Abstract
Accumulation of cholesterol in arterial macrophages may contribute to diabetes-accelerated atherosclerotic cardiovascular disease. The ATP-binding cassette transporter ABCA1 is a cardioprotective membrane protein that mediates cholesterol export from macrophages. Factors elevated in diabetes, such as reactive carbonyls and free fatty acids, destabilize ABCA1 protein in cultured macrophages, raising the possibility that impaired ABCA1 plays an atherogenic role in diabetes. We therefore examined the modulation of ABCA1 in two mouse models of diabetes. We isolated peritoneal macrophages, livers, kidneys, and brains from type 1 non-obese diabetic (NOD) mice and mice made diabetic by viral-induced autoimmune destruction of pancreatic β-cells, and we measured ABCA1 protein and mRNA levels and cholesterol contents. ABCA1 protein levels and cholesterol export activity were reduced by 40–44% (P < 0.01) in peritoneal macrophages and protein levels by 48% (P < 0.001) in kidneys in diabetic NOD mice compared with nondiabetic animals, even though ABCA1 mRNA levels were not significantly different. A similar selective reduction in ABCA1 protein was found in peritoneal macrophages (33%, P < 0.05) and kidneys (35%, P < 0.05) from the viral-induced diabetic mice. In liver and brain, however, diabetes had no effect or slightly increased ABCA1 protein and mRNA levels. The reduced ABCA1 in macrophages and kidneys was associated with increased cholesterol content. Impaired ABCA1-mediated cholesterol export could therefore contribute to the increased atherosclerosis and nephropathy associated with diabetes.
Keywords: ATP-binding cassette transporter A1, cardiovascular disease, liver
A hallmark of the developing atherosclerotic lesion is the accumulation of cholesterol in arterial macrophages. An important determinant of macrophage cholesterol content is ABCA1, a sterol-induced membrane protein that mediates the transport of excess cholesterol from cells to lipid-poor apolipoprotein (apo)A-I, the major protein component of HDLs (1). Mutations in human ABCA1 are associated with a severe HDL deficiency, cholesterol deposition in tissue macrophages, and prevalent cardiovascular disease (2). Over-expressing ABCA1 in mice significantly decreases atherosclerosis (3), whereas ablating ABCA1 in stem-cell transferred mouse macrophages increases atherosclerotic lesions (4, 5). Thus, ABCA1 plays a critical role in protecting against cardiovascular disease.
We showed previously that diabetes-associated metabolic factors impair ABCA1 function by destabilizing the protein in vitro. Reactive carbonyl precursors for advance glycation end products (AGEs), which are increased in both types 1 and 2 diabetes (6–9), acutely and severely suppress ABCA1 cholesterol export activity and reduce ABCA1 protein levels in cultured cells (10). Unsaturated fatty acids, which can be elevated in poorly controlled type 1 diabetes and are often elevated in type 2 diabetes and the metabolic syndrome (11–13), increase ABCA1 degradation through a phospholipase D/protein kinase C δ signaling pathway that phosphorylates ABCA1 serines (14–17).
These studies raise the possibility that diabetes impairs the ABCA1 cholesterol export pathway in vivo, leading to increased accumulation of cholesterol in arterial macrophages and enhanced atherogenesis (18–20). In support of this idea are our studies showing that inducing diabetes in cholesterol-fed swine markedly increased atherosclerotic lesion size in association with a dramatic reduction in the level of immunodectable ABCA1 in lesion foam-cell macrophages (10).
Here, we examined the effects of type 1 diabetes on ABCA1 protein and mRNA levels in mouse macrophages and tissues. Results show that inducing diabetes in two different type 1 diabetic mouse models reduced the ABCA1 protein content of peritoneal macrophages and the kidney without reducing ABCA1 mRNA levels. In contrast, diabetes had no effect or slightly increased ABCA1 protein and mRNA levels in the liver and brain. These results are consistent with the idea that diabetes selectively impairs ABCA1 protein expression in a cell-specific manner, which may contribute to the cardiovascular and renal complications associated with diabetes.
METHODS
Animals
Female non-obese diabetic (NOD) mice (Taconic), aged 6–8 weeks, were maintained in a temperature-controlled room (22°C) with a 12 h light/dark cycle and given free access to food and water.
All animal studies were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC), and were performed following the IACUC guidelines for the care and use of laboratory animals.
To accelerate the development of diabetes, 9-week-old female NOD mice received one intra-peritoneal injection of cyclophosphamide (300 mg/kg) (Sigma) in sterile water. Nondiabetic controls were injected with sterile water alone. Diabetes was defined as glucose levels of >250 mg/dl on two consecutive readings. Peritoneal macrophages and tissues were collected 5 days after mice became diabetic (2–6 weeks after injection). With some mice, peritoneal macrophages were elicited by injection of thioglycolate (40 mg per mouse in 1 ml water) 3 days before sacrifice.
Transgenic LDLR−/−; GP mice were generated and diabetes was induced as previously described (21). Briefly, female LDL receptor (LDLR)−/− mice crossed with mice transgenic for the lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP) under control of the insulin promoter were injected with LCMV or a saline solution at 7–10 weeks of age. Regular mouse chow diet (LabDiet®, 5053) and water were provided ad libitum. Tissues and peritoneal macrophages from the diabetic and saline-injected nondiabetic littermates were collected 5 weeks (4 weeks after diabetes developed) after LCMV or saline injection. To elicit peritoneal macrophages, mice were injected with thioglycolate 5 days prior to sacrifice.
Macrophages were collected in PBS by peritoneal lavage and immediately chilled on ice, and cells were centrifuged and stored frozen. Whole livers, kidneys, and brains were removed from the animals and immediately frozen for storage.
Plasma lipids and glucose and tissue cholesterol
Blood was collected from retro-orbital plexus or the saphenous vein. Plasma total cholesterol was measured with colorimetric assay kits (Diagnostic Chemicals Limited). HDL cholesterol was measured after selective precipitation of apoB-containing lipoproteins with polyethylene glycol. Plasma triglycerides were measured using a colorimetric assay kit (Roche Diagnostics). Blood glucose levels were measured using the LifeScan OneTouch Ultra Meter.
To measure the percentage macrophages accumulating neutral lipid droplets, thioglycolate-elicited macrophages were harvested, counted, and plated in serum free RPMI medium. After 1 h, cells were washed with PBS and fixed with 4% PFA for 30 min. They were then stained using Oil Red O for 30 min followed by counterstaining using hematoxylin for 20 min. Oil Red O positive cells were counted as % of total counted cells (always at least 100) in a masked fashion.
Tissue/cell free and esterified cholesterol were measured using a cholesterol oxidase assay as previous described (22) and normalized to cell or tissue protein levels. Proteins were measured by the method of Lowry (22).
ABCA1 and other protein levels
Frozen powdered tissue or pelleted peritoneal macrophages were suspended for 1 h in ice cold TBS buffer (1 X TBS, pH 7.4, 1.0% triton X-100, protease inhibitors). Homogenates were sonicated once for 20 s and centrifuged at 15,000 g at 4°C for 10 min. The protein concentrations of the supernatants were determined, and equal amounts of protein (50 µg for macrophages and 100 µg for tissues) were resolved by 6% SDS-PAGE gel followed by transfer to nitrocellulose membranes. Protein levels were measured by immunoblots using antibodies for ABCA1 and ABCG1 (Novus Biological, 1:1000 dilution), scavenger receptor A1 (Santa Cruz Biotechnology, 1:300 dilution), CD36 (Santa Cruz Biotechnology, 1:100 dilution), and GAPDH (Chemicon International, 1: 10,000 dilution). Immunoreactivity was detected by enhanced chemiluminescence, quantified using the OptiQuant computer program, and normalized to GAPDH levels in each tissue or cell sample.
AGE modified ABCA1 was isolated by immunoprecipitation. Whole kidney or peritoneal macrophage cells were homogenized in TBS buffer, 3 µg anti-AGE antibody (from ABcam) was added into 1ml 1× TBS buffer containing 2000 µg protein from kidney or 350 µg protein from peritoneal macrophages, and incubated overnight in the cold. The immuno-complexes were precipitated using protein G Dynabeads (from Invitrogen) and resolved in 5% SDS-PAGE. AGE-modified ABCA1 was detected by immunoblot analysis using ABCA1 antibody.
ABCA1 and ABCG1 mRNA levels
The one-step real-time RT-PCR was performed to quantify ABCA1 and ABCG1 mRNA using the Mx4000 Multiplex Quantitative PCR System (Stratagene, La Jolla, CA) and the Brilliant Single-step Quantitative RT-PCR Core Reagent Kit (Stratagene). Thermal cycling conditions consisted of an initial reverse transcription step at 45°C for 30 m, followed by 10 m at 95°C, then followed by 40 cycles of amplification. Each cycle of amplification consisted of a denaturizing step at 95°C for 30 s and an annealing/extension step at 60°C for 1 m. The ABCA1 primers used for the RT-PCR were forward: 5′-GGACATGCACAAGGTCCTGA-3′; reverse: 5′-CAGAAAATCCTGGAGCTTCAAA-3′; and the probe was 5′-FAM-AATGTTACGGCAGATCAAGCATCCCAAC-BHQ1–3′. The ABCG1 primers for the RT-PCR were forward: 5′-CCTTCCTCAGCATCATGCG-3′; Reverse: 5′- CCGATCCCAATGTGCGA-3′; and the probe was 5′-6FAM-CTCGGTCCTGACACATCTGCGAATCAC-TARMA-3′. The GAPDH primers for the RT-PCR are forward: 5′-AGCCTCGTCCCGTAGACAAA-3′; reverse: 5′-ACCAGGCGCCCAATACG-3′; and the probe was 5′hEX-AAATCCGTTCACACCGACCTTCACCA-BHQ1–3′.
Cholesterol export activity
Thioglycolate-elicited macrophages were suspended in DMEM medium containing 1 mg/ml BSA, 50 μg/ml acetylated LDL, and 1 μC/ml [3H]cholesterol. After plating for 1 h, cells were washed twice with PBS/albumin and incubated for 2.5 h with DMEM/albumin minus or plus 10 μg/ml apoA-I or 50 μg/ml HDL. The medium was collected, centrifuged to removed detached cells, and assayed for [3H]cholesterol. Acceptor-specific cholesterol efflux was calculated by subtracting values obtained without acceptor from those obtained in the presence of either apoA-I or HDL. HDL was isolated from human plasma by ultracentrifugation and apoA-I was purified from HDL as described (22).
RESULTS
Hyperglycemia and elevated lipids in two mouse models of type 1 diabetes
We examined two different mouse models for the effects of type 1 diabetes on macrophage and tissue ABCA1 levels. We used autoimmune diabetic NOD mice as a model for relatively early stages of diabetes. These mice spontaneously develop type 1 diabetes within several weeks after injection of an antibiotic such as cyclophosphamide. We used LDLR−/−; GP mice as a model for the effects of more sustained diabetes. These mice are a cross between LDLR-deficient mice and rat insulin promoter-LCMV transgenic mice, which express the viral GP as a self antigen under the control of the insulin promoter. After LCMV injection, an immune reaction specifically destroys the GP-expressing pancreatic β cells, and mice develop type 1 diabetes in 1–2 weeks (21). Inducing diabetes in these mice increases atherosclerotic lesions after 12 weeks on either a cholesterol-free or -enriched diet (21). We obtained plasma, macrophages, and tissues from chow-fed diabetic and nondiabetic NOD mice 5 days after they became diabetic (glucose levels of >250 mg/dl) and from LDLR−/−; GP mice 4 weeks after the onset of diabetes.
Measurements of plasma glucose and lipids showed that inducing diabetes dramatically increased glucose and moderately increased triglycerides to comparable levels in each mouse model (Table 1). Because they lack the LDL receptors required for clearing cholesterol-rich lipoproteins, the LDLR−/−; GP mice had higher total cholesterol and lower HDL cholesterol levels than NOD mice. With both mouse models, however, the diabetic mice had significantly higher total cholesterol levels than the controls (Table 1). There was no difference in HDL cholesterol levels between diabetic and control NOD mice, but the diabetic LDLR−/−; GP mice had higher HDL levels compared with controls.
TABLE 1.
Blood glucose and lipid levels in control and diabetic NOD and LDLR−/−;GP mice
| NOD |
LDLR−/−;GP |
|||
|---|---|---|---|---|
| Control (n) | Diabetic (n) | Control (n) | Diabetic (n) | |
| Glucose (mmol/l) | 5.0 ± 1.3 (25) | 22.7 ± 71 (18)* | 8.1 ± 1.2 (5) | 26.1 ± 3.6 (10)* |
| Triglycerides (mmol/l) | 0.88 ± 0.39 (25) | 1.36 ± 0.64 (18)* | 0.64 ± 0.72 (5) | 1.32 ± 0.72 (10)* |
| Cholesterol (mmol/l) | 1.82 ± 0.28 (25) | 2.15 ± 0.53 (18)* | 4.00 ± 0.23 (5) | 5.41 ± 1.56 (10)* |
| HDL-C (mmol/l) | 1.51 ± 0.26 (25) | 1.69 ± 0.49 (18) | 0.18 ± 0.05 (5) | 0.31 ± 0.13 (10)* |
Results are means ± SD.
Numbers of animals per group are in parentheses.
*P < 0.05 compared with controls (Student's t-test).
Diabetes selectively reduces ABCA1 protein and activity in mouse macrophage
Because regulation of ABCA1 expression occurs at both transcriptional and posttranscriptional levels in a tissue-specific manner (23), we measured both ABCA1 protein and mRNA levels in select tissues and cells. ABCA1 protein was first detected by immunoblot analyses of equal amounts of cell or tissue protein and then quantified by scanning densitometry. ABCA1 mRNA was measured by RT-PCR on the same samples. All values for ABCA1 protein and mRNA were normalized to those obtained for GAPDH in the same samples. There were no detectable differences in GAPDH expression between control and diabetic mouse cells and tissues.
Based on our previous studies showing that metabolic factors increased in diabetes reduce ABCA1 protein levels in macrophages (10, 15), we measured ABCA1 expression in freshly isolated peritoneal macrophages. Because of the only 5 day period of diabetes, we initially chose not to elicit macrophages in NOD mice, which necessitated combining cells from two mice to obtain enough material for single protein and mRNA analyses. With LDLR−/−; GP mice, we elicited macrophages for 5 days by thioglycolate injections, which generated enough cells from a single mouse for multiple assays.
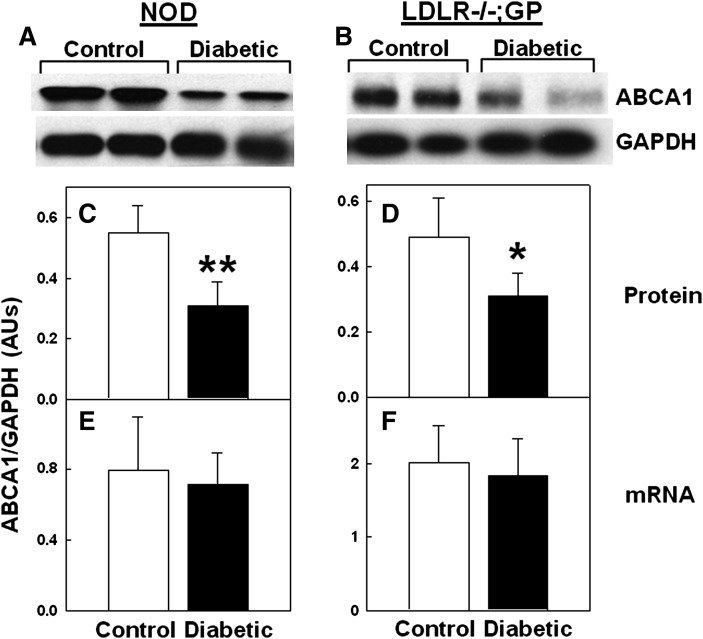
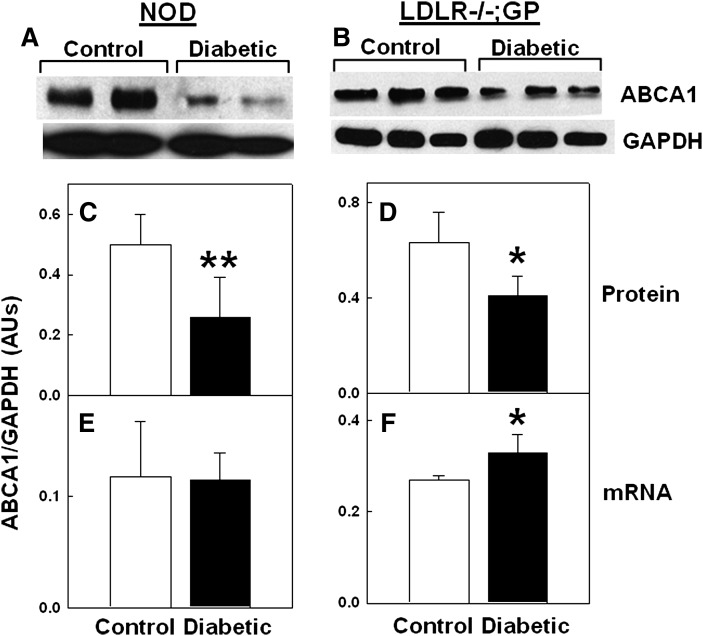
Immunoblots of macrophages from either NOD or LDLR−/−; GP mice revealed that macrophage ABCA1 protein levels per amount of total cell protein were lower in diabetic mice compared with controls, whereas GAPDH levels were unaffected by diabetes (examples in Fig. 1A, B). Quantifying scans showed that inducing diabetes reduced the macrophage ABCA1 to GAPDH protein levels by 44% (P < 0.01) in NOD mice (Fig. 1C) and by 33% (P < 0.05) in LDLR−/−; GP mice (Fig. 1D). ABCA1 mRNA levels, however, were not significantly different between diabetic and nondiabetic mice for both models (Fig. 1E, F). These results show that inducing diabetes in these mice selectively reduced macrophage ABCA1 protein levels.
Fig. 1.
Diabetes-induced reduction in ABCA1 protein but not in mRNA levels in mouse macrophages. Resident (A, C, E) or thioglycollate-elicited (B, D, F) peritoneal macrophages were isolated from control and diabetic mice and assayed for ABCA1 protein and mRNA levels. A, B: Representative sets of ABCA1 and GAPDH immunoblots of the same amount of macrophage protein (50 μg/lane). C, D: Densitometric quantification of ABCA1 relative to GAPDH protein levels. E, F: Real-time RT-PCR quantification of ABCA1 mRNA expressed relative to GAPDH mRNA. Each value is the mean ± SD from seven pairs of mice (14 total) (C, E) or 5 individual mice (D, F). *P < 0.05, **P < 0.01 versus control (Student's t-test).
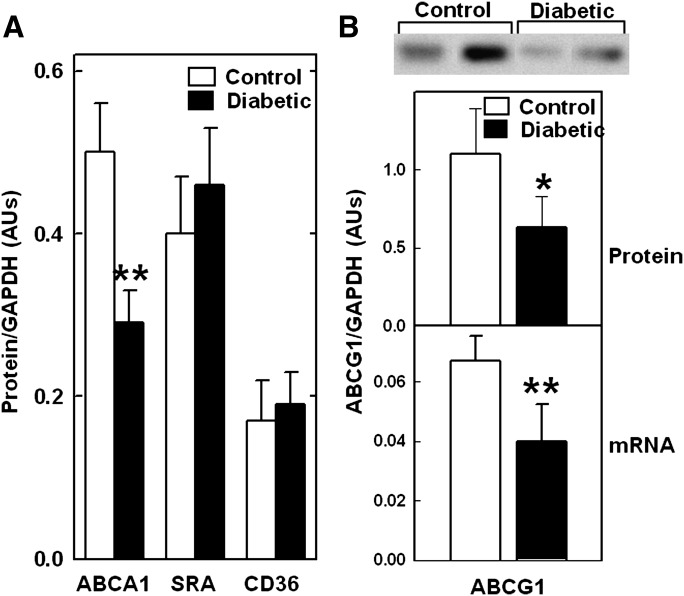
To examine the specificity of the effects of diabetes on macrophage protein expression, we compared ABCA1 protein levels with those of scavenger receptor A1 and CD36, two other plasma membrane proteins that mediate lipoprotein interactions with macrophages (24). To generate enough cells from a single animal for multiple measurements, we elicited peritoneal macrophages in NOD mice by thioglycolate injections 3 days prior to sacrificing the mice. As with resident macrophages (Fig. 1), inducing diabetes reduced ABCA1 protein levels in elicited macrophages by greater than 40% (Fig. 2). In contrast, the protein levels of scavenger receptor A1 and CD36 were not significantly affected by diabetes. We also measured CD36 mRNA levels (not shown) and found no difference between normal and diabetic mice. These results support our previous cell-culture studies showing that ABCA1 protein is uniquely sensitive to diabetes-associated metabolic factors (14, 15, 25).
Fig. 2.
Diabetes-induced reduction in macrophage ABCA1 and ABCG1 but not in scavenger receptor A1 (SRA) and CD36. Thioglycolate-elicited peritoneal macrophages were isolated from control and diabetic NOD mice, (A) ABCA1, scavenger receptor A1 (SRA), CD36, (B) ABCG1, and (A, B) GAPDH protein levels were quantified from scans of immunoblots. B: ABCG1 mRNA was quantified by RT-PCR and expressed relative to GAPDH mRNA. Results are the mean ± SD of the ratio of protein to GAPDH for 5 (protein) or 10 (mRNA) animals per group. *P < 0.04, **P < 0.001 versus control (Student's t-test).
Previous studies showed that transcription of ABCG1, a macrophage transporter that promotes cholesterol efflux to HDL particles, was reduced in mouse models and humans with type 2 diabetes, and this could be mimicked by incubating mouse peritoneal macrophages with high glucose (26–28). We therefore tested if inducing type 1 diabetes in NOD mice had similar effects on ABCG1. Diabetes reduced both ABCG1 protein and mRNA levels in peritoneal macrophages from NOD mice by ∼40% (Fig. 2B). Thus, inducing type 1 diabetes also reduced ABCG1 levels, but as with type 2 diabetes, this appears to occur at the level of transcription.
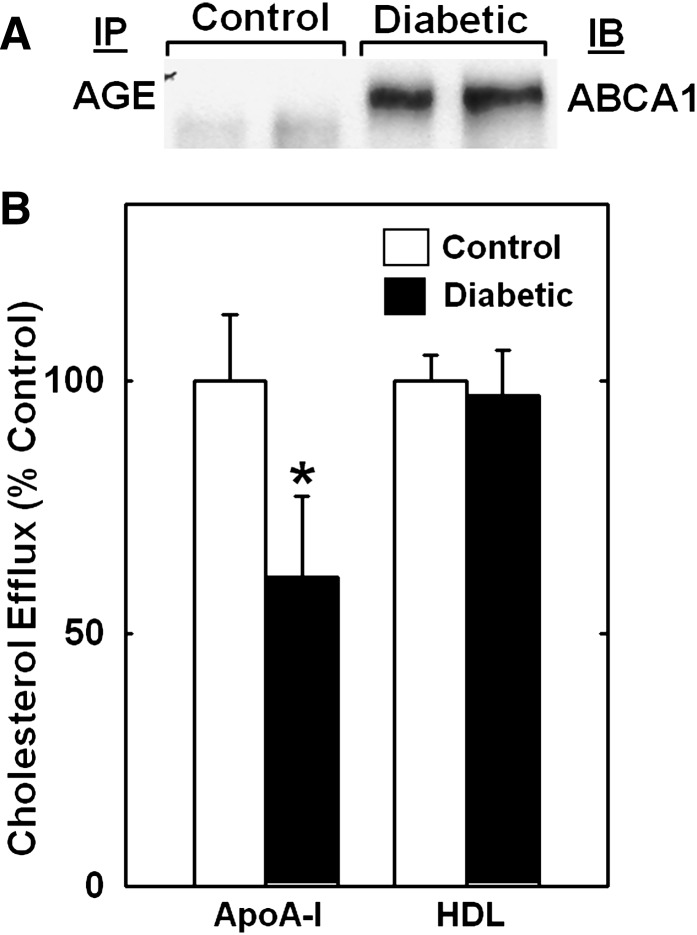
We showed previously that treating cultured macrophages with the reactive carbonyls glycoaldehyde and glyoxal, oxidative products of glucose that are AGE precursors, severely impaired the cholesterol export activity of ABCA1. Because the diabetic NOD mice were markedly hyperglycemic, we tested the possibility that macrophage ABCA1 was modified by AGEs by immunoprecipitating AGE-modified proteins and probing for ABCA1. No detectable ABCA1 was immunoprecipitated with an AGE antibody from macrophages from nondiabetic control NOD mice, but ABCA1 was readily detectable in AGE precipitates from diabetic mice (Fig. 3A). These results support the idea that inducing diabetes in NOD mice increases AGE adducts on macrophage ABCA1.
Fig. 3.
Diabetes-induced AGE modification of macrophage ABCA1 and a reduction in apoA-I-mediated cholesterol efflux from macrophages. A: Thioglycolate-elicited peritoneal macrophages were isolated from control and diabetic NOD mice, AGE proteins were immunoprecipitated (IP) from lysates, proteins were resolved by SDS PAGE and transferred to nitrocellulose, and ABCA1 was detected by immunoblot (IB) analysis. Results represent three separate experiments. B: Thioglycolate-elicited macrophages from NOD mice were plated for 1 h in acetylated LDL/[3H]cholesterol medium and incubated for 2.5 h minus or plus 10 μg/ml apoA-I or 50 μg/ml HDL, and [3H]cholesterol efflux was assayed. Results are the mean ± SD from four control and diabetic NOD mice performed in triplicate and normalized to control values for each mouse. *P < 0.001 versus control (Student's t-test).
To determine if diabetes impaired the cholesterol export activity of the ABCA1 pathway in macrophages, we labeled macrophages freshly isolated from NOD mice with [3H]cholesterol, and measured apoA-I-mediated [3H]cholesterol efflux, which occurs exclusively through ABCA1 (29). As a control, we measured cholesterol efflux in the presence of HDL particles, which occurs by ABCA1-independent processes. To minimize changes in ABCA1 expression during the cell culture incubations, we plated and labeled cells during only 1 h incubations and limited our subsequent cholesterol efflux incubation to 2.5 h. Previous studies with ABCA1-transfected cells showed that we could measure ABCA1-dependent cholesterol efflux when cell membranes were labeled for only 2 h with cholesterol tracer (30). Inducing diabetes in NOD mice reduced the apoA-I-mediated cholesterol efflux by 40% (Fig. 3B), comparable to the level of protein reduction. Thus, diabetes-associated factors impaired ABCA1-dependent cholesterol efflux from macrophages. In contrast, cholesterol efflux to HDL was unaffected by diabetes, despite a reduction in ABCG1 levels. This may have been because the cholesterol efflux assay was too brief (2.5 h) to involve the ABCG1 pathway.
Diabetes reduces ABCA1 protein in mouse kidneys but not in liver or brain
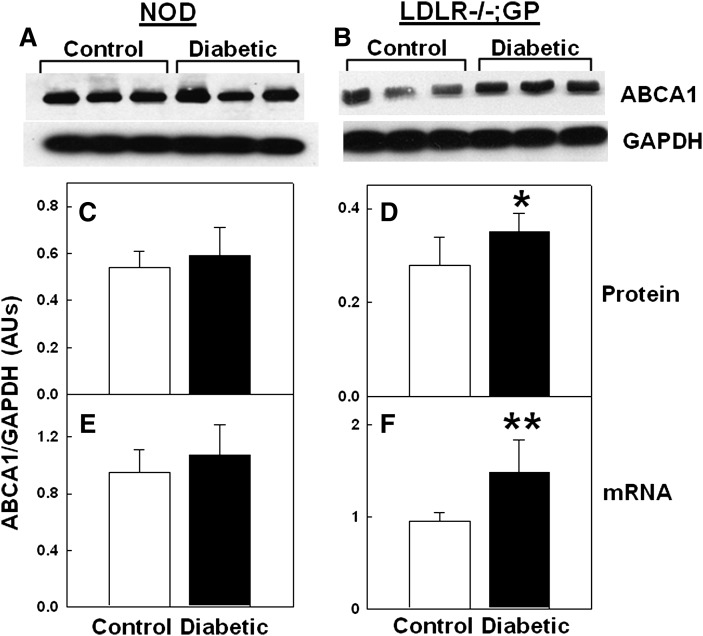
Because hepatic ABCA1 activity is a major determinant of plasma HDL levels in mice (31, 32), we tested the effects of inducing diabetes on ABCA1 expression in mouse. Liver ABCA1 protein and mRNA levels did not differ between diabetic and nondiabetic NOD mice (Fig. 4A, C, E), which is consistent with a lack of a diabetes effect on plasma HDL levels (Table 1). In contrast, the livers of diabetic LDLR−/−; GP mice had significantly higher levels of both ABCA1 protein (P < 0.05) and mRNA (P < 0.02) when compared with control mice (Fig. 4B, D, F), possibly because of longer exposure to elevated blood cholesterol. This may at least partially explain the higher HDL levels in the diabetic LDLR−/−; GP mice (Table 1). These results indicate that diabetes does not selectively reduce ABCA1 protein levels in the liver and may actually increase them.
Fig. 4.
Lack of a diabetes-induced reduction in ABCA1 in mouse livers. Each value is the mean ± SD from 13–15 NOD (C, E) and 5–8 LDLR−/−; GP (D, F) mice. *P < 0.05, **P < 0.02 versus control (Student's t-test).
ABCA1 is also highly expressed in the brain (33) where it may modulate neuronal cholesterol homeostasis. We found no significant differences in ABCA1 protein or mRNA levels in brains from diabetic and nondiabetic NOD mice (data not shown).
Renal disease is a common complication of diabetes (34), and there is evidence that renal function is influenced by the cellular cholesterol content (35). We therefore examined the effects of diabetes on ABCA1 expression in mouse kidneys. Surprisingly, renal ABCA1 protein levels were significantly lower in diabetic NOD (48%, P < 0.001) and LDLR−/−; GP (35%, P < 0.02) mice when compared with nondiabetic controls (Fig. 5A–D). In contrast, ABCA1 mRNA levels did not differ between diabetic and nondiabetic NOD mice (Fig. 5E) and were slightly but significantly (P < 0.02) elevated in diabetic LDLR−/−; GP mice (Fig. 5F). Similar to the data in Fig. 3A, we recovered ABCA1 in immunoprecipitates of AGE-modified proteins from the kidneys of diabetic NOD mice (data not shown). Thus, as with macrophages, inducing diabetes in both NOD and LDLR−/−; GP mice selectively reduced ABCA1 protein levels in the kidney.
Fig. 5.
Diabetes-induced reduction in ABCA1 protein but not mRNA levels in mouse kidneys. Each value is the mean ± SD from 15 NOD (C, E) and 5 LDLR−/−; GP (D, F) mice. *P < 0.02, **P < 0.001 versus control (Student's t-test).
Diabetes increases the cholesterol content of macrophages and kidneys
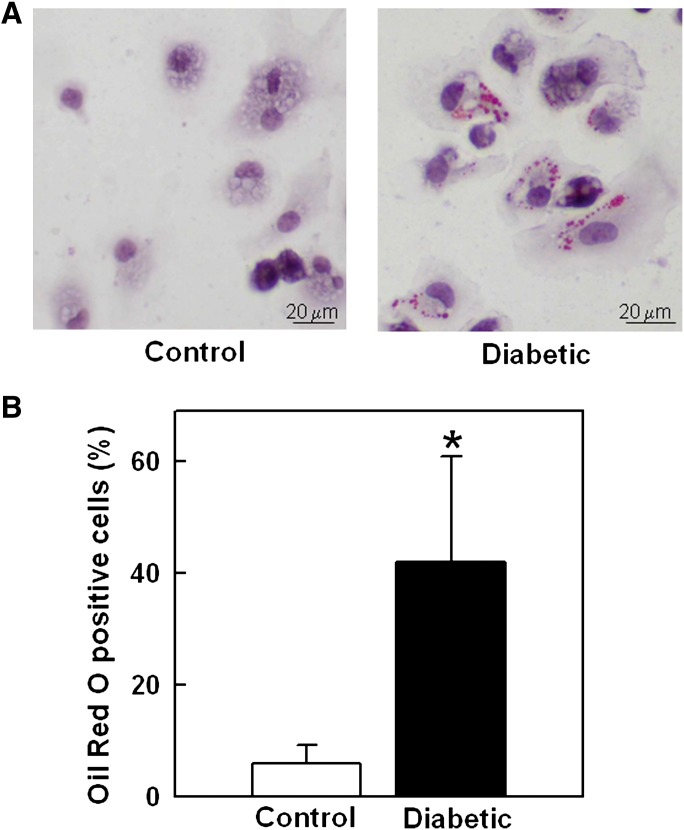
We examined whether the effects of diabetes on ABCA1 protein levels were associated with altered cholesterol homeostasis. When cells accumulate cholesterol in excess of that needed for membrane synthesis, most of it is esterified as neutral lipids. We first examined if inducing diabetes increased neutral lipid droplets in macrophages using an Oil Red O staining protocol. We found that diabetes markedly increased the fraction of peritoneal macrophages from LDLR−/−; GP mice that contained lipid droplets stained with Oil Red O (Fig. 6), consistent with an increased accumulation of cholesteryl esters.
Fig. 6.
Diabetes-induced increased neutral lipid droplets in macrophages from LDLR−/−; GP mice. A: Lipid droplets in peritoneal macrophages from control and diabetic LDLR−/−; GP mice visualized (red) by Oil Red O staining. B: The counted number of Oil Red O positive cells expressed as % of total cells. Results are mean ± SD of values for macrophages from five (non-diabetic) or nine (diabetic) mice plated in two different wells per animal. *P < 0.001 versus control (Student's t-test).
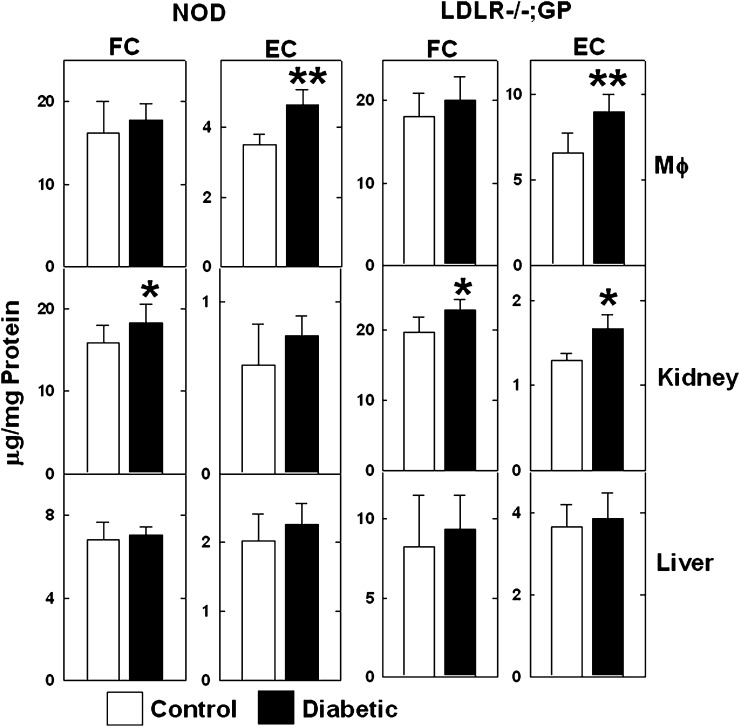
We then examined if diabetes affected the free and esterified cholesterol content of macrophages and tissues. Because these mice were maintained on a low-cholesterol chow diet, the fractional cholesteryl ester content of macrophages and tissues was relatively low (less than 25% of the total cholesterol). However, macrophages from diabetic NOD and LDLR−/−; GP mice had significantly more cholesteryl esters than cells from the nondiabetic controls (Fig. 7). Kidneys from diabetic NOD and LDLR−/−; GP mice had higher levels of free and esterified cholesterol compared with those from control mice, but the difference in cholesteryl esters did not reach significance for NOD mice (Fig. 7). Although there was an upward trend, the free and esterified cholesterol content of livers from diabetic NOD and LDLR−/−; GP mice were not significantly different from those in livers from control mice. These results show that the reduced ABCA1 protein levels in macrophages and kidneys of diabetic mice were associated with increased cellular cholesterol content.
Fig. 7.
Diabetes-induced increased cholesterol content of mouse macrophages and kidneys. The free cholesterol (FC) and esterified cholesterol (EC) mass of macrophages (Mϕ), kidneys, and livers from control and diabetic NOD and LDLR−/−; GP mice were measured and normalized to protein content. Each value is the mean ± SD from 10 NOD and 10 LDLR−/−; GP mice per group. *P < 0.05, **P < 0.02 versus control (Student's t-test).
DISCUSSION
We showed previously that metabolic factors elevated in diabetes, such as reactive carbonyls and free fatty acids, impair ABCA1-dependent cholesterol export from cultured macrophages and other cells by destabilizing ABCA1 protein (10, 14–16). We also showed that inducing diabetes in pigs fed a high-fat/cholesterol diet increased atherosclerosis in association with a dramatic reduction in the ABCA1 immunoreactivity in cholesterol-laden macrophages in atherosclerotic lesions (10). These findings raised the possibility that impaired ABCA1-dependent cholesterol efflux from arterial macrophages contributes to the atherosclerosis caused by diabetes. Here, we provide more evidence to support this idea by showing that diabetes selectively reduces ABCA1 protein levels in peritoneal macrophages from two different mouse models of type 1 diabetes (NOD and LDLR−/−; GP mice) and that this is associated with elevated intracellular cholesteryl esters.
Inducing diabetes significantly reduced the ABCA1 protein content of freshly isolated peritoneal macrophages from both NOD (by 42–44%) and LDLR−/−; GP (by 33%) mice. This was evident whether protein expression was normalized to total cell protein or to GAPDH levels, which were unaffected by diabetes in macrophages and all tissues studied. In contrast, the macrophage ABCA1 mRNA levels were unchanged by diabetes. Diabetes therefore selectively reduced the macrophage ABCA1 protein content, consistent with a posttranscriptional effect. In contrast to ABCA1, diabetes had no effect on the macrophage content of scavenger receptor A and CD36, two other plasma membrane proteins that interact with lipoproteins (24), in support of our cell culture studies showing that ABCA1 protein is uniquely sensitive to diabetic factors (10, 15). Reduced macrophage ABCA1 protein levels were observed after only 5 days (NOD mice) and up to 4 weeks of diabetes (LDLR−/−; GP mice), indicating that this occurred shortly after the onset of diabetes and was sustained for at least several weeks.
Inducing diabetes in NOD mice also reduced apoA-I-mediated cholesterol efflux from freshly isolated peritoneal macrophages to approximately the same extent as it reduced ABCA1 protein levels (40%). In contrast, cholesterol efflux in the presence of HDL, which is ABCA1 independent, was unaffected by inducing diabetes under the conditions of our cholesterol efflux assay. Thus, measurements of both protein mass and cholesterol export activity revealed that macrophage ABCA1 was impaired by diabetes-associated factors.
We also found the inducing type 1 diabetes in NOD mice decreased expression of ABCG1. This was evident by reduced levels of ABCG1 protein and mRNA, indicating that it likely occurred at the level of transcription, as was described previously for the effects of type 2 diabetes on monocyte/macrophage ABCG1 levels (26–28). Thus, diabetes reduces both major macrophage cholesterol exporters but by different mechanisms. It is noteworthy that, of all 49 ABC transporters studied, only expression of ABCA1 and ABCG1 were reduced by high glucose and fatty acids (36).
Tissue-specific expression of ABCA1 in mice revealed that hepatic ABCA1 activity is the major determinant of circulating HDL cholesterol levels (31, 32). We found that diabetes did not affect ABCA1 protein and mRNA levels in livers from NOD mice but significantly increased both protein and mRNA in livers from LDLR−/−; GP mice. Because sterols induce ABCA1, the prolonged exposure of livers in diabetic LDLR−/−; GP mice to higher blood cholesterol levels may have slightly increased ABCA1 transcription. These findings could at least partially explain why inducing diabetes either had no effect or actually increased HDL cholesterol levels, which is also observed in type 1 diabetic humans (37). We also found that inducing diabetes in NOD mice had no effect on ABCA1 protein and mRNA levels in the brain, another tissue that expresses high levels of ABCA1 (33). Thus, ABCA1 in the livers and brains of these mice appears to be resistant to the effects of diabetes.
An unexpected finding from these studies is that diabetes also selectively reduced ABCA1 protein levels in kidneys. ABCA1 is most highly expressed in proximal tubules of mouse kidneys, but it can also be detected in interstitial blood vessel endothelial cells and glomeruli (23). Inducing diabetes decreased kidney ABCA1 protein by nearly 50% in NOD mice and by 35% in LDLR−/−; GP mice. Diabetes did not change ABCA1 mRNA levels in kidneys from NOD mice and actually increased those in LDLR−/−; GP mice, so that the protein to mRNA ratios dropped by identical extents (47%) in each mouse model. Thus, as in macrophages, diabetes selectively reduced posttranscriptional expression of ABCA1 in kidneys.
The selective decrease in ABCA1 protein levels in macrophages and kidneys was associated with increased cellular and tissue cholesterol. Because these mice were maintained on a low-cholesterol chow diet, macrophages did not accumulate cholesteryl esters to levels seen in atherogenic cells. Nevertheless, inducing diabetes in both NOD and LDLR−/−; GP mice significantly increased the cholesteryl ester content of macrophages. In kidneys, which had much lower cholesteryl ester levels compared with macrophages, inducing diabetes in these mice increased both the free and esterified cholesterol content. As with ABCA1 expression, diabetes had no significant effect on the cholesterol content of livers. Impaired ABCA1-dependent lipid export could have contributed to the diabetes-induced accumulation of cholesterol in macrophages and kidneys.
There are several factors that could influence ABCA1 protein levels in diabetic mice, but strong candidates are reactive carbonyls. These carbonyls are formed during glucose oxidation reactions that generate AGEs, modified proteins implicated in the pathogenesis of diabetes, atherosclerosis, and other disorders (6–9). We showed previously that ABCA1 was highly and uniquely sensitive to damage by the carbonyls glyoxal and glycoaldehyde, which severely and acutely reduced ABCA1 protein levels in cultured macrophages without affecting mRNA levels (10). Chronic exposure of ABCA1 to these carbonyls is likely to be increased under the hyperglycemic conditions associated with diabetes. We were able to detect carbonyl-damaged ABCA1 in macrophages and kidneys from diabetic mice using AGE antibodies, consistent with this type of damage contributing to the impaired function of ABCA1.
Elevated free fatty acids could also contribute to the reduced ABCA1 protein levels in macrophages and kidneys from diabetic mice. We showed that high physiologic levels of oleate and other fatty acids destabilize ABCA1 protein through a phospholipase D2/protein kinase C δ signaling pathway that phosphorylates ABCA1 serines (14–17). Diabetic mice have elevated circulating triacylglyceride levels, which could increase flux of free fatty acids into tissues. We are currently investigating the possibility that increased fatty acids could also contribute to the impaired ABCA1.
It is unlikely that inflammation in response to thioglycolate injections could contribute to the effects of inducing diabetes in these animals. ABCA1 levels in tissues from NOD and LDLR−/−; GP mice were affected by diabetes to similar extents, even though only the LDLR−/−; GP mice were treated with thioglycolate. Diabetes reduced ABCA1 protein levels to the same extent in resident or thioglycolate-elicited macrophages isolated from NOD mice.
The lack of changes in liver and brain ABCA1 indicate that there is cell specificity for the effects of diabetes on this protein, which may reflect different sensitivities to carbonyl stress and/or fatty acid load. In addition to enhanced fatty acid production, the oxidative environment surrounding macrophages is likely to generate high levels of reactive carbonyls (38). Renal cells are highly sensitive to oxidative and carbonyl stress, and the kidney is capable of producing large amounts of reactive carbonyls (34). In contrast, the liver and the brain might generate less of these metabolic factors or have greater abilities to quench their effects.
These studies have important implications about diabetic complications in animal models and humans. Inducing diabetes in LDLR−/−; GP mice increases atherosclerosis even when animals are fed a low-cholesterol diet and circulating lipoproteins are unchanged (21). Diabetes accelerates cardiovascular disease in humans with both types 1 and 2 diabetes. An impaired ABCA1-dependent clearance of excess cholesterol from arterial macrophages could contribute to these macrovascular complications. Diabetes causes renal disease in autoimmune diabetic mice (39) and in humans (34), and it has been shown that hypercholesterolemia promotes renal disease in association with accumulation of cholesterol in the kidney (35). A defective ABCA1 cholesterol export pathway in renal cells could therefore promote cholesterol accumulation and enhance diabetic nephropathy. These observations implicate ABCA1 as an important therapeutic target for treating diabetic cardiovascular disease and nephropathy.
Acknowledgments
We thank Dr. Timothy McMillen for helping provide the NOD mice and for technical assistance.
Footnotes
Abbreviations:
- AGE
- advance glycation end product
- apo
- apolipoprotein
- GP
- glycoprotein
- LDLR
- LDL receptor
- NOD
- non-obese diabetic
- LCMV
- lymphocytic choriomeningitis virus
This study was supported by the National Institutes of Health grants DK02456, HL55362, HL079382, HL62887, and HL088627. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Oram J. F., Heinecke J. W. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. [DOI] [PubMed] [Google Scholar]
- 2.Singaraja R. R., Brunham L. R., Visscher H., Kastelein J. J., Hayden M. R. 2003. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler. Thromb. Vasc. Biol. 23: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 3.Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., et al. 2002. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 110: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Eck M., Bos I. S., Kaminski W. E., Orso E., Rothe G., Twisk J., Bottcher A., Van Amersfoort E. S., Christiansen-Weber T. A., Fung-Leung W. P., et al. 2002. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. USA. 99: 6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiello R. J., Brees D., Bourassa P. A., Royer L., Lindsey S., Coskran T., Haghpassand M., Francone O. L. 2002. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 22: 630–637. [DOI] [PubMed] [Google Scholar]
- 6.Baynes J. W., Thorpe S. R. 1999. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 48: 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M., Cerami A., Vlassara H. 1988. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley P. J., Langborg A., Minhas H. S. 1999. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 344: 109–116. [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff S. P., Dean R. T. 1987. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem. J. 245: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passarelli M., Tang C., McDonald T. O., O'Brien K. D., Gerrity R. G., Heinecke J. W., Oram J. F. 2005. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 54: 2198–2205. [DOI] [PubMed] [Google Scholar]
- 11.Haffner S. M. 2006. Risk constellations in patients with the metabolic syndrome: epidemiology, diagnosis, and treatment patterns. Am. J. Med. 119: S3–S9. [DOI] [PubMed] [Google Scholar]
- 12.Seigneur M., Freyburger G., Gin H., Claverie M., Lardeau D., Lacape G., Le Moigne F., Crockett R., Boisseau M. R. 1994. Serum fatty acid profiles in type I and type II diabetes: metabolic alterations of fatty acids of the main serum lipids. Diabetes Res. Clin. Pract. 23: 169–177. [DOI] [PubMed] [Google Scholar]
- 13.Reaven G. M., Hollenbeck C., Jeng C. Y., Wu M. S., Chen Y. D. 1988. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 37: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Kurdi-Haidar B., Oram J. F. 2004. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 45: 972–980. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Oram J. F. 2002. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J. Biol. Chem. 277: 5692–5697. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Oram J. F. 2005. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J. Biol. Chem. 280: 35896–35903. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Oram J. F. 2007. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J. Lipid Res. 48: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 18.Kannel W. B. 2000. The Framingham Study: its 50-year legacy and future promise. J. Atheroscler. Thromb. 6: 60–66. [DOI] [PubMed] [Google Scholar]
- 19.Ruderman N. B., Williamson J. R., Brownlee M. 1992. Glucose and diabetic vascular disease. FASEB J. 6: 2905–2914. [DOI] [PubMed] [Google Scholar]
- 20.Nathan D. M., Lachin J., Cleary P., Orchard T., Brillon D. J., Backlund J. Y., O'Leary D. H., Genuth S. 2003. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 348: 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renard C. B., Kramer F., Johansson F., Lamharzi N., Tannock L. R., von Herrath M. G., Chait A., Bornfeldt K. E. 2004. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J. Clin. Invest. 114: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez A. J., Anantharamaiah G. M., Segrest J. P., Oram J. F. 1994. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J. Clin. Invest. 94: 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellington C. L., Walker E. K., Suarez A., Kwok A., Bissada N., Singaraja R., Yang Y. Z., Zhang L. H., James E., Wilson J. E., et al. 2002. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab. Invest. 82: 273–283. [DOI] [PubMed] [Google Scholar]
- 24.Moore K. J., Freeman M. W. 2006. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 26: 1702–1711. [DOI] [PubMed] [Google Scholar]
- 25.Passarelli M., Oram J. F. 2000. Cellular glycoxidation impairs the ABCA1-mediated lipid secretory pathway. Circulation. 102: II-312. [Google Scholar]
- 26.Zhou H., Tan K. C., Shiu S. W., Wong Y. 2008. Determinants of leukocyte adenosine triphosphate-binding cassette transporter G1 gene expression in type 2 diabetes mellitus. Metabolism. 57: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 27.Mauldin J. P., Srinivasan S., Mulya A., Gebre A., Parks J. S., Daugherty A., Hedrick C. C. 2006. Reduction in ABCG1 in Type 2 diabetic mice increases macrophage foam cell formation. J. Biol. Chem. 281: 21216–21224. [DOI] [PubMed] [Google Scholar]
- 28.Mauldin J. P., Nagelin M. H., Wojcik A. J., Srinivasan S., Skaflen M. D., Ayers C. R., McNamara C. A., Hedrick C. C. 2008. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation. 117: 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oram J. F. 2003. HDL apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 23: 720–727. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan A. M., Oram J. F. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 31.Haghpassand M., Bourassa P. A., Francone O. L., Aiello R. J. 2001. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J. Clin. Invest. 108: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellington C. L. 2004. Cholesterol at the crossroads: Alzheimer's disease and lipid metabolism. Clin. Genet. 66: 1–16. [DOI] [PubMed] [Google Scholar]
- 34.Bohlender J. M., Franke S., Stein G., Wolf G. 2005. Advanced glycation end products and the kidney. Am. J. Physiol. Renal Physiol. 289: F645–F659. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan R., Dodesini A. R., Lepore G. 2006. Lipids and renal disease. J. Am. Soc. Nephrol. 17: S145–S147. [DOI] [PubMed] [Google Scholar]
- 36.Mauerer R., Ebert S., Langmann T. 2009. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp. Mol. Med. 41: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins A. J., Lyons T. J., Zheng D., Otvos J. D., Lackland D. T., McGee D., Garvey W. T., Klein R. L. 2003. Serum lipoproteins in the diabetes control and complications trial/epidemiology of diabetes intervention and complications cohort: associations with gender and glycemia. Diabetes Care. 26: 810–818. [DOI] [PubMed] [Google Scholar]
- 38.Kirkham P. 2007. Oxidative stress and macrophage function: a failure to resolve the inflammatory response. Biochem. Soc. Trans. 35: 284–287. [DOI] [PubMed] [Google Scholar]
- 39.Wilson K. H., Eckenrode S. E., Li Q. Z., Ruan Q. G., Yang P., Shi J. D., Davoodi-Semiromi A., McIndoe R. A., Croker B. P., She J. X. 2003. Microarray analysis of gene expression in the kidneys of new- and post-onset diabetic NOD mice. Diabetes. 52: 2151–2159. [DOI] [PubMed] [Google Scholar]