Abstract
Lecithin:retinol acyltransferase (LRAT) is an enzyme that converts retinol (vitamin A) to retinyl esters. Its expression is often reduced in human cancers, including oral cavity cancers. We investigated the effects of ectopic expression of human lecithin:retinol acyltransferase (LRAT) on murine oral cavity carcinogenesis induced by the carcinogen 4-nitroquinoline 1-oxide (4-NQO). We targeted human LRAT expression specifically to the basal layers of mouse skin and oral cavity epithelia by using a portion of the human cytokeratin 14 (K14) promoter. High levels of human LRAT transgene transcripts were detected in the tongues and skin of adult transgenic positive (TG+) mice, but not in transgenic negative (TG-) mice. The retinyl ester levels in skin of LRAT TG+ mice were 32% ± 5.4% greater than those in TG- mice, and topical treatment of the back skin with retinol resulted in greater increases in retinyl esters (from 6.9 to 14.3 fold in different TG+ mice) in TG+ mouse skin than in TG- mouse skin (1.3 fold). While carcinogen (4-NQO) treatment induced multifocal precancerous and cancer lesions in the tongues of both TG positive (n=16) and negative mice (n=22), higher percentages of transgenic positive mice (62.5%) developed more severe tongue lesions (grades 3 and 4) than transgenic negative mice (24.8%) after 4-NQO treatment (p < 0.05). Carcinogen treatment also resulted in greater percentages of transgenic positive mouse tongues with hyperplasia (71.4%), dysplasia (85.7%, p < 0.05), and carcinoma (28.6%) than transgenic negative mouse tongues (53.3%, 46.7%, and 20%, respectively). Moreover, we observed higher cyclooxygenase-2 (Cox-2) and lower RARβ2 mRNA levels in TG+ as compared to TG- mouse tongues after 4-NQO treatment (p < 0.05). Taken together, these data show that overexpression of human LRAT specifically in oral basal epithelial cells makes these cells more sensitive to carcinogen induced tumorigenesis.
Keywords: cancer stem cells, Cox-2, cytokeratin14 (K14), head and neck squamous cell carcinoma (HNSCC), keratinocyte, LRAT, 4-NQO, oral cancer, RARβ2, retinoids, tongue lesions
INTRODUCTION
Oral squamous cell carcinoma (SCC) is one of the most common cancers in the world (Jemal et al., 2007). Even though the cure rate for small primary tumors is high, many patients will develop second primary tumors and the long-term survival rate for this cancer is lower than 60% (Jemal et al., 2007). Two major etiological factors in oral cavity SCC are the use of tobacco and alcohol, and malignant transformation of the oral cavity tissue is thought to be related to exposure to certain carcinogens (Argiris et al., 2008; Binnie et al., 1983). Oral cavity squamous cell carcinoma (SCC) development is a complicated, multi-step process which involves genetic, epigenetic, and metabolic changes (Brinkman and Wong, 2006). Therefore, in addition to treatment, prevention of oral cancer is very important.
Retinoids, a group of natural and synthetic small, lipophilic molecules which are vitamin A (retinol) derivatives, regulate cell proliferation and differentiation (Gudas et al., 1994). All-trans retinoic acid (ATRA), one of the metabolites of vitamin A, regulates gene expression by binding and activating retinoic acid receptors (RARs) and retinoid X receptors (RXRs) (Chambon, 1996). Epidemiological studies on human populations have demonstrated that increasing vitamin A in the diet is beneficial for the inhibition of the progression of carcinogenesis of the lung, breast, cervix, prostate, gastrointestinal tract, kidney, and oral cavity (Clarke et al., 2004; Hong and Itri, 1994; Lotan, 1996; Niles, 2000). In mammalian cells, retinol is taken up via STRA6, a membrane receptor for retinol binding protein 4 (RBP4) (Kawaguchi et al., 2007), and retinol can be metabolized to polar products, such as all-trans retinoic acid (ATRA); alternatively, retinol can be converted to all-trans retinyl esters (storage forms of retinol) by lecithin:retinol acyltransferase (LRAT) (Blaner and Olson, 1994; Ruiz et al., 1999). LRAT, retinyl ester hydrolases, and STRA6 play important roles in maintaining a stable retinol concentration in serum when the intakeof retinoids in the diet fluctuates (Blaner, 2007; Kawaguchi et al., 2007; Liu and Gudas, 2005; O'Byrne et al., 2005), and LRAT null mice exhibit a much higher sensitivity to vitamin A deficiency (Liu and Gudas, 2005). In retinal pigment epithelial cells, and possibly in other cell types as well, LRAT also functions as a palmitoyl transferase to catalyze the conversion of the pigment epithelial protein RPE65 from a membrane bound form to a solubilized form in the visual cycle (Xue et al., 2004).
Recent reports have shown that vitamin A (retinol) metabolism may be altered in some types of cancer, such as oral cavity cancer, prostate cancer, skin cancer, and breast cancer (Guo and Gudas, 1998; Guo et al., 2002; Guo et al., 2001; Guo et al., 2000; Mongan and Gudas, 2007). In addition, we observed that the esterification of retinol to retinyl esters and the levels of LRAT were greatly decreased in human oral cancer cells (Guo and Gudas, 1998). However, the relationship of LRAT to the process of tumorigenesis has not been defined.
Several animal models which mimic certain aspects of human oral cancer have been established, including hamster, rat, and mouse models (Gimenez-Conti and Slaga, 1992; Gimenez-Conti and Slaga, 1993; Kanojia and Vaidya, 2006; Suzuki et al., 2006; Tanaka et al., 1991; Tang et al., 2004). The oral SCCs induced by the carcinogen 4-nitroquinoline 1-oxide (4-NQO) in the drinking water in the mouse model previously generated in our laboratory demonstrate similarities to human oral tumors in terms of their morphological, histopathological, and molecular characteristics (Tang et al., 2004; Vitale-Cross et al., 2009). Therefore, in order to test the function of LRAT in the carcinogenesis process, we generated transgenic mice in which the human LRAT gene was ectopically expressed in the basal layer of the epithelial cells in the skin and tongue by using the human cytokeratin 14 (K14) promoter, and we investigated the effects of overexpression of LRAT on the process of oral cavity carcinogenesis.
MATERIALS AND METHODS
Synthesis of transgene constructs
The human K14 promoter containing plasmid (Arnold and Watt, 2001; Frye et al., 2003; Tumbar et al., 2004) was provided by Dr. Elaine Fuchs, Rockefeller University. This portion of the human K14 promoter functions in the mouse and its activity is not greatly affected by its chromosomal location in transgenic mice (Vassar et al., 1989). Because expression of transgenes driven by the K14 promoter is limited to the basal layer of the tongue, esophagus, and skin,this truncated K14 promoter has been widely used in previous studies to express ectopic proteins in the basal layer of stratified squamous epithelia of the skin, tongue, and esophagus of mice (Arnold and Watt, 2001; Nowak et al., 2008; Tang et al., 2008; Tumbar et al., 2004; Vassar et al., 1989). A human LRAT (hLRAT) cDNA of 693 bp (Locuslink No AF 071510) corresponding to its protein coding region was amplified by polymerase chain reaction (PCR) with the primer pairs listed in Table 1A. After PCR, the Sal I and BamHI restriction sites GTCGACGGATCC were added at both ends of the construct, with a FLAG tag ATGGACTAC AAAGACGATGACGATAAAGC added at the N-terminus prior to the hLRAT coding region. The FLAG-hLRAT fragment was subcloned into the human K14 promoter containing plasmid by standard molecular cloning techniques. Briefly, the promotercontaining vector was digested, dephosphorylated, and ligated to the FLAG-hLRAT cDNA. Then the expression vectors were sequenced by the Bioresource Center of Cornell University to verify that the sequences were correct.
Generation of transgenic mice
The K14/FLAG-hLRAT (4.7 Kb) fragment was liberated from the vector sequence by the Kpn1/HindIII unique sites and given to the Weill Cornell Medical College/Memorial Sloan-Kettering Transgenic Facility for transgenic mice generation. This construct was injected into the pronuclei of one-cell fertilized eggs by standard methods. Positive founders were identified by Southern blot analysis using a probe corresponding to the human LRAT cDNA and were crossed into a C57BL/6J background. All procedures using mice were approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medical College, and were conducted in accordance with the principles outlined in the NIH guidelines for the care and use of experimental animals.
Identification of transgenic mice by Southern blot
Mice carrying the K14/FLAG-hLRAT transgene were identified by Southern blot. Mouse tail genomic DNA samples were digested with BamHI, separated by 1% agarose gel electrophoresis by size, transferred to nylon membranes, and hybridized to the [32P] labeled human LRAT probe. The specific signal was visualized by exposing the nylon membrane to an X-ray film. Based on the results of Southern blot analysis, several independent K14/FLAG-hLRAT transgenic mouse founder lines were established: #33, 54, 55, and 56.
Examination of human LRAT transgene expression by RT-PCR
Ten-week old adult mouse skin and tongue samples were dissected and snap frozen in dry ice. The tissue samples were homogenized in Trizol (Invitrogen, Carlsbad, CA) and RNA was extracted following the manufacturer's instructions. Five μg of total RNA was treated with DNAse I (Invitrogen, Carlsbad, CA) and then 2 μg DNAse I treated RNA was used for reverse transcription in a 20 μl reaction using Super Script™ II Reverse Transcriptase (RT) (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The reverse transcribed cDNA and the same amount of DNAse I digested total RNA (as a negative control) were used for PCR. Primers specific to FLAG and human LRAT were used to determine the level of the FLAG-hLRAT transcript. The conditions for PCR reactions were: 95 °C for 5 minutes, followed by 35 cycles at 94 °C for 30 sec; primer annealing at 58 °C for 30 sec; product extension at 72 °C for 80 sec, plus a final extension at 72 °C for 10 min. Mouse β-actin was used as a loading control. The primer sequences are listed in Table 1B.
Examination of retinoid metabolism in mice
The skin and tongue samples from K14/FLAG-hLRAT transgenic positive (TG+) and negative (TG-) mice were dissected, weighed, and homogenized in cold phosphate-buffered saline (PBS) (100 μl of PBS per 100 mg of tissue) with an electrical homogenizer (Ultra-turrax T25, Jankel and Kunkel Laboratories Techniques). The volumes were adjusted to 500 μl with cold PBS. Alternatively, the back skin areas (an area of 3 × 3 cm) of K14/FLAG-hLRAT transgenic positive and negative mice were shaved and mice were topically treated with a single daily dose of ATRA or retinol (40 nmoles) in 400 μl acetone (vehicle) for 5 consecutive days following the method described previously (Tang et al., 2008). Then the mouse skin topically treated with ATRA or retinol was dissected and homogenized as described above.
The retinoids were extracted as described previously (Tang et al., 2007). Briefly, 350 μl of acetoneitrile:butanol (50:50,v/v) and 0.1 % butylated hydroxytoluene were added to 0.5 ml of homogenized tissues. The mixture was vortexed thoroughly for 30 seconds. After addition of 300 μl of a saturated K2HPO4 solution and thorough mixing, the samples were centrifuged for 10 min at 12,000 × g. The upper organic layer was collected for automated high-performance liquid chromatography (HPLC) analysis. The HPLC analysis was performed using a Waters Millennium system (Waters Corp., Milford, MA) to separate the various retinoids as previously described (Guo and Gudas, 1998; Guo et al., 2000; Tang et al., 2007). Samples were applied to an analytical 5-μm reverse-phase C18 column (Vydac, Hesperia, CA) at a flow rate of 1.5 ml/min. Retinoids were identified by HPLC based on two criteria: an exact match of the retention times of unknown peaks with those of authentic retinoid standards and identical UV spectra (220–400 nm) of unknowns against spectra from authentic retinoid standards during HPLC by the use of a photodiode array detector. The retinoid levels were calculated from the areas under the peaks detected at the wavelength of 340 nm. The levels of retinol and retinyl esters were normalized to the tissue weights.
Tumor development in the mouse oral cavity
Six week old K14/FLAG-hLRAT positive and negative mice were used for this study. The experiments were carried out under controlled conditions with a 12 hour light/dark cycle. Animals were maintained on a normal chow (Lab-diet with constant nutrition, Lab-Diet Co, St. Louis, MO). The mice were treated with 400 μl propylene glycol (vehicle as a control) or 100 μg/ml carcinogen 4-nitroquinoline-1-oxide (4-NQO, Sigma, St Louis, MO), made up in vehicle, in the drinking water as previously described (Tang et al., 2004) for 10 weeks. Mice were allowed access to the drinking water at all times during the treatment. After the carcinogen treatment, mice were analyzed for precancerous and cancerous lesions in their oral cavities at different times for up to 15 weeks, or until signs of sickness or weight loss.
Tissue dissection and pathological examination
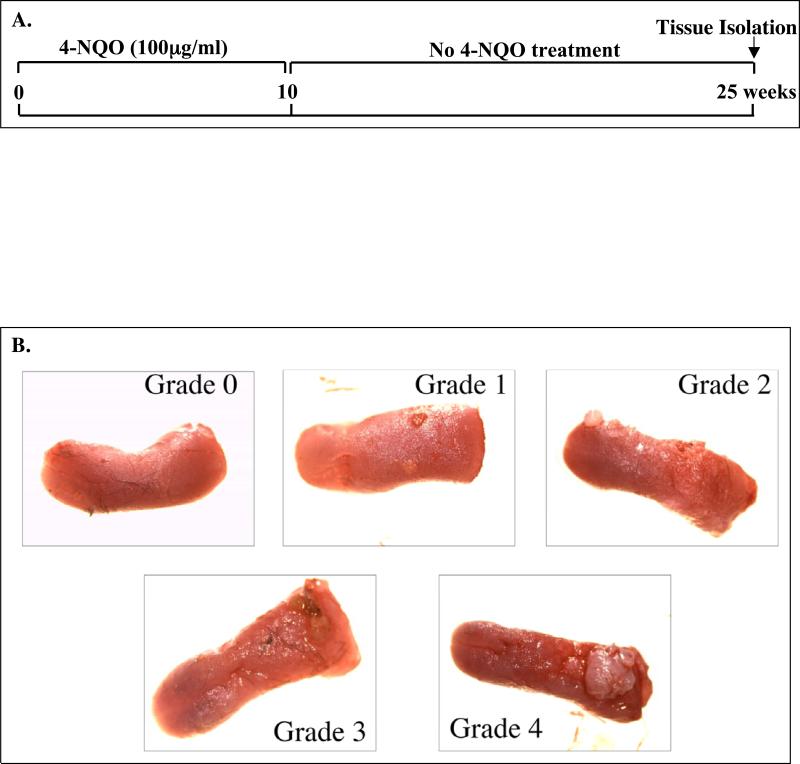
The mouse body weights were measured before the tongues of mice were dissected, immediately after cervical dislocation. Gross lesions were identified and photographed, and tumors on the tongues with diameters of 1–2 mm or greater were counted. The gross lesions on the mouse tongues were quantified by a grading system that included 0 (no lesion), 1 (mild lesion), 2 (intermediate lesion), 3 (severe lesion), and 4 (most severe lesion) in a double blinded manner. Mouse tongues were cut in half longitudinally. Half of each tissue was fixed in freshly made 4% paraformaldehyde overnight at 4°C, embedded in paraffin, and sectioned into 5-μm sections. The other half of each tongue tissue was immediately placed in RNAlater solution (Ambion, Austin, TX) and stored at 4°C overnight before being moved to -20°C for long storage. The histological diagnosis of squamous neoplasia was performed by a pathologist (T. S.) on the hematoxylin and eosin (H & E) stained, sectioned tissue samples. The lesions observed were classified into four types: epithelial hyperplasia; dysplasia; carcinoma in situ; and invasive carcinoma, as described previously (Tang et al., 2004).
Immunohistochemistry
Paraffin embedded sections were deparaffinized and rehydrated, and antigen was retrieved by using Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) in a pressure cooker for 2 min. The tissue sections were stained by using the M.O.M kit (when the primary antibody was mouse IgG, except for the PCNA Ab) (Vector Laboratories, Burlingame, CA) or the Zymed Superpicture kit (when the primary antibody was rabbit IgG) (Zymed Laboratories, South San Francisco, CA). For proliferating cell nuclear antigen (PCNA) antibody, the tissue sections were stained by using Envision™ + HRP (DAB+) kit (Dako, Carpinteria, CA). After quenching endogenous peroxidase with 3% H2O2, the tissue sections were blocked with Vector special blocking reagent (from the M.O.M. kit) or 1.5% goat serum. Then the tissue sections were incubated with human LRAT (1:500) antibody (rabbit polycolonal Ab, a custom antibody generated for the Gudas laboratory by Alpha Diagnostic International, San Antonio, TX.), mouse PCNA (1:100) antibody (Cat # M0879, mouse monoclonal Ab, Dako, Carpinteria, CA), p16 (1:200) antibody (Cat # sc-1661, mouse monoclonal Ab, Santa Cruz, Santa Cruz, CA), cyclin D1 (1:100) antibody (Cat # sc-718, rabbit polyclonal Ab, Santa Cruz, Santa Cruz, CA), and phospho-Akt (Ser 473) (1:200) antibody (Cat # 587F11, mouse monoclonal Ab, Cell Signaling, Danvers, MA), respectively, for 60 min at room temperature. Then the sections were incubated with secondary antibodies (1:200, anti-mouse IgG from the Vector M.O.M kit for p16 and phospho-Akt; ready to use anti-rabbit IgG from the Zymed SuperPicture kit for human LRAT and cyclin D1; ready to use anti-mouse IgG from the Dako Envision™ HRP (DAB+) kit for mouse PCNA). As a negative control, sections were stained without incubation with primary antibodies. Finally, signals were visualized based on a peroxidase detection mechanism (manual of the product), and 3,3-diaminobenzidine (DAB) (Zymed SuperPicture kit) was used as the substrate. The cells with distinct nuclear staining were regarded as positive for PCNA, p16 and cyclin D1. Percentages (indices) of PCNA, p16, and cyclin D1 positive cells were determined by the number of these positive nuclei divided by the total number of basal layer epithelial cell nuclei in the control mouse tongues (not 4-NQO treated) or in the lesions. The intensity of phospho-Akt staining was quantified by using a grading system including 0 (no staining), 1 (weak staining), 2 (intermediate staining), and 3 (intensive staining) in a double blinded manner.
Real time RT-PCR analysis
Total RNA from mouse tongues was extracted and reverse transcribed to cDNA as described above. Real-time PCR was performed using gene-specific oligonucleotide primers for mouse genes. These primers were designed to generate cDNA fragments that cross an intron-exon boundary in the genomic DNA. Real-time PCR was performed on a MyiQ real time PCR detection system (Bio-Rad Laboratories, Hercules, CA) with an iQ SYBR Green Supermix. For every primer set, a series of dilutions of a sample with the highest gene expression, from semiquantitative RT-PCR, was used to generate a standard curve. The conditions for the PCR were as follows: 95 °C for 3 min to activate the DNA polymerase, followed by 50 cycles at 94°C for 30 s, primer annealing at 58°C for 30 s, and product extension at 72 °C for 30 s. After each cycle, fluorescence was read at 84 °C. 36B4 was used as a control (Gillespie and Gudas, 2007a; Gillespie and Gudas, 2007b). The primer sequences are listed in Table 1C.We used the University of California, Santa Cruz In-Silico PCR program (http://genome.ucsc.edu/cgi-bin/hgPcr) to ensure that the PCR primers were not homologous to pseudogene sequences.
Statistical analysis of the data
The statistical analysis of the results was carried out using one way ANOVA test, Fisher's exact probability test, and Wilcoxon rank sum test for multiple comparisons. Differences with a p < 0.05 were considered statistically significant.
Results
Generation of transgenic mice that contain the K14/FLAG-hLRAT transgene (TG)
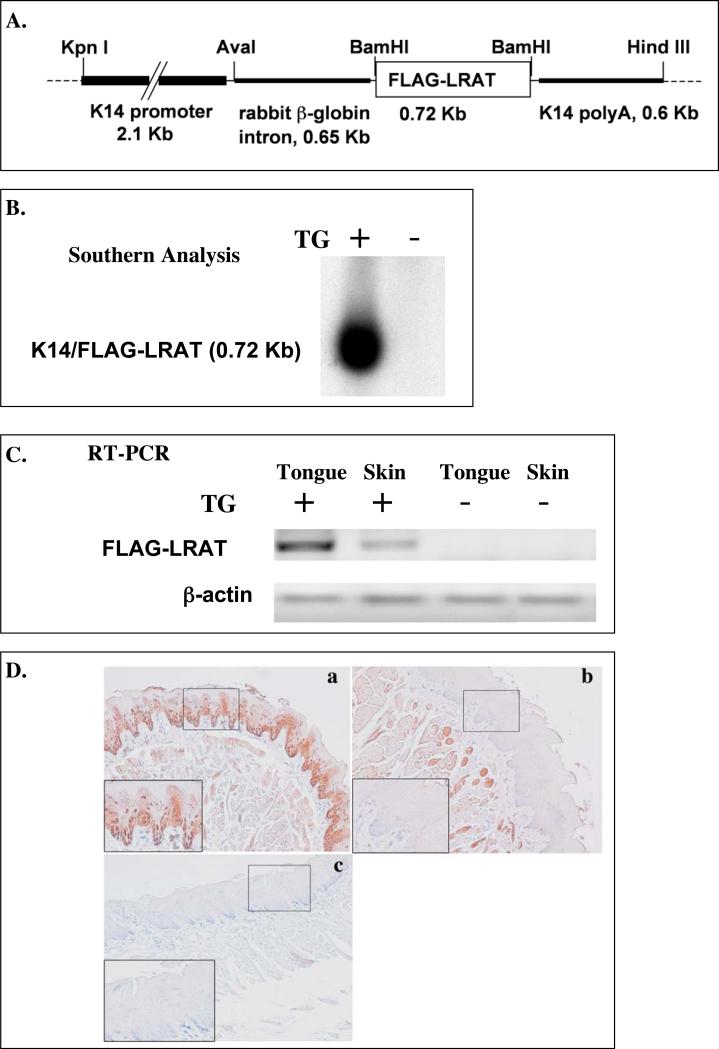
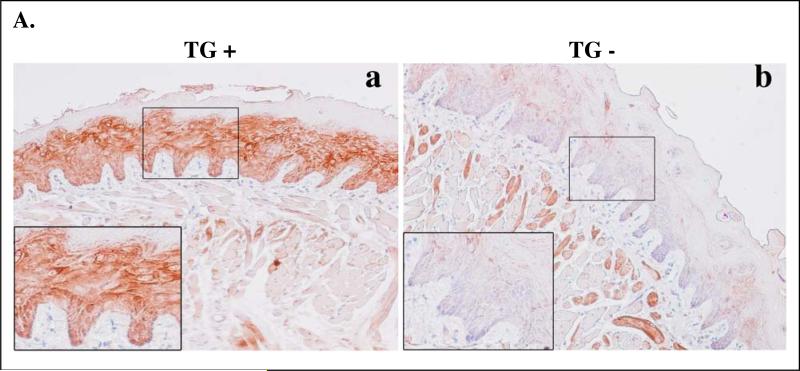
The K14/FLAG-hLRAT (Fig. 1A) fragment was injected into fertilized mouse oocytes and these 1-cell stage embryos were transplanted into pseudopregnant female mice. Four independent lines of transgenic mice (#33, 54, 55, and 56) carrying the transgene (TG) human LRAT were established based on the Southern blot analysis (Fig. 1B). RT-PCR analysis results show that FLAG-hLRAT mRNA was expressed in the skin and tongues of TG positive progeny, but not in TG negative progeny (Fig. 1C). We used a human LRAT antibody that does not cross-react with the endogenous mouse LRAT to examine human LRAT protein expression in mouse tongues. The hLRAT protein was detected in the tongue epithelial basal layer of TG positive mice but not in that of TG negative mice (Fig. 1 D), and its expression pattern was similar to previously described expression patterns of K14 protein in mouse tongues (Tang et al., 2004).
Figure 1. Targeted overexpression of the transgene FLAG-hLRAT to the mouse epithelial basal layer cells.
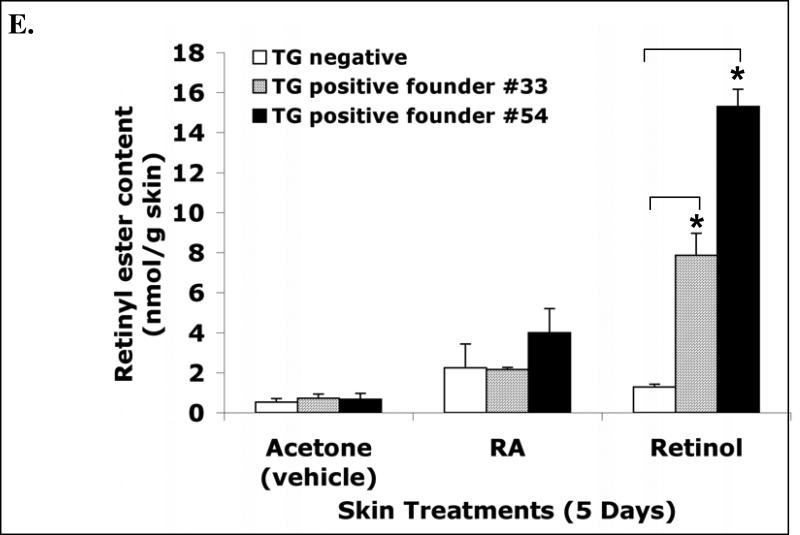
A, The schematic structure of the transgene cassette. B, Southern blot result shows the presence of the transgene in the mouse genomic DNA. C, RT-PCR analysis of the FLAG-hLRAT transgene mRNA in the tongue and skin of K14/FLAG-hLRAT Southern blot transgenic positive (TG+) and negative (TG-) mice from founder #54. D, The expression of the transgene human LRAT protein in the tongue epithelial basal layer cells of K14/FLAG-hLRAT transgenic positive (TG+) and negative (TG-) mice from founder #54 (200 ×). K14/FLAG-hLRAT TG+ and TG- mice were sacrificed, and the tongues were fixed, embedded, sectioned, and stained with anti-human LRAT antibody (see “Materials and Methods”). a, TG positive mouse tongue; b, TG negative mouse tongue; c, negative control, tongue stained only with a secondary antibody; the inset in each picture is 3 × the digital magnification of the small boxed area in the picture. E, the levels of retinyl esters in the skin of K14/FLAG-hLRAT TG positive and negative mice examined by HPLC after a 5 day topical treatment with 40 nmoles (volume 400 μl) of all-trans retinoic acid (ATRA) or retinol per day. Drugs were dissolved in acetone that was the vehicle (see “Materials and Methods”). TG+ #33 and #54 are two TG positive founder lines. The differences among different treatment groups were analyzed by using a one way ANOVA test. Differences with a p value of < 0.05 (marked with an asterisk) in a comparison of retinyl ester levels in retinol treated TG+ mouse skin to that in retinol treated TG- mouse skin were considered to be statistically significant.
Then the retinoid levels in the skin and tongues of TG positive and negative mice were measured by high-performance liquid chromatography (HPLC) as described in the Materials and Methods. We detected trace amounts of retinyl esters in the tongues from the TG positive mice and TG negative littermates (data not shown); the retinyl ester levels in the skin of TG positive mice (from founders #33 and 54) were 32% ± 5.4% greater than those in TG negative mice (Fig. 1E). After a 5-day ATRA or retinol topical treatment of the skin at a daily dose of 40 nmoles, the retinyl ester levels in the skin of TG positive mice were much greater than those in TG negative mice (Fig. 1E). Retinol topical treatment resulted in 6.9 and 14.3 fold increases in skin retinyl ester levels in mice from founders #33 and 54, respectively, as compared to a 1.3 fold increase in TG negative mice (Fig. 1E). These data demonstrate that the FLAG-hLRAT transgene is expressed and is functional in the skin and also in the tongue, because the hLRAT protein expression in the skin and tongue of the transgenic positive mice is the same. The TG positive mice did not show any obvious phenotype. In addition, expression of the human LRAT transgene (TG) in the basal layer of the skin and tongue epithelia did not cause significant morphological abnormalities in adult skin and tongue epithelia in these TG positive mice (data not shown). In the following studies, we used the progeny from founder #54 for the oral cavity carcinogenesis study.
Oral cavity and esophageal carcinogenesis
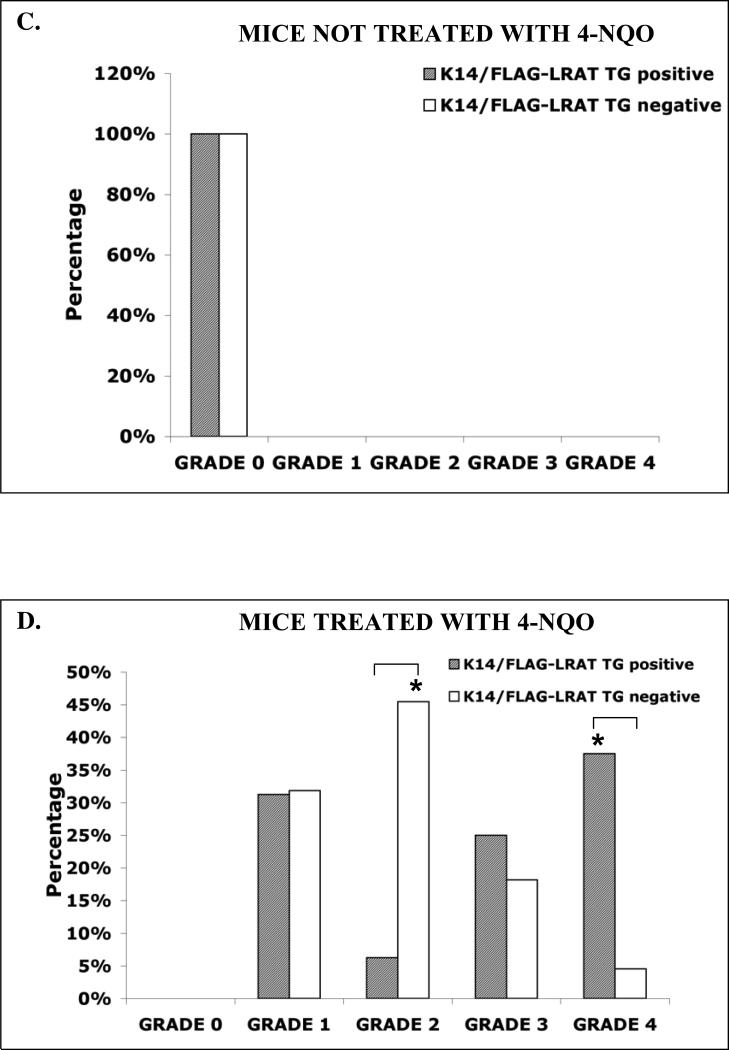
During the period of carcinogen treatment TG positive and TG negative mice consumed similar amounts of drinking water. All of the K14/FLAG-hLRAT TG positive and negative mice survived the 10 week 4-NQO treatment, and more than 90% of the mice survived throughout the 15 week post-treatment period (Fig. 2A). Consistent with our previous study (Tang et al., 2004), we did not observe any gross lesions immediately after the end of the 10 week 4-NQO treatment. The precancerous and cancerous lesions developed during the 15week post-treatment period. The carcinogen 4-NQO induced multifocal precancerous and cancerous lesions in the tongues of both TG positive (n=16) and negative mice (n=22) (Fig. 2B). In contrast, no visible lesions (grade 0) were detected in the control TG positive (n=10) and negative mice (n= 8) not treated with 4-NQO (Fig. 2B and C). The body weights of the control (not treated with 4-NQO) TG positive and negative mice of each sex did not differ significantly. However, after 4-NQO treatment the body weights of TG positive mice were lower than those of the TG negative mice of each sex (Table 2). After 4-NQO treatment, the incidenceof grade 1 tongue lesions in TG positive mice (31.3%) and TG negative mice (31.8%) did not differ; the incidence of grade 2 tongue lesions in TG positive mice (6.3%) was significantly lower (p < 0.05) than that in TG negative mice (45.5%); however, the percentages of TG positive mice with grade 3 (25%) and grade 4 (37.5%, p < 0.05) tongue lesions were greater than those of TG negative mice (18.2% and 4.6%, respectively) (Fig. 2D). These data indicate that the lower body weights of TG positive mice after 4-NQO treatment resulted from more severe tongue lesions.
Figure 2. 4-NQO carcinogen treatment of K14/FLAG-hLRAT TG positive and negative mouse tongues.
K14/FLAG-hLRAT TG positive and negative mice were treated with 4-NQO (100 μg/ml) in the drinking water (see “Materials and Methods”) for 10 weeks and then maintained for another 15 weeks. A, a brief diagram of the experimental protocol. B, representative gross morphological observations of the mouse tongues and the gross tongue lesions grading system (10 ×). C, the grading of tongue lesions in K14/FLAG-hLRAT TG positive and negative control (not treated with carcinogen) mice. D, the grading of tongue lesions in K14/FLAG-hLRAT TG positive and negative mice treated with 4-NQO. The data (percentages in different grades) were analyzed by using a Fisher's exact probability test. Differences with a p value of < 0.05 (marked with an asterisk) between TG+ and TG- mouse tongue lesion grades were considered to be statistically significant.
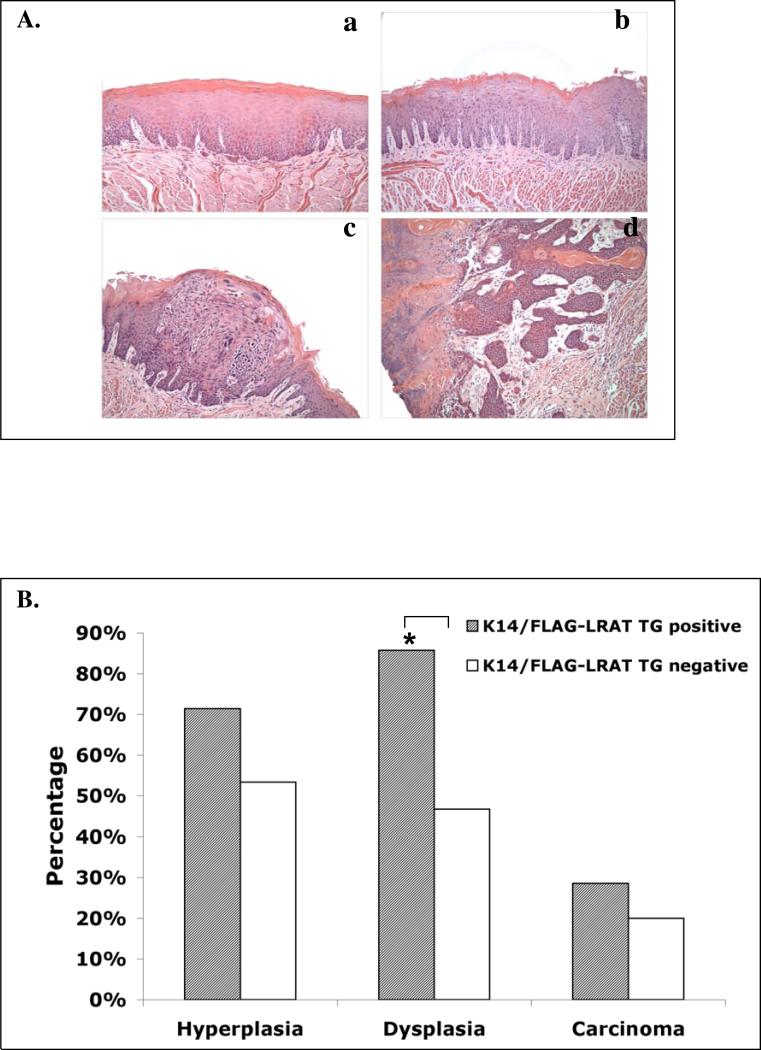
We also observed precancerous and cancerous lesions in mouse esophagi, as described earlier (Tang et al., 2004). In this paper we have focused on oral cavity carcinogenesis. Pathological analyses show evidence of carcinogenesis induced by 4-NQO treatment in both TG positive and TG negative mice, as representative pictures shown in Fig. 3A, including hyperplasia, dysplasia, and carcinoma (including carcinoma in situ and invasive carcinoma), and in some samples multiple types of lesions were present. After 4-NQO treatment, the tongues of TG positive mice displayed a greater incidence of hyperplasia (71.4%), dysplasia (85.7%), and carcinoma (28.6%) than those of TG negative mice (hyperplasia (53.3%), dysplasia (46.7%), and carcinoma (20%), respectively), especially dysplasia (p < 0.05) (Fig. 3B). These data indicate that overexpression of the human LRAT transgene in the mouse epithelial basal layer cells makes mice more sensitive to 4-NQO treatment induced oral cavity carcinogenesis.
Figure 3. Pathological evidence of carcinogenesis in mouse tongues after carcinogen treatment.
K14/FLAG-hLRAT TG positive and negative mice were treated with 4-NQO (100 μg/ml) in drinking water (see “Materials and Methods”) for 10 weeks and then maintained for another 15 weeks. The mice were sacrificed, and the tongues were fixed, embedded, sectioned, and stained with H&E. A, representative pictures of pathology (200 ×): a, hyperplasia with marked hyperkeratosis; b, mild dysplasia; c, severe dysplasia / carcinoma in situ; d, invasive carcinoma with tumor cells invading the skeletal muscle fibers of the tongue. B, Percentage of mouse tongue samples (each sample was from a different mouse) at different carcinogenesis stages. The data were analyzed by using a Fisher's exact probability test. Differences with a p value of < 0.05 (marked with an asterisk) were considered to be statistically significant.
Cox-2 and RARβ2 mRNA levels in mouse tongues
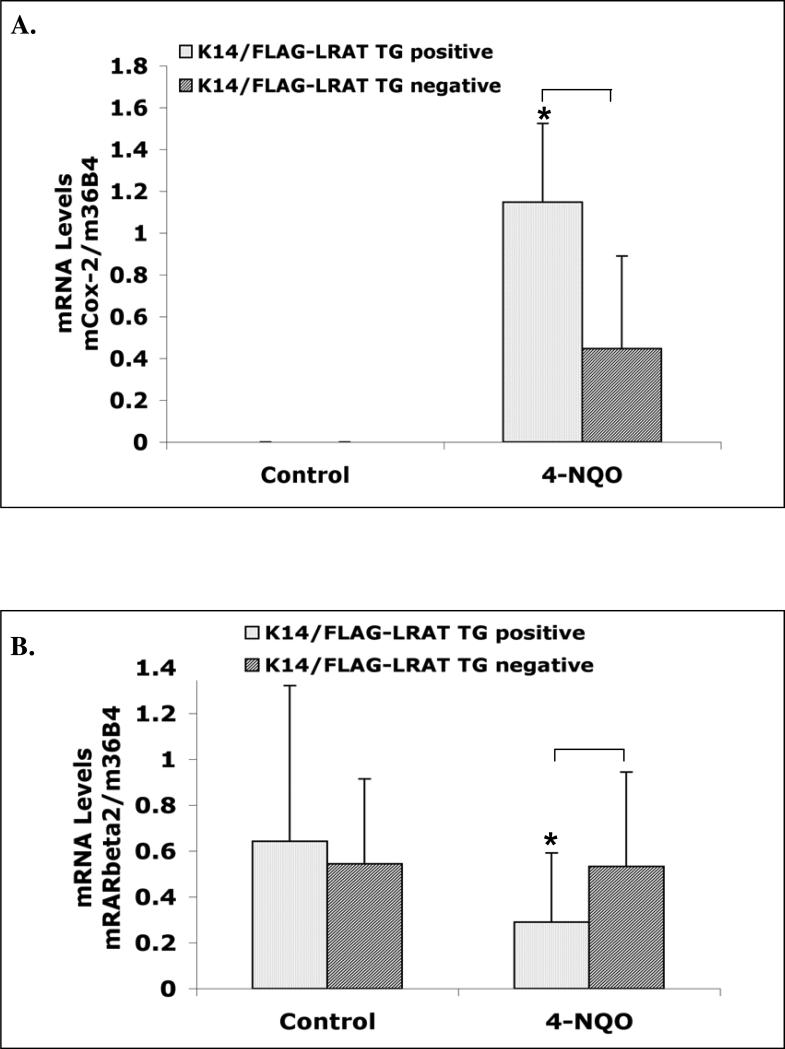
Cyclooxygenases (Cox) are enzymes that synthesize prostaglandins from arachidonic acid. Cox-1 is constitutively expressed in almost all mammalian cells. Cox-2 is usually undetectable in normal tissues, but its expression can be rapidly induced by many signaling molecules involved in the carcinogenesis process, such as growth factors, oncogenes, and tumor promoters (Smith et al., 2000). Overexpression of Cox-2 has been reported in many types of human cancer, such as colon, breast, pancreas, and head and neck cancers (Cha and DuBois, 2007; Chan et al., 1999; Dannenberg et al., 2005; Dubois et al., 1998). These findings suggest that the Cox-2 expression level is related to oral cavity cancer development (Chan et al., 1999; Vishwanatha et al., 2003). During oral cavity carcinogenesis, Cox-2 expression is enhanced during malignant transition of the oral epithelium (Lippman et al., 2005). In addition, in two different oral carcinoma animal models premalignant and malignant oral lesions showed Cox-2 overexpression (Nishimura et al., 2004; Suzuki et al., 2006), and inhibitors of Cox-2 suppressed carcinogen-induced tongue carcinogenesis (Nishimura et al., 2004; Yoshida et al., 2003). Therefore, Cox-2 has been proposed to be a promising molecular target for oral cancer prevention (Chen et al., 2004; Lin et al., 2008; Lippman et al., 2005). Because the K14/FLAG-hLRAT TG positive mice showed more severe pathological characteristics than the TG negative mice after the carcinogen treatment (Figs. 2 and 3), we measured Cox-2 mRNA levels in the tongues in control and 4-NQO-treated K14/FLAG-hLRAT TG positive and negative mice by real time RT-PCR. In both TG positive (n=7) and negative (n=6) control (not 4-NQO treated) mouse tongues we did not detect Cox-2 mRNA expression (Fig. 4A). Carcinogen treatment resulted in Cox-2 mRNA expression; seven out of twelve TG positive mouse tongue samples and eight out of sixteen TG negative mouse tongue samples showed Cox-2 mRNA expression. Moreover, after 4-NQO treatment the TG positive mouse tongues showed significantly greater average Cox-2 mRNA levels than TG negative mouse tongues (p < 0.05) (Fig. 4A).
Figure 4. Cox-2 and RARβ2 mRNA levels in control (not treated with 4-NQO) and 4-NQO treated mouse tongues.
K14/FLAG-hLRAT TG positive and negative mice were treated with propylene glycol (vehicle) or 4-NQO (100 μg/ml) in drinking water (see “Materials and Methods”) for 10 weeks and then maintained for another 15 weeks. Total RNA was extracted from mouse tongues and specific mRNA levels were measured by real time RT-PCR. A, Cox-2 mRNA. B, RARβ2 mRNA. The data were analyzed by using a Wilcoxon rank sum test. Differences of mRNA levels between TG+ and TG- mouse tongues with a p value of < 0.05 (marked with an asterisk) were considered to be statistically significant.
RARβ2, one of the isoforms of the retinoic acid receptor RARβ, plays a role in the prevention of head and neck cancer (Hong et al., 1990; Lippman et al., 2005; Lotan, 1996; Lotan et al., 1995). Compared to normal epithelial tissues, RARβ2 mRNA levels are usually decreased or absent in head and neck squamous carcinomas because of hypermethylation of the RARβ2 promoter (Hu et al., 1991; Mongan and Gudas, 2007; Wan et al., 1999; Xu et al., 1994). We assessed RARβ2 mRNA levels in TG positive and negative mouse tongues. In non-carcinogen treated control mice we did not observe statistically significant differences in RARβ2 mRNA levels between TG positive and negative mice (Fig. 4B). In 4-NQO treated animals, the RARβ2 mRNA levels were statistically lower in the TG positive mouse tongues than in the TG negative mouse tongues (p < 0.05) (Fig. 4B).
Immunohistochemistry of hLRAT, PCNA, p16, cyclin D1, and phospho-Akt (Ser 473) in mouse tongues
Because our previous findings showed that the K14 protein expression pattern expanded during the carcinogenesis process (Tang et al., 2004), we examined the transgene hLRAT protein expression since hLRAT expression is driven by the K14 promoter. In mouse tongues after 4-NQO carcinogen treatment hLRAT protein expression was expanded in TG positive mice, but expression of hLRAT was not observed in carcinogen treated TG negative mice, as expected (Fig. 5A).
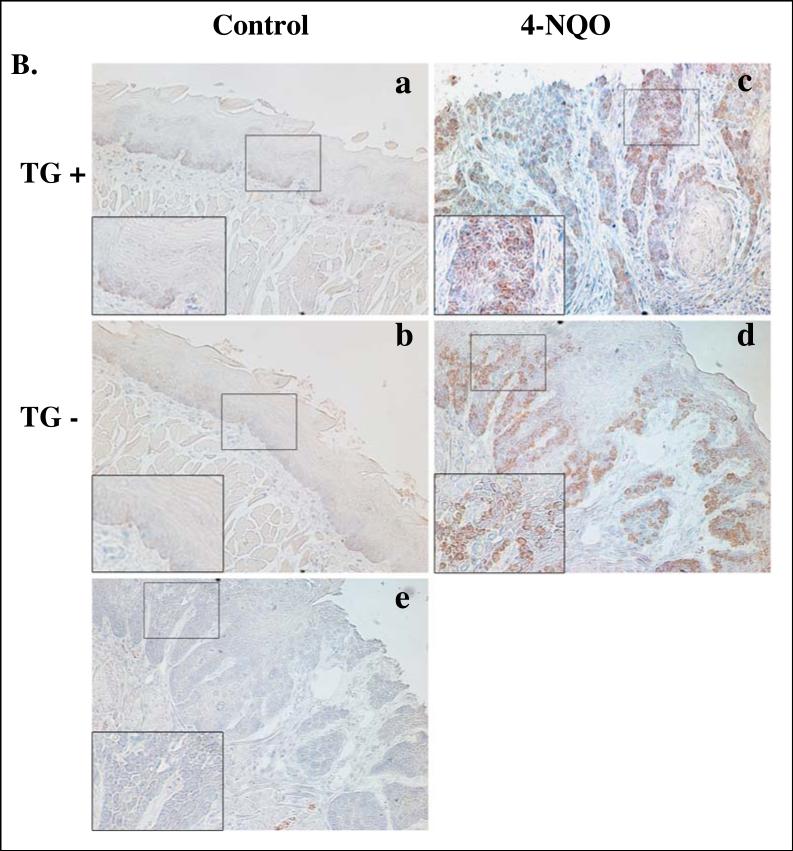
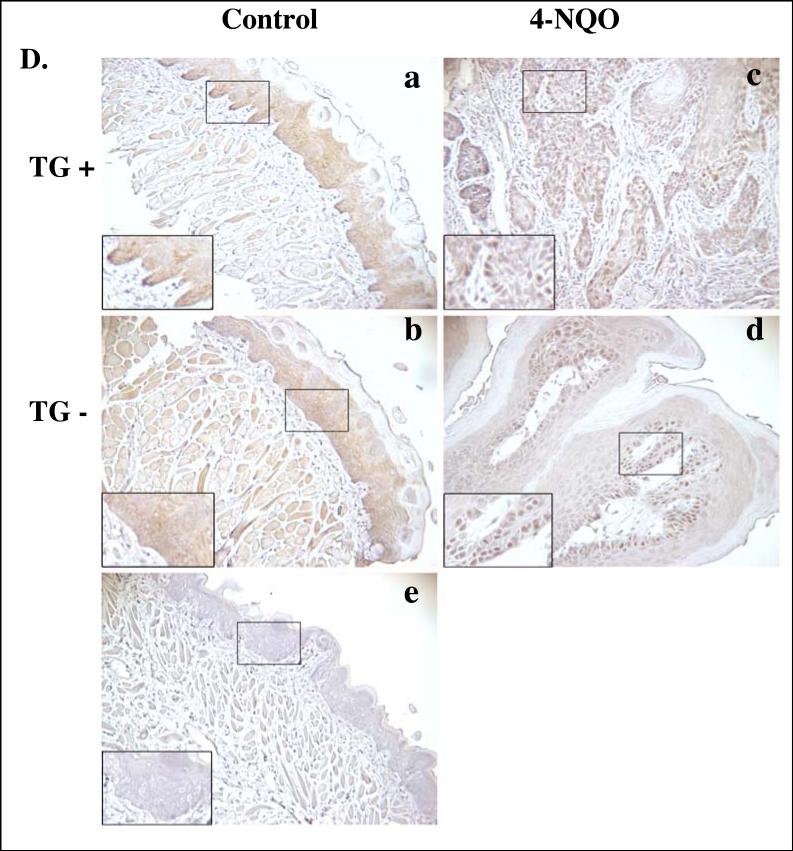
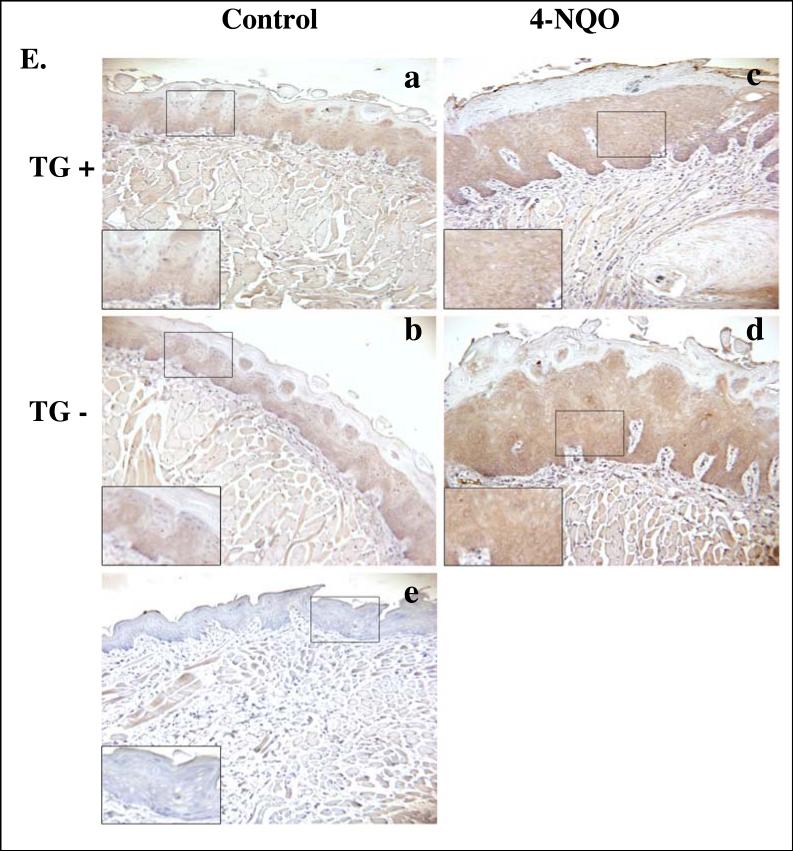
Figure 5. Expression of hLRAT, PCNA, p16, cyclin D1, and phospho-Akt (ser 473) proteins in control (not treated with 4-NQO) and 4-NQO treated mouse tongues.
K14/FLAG-hLRAT TG positive and negative mice were treated with propylene glycol (vehicle) or 4-NQO (100 μg/ml) in drinking water for 10 weeks and then maintained for another 15 weeks. Mice were sacrificed, and the tongues were fixed, embedded, sectioned, and stained with anti-hLRAT, anti-PCNA, anti-p16, anti-cyclin D1, and anti-phospho-Akt (Ser 473) antibodies, respectively (see “Materials and Methods”) (200 ×). A, hLRAT staining of 4-NQO treated mouse tongues (a and b TG+; c, TG-; a and c, before tumor appears; b, tumor). B, PCNA staining of mouse tongues. C, p16 staining of mouse tongues. D, cyclin D1 staining of mouse tongues. E, phospho-Akt (Ser 473) staining of mouse tongues. In panels B to E: a and c, TG positive mouse tongues; b and d, TG negative mouse tongues. a and b, control (not treated with 4-NQO) mouse tongues; c and d, 4-NQO treated mouse tongues. e, negative control, a mouse tongue stained only with a secondary antibody. The inset in each picture is 3 × the digital magnification of the small boxed area in the picture.
Proliferating cell nuclear antigen (PCNA) is a protein which is expressed in the nuclei of cells during the S phase of cell cycle, and this protein is needed for DNA polymerase delta to bind DNA (Moldovan et al., 2007). Therefore, PCNA is a marker of cell proliferation. We measured the PCNA protein levels in mouse tongues by immunostaining. Very few nuclei in the tongue epithelia from TG positive and TG negative control mice showed PCNA positive cells. In contrast, after carcinogen treatment the numbers of PCNA positive nuclei increased in the tongue epithelial lesions in both K14/FLAG-hLRAT TG positive and TG negative mice (Fig. 5B and Table 3).
During the process of carcinogenesis, dysregulation of the cell cycle is a critical event (Sherr, 2004). One of the cell cycle inhibitors is p16, also known as MTS-1 (major tumor suppressor 1), which inhibits CDK4/6 (Coleman et al., 1997; Morisaki et al., 1999). The loss of p16 protein expression occurs frequently in human oral cancers (Todd et al., 2002). The loss of expression of p16 has been observed in oral premalignant lesions and primary tumors of the oral cavity (Hasegawa et al., 2002; Hunter et al., 2006; Kresty et al., 2002; Papadimitrakopoulou et al., 1997). We examined mouse p16 protein expression in tongues of control (not 4-NQO treated) and 4-NQO treated TG positive and TG negative mice by immunohistochemistry. In control mice, p16 nuclear staining was observed in the epithelial basal and suprabasal layers, but primarily in the basal layer, in both TG positive and negative mice (Fig. 5C). After 4-NQO treatment the nuclear p16 protein levels in mouse tongue lesions were reduced in both TG positive and TG negative mice to similar degrees (Fig. 5C and Table 3).
In mammalian cells two families of cyclins are active key proteins during the transition from G1 phase to S phase of the cell cycle, the D family (D1, D2, and D3) and the E family (E1 and E2). The synthesis of D cyclins depends on extracellular mitogenic factors instead of being regulated by cell cycle progression (Sherr, 2000b; Sherr and Roberts, 1999). The overexpression of D type cyclins, especially cyclin D1, has been reported in a number of human cancers, such as head and neck cancer, lung cancer, stomach cancer, and breast cancer (Sherr, 2000a; Sherr, 2000b). Overexpression of cyclin D1 has been observed both in rat oral tumors induced by 4-nitroquinoline 1-oxide (4-NQO) and in human oral cancer (Nakahara et al., 2000; Nakayama et al., 1996; Schoelch et al., 1999; Todd et al., 2002; Yoshida et al., 2005). In control (not carcinogen treated) mouse tongues very few cyclin D1 stained nuclei were seen in the epithelial basal layer cells of both TG positive and negative mice (Fig. 5D). Treatment with 4-NQO increased the numbers of cyclin D1 positive nuclei in mouse tongue tumors from both TG positive and negative animals to similar degrees (Fig. 5D and Table 3).
The phosphoinositide-3-kinase (PI3K)/Akt pathway plays a crucial role in the survival, proliferation, migration, and differentiation of human cancer cells (Toker and Cantley, 1997). Specific inhibition of Akt suppresses proliferation and causes apoptosis and anoikis in human head and neck squamous cancer cells (Mandal et al., 2006). The activation of Akt, a serine/threonine kinase, is through the phosphorylation of its amino acid residues Thr308 and Ser473 (Alessi et al., 1996; Jacinto et al., 2006; Sarbassov et al., 2005). Carcinogen exposure can activate Akt and this may be a critical event in oral cavity carcinogenesis (West et al., 2003; Xu et al., 2007). Constitutively activated Akt has been detected during the progression of human head and neck squamous cell carcinoma, and the level of activation of Akt was inversely correlated with the survival rates of tongue cancer patients (Amornphimoltham et al., 2004; Massarelli et al., 2005). In our TG positive and negative control (not carcinogen treated) mouse tongues, we observed phospho-Akt (Ser 473) cytoplasmic staining mainly in the epithelial basal layer cells (Fig. 5E). Carcinogen treatment caused phospho-Akt staining to spread across the entire epithelial layers of both TG positive and negative mouse tongues (Fig. 5E).
Discussion
LRAT mRNA and protein expression has been shown to be greatly reduced in human oral cancer cells (Guo and Gudas, 1998). Understanding the role of LRAT in oral cavity carcinogenesis will provide useful information for future prevention and treatment of this disease. Because cytokeratin 14 (K14) is a specific marker for cells in the epithelial basal layer, which contains proliferating cells (Fuchs, 1991; Fuchs and Green, 1980; Tang et al., 2008 Submitted; Tumbar et al., 2004), we investigated the effect of ectopic expression of human LRAT in mouse basal epithelial cells (Fig. 1D) on oral cavity carcinogenesis using a mouse model previously generated in our laboratory (Tang et al., 2004). Human LRAT protein shares 80% homology with mouse LRAT protein, including the region necessary for LRAT activity (Moise et al., 2007; Ross and Zolfaghari, 2004). Our results show that carcinogen treated K14/FLAG-hLRAT TG positive mice exhibit more severe tongue lesions (Fig. 2D), lower body weights, and greater incidences of dysplasia that can progress to carcinoma than TG negative mice (Fig. 3B). These data suggest that overexpression of LRAT in the epithelial basal layer makes mice more susceptible to oral cavity carcinogenesis.
Intracellular LRAT levels in the mouse tongue epithelial basal cells and oral cavity carcinogenesis
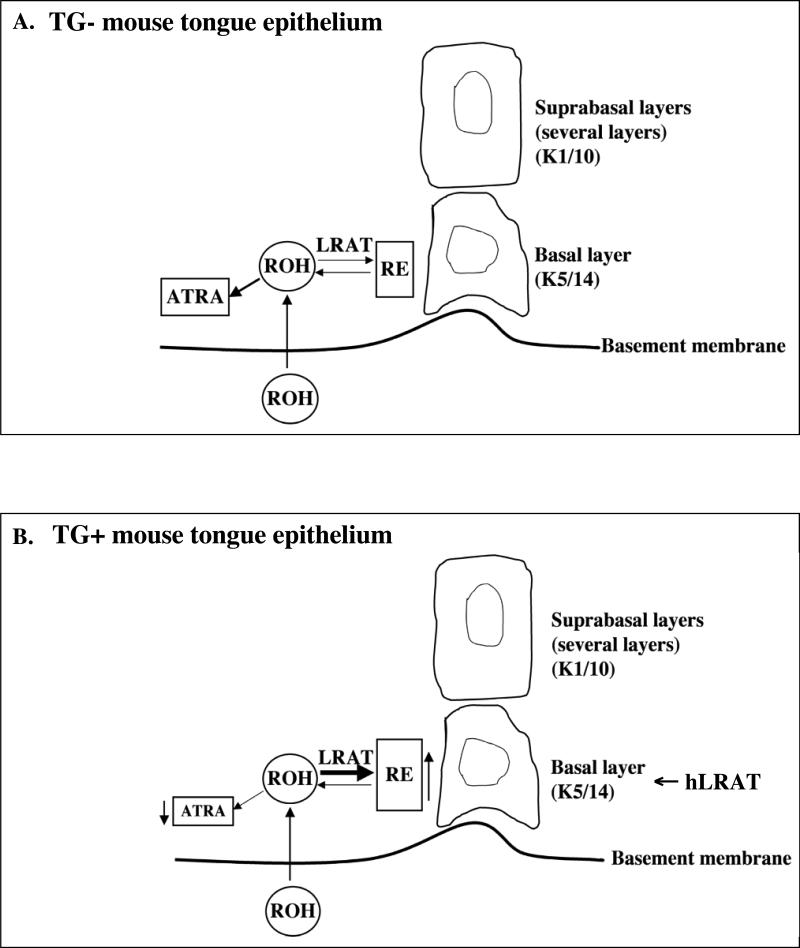
Human head and neck tumors contain heterogeneous cell populations, including tumor cells, stromal cells, and inflammatory cells. Among these cell populations, only a specific subpopulation of cancer cells, cancer stem cells, is thought to drive the growth of the tumors (Dalerba et al., 2007; Prince and Ailles, 2008). Prince et al. (2007) identified cancer stem cells in human head and neck carcinoma. These cancer stem cells express cytokeratin 5/14 and were detected in the basal layer of the squamous epithelium (Prince and Ailles, 2008; Prince et al., 2007). Retinoids, especially all-trans retinoic acid (ATRA), have been regarded as candidates for cancer chemoprevention and chemotherapy because they induce cells to differentiate, growth arrest, and/or undergo apoptosis (Clarke et al., 2004; Fields et al., 2007; Mongan and Gudas, 2007). Our results (Figs. 1 D and E) indicate that in K14/FLAG-hLRAT TG positive mice, the skin epithelia in which the human LRAT protein was expressed contained more retinyl esters than the epithelial cells from TG negative mouse skin. The proteins LRAT and STRA6 both increase retinol uptake, and STRA6 is the RBP4 / retinol receptor (Kawaguchi et al., 2007), and a stable intracellular retinol concentration is maintained by LRAT, retinyl ester hydrolases, and Stra6 (Blaner, 2007; Kawaguchi et al., 2007; Liu and Gudas, 2005; O'Byrne et al., 2005). During human oral cancer development the reduction of LRAT expression in oral epithelial cells might result in less retinol uptake into these cells, which impairs normal retinoid signaling. Conversely, it might be expected that higher levels of LRAT would be protective with respective to cancer because the basal epithelial cells would take up more retinol. Although there has been no report of a relationship between the expression of retinyl ester hydrolases and vitamin A status, Matsuura et al. (1997) showed that intracellular vitamin A status does not affect specific activities of retinyl ester hydrolases (Matsuura et al., 1997). Because LRAT is critical in determining intracellular retinol availability, which in turn affects the levels of all-trans-retinoic acid, our data here suggest that the levels of LRAT must be balanced appropriately with the levels of the various retinyl ester hydrolases (Harrison and Gad, 1989; Kurlandsky et al., 1996; Linke et al., 2005) in order for retinoid signaling to function properly (Fig. 6).
Figure 6. Model of retinoid actions in K14/FLAG-hLRAT transgenic negative and positive mouse tongue epithelia.
A, transgenic negative mouse tongue epithelium. B, transgenic positive mouse tongue epithelium. The thicker arrow under LRAT indicates a greater expression level of LRAT. ATRA, all-trans retinoic acid; RE, retinyl esters; ROH, retinol; TG-, transgenic negative; TG+, transgenic positive.
Cox-2 and RARβ2 mRNA levels in K14/FLAG-hLRAT transgenic positive and negative mouse tongues
Previous studies have shown that both Cox-2 mRNA and protein are undetectable in normal tissues and are upregulated in human cancers, including head and neck cancer (Cha and DuBois, 2007; Chan et al., 1999; Chen et al., 2004; Smith et al., 2000). Similarly, we show here that Cox-2 mRNA is not detected in the control (not carcinogen treated) tongues of both TG positive and negative mice, and that 4-NQO treatment increases Cox-2 mRNA levels (Fig. 4A). Clinical studies show that Cox-2 expression is generally found in DNA aneuploid oral dysplastic lesions which can further develop into malignant carcinoma (Lippman et al., 2005). We found that the incidence of dysplasia in K14/FLAG-hLRAT TG positive mouse tongues is greater than that in TG negative mouse tongues (Fig. 3B). Thus, our Cox-2 mRNA results in mouse tongues treated with 4-NQO (Fig. 4A) correlate with our pathological data (Figs. 2D and 3B). Mestre et al. (1997) (Mestre et al., 1997) reported that ATRA inhibited Cox-2 mRNA and protein expression in human oral squamous carcinoma cells. The level of LRAT is critical for the regulation of intracellular retinol and therefore ATRA availability, which could in turn affect the Cox-2 mRNA level.
The decrease in RARβ2 mRNA level has been reported to correlate with the progression of human oral squamous carcinoma (Hu et al., 1991), and the loss of RARβ2 expression may be caused by abnormal retinoic acid signaling (Ren et al., 2005). Therefore, our results that after 4-NQO treatment RARβ2 mRNA levels in TG positive mouse tongues were lower than those in TG negative mouse tongues (Fig. 4B) are consistent with our pathological data (Figs. 2D and 3B). In addition, the variability of RARβ2 mRNA levels in the mouse tongues was at least in part from the differences in grades of these lesions.
Taken together, our data show that although LRAT expression is reduced in human oral carcinoma cells, very high, ecotopic LRAT expression in oral epithelial basal cells makes these cells more sensitive to carcinogen induced tumorigenesis. Thus, for prevention of oral cavity carcinogenesis it is important to maintain an appropriate LRAT level in the basal cells of the oral epithelium.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Elaine Fuchs for the K14 promoter construct; Dr. Kathy Zhou, a biostatistician in the Department of Public Health of Weill Cornell Medical College, for advice on the statistical analyses; the insightful scientific input provided by the members of the Gudas laboratory; and Christopher Kelly for editorial assistance. This research was supported primarily by NIH grant DE10389 to LJG.
Abbreviations
- ATRA
all-trans retinoic acid
- Cox
cyclooxygenases
- DAB
3, 3'-diaminobenzidine tetrahydrochloride
- H & E
hematoxylin and eosin
- HPLC
high-performance liquid chromatography
- K14
cytokeratin 14
- LRAT
lecithin:retinol acyltransferase
- 4-NQO
4-nitroquinoline 1-oxide
- PCNA
proliferating cell nuclear antigen
- RAR
retinoic acid receptor
- ROH
retinol
- RXR
retinoid X receptor
- TG
transgene
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA, Gutkind JS. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10:4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Binnie WH, Rankin KV, Mackenzie IC. Etiology of oral squamous cell carcinoma. J Oral Pathol. 1983;12:11–29. doi: 10.1111/j.1600-0714.1983.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Blaner WS. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 2007;5:164–6. doi: 10.1016/j.cmet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. Raven Press; New York: 1994. pp. 229–256. [Google Scholar]
- Brinkman BM, Wong DT. Disease mechanism and biomarkers of oral squamous cell carcinoma. Curr Opin Oncol. 2006;18:228–233. doi: 10.1097/01.cco.0000219250.15041.f8. [DOI] [PubMed] [Google Scholar]
- Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–52. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–4. [PubMed] [Google Scholar]
- Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, Shin DM. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5930–9. doi: 10.1158/1078-0432.CCR-03-0677. [DOI] [PubMed] [Google Scholar]
- Clarke N, Germain P, Altucci L, Gronemeyer H. Retinoids: potential in cancer prevention and therapy. Expert Rev Mol Med. 2004;6:1–23. doi: 10.1017/S1462399404008488. [DOI] [PubMed] [Google Scholar]
- Coleman KG, Wautlet BS, Morrissey D, Mulheron J, Sedman SA, Brinkley P, Price S, Webster KR. Identification of CDK4 sequences involved in cyclin D1 and p16 binding. J Biol Chem. 1997;272:18869–74. doi: 10.1074/jbc.272.30.18869. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–66. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J. 1998;12:1063–73. [PubMed] [Google Scholar]
- Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–98. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- Frye M, Gardner C, Li ER, Arnold I, Watt FM. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130:2793–808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Keratin genes, epidermal differentiation and animal models for the study of human skin diseases. Biochem Soc Trans. 1991;19:1112–5. doi: 10.1042/bst0191112. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–42. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoic Acid receptor isotype specificity in f9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic Acid response elements. J Biol Chem. 2007a;282:33421–34. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol. 2007b;372:298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch model of carcinogenesis and chemoprevention. Adv Exp Med Biol. 1992;320:63–7. doi: 10.1007/978-1-4615-3468-6_9. [DOI] [PubMed] [Google Scholar]
- Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch carcinogenesis model. J Cell Biochem Suppl. 1993;17F:83–90. doi: 10.1002/jcb.240531012. [DOI] [PubMed] [Google Scholar]
- Gudas LJ, Sporn MB, Roberts A. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. Raven Press; New York: 1994. pp. 443–520. [Google Scholar]
- Guo X, Gudas LJ. Metabolism of all-trans-retinol in normal human cell strains and squamous cell carcinoma lines from the oral cavity and skin: reduced esterification of retinol in SCC lines. Cancer Res. 1998;58:166–176. [PubMed] [Google Scholar]
- Guo X, Knudsen BS, Peehl DM, Ruiz A, Bok D, Rando RR, Rhim JS, Nanus DM, Gudas LJ. Retinol metabolism and lecithin:retinol acyltransferase levels are reduced in cultured human prostate cancer cells and tissue specimens. Cancer Res. 2002;62:1654–61. [PubMed] [Google Scholar]
- Guo X, Nanus DM, Ruiz A, Rando RR, Bok D, Gudas LJ. Reduced levels of retinyl esters and vitamin A in human renal cancers. Cancer Res. 2001;61:2774–2781. [PubMed] [Google Scholar]
- Guo X, Ruiz A, Rando RR, Bok D, Gudas LJ. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–33. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- Harrison EH, Gad MZ. Hydrolysis of retinyl palmitate by enzymes of rat pancreas and liver. Differentiation of bile salt-dependent and bile salt-independent, neutral retinyl ester hydrolases in rat liver. J. Biol. Chem. 1989;264:17142–14147. [PubMed] [Google Scholar]
- Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–6. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- Hong WK, Itri LM. Retinoids and human cancer. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. Raven Press; New York: 1994. pp. 597–630. [Google Scholar]
- Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- Hunter KD, Thurlow JK, Fleming J, Drake PJ, Vass JK, Kalna G, Higham DJ, Herzyk P, Macdonald DG, Parkinson EK, et al. Divergent routes to oral cancer. Cancer Res. 2006;66:7405–13. doi: 10.1158/0008-5472.CAN-06-0186. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655–67. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–5. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kresty LA, Mallery SR, Knobloch TJ, Song H, Lloyd M, Casto BC, Weghorst CM. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62:5295–300. [PubMed] [Google Scholar]
- Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J. Biol. Chem. 1996;271:15346–15352. doi: 10.1074/jbc.271.26.15346. [DOI] [PubMed] [Google Scholar]
- Lin HY, Sun M, Tang HY, Simone TM, Wu YH, Grandis JR, Cao HJ, Davis PJ, Davis FB. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J Cell Biochem. 2008;104:2131–2142. doi: 10.1002/jcb.21772. [DOI] [PubMed] [Google Scholar]
- Linke T, Dawson H, Harrison EH. Isolation and characterization of a microsomal acid retinyl ester hydrolase. J Biol Chem. 2005;280:23287–94. doi: 10.1074/jbc.M413585200. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23:346–56. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin a deficiency. J Biol Chem. 2005;280:40226–34. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–10. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O, McMurphey A, Ludwick J, El-Naggar AK, Bucana C, et al. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–9. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarelli E, Liu DD, Lee JJ, El-Naggar AK, Lo Muzio L, Staibano S, De Placido S, Myers JN, Papadimitrakopoulou VA. Akt activation correlates with adverse outcome in tongue cancer. Cancer. 2005;104:2430–6. doi: 10.1002/cncr.21476. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Gad MZ, Harrison EH, Ross AC. Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocyte and nonparenchymal cell fractions of rat liver. J Nutr. 1997;127:218–24. doi: 10.1093/jn/127.2.218. [DOI] [PubMed] [Google Scholar]
- Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Dannenberg AJ. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890–5. [PubMed] [Google Scholar]
- Moise AR, Golczak M, Imanishi Y, Palczewski K. Topology and membrane association of lecithin: retinol acyltransferase. J Biol Chem. 2007;282:2081–90. doi: 10.1074/jbc.M608315200. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–79. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–70. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Morisaki H, Ando A, Nagata Y, Pereira-Smith O, Smith JR, Ikeda K, Nakanishi M. Complex mechanisms underlying impaired activation of Cdk4 and Cdk2 in replicative senescence: roles of p16, p21, and cyclin D1. Exp Cell Res. 1999;253:503–10. doi: 10.1006/excr.1999.4698. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Shintani S, Mihara M, Kiyota A, Ueyama Y, Matsumura T. Alterations of Rb, p16(INK4A) and cyclin D1 in the tumorigenesis of oral squamous cell carcinomas. Cancer Lett. 2000;160:3–8. doi: 10.1016/s0304-3835(00)00546-2. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Niles RM. Vitamin A and cancer. Nutrition. 2000;16:573–576. doi: 10.1016/s0899-9007(00)00347-6. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Urade M, Hashitani S, Noguchi K, Manno Y, Takaoka K, Sakurai K. Increased expression of cyclooxygenase (COX)-2 in DMBA-induced hamster cheek pouch carcinogenesis and chemopreventive effect of a selective COX-2 inhibitor celecoxib. J Oral Pathol Med. 2004;33:614–21. doi: 10.1111/j.1600-0714.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem. 2005;280:35647–57. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitrakopoulou V, Izzo J, Lippman SM, Lee JS, Fan YH, Clayman G, Ro JY, Hittelman WN, Lotan R, Hong WK, et al. Frequent inactivation of p16INK4a in oral premalignant lesions. Oncogene. 1997;14:1799–803. doi: 10.1038/sj.onc.1201010. [DOI] [PubMed] [Google Scholar]
- Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871–5. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Pozzi S, Bistulfi G, Somenzi G, Rossetti S, Sacchi N. Impaired retinoic acid (RA) signal leads to RARbeta2 epigenetic silencing and RA resistance. Mol Cell Biol. 2005;25:10591–603. doi: 10.1128/MCB.25.23.10591-10603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: perspectives from studies on vitamin A status. J Nutr. 2004;134:269S–275S. doi: 10.1093/jn/134.1.269S. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schoelch ML, Regezi JA, Dekker NP, Ng IO, McMillan A, Ziober BL, Le QT, Silverman S, Fu KK. Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:333–42. doi: 10.1016/s1368-8375(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cell cycle control and cancer. Harvey Lect. 2000a;96:73–92. [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000b;60:3689–95. [PubMed] [Google Scholar]
- Sherr CJ. Principles of Tumor Suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Suzui M, Yoshimi N, Tsuda H, Wakabayashi K, Tanaka T. An animal model for the rapid induction of tongue neoplasms in human c-Ha-ras proto-oncogene transgenic rats by 4-nitroquinoline 1-oxide: its potential use for preclinical chemoprevention studies. Carcinogenesis. 2006;27:619–30. doi: 10.1093/carcin/bgi241. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kojima T, Okumura A, Yoshimi N, Mori H. Alterations of the nucleolar organizer regions during 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Carcinogenesis. 1991;12:329–33. doi: 10.1093/carcin/12.2.329. [DOI] [PubMed] [Google Scholar]
- Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- Tang XH, Su D, Albert MR, Scognamiglio T, Gudas LJ. Overexpression of lecithin:retinol acyltransferase in epithlial basal layer makes mice more sensitive to oral cavity carcinogenesis induced by a carcinogen. 2008. Submitted. Submitted. [DOI] [PMC free article] [PubMed]
- Tang XH, Suh MJ, Li R, Gudas LJ. Cell proliferation inhibition and alterations in retinol esterification induced by phytanic acid and docosahexaenoic acid. J Lipid Res. 2007;48:165–76. doi: 10.1194/jlr.M600419-JLR200. [DOI] [PubMed] [Google Scholar]
- Tang XH, Vivero M, Gudas LJ. Overexpression of CRABPI in suprabasal keratinocytes enhances the proliferation of epidermal basal keratinocytes in mouse skin topically treated with all-trans retinoic acid. Exp Cell Res. 2008;314:38–51. doi: 10.1016/j.yexcr.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Todd R, Hinds PW, Munger K, Rustgi AK, Opitz OG, Suliman Y, Wong DT. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002;13:51–61. doi: 10.1177/154411130201300106. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the Epithelial Stem Cell Niche in Skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:1563–7. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanatha JK, Swinney R, Banerjee AG. Modulation of annexin I and cyclooxygenase-2 in smokeless tobacco-induced inflammation and oral cancer. Mol Cell Biochem. 2003;248:67–75. doi: 10.1023/a:1024153431272. [DOI] [PubMed] [Google Scholar]
- Vitale-Cross L, Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Chemical carcinogenesis models for evaluating molecular-targeted prevention and treatment of oral cancer. Cancer Prevention Res. 2009 doi: 10.1158/1940-6207.CAPR-09-0058. In press. [DOI] [PubMed] [Google Scholar]
- Wan H, Oridate N, Lotan D, Hong WK, Lotan R. Overexpression of retinoic acid receptor β in head and neck squamous cell carcinoma cells increases their sensitivity to retinoid-induced suppression of squamous differentiation by retinoids. Cancer Res. 1999;59:3518–3526. [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Huang H, Pan C, Zhang B, Liu X, Zhang L. Nicotine inhibits apoptosis induced by cisplatin in human oral cancer cells. Int J Oral Maxillofac Surg. 2007;36:739–44. doi: 10.1016/j.ijom.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–7. [PubMed] [Google Scholar]
- Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004;117:761–71. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Tanaka T, Hirose Y, Yamaguchi F, Kohno H, Toida M, Hara A, Sugie S, Shibata T, Mori H. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005;221:29–39. doi: 10.1016/j.canlet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Tanaka T, Kohno H, Sakata K, Kawamori T, Mori H, Wakabayashi K. A COX-2 inhibitor, nimesulide, inhibits chemically-induced rat tongue carcinogenesis through suppression of cell proliferation activity and COX-2 and iNOS expression. Histol Histopathol. 2003;18:39–48. doi: 10.14670/HH-18.39. [DOI] [PubMed] [Google Scholar]