access to the cranial cavity. The optic nerves were clipped and the brain removed with a blunt forceps and placed into 100-mm ice-cold phosphate buffered saline (PBS). Whole neocortices were dissected and the hippocampus and internal structures were removed to leave only the cortical sheets. The meninges were stripped away, and the cortical sheets were minced with a scalpel and digested for 30 min at 37°C in Dulbecco’s modified essential media (DMEM) containing 0.1% collagenase (150 U/ml; Worthington Biochemical Corp., Lakewood, NJ) and 40 µg/ml deoxyribonuclease (Sigma Chemical Co., St. Louis, MO). The cortical tissue was then triturated in DMEM containing 10% FBS and filtered through a 50-µm sterile mesh. The resulting single-cell suspension was plated onto 75 cm2 tissue culture flasks that had been previously coated with 5 µg/ml mouse laminin (Sigma). The cells were allowed to grow for 7 days in DMEM containing 10% FBS and supplements (see above) in an atmosphere of 8% CO2 (to achieve proper buffering of pH at 33°C). At this time, astroglial cells formed a confluent monolayer with neurons, oligodendrocytes, and fibroblasts growing on top. These contaminating cells were removed by rotary shaking the flasks overnight at 250 revolutions per minute in a warm room. The resulting cultures were composed of more than 95% astrocytes as determined by immunoreactivity with antibodies directed against GFAP. These cultures were expanded and the procedure was repeated and the percentage of astrocytes in these cultures was determined to exceed 99%.
Double Immunofluorescence Staining for CD68 and GFAP and Confocal Imaging of Brain Metastases
Frozen tissues were used to identify populations of cells that expressed GFAP and CD68. The tissues were sectioned (8–10 µm), mounted on positively charged slides, and air-dried for 30 min. Tissue fixation was carried out by subjecting the tissues to three sequential immersions in ice-cold solutions containing acetone, acetone-chloroform (50:50 v/v), and acetone (5 min each). The samples were blocked briefly in 5% normal horse serum and 1% goat serum solution and incubated with antibody directed against GFAP at 4°C overnight. The slides were washed three times in PBS and then incubated with Cy3-conjugated goat anti-rabbit antibody for 1 h at room temperature. The samples were washed three times in PBS and then incubated with antibody targeted against CD68 at 4°C overnight. The samples were rinsed in PBS and then incubated with Cy5-conjugated corresponding secondary antibody for 1 h. Samples were rinsed and briefly incubated with Sytox green (Molecular Probes) to visualize the cell nuclei. The slides were mounted with a glycerol/PBS solution containing 0.1M propyl gallate (Sigma) to minimize fluorescent bleaching and covered with a glass coverslip (Fischer Scientific, Pittsburg, PA).
Confocal fluorescence images were collected using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss, Thornwood, NY) equipped with an argon laser (458/477/488/514 nm, 30 mW), HeNe lasers (543 nm, 1mW and 633 nm, 5 mW), LSM510 control and image acquisition software, and appropriate filters (Chroma Technology Corp., Brattleboro, VT). Collected images were exported to Adobe Photoshop software and prepared for publication. Astrocytes were identified by blue fluorescence, while CD68-positive cells were identified by red fluorescence.
Immunohistochemical Analysis of Astrocytes in Cell Culture
The expression of GFAP, glutamate receptors (glutatmate receptor 1, NMDA receptor) and glutamate transporters (EAAT1 and EAAT2) was determined by immunofluorescence staining. Astrocytes were plated onto four-chamber slides at a density of 3 × 104 cells/well in 10% DMEM and allowed to incubate overnight at 37°C. At this time, the incubation media was aspirated and replaced with DMEM containing 10% FBS for 72 h. The cells were washed once and then fixed in acetone for 15 min. The slides were blocked in PBS containing 5% normal horse serum and 1% normal goat serum. The slides were incubated in primary antibody (all primary antibodies used at 1:100 dilution) overnight at 4°C, rinsed three times with PBS, and the sample coated in protein blocking solution for 10 min. A peroxidase-conjugated antibody that reacted with the primary antibody was then added for 45 min. The slides were rinsed and then incubated in a biotin tyramide solution for 10 min, washed twice, and incubated for 45 min in streptavidin-conjugated Alexa fluorescent 594. Slides were washed twice in PBS and the cell nuclei stained with Hoechst 33342 (Polysciences, Inc., Warrington, PA) for 2 min followed by washing in PBS. Immunofluorescence microscopy was performed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 100-W Hg-lamp and narrow band pass excitation filters (Chroma Technology Corp., Brattleboro, VT). Images were captured with a cooled charged coupled device Hamamatsu C5810 camera (Hamamatsu Photonics K.K., Bridgewater, NJ) and ImagePro software (Media Cybernetics Inc., Silver Spring, MD). Composite photographs were made using Adobe PhotoShop software.
Western Blot Analysis
The kinetics of expression of the temperature-sensitive SV40 large T antigen were measured as described previously (25). In brief, 4 × 105 astrocytes were plated onto a series of 10-cm culture dishes in DMEM containing 10% FBS and placed in an incubator adjusted for 33°C for 72 h, or an incubator adjusted for 37°C for 24, 48, or 72 h. At each time point, the cells were washed with buffer [20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, 20 µM leupeptin, and 0.15 U/ml aprontinin]. Protein concentrations were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA), and 50 µg of total protein resolved in 10% SDS-PAGE under reducing conditions were transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% (w/v) non-fat dried milk in 0.1% Tween 20 (Sigma) in PBS for 1 h and then incubated overnight at 4°C with an antibody directed against the SV40 large T antigen. Immunodetection was performed using the corresponding secondary horseradish peroxidase-conjugated antibodies. Horseradish peroxidase activity was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Determination of Doubling Time
Astrocytes were plated onto 96-well plates at a density of 1000 cells/well in DMEM containing 10% FBS. Cell growth was evaluated under both permissive (33°C) and non-permissive (37°C) conditions. The proliferative activity was determined every 24 h by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenol-tetrazolium bromide assay using a Dynatech MR-5000 96-well microtiter plate reader set at 570 nm. Increase in absorbance was considered a measure of cell proliferation.31
Astroycte/PC14-PE6 Tumor Cell Co-Culture
Astrocytes were plated at a density of 4 × 105 cells in 1 ml of DMEM containing 10% FBS onto sterile 0.4 µm cell culture inserts (Becton Dickinson Labware, Franklin Lakes, NJ). The inserts were gently transferred to empty 6-well plates and placed into a 37°C incubator. PC14-PE6 lung adenocarcinoma cells were plated in 6-well plates at a density of 5 × 105 cells/well in 2 mls of DMEM containing 10% FBS. After an overnight incubation, the medium from astrocytes and tumor cells was aspirated and replaced with serum-free medium. The astrocyte containing inserts were then transferred to the 6-well plates of tumor cells and the two cell types were allowed to co-incubate for either 24 h or 48 h. Control samples consisted of cell-free inserts placed into 6-well plates that contained tumor cells. At each time point the inserts were discarded and the tumor cells were washed with ice-cold PBS and lysed with buffer. The extent of Akt and ERK1/2 phosphorylation was evaluated by Western analysis.
Results
Expression of GFAP and CD68 in Experimental Lung Adenocarcinoma (PC14-PE6) and Melanoma (K-1735) Brain Metastases
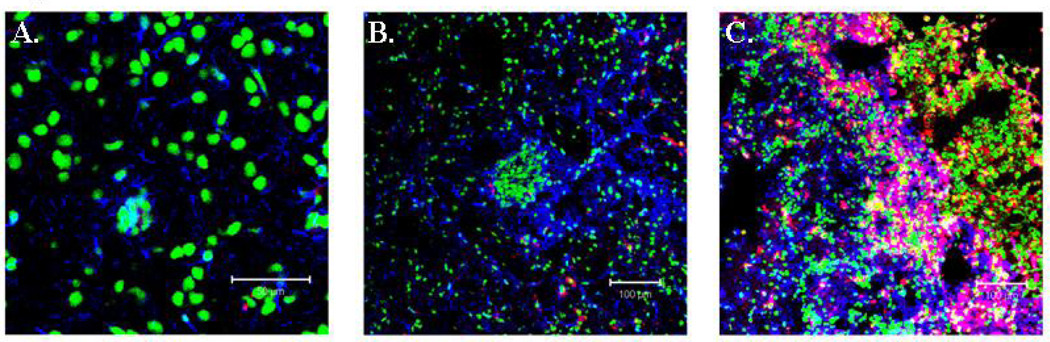
Routine staining performed on a large series of human brain metastases cases indicate that reactive astrogliosis is a distinctive feature of the disease (30). To determine whether reactive gliosis is characteristic of metastases in the mouse brain, we injected nude mice with human PC14-PE6 lung adenocarcinomas cells and C3H mice with K-1735 mouse melanoma cells into the common carotid artery of mice. Mice were divided into three groups and were euthanized at either 7, 14, or 28 days following the injection of tumor cells. The incidence of brain metastasis at each individual time point was 100%, as determined by routine histology in both of the experimental metastasis models (data not shown). Even in small developing metastases (Day 7 and Day 14), we observed reactive astrocytes adjacent to the tumor cells (Figure 1A and 1B). In contrast, no GFAP-positive astrocytes were detected in similar locations of the brain in non-tumor-bearing mice (data not shown). Reactive astrocytes were also observed at the tumor-brain interface in more advanced experimental metastases (Figure 1C).
Figure 1.
Immunofluorescent staining of GFAP and CD68 in PC14-PE6 lung adenocarcinoma brain metastases. Astrocytes are labeled with GFAP (blue) and macrophage cells are labeled with CD68 (red). (A) Reactive astrocytes are detected in small metastases. (B) More astrocytes become activated as the tumor cell population increases. Macrophages begin to localize to the tumor. (C) Reactive astrocytosis in large metastasis. Macrophage trafficking to metastases is considerably increased.
CD68 is a widely used marker for the identification of macrophages and microglia (32). In order to determine the extent that these two cell populations localized to brain metastases, we stained sections with anti-CD68 antibodies. Only an occassional CD68 positive cell was detected in small metastases (Figure 1A and 1B). However, we noted a significant increase in the number of CD68 cells at the tumor border in more advanced tumors (Figure 1C). Astrocytes did not label with the CD68 antibody. Morphologically, microglial cells are characterized by a scant cytoplasm and a number of highly branched processes and while we did detect an occasional cell that fit this description, the majority of the CD68-positive cells possessed a round structure. Based on this observation, we concluded that the majority of the CD68 positive cells in the mouse brain metastases were macrophages that were recruited from the peripheral circulation.
Immunohistochemical Analysis of Isolated Immortal Astrocytes
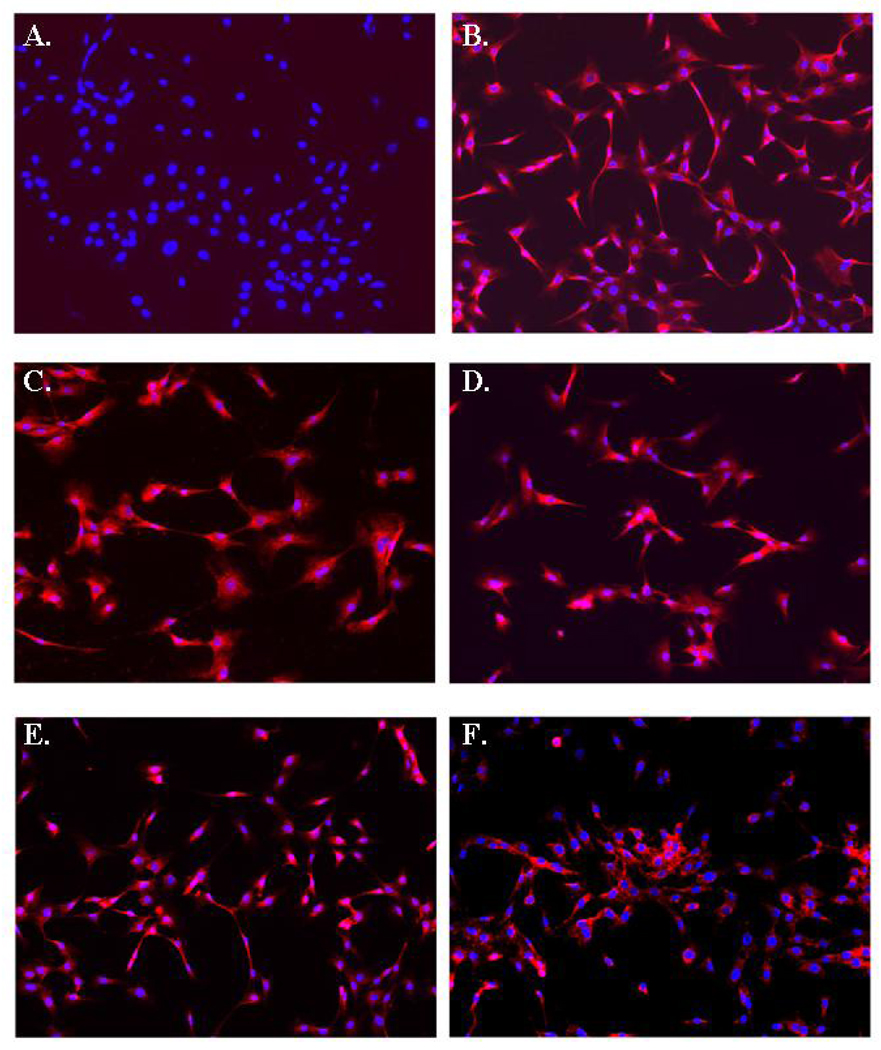
Immunofluorescence staining was used to assess the expression of GFAP, glutamate receptors (glutatmate receptor 1, NMDA receptor) and glutamate transporters (EAAT1 and EAAT2) (Figure 2A–F). We noted that the experimental approach used for astrocyte selection produced a pure population of astrocytes based on the homogenous labeling for GFAP (Figure 2B). Indeed, only a rare cell failed to express high levels of GFAP and we estimated that more than 99% of the cells that had undergone the selection approach were astrocyte in origin. Similarly, we found that the astrocytes were uniformly positive for glutamate receptors (Figure 2C–D) and the glial-specific transporters EAAT1 and EAAT2 (Figure 2E–F).
Figure 2.
Immunohistochemical analyses of GFAP, Glutamate Receptor 1, NMDA Receptor, EAAT1, and EAAT2 on astrocytes isolated from H-2Kb-tsA58 mice. (A) Control labeling with secondary antibody only. (B) All astrocytes stain positive for GFAP expression. (C) Homogenous staining was observed for glutamate receptor 1 and (D) NMDA receptor. (E) EAAT1 and (F) EAAT2 expression on cultured astrocytes. Original magnification × 200.
Astrocyte Growth Characteristics
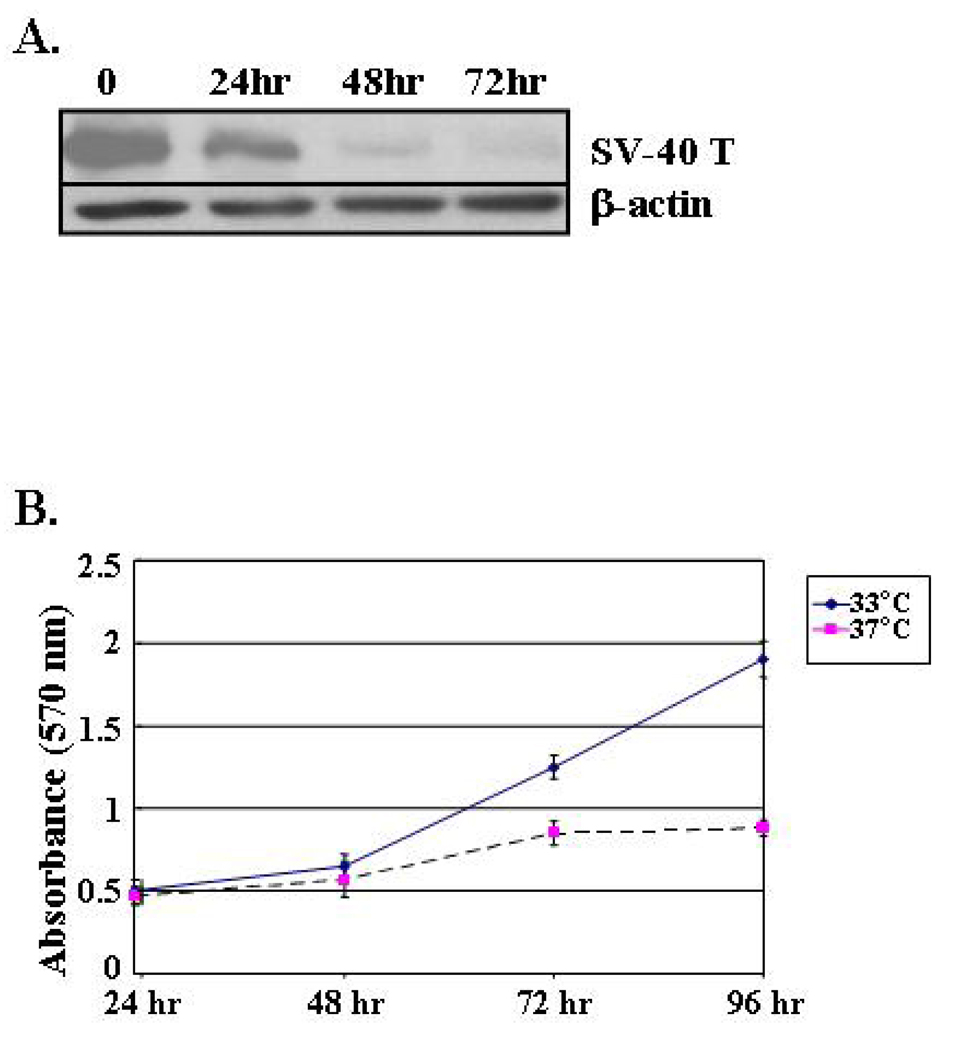
Western blot analysis was carried out to determine the kinetics of SV40 large T antigen in the immortalized astrocytes. Astrocytes growing under the permissive 33° temperature expressed robust levels of the SV40 large T antigen (Figure 3A). Expression of the SV40 large T antigen decreased in a time-dependent manner in astrocytes that were placed in a 37°C incubator and levels were negligible after 72 h of culture (Figure 3A). Astrocyte cell division paralleled expression of the SV40 large T antigen (Figure 3B). We noted that astrocytes growing in a 33°C environment remained in log phase growth and doubled their population in as little as 36 h. In contrast, astrocytes growing in 37°C conditions experienced a slight increase in number immediately after plating, but their growth started to plateau within 72 h.
Figure 3.
Cell proliferation of astrocytes growing in DMEM containing 10% FBS at 33°C (permissive) or 37°C (non-permissive) conditions. (A) Western blot analysis of SV40 large T antigen expression in H-2Kb-tsA58 mouse-derived astrocytes. Protein lysates were obtained at the indicated time points. β-actin is shown as a loading control. (B) Astrocytes were plated at a density of 1 × 103 cells/well in a 96-well plates and cell proliferation was determined in cells growing in 33°C and 37°C every 24 hours by MTT analysis.
Astroycte/PC14-PE6 Tumor Cell Co-Culture
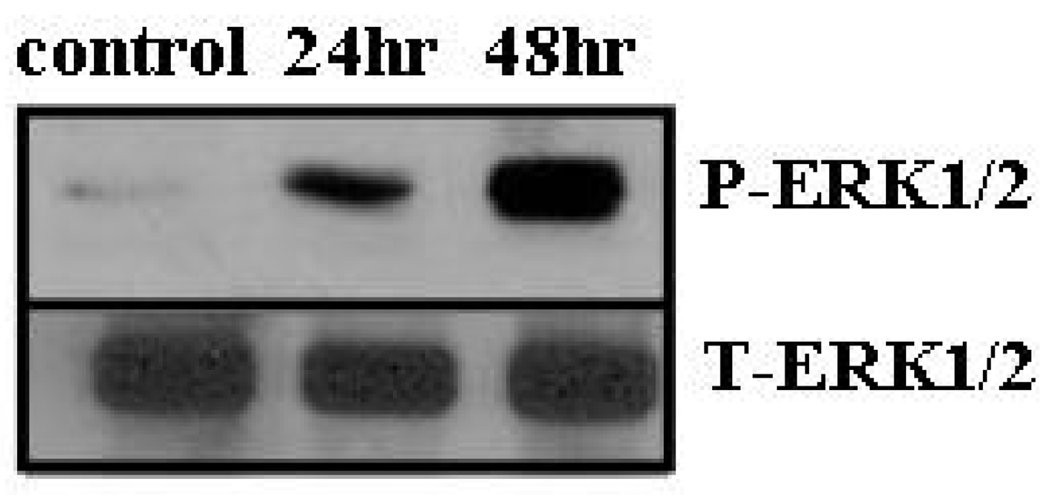
To determine if astrocytes could affect intracellular signaling pathways that regulate cell division and survival programs of tumor cells, we co-cultured astrocytes with PC14-PE6 lung cancer cells. We noted that ERK1/2 was phosphorylated on tumor cells that had been co-cultured with astrocytes for a period of 24 h or 48 h (Figure 4). In contrast, no phosphorylation was evident on lung tumor cells that were cultured with empty inserts. Akt was constitutively phosphorylated in PC14-PE6 cells that were cultured with inserts only and expression did not increase in PC14-PE6 cells that were cultured with astrocytes.
Figure 4.
Expression of phosphorylated ERK1/2 on PC14-PE6 lung adenocarcinoma cells that are co-cultured with mouse astrocytes. Astrocytes were plated in the upper compartment of transwell chambers and then co-incubated with PC14-PE6 cancer cells for either 24 or 48 hours. Control samples consisted of tumor cells incubated with empty inserts. At each time point, the chambers containing astrocytes were discarded and the tumor cells were lysed and examined by Western blotting analysis.
Discussion
The regulatory role of astrocytes in maintaining the constancy of the CNS has been appreciated for some time now, and more recent investigations of astrocytes have been directed toward determining the contribution of these cells to the pathogenesis of disease in the brain. Considerable information has been generated from examinations conducted on astrocytes growing in cell culture. The objective of this study was to establish a conditionally immortalized astrocyte cell line from H-2Kb-tsA58 mice that could be used for future studies designed to determine the role of astrocytes in the progression of brain metastasis.
The results from our experimental brain metastasis studies show that astrocytes become activated in response to very small tumor foci (Figure 1A), and that the number of reactive astrocytes associated with a given tumor is directly related to the size of the tumor (Figure 1B). As the size of metastasis increases, a population of CD68-positive macrophages joins the astrocytes, and both cell types can be observed congregating at the interface between the tumor and normal tissue (Figure 1C). Very limited information exists concerning the cellular and molecular mechanisms that promote astrocyte activation in response to tumor cells. However, there are some data in the literature to suggest that the stimuli that provoke mechanical disturbances (e.g., stretch) in the brain are responsible for eliciting reactive gliosis (33). Thus, one possibility exists that astrocyte mechanoreceptors sense the physical distortion caused by an expanding tumor mass and respond by becoming activated and upregulating expression of GFAP. In any event, the consequences of astrocyte reactivity to metastasis progression remain unclear. Based on the in vivo data, we determined that the generation of an immortalized astrocyte cell line could prove valuable in improving our understanding of the role, if any, of astrocytes in the progression of brain metastasis.
As previously mentioned, one of the primary motivations for employing the H-2Kb-tsA58 mouse line for our astrocyte isolation strategy was that the temperature-sensitive large T antigen in these cells allows the user to control the level of cell differentiation (22). In this regard, we noted that the rate of astrocyte cell division is closely coupled with expression levels of the SV40 large T antigen. For example, SV40 large T antigen expression is maximal in cells growing at 33°C, and the cells are in log phase growth. However, expression of the SV40 large T antigen declines dramatically in cells that are subjected to three days in a 37°C environment and this time point coincides with a plateau in cell division. The alterations in astrocyte SV40 large T antigen expression in response to changes in temperature are virtually identical to those reported on a lymphatic endothelial cell line from the same animal (25). The results of these experiments indicate that the immortal astrocyte cell line may be useful for examining rapidly dividing astrocytes, as well as astrocytes that are in a more differentiated state.
Immunohistochemical staining for GFAP on the cell isolates suggested that the methodology that we employed for cell selection yields a pure population of astrocytes. GFAP is the major intermediate filament protein in the CNS of adults that lack any underlying cerebral pathology, and expression levels are enhanced in response to a variety of neurological disorders (34). In fact, overexpression of GFAP by astrocytes is considered to be one of the hallmarks of reactive gliosis (34). We also found that the astrocytes exhibited homogenous expression of glutamate receptors (GluR1 and NMDA) and transporters (EAAT1 and EAAT2). Astrocytes are one of the central regulators of glutamate-mediated activities in the brain and are responsible for the formation of the precursors for glutamate synthesis, the reuptake of released transmitter, and the disposal of excess glutamate (35). There are reports showing that activation of NMDA receptors on tumor cells can stimulate the growth of primary brain tumors (36), but the role of glutamate signaling in brain metastases has not been studied.
Similarly, very little is known regarding any reciprocal transfer of information that may occur between astrocytes and metastatic tumor cells. We wanted to determine whether astrocytes could activate programs in tumor cells that regulate cell proliferation and survival. We chose a transwell co-culture system for a model and elected to include lung cancer cells in the experiment given the fact that lung cancers are the primary source of brain metastases (37). We found that co-incubation of astrocytes with PC14-PE6 lung tumor cells for either 24 or 48 hours stimulates phosphorylation of ERK1/2 on the tumor cells. ERK signaling is activated by a variety of extracellular signals and phosphorylated ERK may regulate a number of cellular responses that favor tumor progression including, proliferation, survival, migration, and angiogenesis (38). Because the chamber that we used allows communication through soluble factors and cell-cell contact, it will be important to determine the mechanism of MAPK activation. More importantly, studies in the whole animal are needed to confirm the in vitro data. Nevertheless, this experiment demonstrates one of the potential uses for the astrocyte cell line.
It is becoming increasingly clear that metastatic progression is regulated, in large part, by interactions that take place between tumor cells and non-tumor cells residing in the organ microenvironment (39,40). For example, fibroblasts residing in invasive breast carcinomas have been shown to increase the microvascular surface area of tumors (41) and by doing so, increase the possibility that tumor cells will gain access into the systemic circulation. Tumors that preferentially spread to the lung were found to signal to pulmonary endothelial cells during the premetastic phase to increase the production of matrix metalloproteinases in the lung in order to prepare the tissue for invasion (42). However, few studies have examined the contribution of astrocytes to tumor metastasis. We anticipate that the immortalized astrocyte cell line described in this report will significantly contribute to our studies of brain metastasis.
Acknowledgments
Cancer Center Support Core Grant CA16672 and SPORE in Prostate Cancer Grant CA902701 from the National Cancer Institute, National Institutes of Health supported this study. We thank Arminda Martinez for expert assistance with the preparation of this manuscript.
References
- 1.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ. Astrocyte-endothelial cell interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mucke L, Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB. 1993;7:1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- 4.Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007;210:651–660. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 7.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disase. Nature Neuroscience. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kunci RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki S, Komori T, Iwata M. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol. 2000;100:138–144. doi: 10.1007/s004019900159. [DOI] [PubMed] [Google Scholar]
- 10.Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- 11.Watkins LR, Maier SF. Glia: A novel drug discovery target for clinical pain. Nature Reviews Drug Discovery. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 12.Miller G. The dark side of glia. Science. 2005;308:778–781. doi: 10.1126/science.308.5723.778. [DOI] [PubMed] [Google Scholar]
- 13.DeKroon RM, Armani PJ. The endosomal trafficking of apolipoprotein E3 and E4 in cultured human brain neurons and astrocytes. Neurobiol Dis. 2001;8:78–89. doi: 10.1006/nbdi.2000.0362. [DOI] [PubMed] [Google Scholar]
- 14.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radhakrishnan S, Otte J, Enam S, Del Valle L, Khalili K, Gordon J. JC virus-induced changes in cellular gene expression in primary human astrocytes. J Virol. 2003;77:10638–10644. doi: 10.1128/JVI.77.19.10638-10644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aloisi F, Borsellino G, Samoggia P, Testa U, Chelucci C, Russo G, Peschle C, Levi G. Astrocyte cultures from human embryonic brain: characterization and modulation of surface molecules by inflammatory cytokines. J Neurosci Res. 1992;32:494–506. doi: 10.1002/jnr.490320405. [DOI] [PubMed] [Google Scholar]
- 17.Rivieccio MA, John GR, Song X, Suh HS, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1 beta activates IFN response factor 3 in human fetal astrocytes in culture. J Immunol. 2005;174:3719–3726. doi: 10.4049/jimmunol.174.6.3719. [DOI] [PubMed] [Google Scholar]
- 18.Terasaki T, Ohtsuki S, Hori S, Takanaga H, Nakashima E, Hosoya K. New approaches to in vitro models of blood-brain barrier drug transport. Drug Disc Today. 2003;8:944–954. doi: 10.1016/s1359-6446(03)02858-7. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 20.Muruganandam A, Herx LM, Monette R, Durkin JP, Stanimirovic DB. Development of immortalized human cerebromicrovascular endothelial cell line as an in vitro model of the human blood-brain barrier. FASEB. 1997;11:1187–1197. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 21.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 22.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2Kb-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- 23.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langley RR, Fan D, Tsan RZ, Rebhun R, He J, Kim SJ, Fidler IJ. Activation of the platelet-derived growth factor-receptor enhances survival of murine bone endothelial cells. Cancer Res. 2004;64:3727–3730. doi: 10.1158/0008-5472.CAN-03-3863. [DOI] [PubMed] [Google Scholar]
- 25.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8:747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by ovarian tumor cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Langley RR, Wu Q, Wu W, Feng J, Tsan R, Fan D, Fidler IJ. Construction of a novel constitutively active chimeric EGFR to identify new targets for therapy. Neoplasia. 2005;7:1065–1072. doi: 10.1593/neo.05553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano S, Herbst RS, Shinohara H, Knighton B, Bucana CD, Killion JJ, Wood J, Fidler IJ. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin Cancer Res. 2000;6:957–965. [PubMed] [Google Scholar]
- 29.Kripke ML. Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst. 1979;63:541–548. doi: 10.1093/jnci/63.3.541. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J Neurol Sci. 1995;134:26–32. doi: 10.1016/0022-510x(95)00227-9. [DOI] [PubMed] [Google Scholar]
- 31.Fan D, Bucana CD, O’Brian CA, Zwelling L, Scid C, Fidler IJ. Enhancement of murine tumor cell sensitivity to adriamycin by presentation of the drug in phosphatidylcholine-phosphatidylserine in liposomes. Cancer Res. 1990;50:3619–3626. [PubMed] [Google Scholar]
- 32.Ulvestad E, Williams K, Mørk S, Antel J, Nyland H. Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol. 1994;53:492–501. doi: 10.1097/00005072-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes-hypothetical roles in CNS pathophysiology. Brain Res Rev. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 35.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. TRENDS Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nature Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 37.DeAngelis LM, Posner JB. Brain Metastasis. In: Kuff DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E, editors. Cancer Medicine. Hamilton, Ontario, Canada: BC Decker; 2003. pp. 1227–1231. [Google Scholar]
- 38.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signaling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 39.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocrine Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 40.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Orimo A, Gupta PB, Sqroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]