Abstract
Sulfonate sphingoids or sulfonolipids are bioactive unusual compounds found in members of the Bacteroidetes family. The present report describes the structures of sulfonolipids of halophilic bacteria, sharing structural similarity with compounds of fungal origin inhibiting the serine palmitoyl transferase and with capnines, known as antagonists of von Willebrandt factor. Two sulfonolipids (SL1 and SL2) were isolated from the lipid extract of the halophile Salisaeta longa and analyzed by ESI-MS/MS. SL1 and SL2 structures have in common the long chain aminosulfonate 2-carboxy-2-amino-3,4-hydroxy-17 methyloctadec-5-ene-1-sulfonic for which the common name of halocapnine is suggested. The hydroxyl group on carbon 3 of aminosulfonate moiety is acylated: iso C15 and iso hydroxy C15 chains are present in SL1 and SL2, respectively. The levels of the two different sulfonolipids in the bacterium were found to be modulated by the proportion of sodium and magnesium ions in the environment.
Keywords: sulfonolipids, capnine, halocapnine, Bacteroidetes
Most extremely halophilic aerobic prokaryotes known belong to the domain Archaea, order Halobacteriales. However, a few years ago, a novel type of halophile belonging to the Bacteroidetes branch of the Bacteria was isolated: the rod-shaped, red-colored Salinibacter ruber, found in saltern crystallizer ponds all over the world (1, 2).
Salinibacter is no less halophilic than many of the archaeal halophiles, and the haloadaptation mechanism used by this organism is similar to that of the extremely halophilic Archaea. Salinibacter cells contain very high concentrations of K+ ions in their cytoplasm and they do not accumulate organic osmotic solutes such as are used by all other known halophilic and halotolerant aerobic members of the Bacteria (3). The proteome of S. ruber is highly acidic, like that of the members of the Halobacteriales (4).
The membrane lipids of S. ruber are typical for the bacterial domain, with glycerophospholipids containing ester-linked fatty acyl chains and not ether-linked phytanyl chains characteristic of the archaeal halophiles (5). Modification of the membrane lipid composition is an important aspect of haloadaptation in Bacteria to preserve membrane integrity and function at high salt concentrations (6).
Electrospray ionization-mass spectrometry (ESI-MS) analyses (negative ion) of the total lipid extract of S. ruber showed a prominent peak in the high-mass range of the spectrum at m/z 660.7, which was identified as a novel sulfonolipid (7). This lipid, which represents about 10% of the total cellular lipids, appears to be a structural variant of the sulfonolipids, collectively called capnoids, found as main components of the cell envelope of gliding bacteria of the genus Cytophaga and closely related genera (8) and of diatoms (9). Thus far, this novel sulfonolipid had only been found in Salinibacter.
We have recently isolated a novel halophilic representative of the Bacteroidetes from brine collected from an experimental mesocosm at the Dead Sea Works, Sedom, containing a Dead Sea-Red Sea water mixture (10). Plated samples gave rise to the development of a variety of Archaea, but one colony had the typical orange color of Salinibacter and consisted of very long (15–30 μm) slender rods. It grows in the presence of 50–200 g/l NaCl with an optimum at 100 g/l NaCl + 50 g/l MgCl2.6H2O, and is thus less halophilic than Salinibacter, which requires a minimum of 150 g/l NaCl and grows optimally at 200–300 g/l (1). Characterization of this distant relative of Salinibacter showed the presence of bacterial rather than archaeal lipids. Its main fatty acids are 16:0 iso and 16:1 cis 9, followed by 15:0 iso and 15:0 anteiso. Its red-orange pigment has an absorption spectrum similar to salinixanthin, the carotenoid pigment of Salinibacter (11). Intracellular K+ ion concentrations of the new isolate were about as high as those reported for Salinibacter and Halobacterium salinarum cells, despite its lower salt requirement for growth (12). The organism has been described as a new species belonging to a new genus, Salisaeta longa (13).
In view of the phylogenetic affiliation of Salisaeta with Salinibacter and in view of the earlier finding of unusual sulfonolipids in Salinibacter (7), we have studied the lipid composition of Salisaeta. Here, we document the presence of two sulfonolipids in Salisaeta, one of which is identical to the Salinibacter sulfonolipid, the second being a hydroxyl derivative of the first.
EXPERIMENTAL PROCEDURES
Materials
S. longa strain S4-4T (DSM 21114T; CECT 7354T) was grown in a rotary shaker (100 rpm) at 37°C in 2-l Erlenmeyer flasks containing 1 l of medium of the following composition (g/l): NaCl, 100; MgCl2·6H2O, 50; K2SO4, 5; CaCl2·2H2O, 0.1; yeast extract, 1; casamino acids, 1; and soluble starch, 2. The pH was adjusted to 7.0 before autoclaving. When indicated, the concentration of NaCl was modified (50, 150, or 200 g/l) and/or the MgCl2·6H2O was increased to 100 g/l. Cells in the late exponential growth phase were harvested by centrifugation (30 min, 5,500 g, 4°C) and dried by lyophilization.
S. ruber M31T (DSM 13855T), used for comparison in a number of experiments, was grown under similar conditions in medium of the following composition (g/l): NaCl, 195; MgSO4·7H2O, 25; MgCl2·6H2O, 16.3; CaCl2·2H2O, 1.25; KCl, 5.0; NaHCO3, 0.25; NaBr, 0.625; and yeast extract, 1.0, pH 7.0.
Lipid extraction
Total lipids were extracted using the Bligh and Dyer method (14), as modified for the extreme halophiles (15). Dried cells (about 200 mg) were rehydrated in 4 ml of water. For the lipid extraction, 15 ml of methanol-chloroform (2:1, v/v) was added to cell suspension and the mixture was gently shaken for 15 min; after centrifugation, the supernatant extract was decanted into a separatory funnel. The residual pellet was resuspended in 7.6 ml of chloroform-methanol-water (1:2:0.8, v/v/vol); the mixture was then shaken and centrifuged. Seven ml each of chloroform and KCl 0.2 M were added to the combined supernatants in the separatory funnel to obtain a two-phase system. After complete phase separation (requiring a few hours at room temperature), the chloroform phase, with the addition of benzene (about 10% of the volume of the chloroform phase) was brought to dryness under nitrogen. Dried lipids were weighted and then resuspended in a small volume of chloroform and stored at −20°C. All organic solvents used throughout the study were commercially distilled and of the highest available purity (Sigma-Aldrich).
TLC analyses
Total lipid extracts were analyzed by one-or two-dimensional TLC on silica gel 60A plates (Merck, 20 × 10 cm, layer thickness 0.2 mm). The plates were washed twice with chloroform-methanol (1:1, v/v) and activated at 180°C before use. Total lipids were eluted with Solvent A (chloroform-methanol-acetic acid-water 85:15:10:3.5, v/v), for one-dimensional TLC, or first with Solvent B [chloroform-methanol-29.9% (wt/vol) ammonia, 65:35:5, v/v/v] and then with solvent A for two-dimensional TLC. The following stains were used for the detection of lipids: a) 5% sulfuric acid in water, followed by charring at 120°C for all lipids (15); b) iodine vapor for all lipids; c) molybdenum blue spray reagent (Sigma) specific for phospholipids; d) 0.5% α-naphtol in methanol-water (1:1, v/v) and 0.5% orcinol in 2.1 M sulfuric acid specific for glycolipids (16); and e) 2% azure A in 1 mM sulfuric acid for sulfatides (17). Phospholipids were quantified using lipid analysis after separation by TLC. Individual lipid spots on thin layer chromatograms, identified using iodine vapor, were scraped into digestion tubes, and the lipid phosphorus released as inorganic phosphate was measured colorimetrically (15).
Isolation and purification of individual lipids by preparative TLC
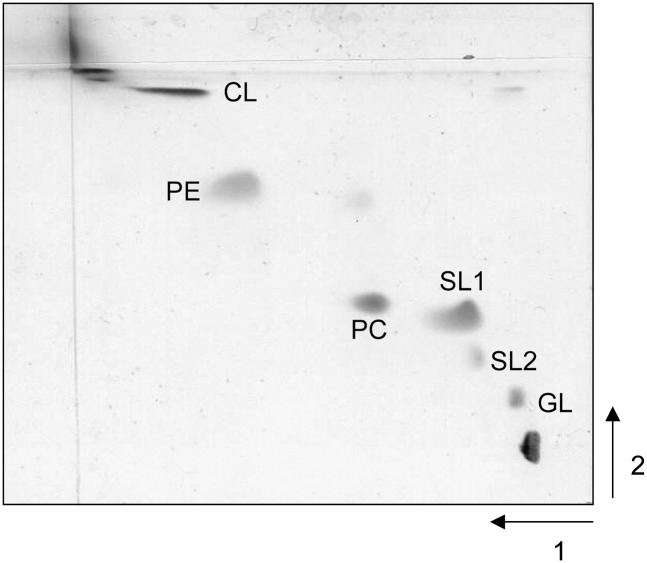
Cellular lipids were separated by preparative TLC on silica gel 60A plates (20 × 20 cm, layer thickness 0.5 mm) and eluted with chloroform-methanol-acetic acid-water (85:15:10:3.5, v/v). Five bands of polar lipids were observed. After scraping the silica in each band from the plate, the lipid were extracted five times with chloroform-methanol (1:1, v/v), and the combined supernatants were brought to dryness under a stream of nitrogen (15). The five lipid bands were then rechromatographed. Band 1 contained a glycolipid, band 2 contained a sulfonolipid (SL2), band 3 contained the sulfonolipid (SL1) plus phosphatidylcholine (PC), band 4 contained phosphatidylethanolamine (PE), and finally, band 5 contained cardiolipin or bisphosphatidylglycerol (CL). Further experiments are in progress to elucidate the structure of the lipid that gives rise to spot 1 in the one-dimensional TLC. The lipids in the five bands were further reanalyzed by TLC using chloroform-methanol-29.9% (w/v) ammonia, 65:35:5, v/v/v. The components of band 3 and 4 were well separated by the rechromatography, whereas from the analyses of bands 1, 2, and 5 the presence of only one lipid component was confirmed.
Mass spectrometry analyses
All MS analyses were performed on a Q TRAP® MS/MS system, from Applied Biosystems (Foster City, CA) equipped with an ESI interface.
Analyses were carried out in the loop injection mode with dried lipid extracts dissolved in chloroform-methanol (1:1, v/v). Samples (2 µl), injected via a 10 µl loop, were transferred to the MS electrospray interface (ESI) at a flow rate of 0.01 ml/min of chloroform-methanol (1:1, v/v) delivered by a Harvard infusion pump (model 11, South Natick, MA). Interface parameters were as follows: temperature, 200°C, curtain gas (nitrogen), 20 psi, nebulizer gas (air), 5 psi, heater gas (air), 15 psi, ionspray voltage + 4500 V or −4500 V, for positive and negative ions, respectively. MS/MS measurements were carried out by fragmenting the target ions at proper collision energy between 20 and 60 eV.
For the acquisition of positive ion mass spectra, lipids were dissolved in chloroform-1 mM ammonium acetate in methanol (1:3, v/v) at a concentration of 5 mg/ml. For negative ion mass spectra, lipids were first converted to the ammonium salt form as described by Kates (15). Typically, 4.5 ml of 0.2 N HCl was added to a solution containing about 10 mg of lipids in 5 ml of chloroform-methanol (1:1, v/v). The biphasic system was mixed and centrifuged. After removal of upper phase, the lower chloroform phase was washed twice with 4 ml of methanol-water (10:1 v/v). The chloroform phase containing the free acid form of lipids were immediately neutralized by addition of ammonium hydroxide to pH 7–8 and brought to dryness. The residue was dissolved in 0.5 ml of chloroform-methanol (1:1, v/v) and diluted with 10 ml of acetone. After cooling overnight at −20°C, lipids in the ammonium salt form were collected by centrifugation, dried, and dissolved in chloroform-methanol (1:1, v/v).
RESULTS
Two-dimensional TLC lipid profile of S. longa
Figure 1 shows the two-dimensional TLC of a total lipid extract of S. longa. Individual polar lipid components were identified by staining behavior, by comparison of their Rf values with those of the total lipid extract of the previously characterized extremely halophilic bacterium S. ruber (5), and by ESI-MS analyses. The main polar lipid components are a glycolipid, the sulfonolipid (SL1), PC, PE, and CL. In a previous study on the lipid composition of S. ruber, the sulfonolipid SL1 has been characterized as a capnine derivative (7). This lipid, the iso C15:0 fatty acid ester of 2-carboxy-2-amino-3,4-hydroxy-17-methyloctadec-5-ene-1-sulfonic acid, is a structural variant of the sulfonolipids found in gliding bacteria belonging to the genus Cytophaga and other closely related genera (18); a similar sulfonolipid has also been found in the diatom Nitzschia alba (9, 19). Interestingly, the staining of the two-dimensional TLC with the azure A reagent (specific for sulfate and sulfonate groups) revealed that close to the SL1 band, another positive band is present (named SL2).
Fig. 1.
Two-dimensional TLC of the total lipid extract of S. longa.
ESI-MS analyses of total lipid extract
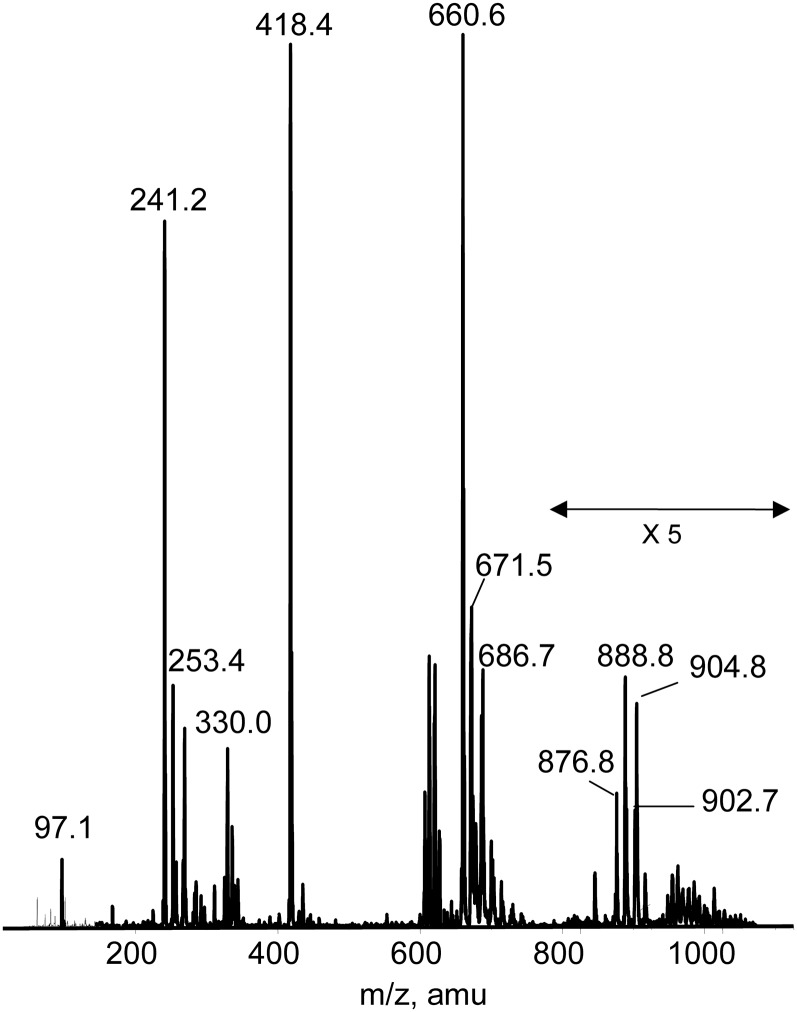
The ESI-MS spectrum (negative ion) of the total lipid extract of S. longa (Fig. 2) showed three main peaks at m/z 660.6, 418.4, and 241.2. The prominent peaks at m/z 660.6 and 418.4 are diagnostic for the presence of the sulfonolipid previously found in S. ruber; they correspond to the molecular ion [M-H]− and to the fragment arising from the loss of the C15:0 fatty acyl moiety, respectively. The peak at 671.5 is bicharged and is attributable to the major cardiolipin species present in the lipid extract. Furthermore, the peak at m/z 686.7 corresponds to the major species of PE. In the mass range of 200–300 amu, the characteristic peaks of fatty acid anions formed during the fragmentation of molecular ions of different lipids are present. The peaks at m/z 241.2 and 253.4 indicate the presence of C15:0 and C16:1 fatty acid, respectively. Previous GC-MS analyses indicated that C15:0 and C16:0 fatty acid chains are mainly in the iso-configuration (13).
Fig. 2.
ESI MS (negative ion) lipid profile of the total lipid extract of S. longa. In the m/z range 800–1000, the heights of the reported signals have been multiplied ×5.
Preparative TLC and ESI-MS analyses of individual lipid components
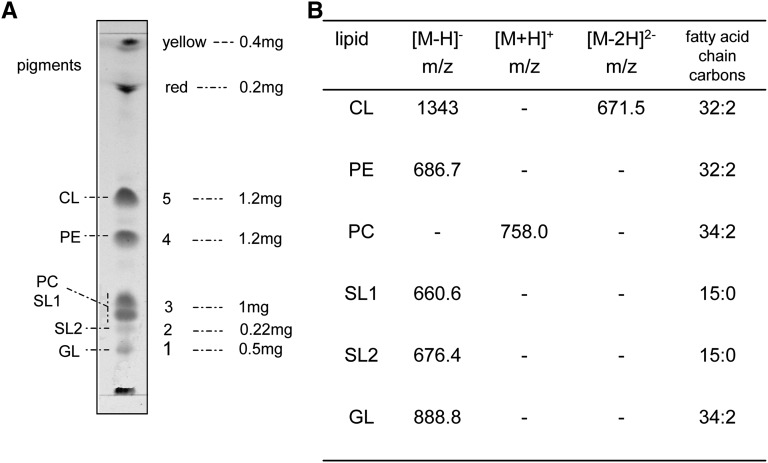
For a more detailed study of the polar lipids of S. longa, different polar lipid classes were characterized by combining TLC and ESI-MS analyses. Lipid components were isolated by preparative TLC in solvent system A. Five main bands plus pigments close to the solvent front were obtained (Fig. 3A); then the lipid material was extracted from the silica gel. The purity and/or the presence of multiple components in each band were checked by chromatography in the neutral solvent system B.
Fig. 3.
A: Lipid profile of preparative TLC. Five mg of lipid extract was typically loaded on a 20 × 20 TLC plate; the amount of lipid material extracted from each TLC band is reported; total yield was 94%. B: ESI-MS analyses (both in the positive and negative modes) of individual lipid components isolated and purified by preparative analyses.
The purified individual lipid components corresponding to bands 1–5 were first characterized by their reaction with different staining reagents. Molybdenum blue and azure A were used to detect the presence of phosphate and sulfate (or sulfonate) respectively, and orcinol for glycolipids. A glycolipid is present in band 1; PE and CL were found in band 4 and 5, respectively; band 2 contains the novel sulfur-containing lipid; and band 3 is a mixture of SL1 and PC.
To obtain detailed structural information, lipids extracted from the silica gel were analyzed by ESI-MS. The ESI-MS (negative ion) of band 1 showed a cluster of peaks centered at m/z 888.8, attributable to the molecular ion [M-H]−. The other peaks of the cluster at 904.8 and 876.8 m/z differ from 888.8 molcular ion by +16 and −12 amu, respectively; these differences could result from double bond composition and acyl chain length variations and, in particular, the +16 difference can also be attributed to the presence of an additional hydroxyl group. The spectrum also showed a peak at 902.7 m/z that is also present in the total lipid extract of S. ruber (5). The ESI-MS/MS spectrum of the molecular ion [M-H]− at 888.8 showed a peak at 179.0 m/z attributable to the deprotonated molecule of hexose, in agreement with results obtained by orcinol staining. The peak at 269.3 is diagnostic for the presence of C17:1 fatty acid. Further experiments are in progress to elucidate the complete structure of this lipid.
Staining with azure A confirmed that the purified band 2 contains a sulfate or sulfonate group. The ESI-MS spectrum (negative ion) showed a molecular ion peak [M-H]− at 676.4 m/z that differed from the molecular ion [M-H]− at 660.6 of SL1 by 16 amu, compatible with the presence of an additional OH− residue in the sulfonolipid SL2.
The rechromatography of isolated band 3 in Solvent B revealed presence of two components that stained with azure A and molybdenum blue, respectively. Quantitative analyses showed that SL1 represents about 10% of the total lipid extract of S. longa.
Further information on the nature of these fractions was obtained from the ESI-MS spectrum (negative and positive ion). The ESI-MS spectrum (negative ion) of band 3 showed the prominent peak at m/z 660.6 and the peak of m/z 418.3 diagnostic for the presence of the molecular ion [M-H]− of the sulfonolipid SL1 and a fragment arising from the loss of the C15:0 fatty acyl moiety (peak of m/z 241.3). The ESI-MS/MS of the peak at 660.6 confirmed this structure. The ESI-MS spectrum (positive mode) of band 3 showed a molecular ion [M+H]+ at m/z 758.0, consistent with the molecular weight of a PC 34:2. The ESI-MS spectrum of band 4 showed a peak at 686.7 m/z, assigned to the molecular ion [M-H]− of a PE 32:2.
Finally, band 5 contained a CL. Having two phosphate groups in the polar head, CLs have a greater tendency to form sodium adducts as compared with other lipids. Thus, before the ESI-MS analyses the isolated lipid was converted to the ammonium salt, which is an easily ionizable form. Due to the presence of the two phosphate groups in the molecule, CL forms a bicharged molecular ion [M-2H]2− during the ionization phase. This characteristic behavior allows easy identification of CL peaks in the mass spectrum of lipid extract. A bicharged peak at m/z 671.5 strongly supported the presence of a CL having a molecular weight of 1343. The peaks at m/z 253.3 in the low mass range of the spectrum indicated the presence of C16:1 fatty acid. Therefore, unlike the CL of S. ruber, which is not a symmetrical molecule, CL of S. longa presents four C16:1 fatty acid chains as hydrophobic chains.
The main ESI-MS peaks of the polar lipids identified in the total lipid extract of S. longa are reported in Fig. 3B.
ESI-MS/MS analyses of the minor sulfonolipid of S. longa
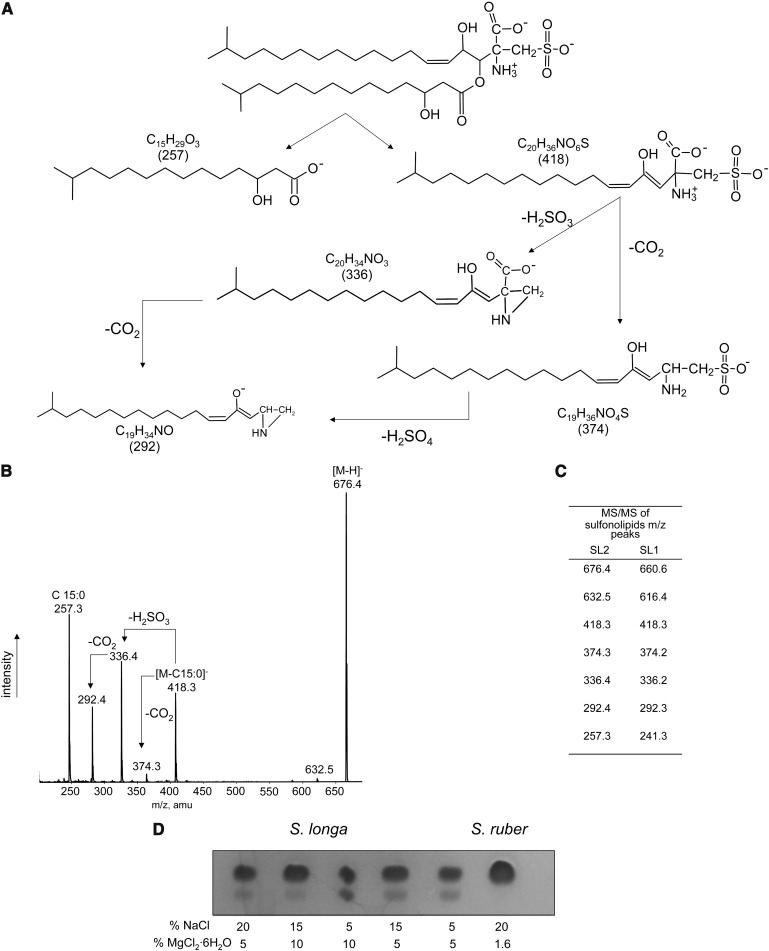
The proposed structure for SL2 together with its fragmentation pathway is shown in Fig. 4A, and the MS/MS spectrum of the isolated and purified band 2 lipid is presented in Fig. 4B. The ESI-MS/MS spectrum of the peak at 676.4 showed a peak at m/z 257.3, representing a C15:0 fatty acid with an OH group that may be bound in position 2 or 3 of the iso chain; iso-branched 2- and 3-hydroxy fatty acids are indeed abundant in representatives of the genera of the Cytophaga-Flexibacter group (20). The peak at 418.3 is a fragment arising from the loss of the fatty acid moiety and is identical to that obtained by fragmentation of the sulfonolipid SL1. This fragment has the structure of a capnine analog, which until now has been found only in Salinibacter. Furthermore, it can be seen that the peak at m/z 374.3 corresponds to the neutral loss of CO2 from the peak at 418.3 having a capnine like structure, the peak at 336.4 arises from the same fragment after neutral loss of H2SO3, and that the peak at 292.4 can be explained by the neutral loss of both CO2 and H2SO3. Also these fragments are present in the MS/MS profile of SL1 (Fig. 4C).
Fig. 4.
The minor sulfonolipid of S. longa. A: Fragmentation pathways; B: ESI MS/MS of the isolated and purified minor sulfonolipid SL2; C: comparison of the ESI MS/MS peaks of the two sulfonolipids SL1 and SL2; D: modulation of the membrane level of the sulfonolipid SL2 by the concentrations of magnesium and sodium ions in the growth medium.
The level of the minor sulfonolipid SL2 is modulated by the proportion of sodium and magnesium ions in the culture medium. Cultivation of S. longa in a medium containing relatively low concentrations of sodium and high magnesium ions concentration led to an increase in the amount of the minor sulfonolipid SL2 in the total lipid extract (Fig. 4D).
DISCUSSION
The data presented above document the lipid composition of S. longa, a halophilic member of the Bacteroidetes (13), isolated from a mesocosm experimental system filled with a mixture of Dead Sea and Red Sea water (10). Salisaeta is a distant relative of Salinibacter, an organism widespread in saltern crystallizer ponds worldwide (1, 2). S. longa grows optimally at 100 g/l NaCl and is therewith markedly less halophilic than S. ruber, which prefers salt concentrations approaching saturation.
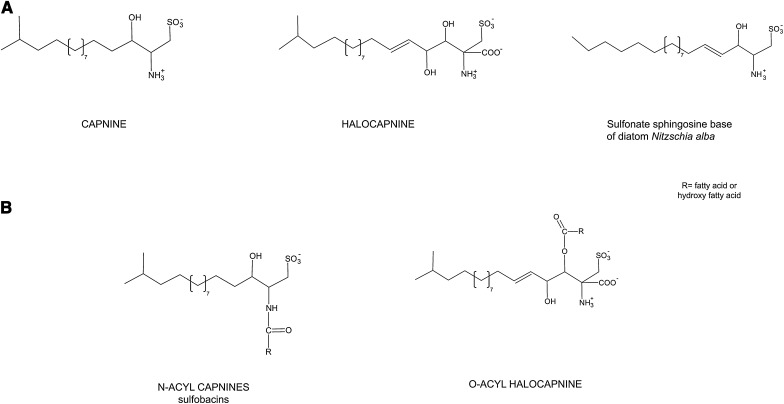
The most characteristic lipids of these two halophilic members of the Bacteroidetes are undoubtedly the sulfonolipids, which are analogs of capnine. Capnine can be considered as the sulfonic acid derivative of an iso-branched sphingoid base, in which the -CH2-SO3 group replaces the −CH2OH group. In contrast to the sulfolipids-sulfatated lipid compounds in which sulfate is bound by ester linkage, capnine and capnoids (i.e., capnine derivatives) contain sulfur in direct covalent linkage with carbon. Such compounds are commonly named sulfonolipids.
Capnine was first discovered in large quantities in bacteria of the genus Capnocytophaga, which are rod-shaped, chemoorganotrophic members of the Bacteroidetes, requiring CO2 for growth and exibiting gliding motility (18). Earlier studies of the lipids of the diatom Nitzschia alba had revealed the presence of a sulfonolipid closely resembling capnoids, having the structure of 1-deoxyceramide-1-sulfonic acid (9) (Fig. 5). More recently, N-acyl capnine was found in the soil bacterium Chryseobacterium sp. and in a marine Flavobacterium sp. (21). Such compounds, also named sulfobacins, can also easily be synthesized (22). Interestingly, both diatoms and representatives of Flavobacteria display gliding motility. The localization of N-acyl derivatives of capnine in the outer membranes of bacteria was brought forward as an argument in favor of the possible role of this lipid in the bacterial motility (23, 24).
Fig. 5.
The structures of capnine, halocapnine, N-acyl capnines and O-acyl halocapnines.
Table 1 summarizes the information available on the different acylated capnines detected in the microbial world, and Fig. 5 illustrates their structures. The capnine analog constituting the backbone of the two sulfonolipids of the red halophilic members of the Bacteroidetes is slightly different from the capnine of Capnocytophaga and Flexibacter (Fig. 5), as it corresponds to an iso branched unsaturated sphingoid base containing an additional hydroxyl group in C4 and an additional COO− group in C2. To distinguish the capnine of the halophilic bacteria from those previously found in other organisms, we propose the common name of halocapnine. The polar head of the sphingoid base of halophile members of Bacteroidetes shows features similar to those of sulfamisterin, an inhibitor of serine palmitoyl transferase, the key enzyme in the route of sphingoid biosynthesis (25). Both sulfonolipids of Salisaeta are fatty acid esters (and not amides) of halocapnine. One is identical to that of Salinibacter, the other contains an additional hydroxyl group on the fatty acid moiety and is more abundant in bacteria grown at high magnesium concentrations. Hydroxylated fatty acids have also been found in the N-acyl capnine of Flavobacterium (26, 27). Unusually long-chain sulfur-containing compounds were also found in the phytoflagellate Ochromonas danica (a chrysophyte alga) (28).
TABLE 1.
Reports on the occurrence of acylated capnines in the microbial world
| Species | Compounds | References | |
|---|---|---|---|
| Diatoms, marine | |||
| Nitzschia alba | Ceramide sulfonic acid | (9,19) | |
| Bacteroidetes non -halophilic or marine | Capnocytophaga ochracea Capnocytophaga sputigena Capnocytophaga gingivalis | Capnine, N-acyl capnine | (8,18,30,31) |
| Sporocytophaga myxococcoides Flexibacter sp. Flavobacterium spp. | N-acyl capnine | (8,30) | |
| Flectobacillus major | Sulfonic acid analogs of ceramide | (32) | |
| Chryseobacterium sp. | Sulfobacins | (21) | |
| Bacteroidetes, halophilic | |||
| Salinibacter ruber | Acylhalocapnine | (7) | |
| Salisaeta longa | Acylhalocapnine | this study | |
The interest in sulfonolipids stems not only from the fact of these being relatively rare lipid compounds, but for other reasons as well. First, sulfonates are frequently toxic to animal cells; it has been proposed that sulfonate lipids might play a role in the inflammation of oral cavity caused by Capnocytophaga sp. (18); sulfonate glycolipids have been shown to be potent inhibitors of mammalian DNA polymerases (29). Second, due to their peculiar chemical structures, capnine derivatives may confer special properties to the bacterial membrane.
The presence of the sulfonolipids among the cellular lipids of Salinibacter and Salisaeta raises questions regarding their precise localization and their possible functional role. It is as yet unknown whether these sulfonolipids are located in the outer membrane. A function in cellular motility is not very probable. S. ruber is motile by means of flagella (4) and not by a gliding mechanism as displayed by Cytophaga, Flexibacter, and relatives; no motility has ever been observed in S. longa. Whatever their importance to the cell may be, the discovery of the halocapnines in the first two truly halophilic representatives of the Bacteroidetes shows that more unusual types of lipids may be waiting to be discovered in this physiologically and ecologically disparate group of prokaryotes.
Footnotes
Abbreviations:
- CL
- cardiolipin or bisphosphatidylglycerol
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
This work was supported by the Ministero Italiano dell'Università e della Ricerca (Fondi Ateneo), the Ministero Italiano della Difesa (contract no. 1999/13.12.2005). The work of A.O. and N.V. was supported by the Israel Science Foundation (grant no. 617/07). N.V. further acknowledges support from the Yael Piton Memorial Fund.
REFERENCES
- 1.Antón J., Oren A., Benlloch S., Rodríguez-Valera F., Amann R., Rosselló-Mora R. 2002. Salinibacter ruber gen. nov., sp. nov., a novel extreme halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Microbiol. 52: 485–491. [DOI] [PubMed] [Google Scholar]
- 2.Rosselló-Mora R., Lucio M., Peña A., Brito-Echeverría J., López-López A., Calens-Vadell M., Frommberger M., Antón J., Schmitt-Kopplin P. 2008. Metabolic evidence for biogeographic isolation of the extremophilic bacterium Salinibacter ruber. ISME J. 2: 242–253. [DOI] [PubMed] [Google Scholar]
- 3.Oren A., Heldal M., Norland S., Galinski E. A. 2002. Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles. 6: 491–498. [DOI] [PubMed] [Google Scholar]
- 4.Mongodin E. F., Nelson K. E., Duagherty S., DeBoy R. T., Wister J., Khouri H., Weidman J., Balsh D. A., Papke R. T., Sanchez Perez G., et al. 2005. The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc. Natl. Acad. Sci. USA. 102: 18147–18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lattanzio V. M. T., Baronio M., Oren A., Russell N. J., Corcelli A. 2009. Characterization of polar membrane lipids of the extremely halophilic bacterium Salinibacter ruber and possible role of cardiolipin. Biochim. Biophys. Acta. 1791: 25–31. [DOI] [PubMed] [Google Scholar]
- 6.Russell N. J. 1993. Lipids of halophilic and halotolerant microorganisms. In The Biology of Halophilic Bacteria Vreeland R. H., Hochstein L. I., CRC Press, Boca Raton: 163–210. [Google Scholar]
- 7.Corcelli A., Lattanzio V. M. T., Mascolo G., Babudri F., Oren A., Kates M. 2004. Novel sulfonolipid in the extremely halophilic bacterium Salinibacter ruber. Appl. Environ. Microbiol. 70: 6678–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godchaux W., III, Leadbetter E. R. 1984. Sulfonolipids of gliding bacteria. J. Biol. Chem. 259: 2982–2990. [PubMed] [Google Scholar]
- 9.Anderson R., Kates M., Volcani B. E. 1978. Identification of the sulfolipids in the non-photosynthetic diatom Nitzschia alba. Biochim. Biophys. Acta. 528: 89–106. [DOI] [PubMed] [Google Scholar]
- 10.Oren A., Gavrieli I., Gavrieli J., Lati J., Kohen M., Aharoni M. 2004. Biological effects of dilution of Dead Sea water with seawater: implications for the planning of the Red Sea – Dead Sea “Peace Conduit”. J. Mar. Syst. 46: 121–131. [Google Scholar]
- 11.Lutnæs B. F., Oren A., Liaaen-Jensen S. 2002. New C40-carotenoid acyl glycoside as principal carotenoid of Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 65: 1340–1343. [DOI] [PubMed] [Google Scholar]
- 12.Vaisman N. 2009. Isolation and Characterization of Novel Halophilic Prokaryotes from the Dead Sea and from Experimental Mesocosms Containing Mixtures of Dead Sea and Red Sea Water. M.Sc. Thesis, The Hebrew University of Jerusalem. [Google Scholar]
- 13.Vaisman N., Oren A. 2009. Salisaeta longa gen. nov., sp. nov., a red halophilic member of the Bacteroidetes. Int. J. Syst. Evol. Microbiol. 59: 2571–2574. [DOI] [PubMed] [Google Scholar]
- 14.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 15.Kates M. 1986. Techniques of Lipidology. In Laboratory Techniques in Biochemistry and Molecular Biology Burdon R. H., van Knippenberg P. H., Elsevier, Amsterdam: 100–110. [Google Scholar]
- 16.Siakotos A. N., Rouser G. 1965. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J. Am. Oil Chem. Soc. 42: 913–919. [DOI] [PubMed] [Google Scholar]
- 17.Kean E. L. 1968. Rapid sensitive spectrophotometric method for quantitative determination of sulfatides. J. Lipid Res. 9: 319–327. [PubMed] [Google Scholar]
- 18.Godchaux W., III, Leadbetter E. R. 1980. Capnocytophaga spp. contain sulfonolipids that are novel in Procaryotes. J. Bacteriol. 144: 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson R., Livermore B. P., Volcani B. E., Kates M. 1975. A novel sulfonolipid in diatoms. Biochim. Biophys. Acta. 409: 259–263. [DOI] [PubMed] [Google Scholar]
- 20.Reichenbach H. 2006. The order Cytophagales. In The Prokaryotes. A Handbook on the Biology of Bacteria: Ecophysiology and Biochemistry Vol. 7 Dworkin M., Falkow S., Rosenberg E., Schleifer K-H., Stackebrandt E., Springer, New York: 549–590. [Google Scholar]
- 21.Kamiyama T., Umino T., Itezono Y., Nakamura Y., Satoh T., Yokose K. 1995. Sulfobacin-A and sulfobacin-B, novel von-Willebrand-factor receptor antagonists. 2. Structural elucidation. J. Antibiot. 48: 929–936. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A., Gamre S., Chattopaddhyay S. 2007. An asymetric synthesis of sulfobacin A. Tetrahedron Lett. 48: 3705–3707. [Google Scholar]
- 23.Godchaux W., III, Leadbetter E. R. 1988. Sulfonolipids are localized in the outer membrane of the gliding bacterium Cytophaga johnsonae. Arch. Microbiol. 150: 42–47. [Google Scholar]
- 24.Abbanat D. R., Leadbetter E. R., Godchaux W., III, Escher A. 1986. Sulphonolipids are molecular determinants of gliding motility. Nature. 324: 367–369. [Google Scholar]
- 25.Pruett S. T., Bushnev A., Hagedorn K., Adiga M., Haines C. A., Sullards M. C., Liotta D. C., Merril A. H., Jr 2008. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 49: 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano I., Ohno M., Masui M., Kato K., Yabuuchi E., Ohyama A. 1976. Occurence of 2-and 3-hydroxy fatty acid in high concentrations in the extractable and bound lipids of Flavobacterium meningosepticum and Flavobacterium IIb. Lipids. 11: 685–689. [DOI] [PubMed] [Google Scholar]
- 27.Fautz E., Rosenfelder G., Grotjahn L. 1979. Iso-branched 2-and 3- hydroxy fatty acid as characteristic lipid constituents of some gliding bacteria. J. Bacteriol. 140: 852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L. L., Pousada M., Haines T. H. 1976. The flagellar membrane of Ochromonas danica. Lipid composition. J. Biol. Chem. 251: 1835–1842. [PubMed] [Google Scholar]
- 29.Murakami C., Yamazaki T., Hanashima S., Takahashi S., Ohta K., Yoshida H., Sugawara F., Sakaguchi K., Mizushina Y. 2002. Structure-function relationship of synthetic sulfoquinovosyl-acyl- glycerols as mammalian DNA polymerase inhibitors. Arch. Biochem. Biophys. 403: 229–236. [DOI] [PubMed] [Google Scholar]
- 30.Godchaux W., III, Leadbetter E. R. 1983. Unusual sulfonolipids are characteristic of the Cytophaga-Flexibacter group. J. Bacteriol. 153: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leadbetter E. R. 2006. The genus Capnocytophga. In The Prokaryotes. A Handbook on the Biology of Bacteria: Ecophysiology and Biochemistry, Vol. 7 Dworkin M., Falkow S., Rosenberg E., Schleifer K-H., Stackebrandt E., E., editors Springer, New York: 709–711. [Google Scholar]
- 32.Batrakov S. G., Mosezhnyi A. E., Ruzhitsky A. O., Sheichenko V. I., Nikitin D. I. 2000. The polar lipid composition of the sphingolipid-producing bacterium Flectobacillus major. Biochim. Biophys. Acta. 1484: 225–240. [DOI] [PubMed] [Google Scholar]