Abstract
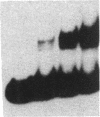
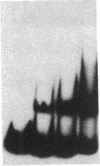
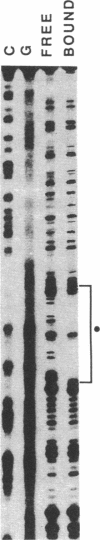
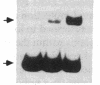
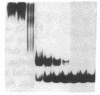
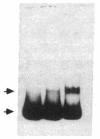
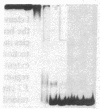
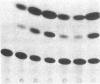
To define the molecular mechanism(s) that activate insulin receptor gene transcription during cell differentiation, we tested nuclear extracts from BC3H-1 muscle cells for their binding to the 5'-flanking region of the human insulin receptor gene. DNA binding activity of nuclear extracts was low in undifferentiated BC3H-1 cells and increased significantly during differentiation. Gel retardation assays, combined with DNase I footprinting, showed that the increased insulin receptor gene transcription occurring during differentiation was directly correlated with the appearance of DNA binding proteins that specifically interacted with two AT-rich sequences of the regulatory region of the insulin receptor gene. Fibroblast growth factor, a known inhibitor of the transcription of muscle-specific DNA binding proteins, did not inhibit the appearance of these insulin receptor DNA binding proteins. When 3T3-L1 cells differentiate from preadipocytes to adipocytes, insulin receptor gene transcription significantly increases. In differentiated adipocytes, the same two insulin receptor DNA binding proteins markedly increased. Reporter gene analysis with the two AT-rich sequences demonstrated that both of these regions of the insulin receptor gene had the characteristics of promoter rather than enhancer elements. Thus, these proteins interacting with these AT-rich sequences may have major importance in regulating the expression of the insulin receptor in target tissues.
Full text
PDF


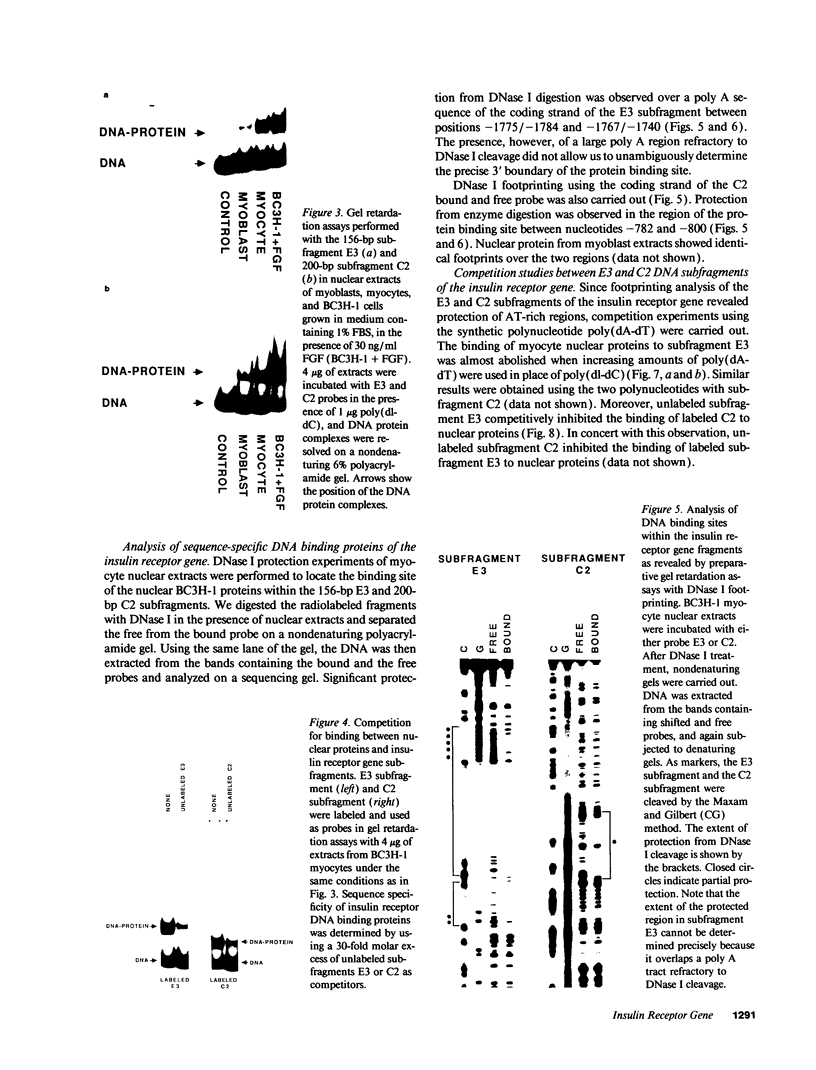

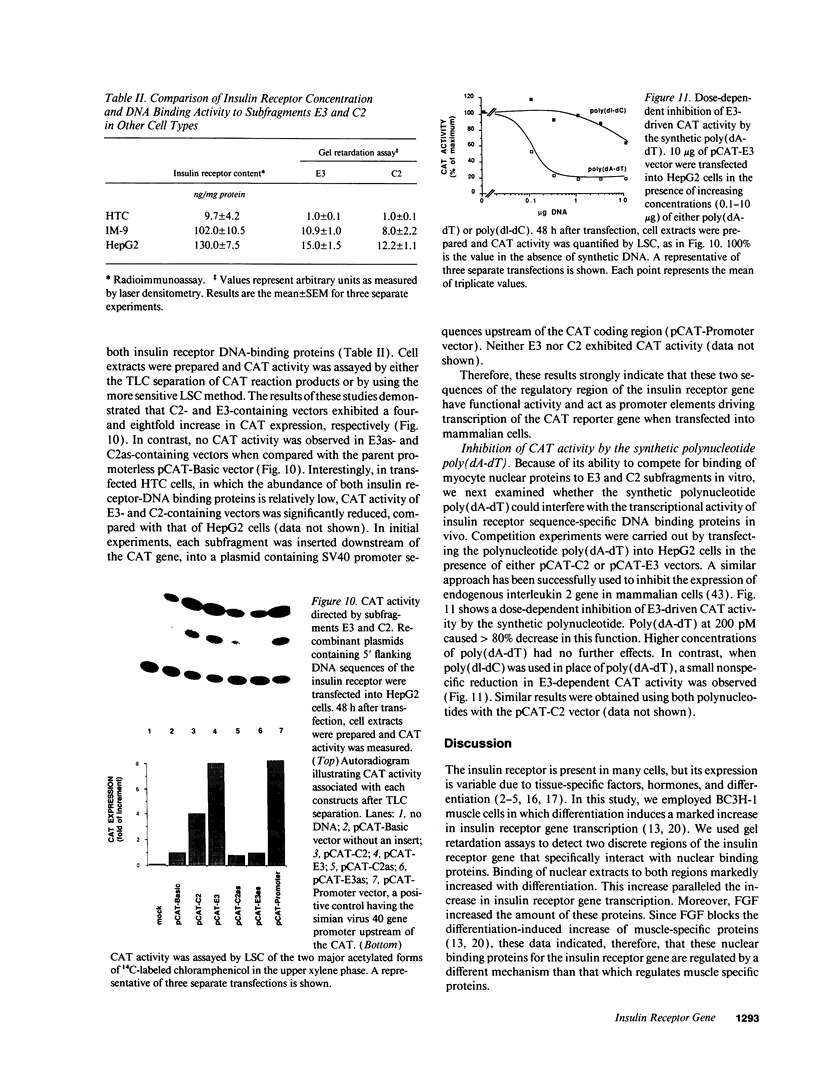


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki E., Shimada F., Uzawa H., Mori M., Ebina Y. Characterization of the promoter region of the human insulin receptor gene. Evidence for promoter activity. J Biol Chem. 1987 Nov 25;262(33):16186–16191. [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., Smith R. J. The development of insulin receptors and responsiveness is an early marker of differentiation in the muscle cell line L6. Endocrinology. 1986 Jan;118(1):446–455. doi: 10.1210/endo-118-1-446. [DOI] [PubMed] [Google Scholar]
- Bielinska A., Shivdasani R. A., Zhang L. Q., Nabel G. J. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990 Nov 16;250(4983):997–1000. doi: 10.1126/science.2237444. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunetti A., Goldfine I. D. Differential effects of fibroblast growth factor on insulin receptor and muscle specific protein gene expression in BC3H-1 myocytes. Mol Endocrinol. 1990 Jun;4(6):880–885. doi: 10.1210/mend-4-6-880. [DOI] [PubMed] [Google Scholar]
- Brunetti A., Goldfine I. D. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem. 1990 Apr 15;265(11):5960–5963. [PubMed] [Google Scholar]
- Brunetti A., Maddux B. A., Wong K. Y., Goldfine I. D. Muscle cell differentiation is associated with increased insulin receptor biosynthesis and messenger RNA levels. J Clin Invest. 1989 Jan;83(1):192–198. doi: 10.1172/JCI113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Konda T. S., Standaert M. L., Davis J. S., Pollet R. J., Farese R. V. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987 Mar 15;262(8):3633–3639. [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Barnes D. E., Davis J. S., Standaert M. L., Pollet R. J. Effects of insulin and protein synthesis inhibitors on phospholipid metabolism, diacylglycerol levels, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. J Biol Chem. 1984 Jun 10;259(11):7094–7100. [PubMed] [Google Scholar]
- Gartenberg M. R., Crothers D. M. Synthetic DNA bending sequences increase the rate of in vitro transcription initiation at the Escherichia coli lac promoter. J Mol Biol. 1991 May 20;219(2):217–230. doi: 10.1016/0022-2836(91)90563-l. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocr Rev. 1987 Aug;8(3):235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- Goodman P. A., Sbraccia P., Brunetti A., Wong K. Y., Carter J. D., Rosenthal S. M., Goldfine I. D. Growth factor receptor regulation in the Minn-1 leprechaun: defects in both insulin receptor and epidermal growth factor receptor gene expression. Metabolism. 1992 May;41(5):504–509. doi: 10.1016/0026-0495(92)90209-s. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hawley D. M., Maddux B. A., Patel R. G., Wong K. Y., Mamula P. W., Firestone G. L., Brunetti A., Verspohl E., Goldfine I. D. Insulin receptor monoclonal antibodies that mimic insulin action without activating tyrosine kinase. J Biol Chem. 1989 Feb 15;264(5):2438–2444. [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocr Rev. 1981 Summer;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Merlino G. T., Pastan I. Epidermal growth factor (EGF) receptor gene transcription. Requirement for Sp1 and an EGF receptor-specific factor. J Biol Chem. 1988 May 5;263(13):6329–6336. [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Grunfeld C., Kahn C. R., Roth J. Regulation of insulin receptors and insulin responsiveness in 3T3-L1 fatty fibroblasts. Endocrinology. 1979 May;104(5):1383–1392. doi: 10.1210/endo-104-5-1383. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Imamoto F., Merlino G. T., Pastan I., Ishii S. Cooperative function of two separate enhancers of the human epidermal growth factor receptor proto-oncogene. J Biol Chem. 1989 Apr 5;264(10):5488–5494. [PubMed] [Google Scholar]
- Mamula P. W., McDonald A. R., Brunetti A., Okabayashi Y., Wong K. Y., Maddux B. A., Logsdon C., Goldfine I. D. Regulating insulin-receptor-gene expression by differentiation and hormones. Diabetes Care. 1990 Mar;13(3):288–301. doi: 10.2337/diacare.13.3.288. [DOI] [PubMed] [Google Scholar]
- Mamula P. W., Wong K. Y., Maddux B. A., McDonald A. R., Goldfine I. D. Sequence and analysis of promoter region of human insulin-receptor gene. Diabetes. 1988 Sep;37(9):1241–1246. doi: 10.2337/diab.37.9.1241. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Caldwell K. L., Glaser L. Multiple controls for the synthesis of muscle-specific proteins in BC3H1 cells. J Cell Biol. 1982 Feb;92(2):350–356. doi: 10.1083/jcb.92.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N., Ercolani L., Denaro M., Kong X. F., Kang I., Alexander M. An insulin response element in the glyceraldehyde-3-phosphate dehydrogenase gene binds a nuclear protein induced by insulin in cultured cells and by nutritional manipulations in vivo. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5273–5277. doi: 10.1073/pnas.87.14.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Caldwell K. L., Gordon J. I., Glaser L. Regulation of creatine phosphokinase expression during differentiation of BC3H1 cells. J Biol Chem. 1983 Feb 25;258(4):2644–2652. [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Lindstrom J. Expression of acetylcholine receptor alpha-subunit mRNA during differentiation of the BC3H1 muscle cell line. J Biol Chem. 1984 Mar 10;259(5):3330–3336. [PubMed] [Google Scholar]
- Pezzino V., Papa V., Trischitta V., Brunetti A., Goodman P. A., Treutelaar M. K., Williams J. A., Maddux B. A., Vigneri R., Goldfine I. D. Human insulin receptor radioimmunoassay: applicability to insulin-resistant states. Am J Physiol. 1989 Sep;257(3 Pt 1):E451–E457. doi: 10.1152/ajpendo.1989.257.3.E451. [DOI] [PubMed] [Google Scholar]
- Podskalny J. M., Takeda S., Silverman R. E., Tran D., Carpentier J. L., Orci L., Gorden P. Insulin receptors and bioresponses in a human liver cell line (Hep G-2). Eur J Biochem. 1985 Jul 15;150(2):401–407. doi: 10.1111/j.1432-1033.1985.tb09034.x. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Cox D., Williamson V. M., Young E. T. DNA sequences of two yeast promoter-up mutants. Nature. 1983 Aug 18;304(5927):652–654. doi: 10.1038/304652a0. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Nishi S., Bell G. I. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A. 1989 Jan;86(1):114–118. doi: 10.1073/pnas.86.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Bell G. I. Rsa1 polymorphism at the insulin receptor locus (INSR) on chromosome 19. Nucleic Acids Res. 1985 Dec 9;13(23):8661–8661. doi: 10.1093/nar/13.23.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley E., Kastelic T., Kelly T. J., Lane M. D. Characterization of the mouse insulin receptor gene promoter. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9732–9736. doi: 10.1073/pnas.86.24.9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Strauch A. R., Rubenstein P. A. Induction of vascular smooth muscle alpha-isoactin expression in BC3H1 cells. J Biol Chem. 1984 Mar 10;259(5):3152–3159. [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D. S., Cook D. M., Taub R. Characterization of the promoter region and 3' end of the human insulin receptor gene. J Biol Chem. 1989 Sep 25;264(27):16238–16245. [PubMed] [Google Scholar]
- Wadsworth H. L., Chazenbalk G. D., Rapoport B. Thyrotropin can increase the steady state level of a messenger ribonucleic acid species (glyceraldehyde-3-phosphate dehydrogenase) in thyroid cells by decreasing its rate of disappearance. Endocrinology. 1990 Jul;127(1):5–9. doi: 10.1210/endo-127-1-5. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]