Abstract
Animal experiments show that the kidney contributes to apolipoprotein (apo)A-I catabolism. We tested relationships of HDL cholesterol (HDL-C) and apo-I with kidney function in subjects without severe chronic kidney disease. Included was a random sample of the general population (part of the PREVEND cohort). Kidney function [estimated glomerular filtration rate (e-GFR) by two well-established equations and creatinine clearance], HDL-C, triglycerides, apoA-I and insulin resistance (HOMAir) were measured in 2,484 fasting subjects (e-GFR≥45 ml/min/1.73m2) without macroalbuminuria, cardiovascular disease, diabetes, or the use of anti-hypertensives and/or lipid-lowering agents. HDL-C (r = −0.056 to −0.102, P < 0.01 to < 0.001) and apo A-I (r = −0.096 to −0.126, P < 0.001) were correlated inversely with both GFR estimates and creatinine clearance in univariate analyses. Multiple linear regression analyses also demonstrated inverse relationships of HDL-C and apoA-I with all measures of kidney function even after adjustment for age, sex, waist circumference, HOMAir, triglycerides, and urinary albumin excretion (P = 0.053 to 0.004). In conclusion, HDL-C and apoA-I are inversely related to e-GFR and creatinine clearance in subjects without severely compromised kidney function, which fits the concept that the kidney contributes to apoA-I regulation in humans. High glomerular filtration rate may be an independent determinant of a pro-atherogenic lipoprotein profile.
Keywords: e-GFR, creatinine clearance, HDL cholesterol, apolipoprotein A-II
Epidemiological studies have repeatedly demonstrated an inverse relationship between the risk of atherosclerotic cardiovascular disease and plasma levels of high-density lipoprotein cholesterol (HDL-C), as well as of its major apolipoprotein (apo), apoA-I (1, 2). Despite proven efficacy, primary and secondary prevention trials have shown that there remains a considerable residual cardiovascular risk during cholesterol-lowering treatment (3). In view of anti-atherogenic properties of HDL, this lipoprotein fraction has been identified as a therapeutic target (4–7). Therefore, progress in our understanding of HDL metabolism and HDL function is expected to contribute to the development of novel strategies in order to reduce the burden of atherosclerotic disease in humans (5, 6, 8).
The metabolism of HDL particles, which comprise a heterogeneous group of lipoproteins, is in a complex way regulated by many factors, including lipases, lipid transfer proteins, and cellular receptors (4, 6, 8). The liver and intestine are major sites of apoA-I secretion and hence contribute to HDL particle generation (8–10). Importantly, studies in rats, rabbits, and hamsters have shown that a major fraction of radiolabeled apoA-I is being taken up by the kidney in vivo (11–13), with the proximal renal tubule representing a likely site for apoA-I uptake and degradation (14). A plausible mechanism that may explain the role of the kidney in apoA-I catabolism includes filtration of apoA-I through the glomerular basement membrane and subsequent proximal tubular uptake via the cubilin-megalin-amnionless complex, which enables endocytosis of HDL-derived proteins (9, 10, 15, 16).
Despite the prominent role of the kidney in apoA-I catabolism as inferred from animal experiments (9–14) and the observation that increased catabolism of apoA-I is a predominant kinetic abnormality in humans with low HDL-C (17), the relationship of HDL-C and apoA-I in subjects without severely compromised kidney function has received little attention. In fact, most studies have focused on low HDL-C and apoA-I levels in subjects with severe chronic kidney disease (18–22). Few epidemiological studies have determined the relationship of HDL-C with estimates of glomerular filtration rate (GFR) in populations with low prevalence of severe chronic kidney disease (23–25). Using data from the Kaiser Permanente Renal Registry, it was found that the prevalence of low HDL-C was increased in subjects with an estimated GFR (e-GFR) ≤ 60 ml/min/1.73m2, irrespective of obesity (23). An analysis from the population-based PREVEND (“Prevention of REnal and Vascular ENd stage Disease”) cohort demonstrated the lowest HDL-C in subjects with the highest compared with subjects with the lowest creatinine clearance (24). In apparent contrast, a report from the Multi-Ethnic Study of Atherosclerosis showed among subjects with e-GFR > 60 ml/min/1.73m2 that HDL-C and HDL particle concentrations were related inversely to serum cystatin-C levels, as a measure of renal function (25). Of note, in these studies, the relationship of kidney function with apoA-I was not determined (23–25). Moreover, in several of these reports (24, 25), associations of HDL-C with kidney function were not controlled for factors affecting both kidney function and HDL-C, such as obesity and insulin resistance (26–28).
The present study was initiated to test the hypothesis that HDL-C and apoA-I are inversely associated with GFR in subjects without severely compromised renal function. To this end, we carried out cross-sectional analyses in a random sample of the general population that makes part of the PREVEND cohort. Fasting subjects were included, and we took potential confounding due to obesity, insulin resistance, and serum triglycerides into account.
SUBJECTS AND METHODS
Study participants
This study was conducted among subjects who participate in the Prevention of REnal and Vascular End-stage Disease (PREVEND) study, which began in 1997. This prospective cohort study in the city of Groningen (The Netherlands) investigates the natural course of urinary albumin excretion and its relation to renal and cardiovascular disease. The study was approved by the local medical ethics committee. All participants gave written informed consent. Details of the study protocol have been published elsewhere (29, 30). In summary, all inhabitants of the city of Groningen aged 28–75 years were sent a questionnaire and a vial to collect a first-morning-void urine sample (prescreening). Of these subjects, 40,856 responded (47.8%) and returned a vial to a central laboratory for urinary albumin and urinary creatinine assessment. From these 40,856 subjects, the PREVEND cohort was selected with the aim to create a cohort enriched for the presence of high urinary albumin excretion. After exclusion of insulin-using diabetic patients and pregnant women (defined by self-report), all subjects with a urinary albumin concentration of ≥10 mg/L (n = 7,768) were invited, of whom 6,000 participated. Furthermore, a randomly selected control group with a urinary albumin concentration of <10 mg/L (n = 3,394) was invited, and 2,592 participated. These 8,592 subjects constitute the entire PREVEND cohort and were asked to collect two consecutive 24 h urine samples (baseline screening). In order to compose a “random sample” representative of the Groningen population, we considered a subcohort of the 8,592 subjects. For this purpose, we included all subjects with a urinary albumin concentration of <10 mg/L who completed the first screening (n = 2,592) and added a subset of the “oversampled” subjects whose urinary albumin concentration was >10 mg/L by proportionally taking a computer-generated, random subset (n = 840) (31). After exclusion of 18 participants known to have proteinuria or renal disease, a cohort of 3,414 participants was created. As expected, the characteristics of this “random sample” were similar to that of the original population (n = 40,856) (32).
Because we aimed to test a physiological relationship of HDL-C and apoA-I with kidney function, we selected individuals without manifest disease. Therefore, we excluded subjects with an estimated glomerular filtration rate (e-GFR) ≤ 45 ml/min/1.73 m2 [assessed using the Modification of Diet in Renal Disease (MDRD) equation; see below] and urinary albumin excretion >300 mg/24 h. We also excluded subjects with prior history of cardiovascular disease or with diabetes mellitus (see definitions), as well as subjects using any anti-hypertensives or lipid-lowering agents (including diuretics, angiotensin converting enzyme inhibitors, angiotensin antagonists, statins, and fibrates) in order to avoid bias attributable to associations of prevalent cardiovascular disease with (apo)lipoproteins and interference due to HDL effects of lipid-lowering and anti-hypertensive drugs. Individuals who were nonfasting at the time of screening were not allowed to participate to be able to calculate insulin resistance. Finally, we excluded subjects with missing values for HDL-C, apoA-I and apoA-II, and triglycerides (n = 24), leaving a study population consisting of 2,484 subjects.
Measurements, definitions, and renal function estimates
Participants underwent two visits to the outpatient research unit for the baseline survey. All participants completed a questionnaire on demographics, cardiovascular disease history, and medication use prior to their first visit. Height and weight were measured on the first visit; body mass index (BMI) was calculated as the ratio between weight and height squared (in kg/m2). Waist circumference was measured on the bare skin between the 10th rib and iliac crest. During the first and second visit, blood pressure was measured in supine position every min for 10 min with an automatic device (Dinamap XL Model 9300, Johnson-Johnson Medical, Tampa, FL). Blood pressure values are given as the mean of the last two recordings of both visits. The participants collected two 24 h urine samples for measurement of creatinine and albumin excretion; microalbuminuria and macroalbuminuria were defined as mean urinary albumin excretion between 30 and 300 mg/24 h, and >300 mg/24 h, respectively (30–32). Participants were instructed to remain fasting for at least 8 h before blood sampling, which was done at the second visit. Diabetes mellitus was diagnosed by fasting plasma glucose ≥7.0 mmol/l according to 1997 American Diabetes Association criteria (33) or use of glucose-lowering drugs. Furthermore, information on medication use was checked using pharmacy-dispensing data from all community pharmacies in the city of Groningen, which covers complete information on drug use in 80% of PREVEND participants. Insulin resistance was estimated using homeostasis model assessment (HOMAir), which was quantified as insulin · glucose/22.5 (34).
Three estimates of kidney function were applied: e-GFR calculated with the MDRD equation (35), e-GFR calculated with the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (36) (supplementary Table I) and the mean of two 24 h creatinine clearance measurements. All estimates of kidney function were corrected for 1.73 m2 of body surface area, which was calculated as 0.007184 · (height in cm) 0.725 · (body weight) 0.425 (37).
Laboratory methods
Plasma glucose was measured shortly after blood collection. For other measurements, serum samples were stored at −80°C until analysis. Serum and urinary creatinine, serum total cholesterol, and plasma glucose were measured using Kodak Ektachem dry chemistry (Eastman Kodak, Rochester, NY) with intra- and inter-assay coefficients of variation (CVs) < 3%. HDL-C was measured with a homogeneous method (direct HDL, no. 7D67, AEROSETTM System, Abbott Laboratories, Abbott Park, IL). In this assay system, HDL and apoB-containing lipoproteins are complexed with one reagent, followed by solubilizing HDL particles by another reagens (38). Serum triglycerides were measured enzymatically. Serum apoA-I and apoA-II were determined by nephelometry applying commercially available reagents for Dade Behring nephelometer systems [BN II, Dade Behring, Marburg, Germany; apoA-I test kit, code no. OUED; apoA-II test kit, code no. OQBA, apo B test kit, code no. OSAN (39)]. Intra- and inter-assay CVs of apoA-I, apoA-II, and HDL-C were all <5%. Urinary albumin concentration was determined by nephelometry, with a threshold of 2.3 mg/l and intra- and inter-assay CVs <3% (BNII; Dade Behring).
Statistical analysis
Data are given in mean ± SD for parametrically distributed variables and in median (interquartile range) for variables with a skewed distribution. Univariate correlations were calculated using Pearson's regression coefficients. We additionally estimated the continuous relationships between HDL-C, apoA-I and apoA-II, and eGFR (MDRD) with polynomial regression analysis. Associations of HDL-C, apoA-I, and apoA-II with kidney function were also determined by dividing GFR estimates and creatinine clearance into quintiles using one-way ANOVA. Multiple linear regression analysis was performed to determine independent relationships between variables. In these analyses, logarithmically transformed values of triglycerides, HOMAir, and urinary albumin excretion were used. Two-sided P-values < 0.05 were considered to be statistically significant.
RESULTS
Table 1 shows clinical and laboratory characteristics of this predominantly (>95%) Caucasian population. e-GFR, as determined by the MDRD equation, was between 45 and 60 ml/min/1.73 m2 in 4%, between 60 and 90 ml/min/1.73 m2 in 72.5% and above 90 ml/min/1.73 m2 23.5% of subjects; 4.6% had microalbuminuria. Systolic blood pressure was <140 mm Hg in 84% and diastolic blood pressure was <90 mm Hg in 97.5% of subjects. A total of 32.5% were current smokers.
TABLE 1.
Clinical characteristics, estimates of glomerular filtration rate, urinary albumin excretion, serum lipids, high density lipoprotein cholesterol, apolipoprotein A-I and apolipoprotein A-II in 2,484 individuals
| Characteristic | |
|---|---|
| Age (years) | 47 ± 12 |
| Sex (men/women) | 1411/1073 |
| Body mass index (kg/m2) | 25.5 ± 3.9 |
| Waist circumference (cm) | 86 ± 12 |
| Systolic blood pressure (mm Hg) | 124 ± 17 |
| Diastolic blood pressure (mm Hg) | 72 ± 9 |
| Serum creatinine (μmol/L) | 82 ± 13 |
| Urinary albumin excretion (mg/24 h) | 6.9 (5.4-10.3) |
| e-GFRMDRD (ml/min/1.73 m2) | 81.2 ± 13.3 |
| e-GFRCDK-EPI (ml/min/1.73 m2) | 85.1 ± 14.3 |
| Creatinine clearance (ml/min/1.73 m2) | 92.1 ± 22.8 |
| Plasma glucose (mmol/L) | 4.59 ± 0.62 |
| Serum insulin (mU/L) | 73 (5.2-10.5) |
| HOMAir (mU X mmol/(L2 X 22.5)) | 1.46 (1.00-2.18) |
| Serum total cholesterol (mmol/L) | 5.59 ± 1.12 |
| Serum triglycerides (mmol/L) | 1.07 (0.78-1.51) |
| HDL-C (mmol/L) | 1.40 ± 0.41 |
| Serum apoA-I (g/L) | 1.43 ± 0.29 |
| Serum apoA-II (g/L) | 0.35 ± 0.06 |
Data are given in mean ± SD or in median (interquartile range). Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; HOMAir, homeostasis model assessment estimated insulin resistance; e-GFR, estimated glomerular filtration rate by MDRD and CDK-EPI equations (see Methods).
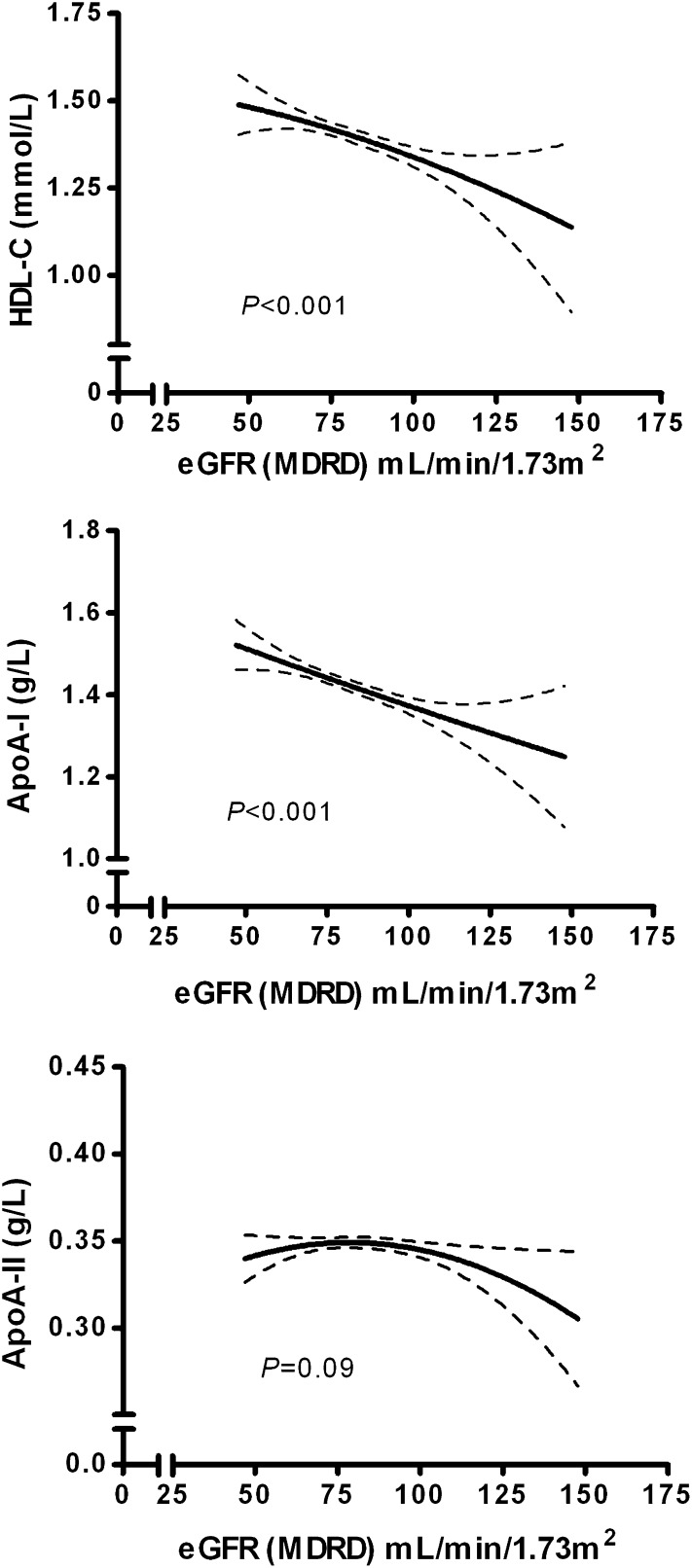
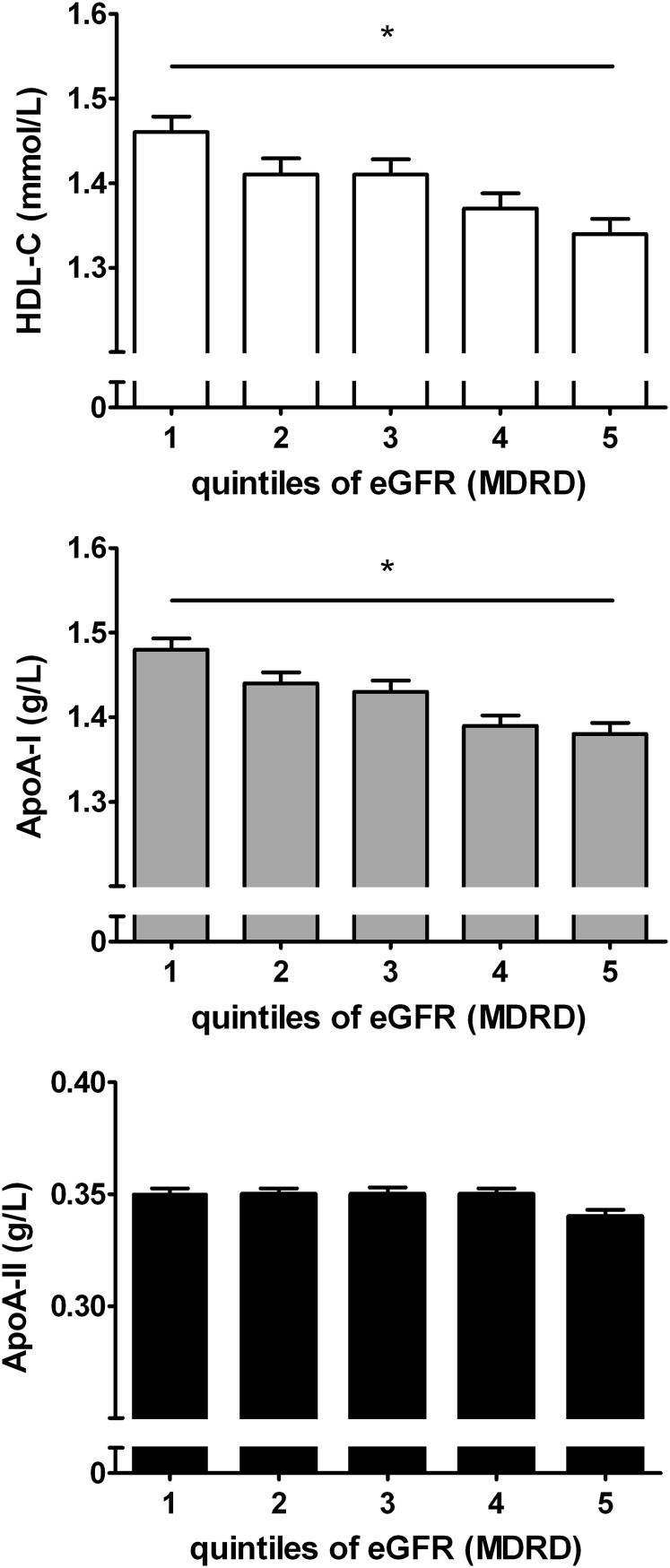
e-GFR calculated using the MDRD equation was strongly correlated with e-GFR calculated using the CKD-EPI equation (r = 0.963, P < 0.001). e-GFR calculated using the MDRD equation and e-GFR calculated using the CKD-EPI equation were also correlated with creatinine clearance, expressed per 1.73 m2 body surface area (r = 0.472, P < 0.001 and r = 0.486, P < 0.001, respectively). HDL-C, apoA-I, and apoA-II levels were strongly interrelated (r = 0.346 to r = 0.712, P < 0.001 for all). As shown in Table 2, HDL-C and apoA-I levels were correlated inversely with e-GFR, calculated using the MDRD and the CKD-EPI equations, as well as with creatinine clearance. HDL-C and apoA-I were also inversely related to waist circumference, HOMAir, triglycerides, and urinary albumin excretion in univariate regression analysis. In contrast, apoA-II was unrelated to e-GFR and creatinine clearance. Likewise, inverse relationships of HDL-C and apoA-I with GFR estimates and creatinine clearance were observed when dividing the participants in normal weight, overweight, and obese individuals (Table 3). The polynomial relationships between HDL-C, apoA-I, and apoA-II with e-GFR, calculated using the MDRD equation, are shown in Fig. 1. Again, inverse relationships of HDL-C and apoA-I but not apoA-II with e-GFR were present. The associations of HDL-C, apoA-I, and apoA-II with quintiles of e-GFR, as estimated by MDRD, are demonstrated in Fig. 2. Both HDL-C and apoA-I were lower in the higher e-GFR quintiles, but this trend was not observed for apoA-II. Similar patterns were observed for the relationship between HDL-C, apoA-I and apoA-II, and e-GFR, as estimated by CKD-EPI, and creatinine clearance (data not shown).
TABLE 2.
Univariate correlations of high density lipoprotein cholesterol, apolipoprotein A-I, and apolipoprotein A-II with renal function, waist circumference, insulin resistance, triglycerides, and urinary albumin excretion in 2,484 individuals
| HDL-C | ApoA-I | ApoA-II | |
|---|---|---|---|
| e-GFRMDRD | −0.102* | −0.126* | −0.022 |
| e-GFRCKD-EPI | −0.056** | −0.096* | 0.001 |
| Creatinine clearance | −0.081* | −0.108* | −0.032 |
| Waist | −0.454* | −0.296* | −0.083* |
| Ln HOMAir | −0.320* | −0.173* | 0.002 |
| Ln Trig | −0.474* | −0.161* | 0.147* |
| Ln UAE | −0.108* | −0.086* | −0.042*** |
Pearson's correlation coefficients are shown. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; HOMAir, homeostasis model assessment estimated insulin resistance; Ln, logarithmically transformed values; Trig, triglycerides; UAE, urinary albumin excretion; e-GFR, estimated glomerular filtration rate by MDRD and CKD-EPI equations (see Methods). e-GFR and creatinine clearance are expressed per 1.73 m2 of body surface area. * P < 0.001; ** P < 0.01; *** P < 0.05.
TABLE 3.
Univariate correlations of high density lipoprotein cholesterol, apolipoprotein A-I, and apolipoprotein A-II with renal function according to obesity category (50 5% nonobese subjects (BMI < 25 kg/m2); 37.8% subjects with overweight (BMI ≥ 25 kg/m2 and < 30 kg/m2); 11.7% obese individuals [BMI ≥ 30 kg/m2)]
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 and < 30 kg/m2 | BMI≥30 kg/m2 | ||||
|---|---|---|---|---|---|---|
| HDL-C | ApoA-I | HDL-C | ApoA-I | HDL-C | ApoA-I | |
| e-GFRMDRD | −0.153* | −0.179* | −0.133* | −0.138* | −0.156** | −0.088 |
| e-GFRCKD-EPI | −0.115* | −0.153* | −0.111* | −0.125* | −0.125*** | −0.068 |
| Creatinine clearance | −0.070*** | −0.104** | −0.095** | −0.122* | −0.147* | −0.121* |
Pearson's correlation coefficients are shown. Apo, apolipoprotein; HDL-C, high density lipoprotein cholesterol; e-GFR, estimated glomerular filtration rate by MDRD and CKD-EPI equations (see methods). e-GFR and creatinine clearance are expressed per 1.73 m2 of body surface area. * P < 0.001; ** P < 0.01; *** P < 0.05.
Fig. 1.
Continuous relationships of HDL-C, apoA-I, and apoA-II with e-GFR, calculated with the MDRD formula, analyzed by polynomial regression analysis. (upper, middle, and lower panel, respectively). Lines of best fit (polynomial quadratic) with 95% confidence intervals are shown.
Fig. 2.
HDL-C, apoA-I, and apoA-II (upper, middle, and lower panel respectively) according to quintiles of eGFR estimated by the MDRD-equation. Ranges of e-GFR in the increasing quintiles were: 47–70, 70–77, 77–84, 84–92 and 92–148 ml/min/1.73 m2, respectively. HDL-C and apoA-I: * P for trend < 0.001 by one-way ANOVA. ApoA-II, P = 0.83.
Multiple linear regression analyses were performed to determine whether the inverse relationships of HDL-C and apoA-I with GFR estimates and creatinine clearance were independent of waist circumference, HOMAIR, and triglycerides. HDL-C and apoA-I were related inversely to both GFR estimates (Tables 4 and 5) as well as to creatinine clearance (Table 6), independently of waist, HOMAIR, and triglycerides in age- and sex-adjusted models. When urinary albumin excretion was also included in the analyses, the strength of the relationships of HDL-C and apoA-I with e-GFR and creatinine clearance remained unchanged. In these models, the independent relationships of HDL-C and apoA-I with urinary albumin excretion did not reach formal statistical significance (Tables 4ndash6, models 2). Furthermore, the independent relationships of HDL-C and apoA-I with GFR estimates and creatinine clearance were unaltered after additional adjustment for smoking and alcohol consumption (data not shown). In women only, HDL-C and apoA-I were correlated inversely with both GFR estimates and with creatinine clearance (β-coefficients ranging from −0.092 to −0.101, P < 0.001 for all; data not shown), independently of waist, HOMAIR, and triglycerides. When men were analyzed separately, HDL-C and apoA-I were also correlated independently and inversely with creatinine clearance (β-coefficient: −0.065, P = 0.013 and β-coefficient: −0.061, P = 0.043, respectively), whereas HDL-C was related inversely with e-GFR according to the CKD-EPI equation (β-coefficient: −0.056, P = 0.038). In men only, the adjusted relationships of apoA-I with e-GFR calculated with the CKD-EPI formula (β-coefficient: −0.040, P = 0.21) and of HDL-C and apoA-I with e-GFR calculated using the MDRD equation (β-coefficient: −0.030, P = 0.260 and β-coefficient: −0.020, P = 0.531, respectively) did not reach significance (data not shown). In these sex-specific analyses, no inverse relationships of apoA-II with any of the GFR estimates and creatinine clearance were observed in women or in men.
TABLE 4.
Multiple linear regression analyses demonstrating relationships of high density lipoprotein cholesterol and apolipoprotein A-I with estimated glomerular filtration rate (e-GFR), creatinine clearance, clinical variables [waist circumference, insulin resistance (HOMAir)], triglycerides, and urinary albumin excretion in 2,484 individuals. Analyses with e-GFR based on MDRD equation (e-GFRMDRD); model 1 without and model 2 with urinary albumin excretion.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| HDL-C |
ApoA-I |
HDL-C |
ApoA-I |
|||||
| β | P | β | P | β | P | β | P | |
| e-GFRMDRD | −0.042 | 0.018 | −0.040 | 0.038 | −0.040 | 0.024 | −0.040 | 0.053 |
| Waist | −0.209 | <0.001 | −0.165 | <0.001 | −0.206 | <0.001 | −0.161 | <0.001 |
| Ln HOMAir | −0.068 | 0.001 | −0.070 | 0.002 | −0.067 | 0.001 | −0.068 | 0.003 |
| Ln Trig | −0.31 | <0.001 | −0.030 | 0.163 | −0.331 | <0.001 | −0.030 | 0.163 |
| Ln UAE | −0.026 | 0.118 | −0.034 | 0.071 | ||||
All models are adjusted for age and sex. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; HOMAir, homeostasis model assessment estimated insulin resistance; Ln, logarithmically transformed values; Trig, triglycerides; UAE, urinary albumin excretion; β, standardized regression coefficient.
TABLE 5.
Multiple linear regression analyses demonstrating relationships of high density lipoprotein cholesterol and apolipoprotein A-I with estimated glomerular filtration rate (e-GFR), creatinine clearance, clinical variables [waist circumference, insulin resistance (HOMAir)], triglycerides, and urinary albumin excretion in 2,484 individuals. Analyses with e-GFR based on CKD-EPI equation (e-GFRCKD-EPI); model 1 without and model 2 with urinary albumin excretion.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| HDL-C |
ApoA-I |
HDL-C |
ApoA-I |
|||||
| β | P | β | P | β | P | β | P | |
| e-GFRCKD-EPI | −0.051 | 0.008 | −0.051 | 0.024 | −0.049 | 0.011 | −0.048 | 0.033 |
| Waist | −0.208 | <0.001 | −0.165 | <0.001 | −0.205 | <0.001 | −0.161 | <0.001 |
| Ln HOMAir | −0.069 | 0.001 | −0.070 | 0.002 | −0.067 | 0.001 | −0.068 | 0.003 |
| Ln Trig | −0.332 | <0.001 | −0.030 | 0.157 | −0.332 | <0.001 | −0.030 | 0.155 |
| Ln UAE | −0.025 | 0.120 | −0.034 | 0.071 | ||||
All models are adjusted for age and sex. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; HOMAir, homeostasis model assessment estimated insulin resistance; Ln, logarithmically transformed values; Trig, triglycerides; UAE, urinary albumin excretion; β, standardized regression coefficient.
TABLE 6.
Multiple linear regression analyses demonstrating relationships of high density lipoprotein cholesterol and apolipoprotein A-I with estimated glomerular filtration rate (e-GFR), creatinine clearance, clinical variables [waist circumference, insulin resistance (HOMAir)], triglycerides and urinary albumin excretion in 2,484 individuals. Analyses with creatinine clearance (in ml/min/1.73 m2); model 1 without and model 2 with urinary albumin excretion.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| HDL-C |
ApoA-I |
HDL-C |
ApoA-I |
|||||
| β | P | β | P | β | P | β | P | |
| Creatinine clearance | −0.048 | 0.004 | −0.050 | 0.011 | −0.048 | 0.012 | −0.043 | 0.033 |
| Waist | — | <0.001 | −0.163 | <0.001 | −0.207 | <0.001 | −0.160 | <0.001 |
| Ln HOMAir | −0.064 | 0.001 | −0.065 | 0.005 | −0.063 | 0.001 | −0.064 | 0.005 |
| Ln Trig | −0.335 | <0.001 | −0.033 | 0.121 | −0.334 | <0.001 | −0.033 | 0.128 |
| Ln UAE | −0.018 | 0.296 | −0.026 | 0.180 | ||||
All models are adjusted for age and sex. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; HOMAir, homeostasis model assessment estimated insulin resistance; Ln, logarithmically transformed values; Trig, triglycerides; UAE, urinary albumin excretion; β, standardized regression coefficient.
In addition, sensitivity analyses were carried out in the subjects with urinary albumin excretion < 30 mg/24 h (n = 2370), as well as in subjects with e-GFR using the MDRD equation ≥ 60 ml/min/1.73 m2 (n = 2385). In subjects with urinary albumin excretion <30 mg/24 h, the independent relationships of HDL-C and apoA-I with both GFR estimates and creatinine clearance were essentially unaltered (β-coefficients ranging from −0.040 to −0.050, P-values ranging from 0.063 to 0.006; data not shown). Likewise in subjects with e-GFR ≥ 60 ml/min/1.73 m2, the relations of HDL-C and apoA-I with GFR estimates and creatinine clearance remained unchanged (β-coefficients ranging from −0.036 to −0.046, P-values ranging from 0.055 to 0.012; data not shown).
DISCUSSION
This population-based study has demonstrated inverse relationships of HDL-C and its qualitatively most abundant apolipopoprotein, apoA-I, with GFR, estimated using the MDRD as well as the CDK-EPI equations in subjects without severe chronic kidney disease. Importantly, similar relationships were found with creatinine clearance, which underscores the robustness of our findings. Such inverse relationships of HDL-C and apoA-I with kidney function were also present in subjects without and with overweight or obesity. Furthermore, multiple linear regression analyses showed inverse relationships of HDL-C and apoA-I with all three measures of kidney function that were independent of central obesity, insulin resistance, and serum triglycerides. Additional analyses in each gender separately yielded essentially similar findings. Thus, the present results support the possibility that renal hemodynamic factors may contribute to apoA-I metabolism, thereby affecting HDL-C levels.
There is a paucity of reports that are primarily aimed to determine the relationships of HDL-C and apoA-I with renal hemodynamics in subjects without severe chronic kidney disease. The inverse relationships of HDL-C and apoA-I with kidney function agree with some reported data obtained in the whole PREVEND cohort (24). Apparently opposite findings were found when stratifying subjects without renal insufficiency according to plasma cystatin-C levels (25), although in adolescent American Aboriginals (40), in subjects with essential hypertension (41), or in type 2 diabetic individuals (42) no significant associations of HDL-C with cystatin-C levels could be demonstrated. It was long believed that cystatin C concentration is a good marker of kidney function, being independent of demographic variables such as muscle mass, weight, or disease states. Recent studies have suggested that cystatin C concentration is not only dependent on kidney function, but also on other factors, such as age, gender, smoking, race and metabolic factors (43–45), which may at least in part explain the discrepancy with the previous report (25).
The kidney represents a major site of apoA-I clearance (11–14), but takes up very little HDL-derived cholesterol (13). It is, therefore, plausible that the kidney metabolizes a lipid-poor form of apoA-I contained in preβ-HDL particles (46). Importantly, evidence is accumulating that proximal renal tubules are able to endocytose HDL-associated proteins, including apoA-I and apoM, as well as other proteins, via the cubulin-megalin-amnionless complex (9, 10, 16, 47). Of note, urinary excretion of apoA-I is strongly increased in dogs and humans with functional cubulin deficiency (15) and in humans with renal tubular reabsorption failure due to Fanconi syndrome (46). Altogether, these findings agree with the concept that in normal circumstances, apoA-I is present in the glomerular ultrafiltrate, and that reabsorption by the proximal renal tubule prevents its urinary excretion (9, 10). Although it is likely that apoA-II, the second most abundant HDL-associated apolipoprotein, is a cubulin ligand as well (48), apoA-II is not present in the urine of Fanconi syndrome patients (46). The strong hydrophobicity of apoA-II probably may impair its dissociation from mature, cholesterol-rich HDL particles, and hence prevents its glomerular passage into the preurine (46). In view of these differences in renal apoA-I and apo-II handling (46), we also determined the relationships of apoA-II with GFR estimates in the present study. Despite expectedly strong interrelationships between apoA-I, apoA-II, and HDL-C, apoA-II was unrelated to any measure of GFR. It is obvious that the mechanisms responsible for the inverse relationship of HDL-C and apoA-I but not apoA-II with GFR, as shown here in the PREVEND population, are not known precisely. We interpret the current findings to be consistent with the hypothesis that glomerular ultrafiltration and subsequent tubular reabsorption and degradation contributes to apoA-I metabolism in humans.
Several methodological aspects of our study need to be considered. First, we decided to test our hypothesis that a higher GFR is a determinant of lower HDL-C and apoA-I levels in a more or less healthy population. Therefore, the present study was carried out in the so-called “random sample” of the PREVEND cohort in order to avoid potential bias due to overrepresentation of microalbuminuric subjects (32). Besides prevalent cardiovascular disease, diabetes, and use of potentially interfering anti-hypertensive and lipid-lowering medications, we also excluded subjects with an estimated GFR ≤ 45 ml/min/1.73 m2, as based on the MDRD formula. This cut-off level is somewhat arbitrary but was also chosen in the Kaiser Permanente Renal Registry report (23). Of note, a sensitivity analysis on subjects with GFR ≥ 60 ml/min/1.73 m2, indicating absence of moderate GFR decrease, yielded similar results. Second, we also carried out a sensitivity analysis in subjects without microalbuminuria. Again, the strength of the relationships of HDL-C and apoA-I with GFR estimates was unaltered. Third, it should be recognized that the MDRD equation has not been developed for estimating kidney function at GFR values > 60 ml/min · 1.73m2 and it's accuracy in this range has been criticized. However, most epidemiological studies have used the MDRD equation for estimating GFR. To make our study comparable to those in the literature (23–25), we decided to adopt e-GFR, calculated using the MDRD equation, as our selection criterium. Recently, the CKD-EPI equation was developed, aiming to provide reliable e-GFR values especially at higher ranges (36). Importantly, our study showed inverse relationships of HDL-C and apoA-I with kidney function, irrespective of GFR being estimated by the MDRD equation, the CDK-EPI equation, or creatinine clearance.
It is increasingly appreciated that kidney function is influenced by metabolic factors such as overweight and insulin resistance (26, 27), which also coincide with lower HDL-C and apoA-I levels (4, 28). Moreover, microalbuminuria has been shown to be associated with higher triglycerides, lower HDL-C, and altered lipoprotein subfraction levels (49). Such interrelationships require that these metabolic factors should be taken into account when addressing the contribution of GFR to HDL-C and apoA-I in a population-based study. It is, therefore, relevant that inverse correlations of HDL-C and apoA-I with kidney function were observed in each weight category, and that these relationships were independent of obesity, insulin resistance, triglycerides, and urinary albumin excretion in multivariable regression analyses.
The inverse relationships of HDL-C and apoA-I with kidney function in subjects without severe renal insufficiency, as shown in this report, clearly opposes the well-established lower levels of HDL-C and apoA-I in subjects with chronic kidney disease (19–22). In this situation, multiple abnormalities in the metabolism of HDL and other lipoproteins have been demonstrated, including impaired cholesterol esterification, increased lipid transfer protein levels, and reduced hepatic lipase activity (18). In experimental chronic renal failure, hepatic apoA-I expression is downregulated (10, 18). Accordingly, apoA-I production may be reduced in humans with chronic kidney disease (50). In contrast, another report suggested increased apoA-I clearance in hemodialysis patients (51). Thus, whereas our findings raise the possibility of an inverse “U-shaped” relationship of HDL-C and apoA-I with glomerular filtration rate along the whole spectrum of kidney function, abnormalities in apoA-I kinetics in subjects with severe chronic kidney disease remain to be defined precisely. Finally, given cardioprotective properties of HDL and apoA-I (1, 2, 4–6), the currently observed inverse relationship of HDL-C and apoA-I with kidney function agrees with the hypothesis that high glomerular filtration rate is a determinant of a pro-atherogenic lipoprotein profile. This concept is in line with the contention that glomerular hyperfiltration may be regarded as an indicator of metabolic risk, including low HDL-C even in apparently healthy adolescent men (52). However, the extent to which lower HDL-C in subjects with high glomerular filtration rate affects HDL's functional properties, and could modify effects of kidney function on (cardiovascular) mortality, awaits further study.
In conclusion, serum HDL-C and apoA-I are inversely associated with glomerular filtration rate in subjects without severely compromised kidney function. These findings fit the concept that the kidney contributes to apoA-I regulation in humans.
Acknowledgments
The laboratory work of J. J. Duker and J. van der Wal-Haneveld is appreciated. We thank Dade Behring (Marburg, Germany) for supplying equipment (Behring Nephelometer II) and analytes for the determination of apolipoproteins and other metabolites.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BMI
- body mass index
- CDK-EPI
- Chronic Kidney Disease Epidemiology Collaboration
- CV
- coefficient of variation
- e-GFR
- estimated GFR
- GFR
- glomerular filtration rate
- HDL-C
- HDL cholesterol
- HOMAir
- homeostasis model assessment of insulin resistance
- MDRD
- Modification of Diet in Renal Disease
This study is supported by a grant from the Netherlands Heart Foundation (grant 2001.005).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a table.
REFERENCES
- 1.Vega G. L., Grundy S. M. 1996. Hypoalphalipoproteinemia (low high density lipoprotein cholesterol) as a risk factor for coronary heart disease. Curr. Opin. Lipidol. 7: 209–216. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto P., Collins R. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 207: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 3.Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. 2002. Circulation. 106: 3143–3421. [PubMed] [Google Scholar]
- 4.Borggreve S. E., de Vries R., Dullaart R. P. F. 2003. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur. J. Clin. Invest. 33: 1051–1069. [DOI] [PubMed] [Google Scholar]
- 5.Linsel-Nitschke P., Tall A. R. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4: 193–205. [DOI] [PubMed] [Google Scholar]
- 6.Singh I. M., Shishehbor M. H., Ansell B. J. 2007. High-density lipoprotein as a therapeutic target: a systematic review. J. Am. Med. Assoc. 298: 786–798. [DOI] [PubMed] [Google Scholar]
- 7.Dullaart R. P. F., Dallinga-Thie G. M., Wolffenbuttel B. H. R., van Tol A. 2007. CETP inhibition in cardiovascular risk management: a critical appraisal. Eur. J. Clin. Invest. 37: 90–98. [DOI] [PubMed] [Google Scholar]
- 8.Lewis G. F., Rader D. J. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 9.Moestrup S. K., Kozyraki R. 2000. Cubilin, a high-density lipoprotein receptor. Curr. Opin. Lipidol. 11: 133–140. [DOI] [PubMed] [Google Scholar]
- 10.Moestrup S. K., Nielsen L. B. 2005. The role of the kidney in lipid metabolism. Curr. Opin. Lipidol. 16: 301–306. [DOI] [PubMed] [Google Scholar]
- 11.Glass C. K., Pittman R. C., Keller G. A., Steinberg D. 1983. Tissue sites of degradation of apoprotein A-I in the rat. J. Biol. Chem. 258: 7161–7167. [PubMed] [Google Scholar]
- 12.Braschi S., Neville T. A., Maugeais C., Ramsamy T. A., Seymour R., Sparks D. L. 2000. Role of the kidney in regulating the metabolism of HDL in rabbits: evidence that iodination alters the catabolism of apolipoprotein A-I by the kidney. Biochemistry. 39: 5441–5449. [DOI] [PubMed] [Google Scholar]
- 13.Woollett L. A., Spady D. K. 1997. Kinetic parameters for high density lipoprotein apoprotein AI and cholesteryl ester transport in the hamster. J. Clin. Invest. 99: 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallinga-Thie G. M., van 't Hooft F. M., van Tol A. 1986. Tissue sites of high density lipoprotein A-IV in rats. Arteriosclerosis. 6: 277–284. [DOI] [PubMed] [Google Scholar]
- 15.Kozyraki R., Fyfe J., Kristiansen M., Gerdes C., Jacobsen C., Cui S., Christensen E. I., Aminoff M., de la Chapelle A., Krahe R., et al. 1999. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat. Med. 5: 656–661. [DOI] [PubMed] [Google Scholar]
- 16.Hammad S. M., Barth J. L., Knaak C., Argraves W. S. 2000. Megalin acts in concert with cubilin to mediate endocytosis of high density lipoproteins. J. Biol. Chem. 275: 12003–12008. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz B. S., Goldberg I. J., Merab J., Vanni T. M., Ramakrishnan R., Ginsberg H. N. 1993. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J. Clin. Invest. 91: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaziri N. D. 2006. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290: F262–F272. [DOI] [PubMed] [Google Scholar]
- 19.Muntner P., Hamm L. L., Kusek J. W., Chen J., Whelton P. K., He J. 2004. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann. Intern. Med. 140: 9–17. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Muntner P., Hamm L. L., Jones D. W., Batuman V., Fonseca V., Whelton P. K., He J. 2004. The metabolic syndrome and chronic kidney disease in US adults. Ann. Intern. Med. 140: 167–174. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak M. G., Fried L. F., Cushman M., Manolio T. A., Peterson D., Stehman-Breen C., Bleyer A., Newman A., Siscovick D., Psaty B. 2005. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 13: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 22.Parikh N. I., Hwang S. J., Larson M. G., Meigs J. B., Levy D., Fox C. S. 2006. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch. Intern. Med. 166: 1884–1891. [DOI] [PubMed] [Google Scholar]
- 23.Lo J. C., Go A. S., Chandra M., Fan D., Kaysen G. A. 2007. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am. J. Kidney Dis. 50: 552–558. [DOI] [PubMed] [Google Scholar]
- 24.Verhave J. C., Hillege H. L., Burgerhof J. G., Gansevoort R. T., de Zeeuw D., de Jong P. E.; PREVEND Study Group. 2005. The association between atherosclerotic risk factors and renal function in the general population. Kidney Int. 67: 1967–1973. [DOI] [PubMed] [Google Scholar]
- 25.de Boer I. H., Astor B. C., Kramer H., Palmas W., Seliger S. L., Shlipak M. G., Siscovick D. S., Tsai M. Y., Kestenbaum B. 2008. Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin. J. Am. Soc. Nephrol. 3: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dengel D. R., Goldberg A. P., Mayuga R. S., Kairis G. M., Weir M. R. 1996. Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension. 28: 127–132. [DOI] [PubMed] [Google Scholar]
- 27.Krikken J. A., Lely A. T., Bakker S. J., Navis G. 2007. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int. 71: 260–265. [DOI] [PubMed] [Google Scholar]
- 28.Borggreve S. E., Hillege H. L., Wolffenbuttel B. H., de Jong P. E., Bakker S. J., van der Steege G., van Tol A., Dullaart R. P.; PREVEND Study Group. 2005. The effect of cholesteryl ester transfer protein -629C->A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J. Clin. Endocrinol. Metab. 90: 4198–4204. [DOI] [PubMed] [Google Scholar]
- 29.Hillege H. L., Janssen W. M., Bak A. A., Diercks G. F., Grobbee D. E., Crijns H. J., Van Gilst W. H., De Zeeuw D., De Jong P. E.; Prevend Study Group. 2001. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 249: 519–526. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Sietsma S. J., Janssen W. M. T., Hillege H. L., Navis G., de Jong P. E. 2000. Urinary albumin excretion is associated with renal functional abnormalities in a non-diabetic population. J. Am. Soc. Nephrol. 11: 1882–1888. [DOI] [PubMed] [Google Scholar]
- 31.Gansevoort R. T., Verhave J. C., Hillege H. L., Burgerhof J. G., Bakker S. J., de Zeeuw D., de Jong P. E.; for the PREVEND Study Group. 2005. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int. Suppl. 94: S28–S35. [DOI] [PubMed] [Google Scholar]
- 32.Lambers Heerspink H. J., Brantsma A. H., de Zeeuw D., Bakker S. J., de Jong P. E., Gansevoort R. T.; PREVEND Study Group. 2008. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am. J. Epidemiol. 168: 897–905. [DOI] [PubMed] [Google Scholar]
- 33.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. 1997. Report of the Expert Committee on the Diagnosis and Classification of diabetes mellitus. Diabetes Care. 20: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 34.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 35.Levey A. S., Bosch J. P., Lewis J. B., Greene T., Rogers N., Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 36.Levey A. S., Stevens L. A., Schmid C. H., Zhang Y. L., Castro A. F., 3rd, Feldman H. I., Kusek J. W., Eggers P., Van Lente F., Greene T., et al. 2009. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois D., DuBois E. F. 1916. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 17: 863–871. [Google Scholar]
- 38.Warnick G. R., Nauck M., Rifai N. 2001. Evolution of methods for measurement of HDL cholesterol: from ultracentrifugation to homogenous assays. Clin. Chem. 47: 1579–1596. [PubMed] [Google Scholar]
- 39.Steinmetz J., Tarallo P., Fournier B., Caces E., Siest G. 1995. Reference limits of apolipoprotein A-I and apolipoprotein B using an IFCC standardized immunonephelometric method. Eur. J. Clin. Chem. Clin. Biochem. 33: 337–342. [DOI] [PubMed] [Google Scholar]
- 40.Retnakaran R., Connelly P. W., Harris S. B., Zinman B., Hanley A. J. 2007. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr. Nephrol. 22: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe S., Okura T., Liu J., Miyoshi K., Fukuoka T., Hiwada K., Higaki J. 2003. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens. Res. 26: 895–899. [DOI] [PubMed] [Google Scholar]
- 42.Connelly P. W., Zinman B., Maguire G. F., Mamakeesick M., Harris S. B., Hegele R. A., Retnakaran R., Hanley A. J. 2009. Association of the novel cardiovascular risk factors paraoxonase 1 and cystatin C in type 2 diabetes. J. Lipid Res. 50: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight E. L., Verhave J. C., Spiegelman D., Hillege H. L., de Zeeuw D., Curhan G. C., de Jong P. E. 2004. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 65: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 44.Stevens L. A., Schmid C. H., Greene T., Li L., Beck G. J., Joffe M. M., Froissart M., Kusek J. W., Zhang Y. L., Coresh J., et al. 2009. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 75: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manetti L., Pardini E., Genovesi M., Campomori A., Grasso L., Morselli L. L., Lupi I., Pellegrini G., Bartalena L., Bogazzi F., et al. 2005. Thyroid function differently affects serum cystatin C and creatinine concentrations. J. Endocrinol. Invest. 28: 346–349. [DOI] [PubMed] [Google Scholar]
- 46.Graversen J. H., Castro G., Kandoussi A., Nielsen H., Christensen E. I., Norden A., Moestrup S. K. 2008. Pivotal role of the human kidney in catabolism of HDL protein components apolipoprotein A-I and A-IV but not of A-II. Lipids. 43: 467–470. [DOI] [PubMed] [Google Scholar]
- 47.Faber K., Hvidberg V., Moestrup S. K., Dahlbäck B., Nielsen L. B. 2006. Megalin is a receptor for apolipoprotein M, and kidney-specific megalin-deficiency confers urinary excretion of apolipoprotein M. Mol. Endocrinol. 20: 212–218. [DOI] [PubMed] [Google Scholar]
- 48.Rousset X., Château D., Pastier D., Klein C., Demeurie J., Cywiner-Golenzer C., Chabert M., Verroust P., Chambaz J., Châtelet F. P., et al. 2007. Apolipoprotein A-II is catabolized in the kidney as a function of its plasma concentration. J. Lipid Res. 48: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 49.de Boer I. H., Astor B. C., Kramer H., Palmas W., Rudser K., Seliger S. L., Shlipak M. G., Siscovick D. S., Tsai M. Y., Kestenbaum B. 2008. Mild elevations of urine albumin excretion are associated with atherogenic lipoprotein abnormalities in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 197: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuh M. M., Lee C. M., Jeng C. Y., Shen D. C., Shieh S. M., Reaven G. M., Chen Y. D. 1990. Effect of chronic renal failure on high-density lipoprotein kinetics. Kidney Int. 37: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 51.Okubo K., Ikewaki K., Sakai S., Tada N., Kawaguchi Y., Mochizuki S. 2004. Abnormal HDL apolipoprotein A-I and A-II kinetics in hemodialysis patients: a stable isotope study. J. Am. Soc. Nephrol. 15: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 52.Tomaszewski M., Charchar F. J., Maric C., McClure J., Crawford L., Grzeszczak W., Sattar N., Zukowska-Szczechowska E., Dominiczak A. F. 2007. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 71: 816–821. [DOI] [PubMed] [Google Scholar]