Abstract
LDL particles that enter the arterial intima become exposed to proteolytic and lipolytic modifications. The extracellular hydrolases potentially involved in LDL modification include proteolytic enzymes, such as chymase, cathepsin S, and plasmin, and phospholipolytic enzymes, such as secretory phospholipases A2 (sPLA2-IIa and sPLA2-V) and secretory acid sphingomyelinase (sSMase). Here, LDL was first proteolyzed and then subjected to lipolysis, after which the effects of combined proteolysis and lipolysis on LDL fusion and on binding to human aortic proteoglycans (PG) were studied. Chymase and cathepsin S led to more extensive proteolysis and release of peptide fragments from LDL than did plasmin. sPLA2-IIa was not able to hydrolyze unmodified LDL, and even preproteolysis of LDL particles failed to enhance lipolysis by this enzyme. However, preproteolysis with chymase and cathepsin S accelerated lipolysis by sPLA2-V and sSMase, which resulted in enhanced fusion and proteoglycan binding of the preproteolyzed LDL particles. Taken together, the results revealed that proteolysis sensitizes the LDL particles to hydrolysis by sPLA2-V and sSMase. By promoting fusion and binding of LDL to human aortic proteoglycans, the combination of proteolysis and phospholipolysis of LDL particles potentially enhances extracellular accumulation of LDL-derived lipids during atherogenesis.
Keywords: proteolytic enzymes, atherosclerosis, low-density lipoprotein
Fractions of circulating LDL particles enter the arterial intima, where they become exposed to proteolytic and lipolytic enzymes and to oxidative agents (1). Indeed, many different proteases and lipases capable of hydrolyzing the protein and lipid components of LDL, respectively, have been detected in atherosclerotic lesions, and experiments in vitro have demonstrated that treatment of LDL with these enzymes can lead to their modification (2). Even minor modifications in the surface structure of LDL particles can result in their aggregation. If the proteolytic or lipolytic modifications are extensive enough, the aggregated LDL particles can become unstable and fuse. The fused particles resemble morphologically the lipid droplets that have been observed in atherosclerotic arterial intima both in humans and in atherosclerotic animal models (2). Importantly, the in vitro modified LDL particles chemically resemble the lipid droplets isolated from atherosclerotic arteries, showing signs of both proteolysis and lipolysis (2). Accordingly, these lipid droplets contain fragmented apolipoprotein B-100 (apoB-100) and have a lower phosphatidylcholine content and a higher ceramide content than native LDL particles (3).
Neutral proteases have been detected in atherosclerotic arterial intima, and they include both plasma-derived and locally synthesized proteases. To the former group belong plasmin, kallikrein, and thrombin (4), and to the latter group the matrix metalloproteinases secreted by intimal macrophages and smooth muscle cells, and chymase, tryptase, and cathepsin G secreted by intimal mast cells (5, 6). In addition, proteases with acidic pH optima, notably the lysosomal proteases cathepsins D, F, K, and S, are secreted by macrophages and smooth muscle cells in atherosclerotic lesions into their extracellular microenvironments (7–9).
Besides the many proteases listed above, also two types of extracellular phospholipolytic enzymes capable of hydrolyzing the surface phospholipids of LDL particles have been detected in atherosclerotic arterial intima. These are (1) the secretory phospholipase A2 enzymes (sPLA2), which hydrolyze phosphoglyceride molecules and generate free fatty acids and lysophospholipids, and (2) the secretory sphingomyelinase (sSMase), which hydrolyzes sphingomyelin molecules into phosphocholines and ceramides (10). Secretory phospholipases A2 of group IIa (sPLA2-IIa) (11–13) and, more recently, of group V (sPLA2-V) (14) and group X (sPLA2-X) (15, 16) are found particularly in the atherosclerotic regions of the arterial intima. Among these phospholipases, sPLA2-V and sPLA2-X are more effective than sPLA2-IIa in hydrolyzing lipoprotein particles, and interestingly, treatment of LDL with sPLA2-V has been shown to induce aggregation and fusion of LDL particles (14).
The presence of secretory acid sphingomyelinase in human atherosclerotic lesions has also been confirmed (17). The human enzyme has an acidic pH optimum and, accordingly, at neutral pH is capable of yielding only a limited degree of hydrolysis of LDL particles (18). On the other hand, hydrolysis of LDL by B. cereus sphingomyelinase, a bacterial sphingomyelinase showing substantial activity at neutral pH, has been shown to induce aggregation and fusion of LDL particles (19), these processes depending on the generation of ceramide on the surface of the modified lipoprotein particles (20).
Based on the above information on the presence of both proteases and phospholipases in atherosclerotic lesions, it is not surprising that apoB-100–containing lipid droplets isolated from such lesions show signs of hydrolysis of both their protein and their phospholipid components. However, no information is available about the effects of LDL proteolysis on subsequent phospholipolysis of the particles. Moreover, the combined effect of proteases and phospholipases on the stability of LDL particles and on their retention to a proteoglycan (PG) matrix is an unexplored research topic. In the present study, we treated LDL with proteases known to be present in the arterial intima and examined the effects of such proteolysis on the ability of sPLA2-IIa, sPLA2-V, and sSMase to hydrolyze phospholipids of the preproteolyzed LDL particles. In addition, the combined effects of proteolysis and lipolysis on LDL fusion and binding to human aortic proteoglycans were evaluated.
METHODS
Isolation and modification of LDL and IDL
Human LDL (d = 1.019-1.050 g/ml) and intermediate density lipoprotein (IDL) (d = 1.006-1.019) were isolated from plasma of healthy volunteers (a kind gift from the Finnish Red Cross) by sequential ultracentrifugation in the presence of 3 mM EDTA (21, 22). The amounts of LDL are expressed in terms of their protein concentrations, which were determined by the method of Lowry et al. (23) with BSA as standard. Each experiment was performed with at least two different LDL preparations.
The apoB-100 components of the lipoproteins were labeled with a 3H-labeling reagent according to the Bolton-Hunter procedure (24). 3H-radiolabeled LDL or 3H-radiolabeled IDL (2 mg/ml) was first incubated for 0.5–18 h at 37°C in 150 mM saline buffered with 20 mM PIPES (pH 7.0) containing either 0.5 mg/ml of α-chymotrypsin (SigmaAldrich), 0.02 mg/ml of human recombinant chymase (Teijin Ltd., Hino, Tokyo, Japan), 40 mU/ml of human plasma-derived plasmin (SigmaAldrich), or 35 µg/ml of human recombinant cathepsin S (Calbiochem). To optimize the activity of cathepsin S, 2.5 mM EDTA was added to the incubation mixtures containing this enzyme. After incubation, proteolysis by α-chymotrypsin, plasmin, or chymase was stopped by adding phenylmethylsulfonyl fluoride (SigmaAldrich) to give a final concentration of 1 mM. To stop the proteolytic degradation by cathepsin S, this enzyme was inhibited by adding E-64 (SigmaAldrich) to give a final concentration of 10 µM. The degree of LDL proteolysis was determined by the trichloroacetic acid (TCA) precipitation method, which measures the amount of radioactive peptides released from the particles (4). The released peptides were separated from the proteolyzed LDL particles by gel filtration chromatography on a Superose 12 PC 3.2/30 column (GE Healthcare), after which the particles and the released peptides were analyzed by SDS-polyacrylamide gel electrophoresis on 4–20% gradient gels. The gels containing radiolabeled LDL or released peptides were either stained with Simply Blue SefaStain (Invitrogen) or each lane was cut into 2-mm sections. To determine the radioactivity of each section of the gel, the pieces were first treated with pronase (SigmaAldrich) to release the radioactively labeled peptides from the gel material.
Preproteolyzed LDL or IDL was incubated for 0–24 h at 37°C with the selected phospholipases in a buffer containing 150 mM NaCl, 2% (w/v) fatty acid free BSA (MP chemicals, Ohio), 2 mM CaCl2, 0.005 mM ZnCl2, and 20 mM PIPES (pH 7.0), which contained 10 µg/ml of human recombinant PLA2 group IIa, 0.1 µg/ml of human recombinant sPLA2 group V (25), or 40 µg/ml of human recombinant acid sphingomyelinase (a kind gift from Genzyme). Because 2.5 mM EDTA was present in the incubation mixtures containing cathepsin S, 4 mM CaCl2 was added to these mixtures before the addition of either phospholipase. Finally, to stop the lipolytic reactions, EDTA was added to give a final concentration of 10 mM. The proteolyzed LDL and IDL particles (1 mg/ml) were allowed to fuse before lipolysis; therefore, they were incubated for 18 h at 37°C before their incubation with the lipases. The degree of sPLA2-induced lipolysis was determined by measuring the amounts of fatty acids in the samples by the NEFA-C-kit (Wako Chemicals, Neuss), and the degree of SMase-induced lipolysis was determined by measuring the amounts of phosphocholine in the samples by Amplex Red phosphorylcholine kit (Molecular Probes, Eugene, Oregon). The average sizes of native and proteolyzed LDL and IDL were measured using “Zetasizer Nano” apparatus (Malvern Instruments). No increase in the amounts of thiobarbituric acid–reactive substances (TBARS) (26) was detected in LDL samples after the incubations, indicating that the LDL particles had not been oxidized during the experiments.
Preparation of human aortic proteoglycans
Proteoglycans from the intima-media of human aortas were obtained at autopsy within 24 h of accidental death and prepared as described previously (27, 28). The Finnish National Authority for Medicolegal Affairs approved the study. The amounts of proteoglycans were determined by quantifying their glycosaminoglycans with the method of Bartold and Page (29).
Binding of LDL to human aortic proteoglycans
Wells of polystyrene 96-well plates (Thermo Labsystems, Vantaa, Finland) were coated with 100 μl of proteoglycans (50 μg/ml in PBS) by incubation at 4°C overnight. The proteoglycan-containing wells were blocked with 3% BSA, 1% fat-free milk powder, and 0.05% Tween 20 in PBS for 1 h at 37°C as described earlier (30). Wells incubated with only blocking buffer served as controls.
100 µl of differentially hydrolyzed LDL (0.1 mg/ml) in a buffer containing 1% BSA, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, and 20 mM PIPES (pH 7.0) were incubated in the wells for 1 h at 37°C. Unbound LDL particles were removed, the wells were washed with PIPES-buffered 50 mM saline, and the amounts of bound LDL particles were determined using the Amplex Red cholesterol kit (Molecular Probes, Eugene, Oregon).
Electron microscopy
LDL particles were negatively stained using 2% neutral uranyl acetate and were then viewed and photographed under a JEOL 1200-EX II transmission electron microscope at the Electron Microscopy Unit, Institute of Biotechnology, University of Helsinki. The diameters of 150 randomly selected lipoprotein particles were measured from the electron micrographs.
Statistical analysis
Statistical analysis of the data was performed with the SPSS software, version 17.0. When analyzing the sizes of LDL particles, the variance between the size distributions of different LDL samples was analyzed with one-way ANOVA with posthoc Bonferroni test, and when analyzing the binding data, the Wilcoxon signed-rank paired test was applied.
RESULTS
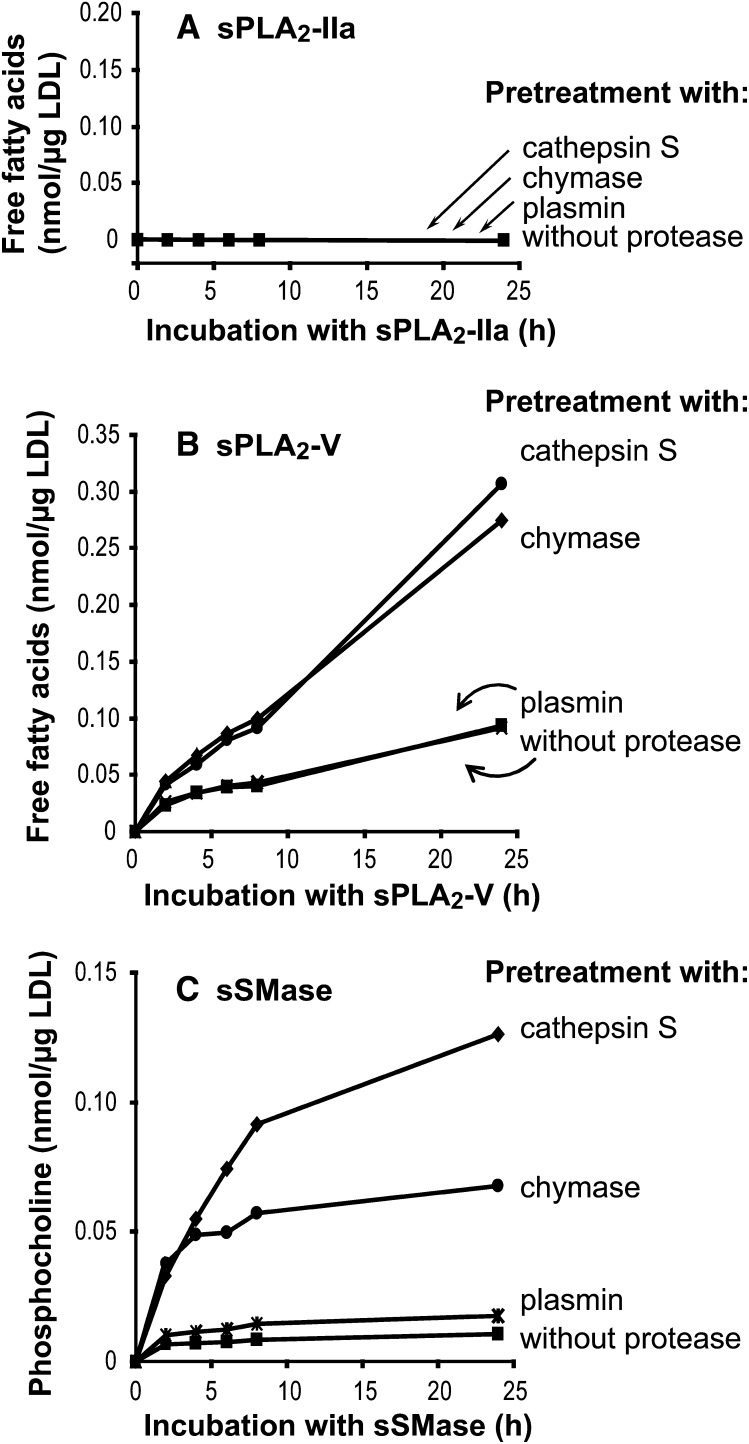
LDL was treated for 18 h with three different proteases, cathepsin S, chymase, or plasmin, after which the differentially proteolyzed LDL samples were incubated for up to 24 h with sPLA2-IIa, sPLA2-V, or sSMase. After incubation, the amounts of released free fatty acids (PLA2) or phosphocholines (SMase) were measured. PLA2-IIa was not able to hydrolyze unproteolyzed LDL, nor had preproteolysis with any of the three proteases any stimulatory effect on subsequent hydrolysis of LDL by this enzyme (Fig. 1A). In contrast, treatment of even unproteolyzed LDL with sPLA2-V led to generation of 0.07 nmol of free fatty acids/µg LDL (panel B), and after pretreatment with either cathepsin S or chymase, sPLA2-V hydrolyzed LDL more efficiently and led to generation of about 0.3 nmol of free fatty acids/µg LDL, which corresponds to hydrolysis of about half of the phosphatidylcholines on LDL particles. In contrast, pretreatment with plasmin had no effect on the ability of PLA2-V to hydrolyze LDL. Secretory SMase was capable of only minimal hydrolysis of unproteolyzed LDL, and plasmin pretreatment failed to stimulate the subsequent treatment with this phospholipase (panel C). In addition, similar to sPLA2-V, sSMase hydrolyzed LDL particles more efficiently after their pretreatment with cathepsin S or chymase. Interestingly, sSMase was able to hydrolyze cathepsin S-treated LDL significantly more efficiently than chymase-treated LDL. Thus, hydrolysis of cathepsin S–treated LDL by SMase led to generation of 0.14 nmol of phosphocholine/µg LDL, which corresponds to hydrolysis of about 40% of sphingomyelin molecules in LDL.
Fig. 1.
Effects of preproteolysis on LDL phospholipolysis. LDL was first incubated for 18 h with chymase, cathepsin S, or plasmin and then treated with either sPLA2-IIa (panel A), sPLA2-V (panel B), or sSMase (panel C). The formation of free fatty acids and phosphocholine, respectively, were measured at the indicated time points. The results are representative of four independent experiments performed with different LDL preparations. sPLA2-IIa, secretory phospholipase A2 group IIa; sPLA2-V, secretory phospholipase A2 group V, sSMase, secretory sphingomyelinase.
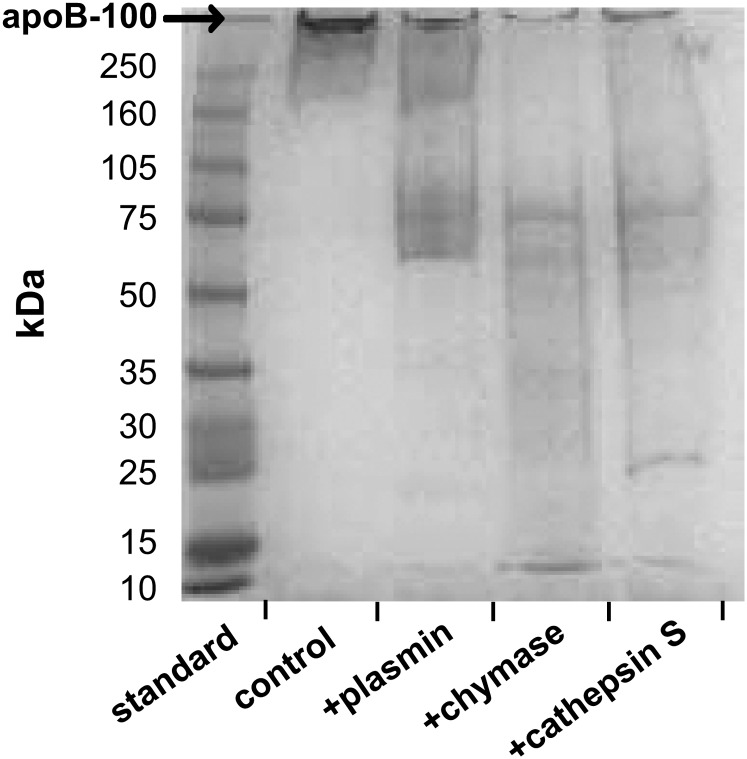
Next, the ability of plasmin, chymase, and cathepsin S to degrade apoB-100 was compared. All three proteases were able to cleave apoB-100 into fragments (Fig. 2), with cathepsin S and chymase leading to more extensive degradation and generation of smaller peptide fragments from LDL than plasmin. The degree of apoB-100 degradation into TCA-soluble products was also determined. It appeared that chymase and cathepsin S led to more extensive release of TCA-soluble products (about 13%) than did plasmin (about 4%) (not shown). Thus, despite the significant difference between the ability of sSMase to lipolyze cathepsin S–treated LDL and chymase-treated LDL, the degrees of proteolysis by these two proteases were comparable when related to the net release of TCA-soluble degradation products.
Fig. 2.
SDS-PAGE of proteolyzed LDL. LDL was first incubated for 18 h with plasmin, chymase, or cathepsin S. The proteolyzed samples were then analyzed by SDS-PAGE on 4–20% gradient gel.
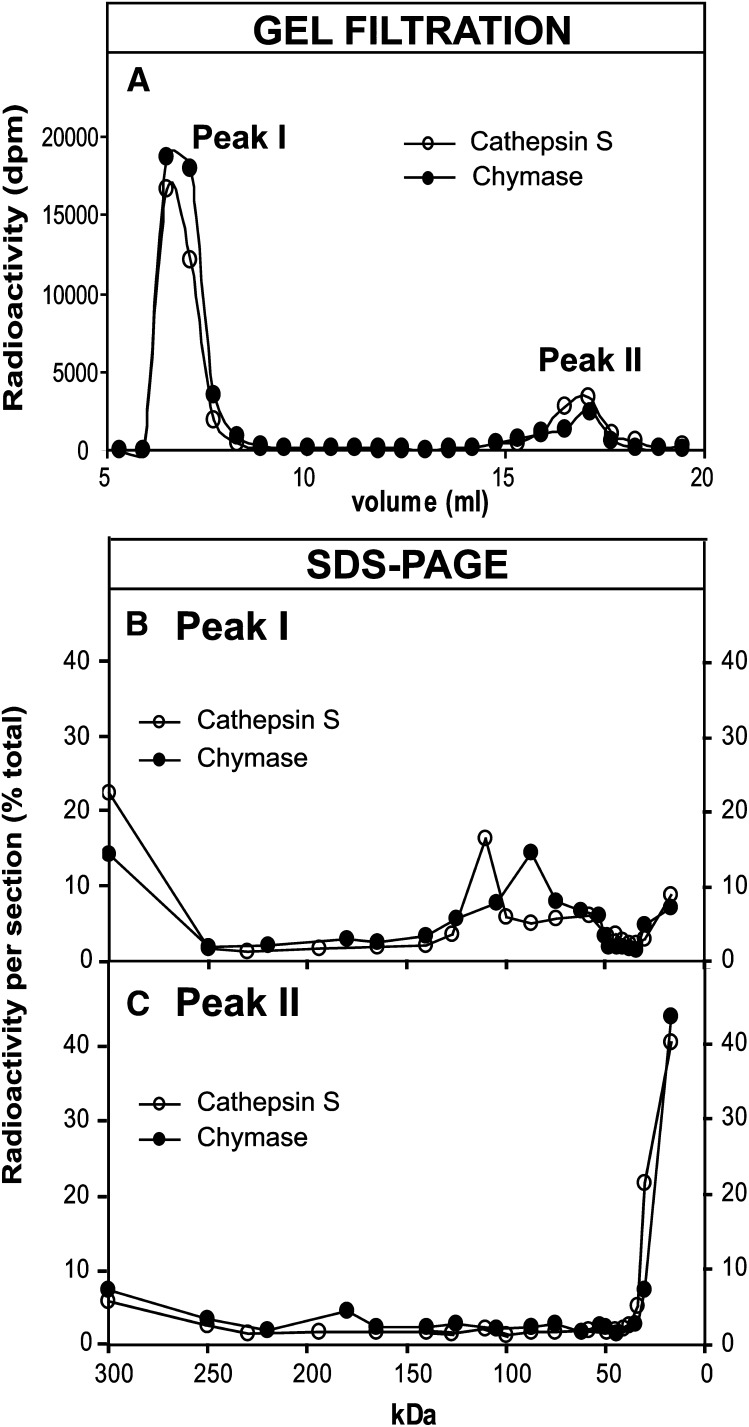
Next, in an attempt to find a reason for the differences in the ability of sSMase to hydrolyze cathepsin S– and chymase-treated LDL, the degradation of apoB-100 and the release of peptide fragments from LDL particles proteolyzed with the two enzymes were analyzed in more detail. Inasmuch as the proteolyzed LDL particles will be precipitated with TCA, peptide fragments of any size that remain particle-bound will be found in the TCA-precipitate, while any TCA-soluble peptides (MW < 5000 kDa) must have been released from the LDL particles. Therefore, samples of LDL particles proteolyzed with either cathepsin S or chymase were subjected to gel filtration on a Superose 12 column. The LDL particles elute in the void volume of the column, whereas the peptide fragments released from LDL can be recovered close to the total volume of the column (Fig. 3A). As expected, all radioactivity in peak I of both samples was TCA-precipitable, while all radioactivity in peak II of both samples was TCA-soluble. To determine the sizes of the peptide fragments that remain bound to LDL particles (peak I) and are released from LDL (peak II), the two peak fractions from each sample were subjected to SDS-PAGE. The gels were sectioned into 2-mm pieces, and the radioactivity of each section measured by liquid scintillation counter (Fig. 3B, C). The major class of peptides that remain bound to both cathepsin S– and chymase- treated LDL is >250 kDa in size. Intact apoB-100 (500 kDa) migrates to the first section (>250 kDa) of the gel, which may contain both undegraded apoB-100 and large apoB-100 fragments. In addition to these fragments, the major class of peptides found in chymase-treated LDL is 80 kDa in size, while that in cathepsin S–treated LDL is about 120 kDa in size. As expected, most of the peptide fragments released from LDL particles are small and are recovered in the last section of the 4–20% gel.
Fig. 3.
Analysis of chymase- and cathepsin S-treated LDL. Radiolabled LDL was first incubated for 18 h with chymase or cathepsin S. The proteolyzed samples were then subjected to gel filtration in a Superose 12 column to separate LDL particles (peak I) from the released peptides (peak II) (panel A). The peptides in each peak fraction were then separated by SDS-PAGE on 4–20% gradient gel, after which the gels were sectioned and the radioacitivity of each section was counted. Panel B shows the peptide fragments remaining bound to LDL particles, and panel C shows the peptide fragments that were released from LDL by the two proteases.
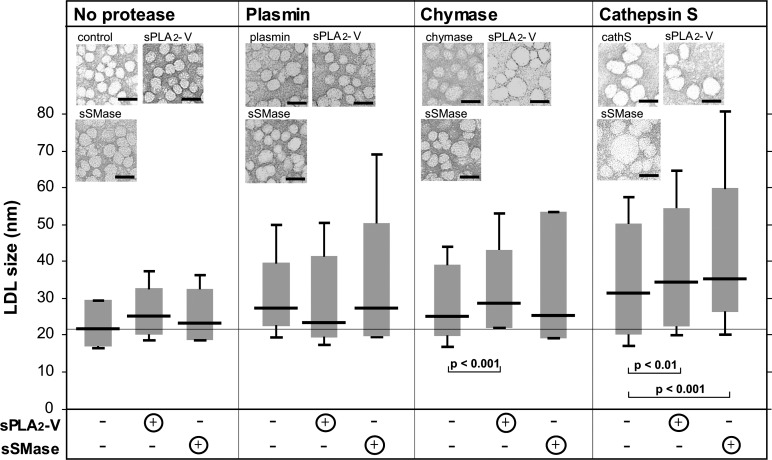
Proteolytic and lipolytic modifications of LDL particles, when examined separately, are known to induce aggregation and fusion of LDL. Next, we examined the effects of combined proteolysis and lipolysis on LDL particle fusion. For this purpose, the differently treated LDL samples were negatively stained and photographed using transmission electron microscopy, and the diameters (sizes) of individual LDL particles were measured. The size distributions of LDL particles in the various LDL samples are shown in Fig. 4. As expected from the minor lipolysis of control LDL that was obtained in our study (see Fig. 1), there was only a minor, nonsignificant change in the size of LDL treated only with sPLA2-V or sSMase, i.e., without a proteolytic pretreatment. However, treatment of LDL with any of the three proteases, but particularly with cathepsin S, led to the formation of enlarged (i.e., fused) LDL particles. Interestingly, lipolysis of plasmin-treated LDL by sPLA2-V led to a nonsignificant decrease in particle size, whereas treatment of chymase- or cathepsin S–treated LDL with the same enzyme led to a significant increase in particle size. Treatment of any of the protease-treated LDL particles with sSMase led to formation of larger LDL particles and to a much more skewed size distribution, particularly in the case of plasmin- and chymase-treated LDL. Thus, while the median of the plasmin- and chymase-treated LDL particles did not change after treatment of the particles with sSMase, some of the LDL particles in the electron micrographs had sizes of >50 nm. Treatment of cathepsin S–treated LDL with sSMase led to an even larger increase in particle size: the median size distribution increased, and particles of up to 80 nm in size were observed.
Fig. 4.
Size distributions of differently modified LDL particles. Differently treated LDL particles were examined by electron microscopy after negative staining, and the size distributions of the particles were determined by measuring the diameters of 150 particles from each photograph. Bars = 50 nm. The size distribution of each sample is shown in the box plot diagram, where 95% of the measured values are placed in the box, and the remaining 2.5% of the largest and smallest measured values are marked as lines above and below the boxes. The horizontal line in each box shows the median of the measured values. sPLA2-IIa, secretory phospholipase A2 group IIa; sPLA2-V, secretory phospholipase A2 group V, sSMase, secretory sphingomyelinase.
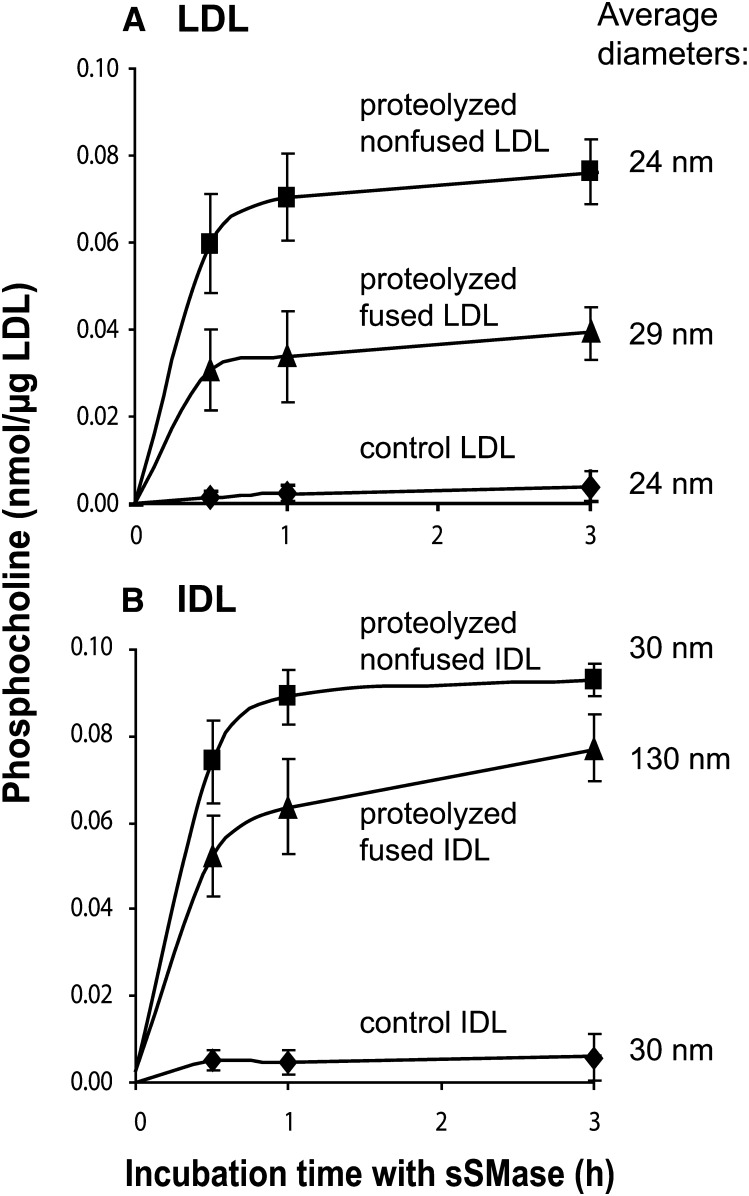
To determine whether particle fusion contributes to the ability of sSMase to hydrolyze LDL, the ability of sSMase to hydrolyze preproteolyzed fused and nonfused LDL particles was compared. For this purpose LDL was first proteolyzed with α-chymotrypsin for 30 min, after which the α-chymotrypsin activity was stopped by the addition of PMSF. Because lipoprotein fusion is a slow process, the portion of fused LDL of total particles after proteolytic degradation for 30 min is negligible (4). The preproteolyzed nonfused LDL was then divided into two fractions. One fraction was immediately subjected to hydrolysis by sSMase. The other fraction was incubated for 18 h at 37°C to allow particle fusion, and then the fused LDL particles were hydrolyzed by sSMase. Unexpectedly, the sSMase hydrolyzed proteolyzed nonfused particles more extensively than fused particles (Fig. 5A). Thus, particle fusion decreased the susceptibility of the proteolyzed lipoproteins to subsequent hydrolysis by sSMase. To test whether the activity of sSMase toward lipoproteins depends on their size, we compared the ability of sSMase to hydrolyze native and proteolyzed lipoproteins of various sizes. The fused LDL particles (29 nm) had a similar size as the nonfused IDL particles (30 nm). As shown in Fig. 5, regardless of the particle size, both the proteolyzed nonfused LDL and IDL particles were avidly hydrolyzed by the sSMase, but particle fusion decreased the ability of sSMase to hydrolyze both lipoprotein types. Thus, the ability of sSMase to hydrolyze the preproteolyzed lipoproteins does not appear to depend on particle size. Instead, it appears that the sphingomyelin molecules on the surface monolayers of the proteolyzed nonfused particles are better substrates for sSMase than those on the surface of fused LDL, where the monolayer is more tightly packed (31).
Fig. 5.
Effect of LDL fusion and size on phospholipolysis. LDL and IDL were first proteolyzed with α-chymotrypsin for 30 min, after which proteolysis was stopped by the addition of PMSF The proteolyzed LDL (panel A) and IDL (panel B) were incubated with sSMase for up to 3 h either directly after proteolysis (proteolyzed nonfused) or after an additional incubation for 18 h at 37°C to allow particle fusion (proteolyzed fused). Formation of phosphoryl choline was measured at the indicated time points. The data shown are means of three independent experiments performed with different LDL preparations ± SEM. IDL, intermediate density lipoprotein; sSMase, secretory sphingomyelinase.
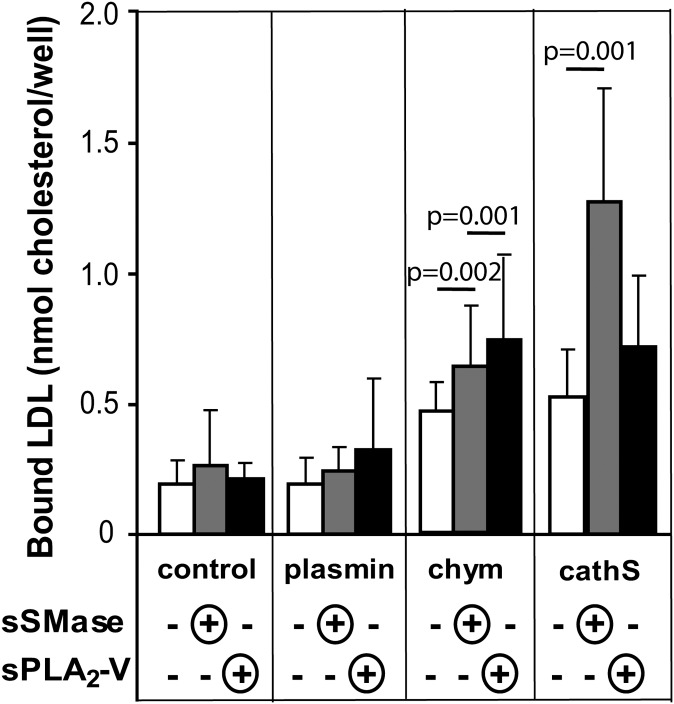
Since aggregated/fused lipoprotein particles bind to proteoglycans more tightly than their monomeric, native-sized counterparts (2), the effects of the different treatments of LDL on the ability of LDL to bind to human aortic proteoglycans were next determined. For this purpose, the differently modified LDL samples were applied to proteoglycan-coated microtiter wells. As shown in Fig. 6, plasmin alone had no effect on binding of LDL to proteoglycans, whereas proteolysis of LDL with chymase or cathepsin S enhanced binding (white columns). Lipolytic modifications (gray columns for sSMase and black columns for sPLA2) further enhanced the binding of chymase-treated and cathepsin S–treated LDL particles to proteoglycans, particularly when cathepsin S–treated LDL particles were subsequently hydrolyzed with sSMase.
Fig. 6.
Binding of modified LDL to human aortic proteoglycans. Microtiter wells were first coated with human aortic proteoglycans, after which modified LDL was added to the wells. The amounts of proteoglycan-bound LDL were determined by measuring the amounts of cholesterol in the wells. The data shown are means of five independent experiments performed with different LDL preparations ± SD. CathS, cathepsin S; Chym, chymase; sPLA2-V, secretory phospholipase A2 group V, sSMase, secretory sphingomyelinase;
DISCUSSION
Chymase, cathepsin S, and plasmin are found in atherosclerotic plaques and are capable of degrading apoB-100 in LDL particles in vitro (2). In this study, we observed that proteolysis by chymase or cathepsin S led to more extensive proteolysis of apoB-100 than proteolysis by plasmin. The plasmin-generated protein fragments were larger (as also observed previously by Piha et al. (4)), and only small amounts of peptides were released from LDL particles (4% TCA-soluble degradation products). In contrast, proteolysis of LDL with the other two enzymes generated larger protein fragments that remained bound to LDL particles, and they also generated small peptides, which were released from LDL particles and could be recovered in the incubation medium as TCA-soluble degradation products (13% TCA-soluble degradation products). Generation of the small peptide fragments and their loss from LDL particles appeared to be a necessary prerequisite to render the particles more susceptible to lipolysis by sPLA2-V and sSMase, as plasmin treatment did not increase the susceptibility of the phosphatidylcholine or sphingomyelin molecules to subsequent lipolysis in the preproteolyzed LDL particles.
On the basis of our findings, it appears that generation of small peptide fragments and their loss from the surface of proteolyzed LDL particles is a critical determinant of the final outcome of the combined action of a protease and a phospholipase on LDL particles. Such loss of protein fragments from the surface of proteolyzed LDL particles is likely to lead to reorganization of the surface lipids and to loosening of the surface monolayer (31). Because changes in surface pressure influence the activity of PLA2 and SMase enzymes (32), a proteolysis-induced decrease in the surface pressure of LDL particles provides a plausible explanation for the increased activity of sPLA2-V and sSMase observed in this study. Similarly, other types of LDL modification (oxidation and PLA2-induced hydrolysis of LDL) that induce changes in the surface of LDL particles have been shown to render the modified particles susceptible to hydrolysis by sSMase at neutral pH (25).
Even extensive proteolysis with alfa-chymotrypsin leading to release of 60% of apoB-100 from LDL particles did not enhance lipolysis of LDL by sPLA2-IIa (data not shown). The binding region of sPLA2-IIa is positively charged, and consequently, this enzyme requires a negatively charged surface for optimal binding (33). As binding of the PLA2 enzymes to lipid surfaces determines the ability of these enzymes to hydrolyze lipids and as proteolysis of apoB-100 does not induce negative charges on LDL particles (4), it is understandable that proteolytic degradation, which only induced minor changes to the net charge of the lipoprotein particles, had no effect on their sPLA2-IIa–induced hydrolysis.
The amounts of lipases that were used in the experiments were selected by testing different concentrations of the enzymes, and as shown previously by others, if higher concentrations of PLA2-V are used, even unproteolyzed LDL can be fully lipolyzed. As this study shows, preproteolysis of LDL renders the LDL particles more susceptible to PLA2-V-induced lipolysis; accordingly, to achieve a maximal degree of lipolysis, smaller amounts of the lipase are needed when preproteolyzed LDL is being lipolyzed. Since the secretory SMase can hardly lipolyze unproteolyzed LDL at neutral pH, increasing the amount of the enzyme to even a 10-fold higher concentration failed to increase the degree of lipolysis significantly (data not shown).
Secretory SMase more avidly hydrolyzed cathepsin S–treated LDL particles than chymase-treated LDL particles, even if the particles had been proteolyzed individually by each protease to yield a similar final degree of proteolysis. Because chymase hydrolyzes apoB-100 on the carbonyl side of aromatic amino acids and cathepsin S favors branched hydrophobic residues in the P2 position, the difference in sSMase activity may depend on differences in the residual lipoprotein-bound apoB-100 fragments. Indeed, the main size class of peptide fragments remaining bound to LDL particles after treatment with cathepsin S was 120 kDa, while the main size class after treatment with chymase was 80 kDa. Thus, the peptide fragments generated by cathepsin S may directly enhance binding of sSMase to LDL particles or change the organization of the surface lipids in a way that increases the accessibility of sphingomyelin molecules to sSMase. Proteolysis of LDL by cathepsin S enhanced particle fusion to a greater extent than did proteolysis by chymase (see Fig. 4). However, proteolytic fusion did not enhance the susceptibility of the lipoproteins for sSMase. This result is in full accordance with previous findings showing that SMase activity decreases with an increasing size of the substrate vesicle (34). The results of our study suggest that it is not the particle fusion, but destabilization of lipoproteins upon proteolysis, that renders the particles susceptible to hydrolysis by sSMase and sPLA2-V. In fact, particle fusion may even stabilize the particles and render them more resistant against a second hydrolytic attack by a phospholipase.
Proteoglycans play the key role in the initial retention and accumulation of LDL in atherosclerosis-prone areas of the arterial intima (35). Native LDL has relatively low affinity for proteoglycans, while proteolytic and phospholipolytic modifications of LDL particles enhance their binding to proteoglycans, particularly if the modifications are extensive enough to induce particle aggregation and fusion (2). Here we have shown that the combined effect of proteases and phospholipases increases the binding of LDL to proteoglycans even further. Such strongly increased binding was seen especially in the LDL preparations in which lipoproteins were fused as a result of the combined action of the two types of hydrolytic enzyme. Indeed, in our earlier work we found that the fused particles bind to proteoglycans more tightly than do monomeric LDL particles because each fused particle contains several copies of apoB-100, allowing polyvalent binding to proteoglycans (2).
To distinguish whether the observed increase in the proteoglycan binding of the proteolyzed and lipolyzed samples resulted from an increase in the size or in the number of bound particles, the relative numbers of PG-bound LDL particles were calculated (Table 1). An increase in the volume of the particles, which reflects the amount of cholesterol in the particles, can be calculated from the average diameters of the particles. Therefore, the relative numbers of PG-bound particles can be calculated from the average diameters and from the average amount of PG-bound cholesterol. It appears that the increased binding of LDL treated with cathepsin S alone and with cathepsin S and sPLA2-V was due to the increased particle size solely, as the number of bound particles was not increased (i.e., it was the same as that of control LDL particles. However, modifications of LDL particles by cathepsin S with sSMase, by chymase with PLA2, by chymase with sSMase, or by chymase alone also increase the number of fused particles bound to the PGs. These results suggest that chymase could induce conformational changes in the peptide fragments that remain bound to the LDL particles and so increase the affinity of the chymase-treated LDL to proteoglycans. Similarly, even proteolytic degradation with rat mast cell chymase without ensuing fusion of the modified LDL particles has been shown to increase the binding of LDL to mast cell heparin proteoglycans (36). The combination of cathepsin S and sSMase, but not cathepsin S alone, may also induce changes in the conformation of apoB-100 peptides that enhance the binding of LDL to proteoglycans.
TABLE 1.
Relative number of protoglycan-bound LDL particles
| Average Size |
Average PG Binding |
Relative Number of PG-Bound LDL Particles | |
|---|---|---|---|
| LDL Treated with | nm | nmol cholesterol/well | |
| Control | 23 | 0.20 | 1 |
| Cathepsin S | 32 | 0.53 | 1 |
| Cathepsin S + sPLA2-V | 35 | 0.72 | 1 |
| Cathepsin S + sSMase | 37 | 1.29 | 1.5 |
| Chymase | 27 | 0.47 | 1.4 |
| Chymase + sPLA2-V | 30 | 0.74 | 1.7 |
| Chymase + sSMase | 28 | 0.64 | 1.7 |
The average sizes of modified LDL samples are the same as shown in Fig. 4, and the results of proteoglycan binding are taken from the experiment shown in Fig. 6. The relative numbers of proteoglycan-bound LDL particles are calculated by calculating the increase in the volume of the particles, which reflects the change in the amount of cholesterol in each particle. This value was then used to calculate the relative number of LDL proteoglycan-bound particles. PG, proteoglycan; sPLA2-IIa, secretory phospholipase A2 group IIa; sPLA2-V, secretory phospholipase A2 group V, sSMase, secretory sphingomyelinase.
LDL-like particles and lipid droplets rich in cholesteryl linoleate isolated from atherosclerotic arteries have features suggesting that they have been derived from LDL particles by modifications (2). These lipid particles show signs of many types of modification, including fragmentation of apoB-100, decreased phosphatidylcholine, and increased ceramide contents. Interestingly, aortic lipid particles can be divided into loosely and tightly bound fractions, the particles bound more tightly to the extracellular matrix of the aorta showing evidence of more extensive modification than the loosely bound particles (37–39). The present approach of double-modification of LDL presents a novel experimental approach to mimic such extensively modified particles in the atherosclerotic arterial intima. Inasmuch as the more extensively modified LDL particles showed increased binding to human aortic proteoglycans, the present approach seems to be a valid surrogate for such experimental studies. Of note, to obtain modification of LDL particles extensive enough to allow their fusion, low, and hence, more physiological concentrations of the lipases are sufficient when the particles are preproteolyzed. Thus, the approach of multiple mild modifications of LDL particles seems to better resemble the conditions in the arterial intima than the approach of single, extensive modification.
CONCLUSION
Our data show that once apoB-100 in LDL particles is partially degraded and a fraction of the formed fragments are released, the proteolyzed lipoprotein particles become more sensitive to phospholipolysis by sPLA2-V and sSMase. Lipolysis of preproteolyzed particles leads to the formation of aggregated and fused particles and enhances their binding to proteoglycans. The combined effects of proteases and lipases lead to formation of fused lipoprotein particles resembling both in size and in composition the tightly bound apoB-100–containing lipid droplets that can be isolated from human atherosclerotic lesions. Thus, the increased ability of both proteolyzed and lipolyzed (i.e., double-modified) particles to bind to proteoglycans suggest a novel and pathophysiologically relevant in vivo operative mechanism by which such lipid droplets are formed and become accumulated extracellularly in atherosclerotic lesions.
Acknowledgments
The Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. The authors gratefully acknowledge the excellent technical assistance of Tuula Järvenpää.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- PG
- proteoglycan
- sPLA2-IIa
- secretory phospholipase A2 group IIa
- sPLA2-V
- secretory phospholipase A2 group V, sSMase, secretory sphingomyelinase
- TCA
- trichloroacetic acid
REFERENCES
- 1.Williams K. J., Tabas I. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Öörni K., Pentikäinen M. O., Ala-Korpela M., Kovanen P. T. 2000. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J. Lipid Res. 41: 1703–1714. [PubMed] [Google Scholar]
- 3.Kruth H. S., Shekhonin B. 1994. Evidence for loss of apo B from LDL in human atherosclerotic lesions: extracellular cholesteryl ester lipid particles lacking apo B. Atherosclerosis. 105: 227–234. [DOI] [PubMed] [Google Scholar]
- 4.Piha M., Lindstedt L., Kovanen P. T. 1995. Fusion of proteolyzed low-density lipoprotein in the fluid phase: a novel mechanism generating atherogenic lipoprotein particles. Biochemistry. 34: 10120–10129. [DOI] [PubMed] [Google Scholar]
- 5.Dollery C. M., Libby P. 2006. Atherosclerosis and proteinase activation. Cardiovasc. Res. 69: 625–635. [DOI] [PubMed] [Google Scholar]
- 6.Mäyränpää M. I., Heikkilä H. M., Lindstedt K. A., Walls A. F., Kovanen P. T. 2006. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron. Artery Dis. 17: 611–621. [DOI] [PubMed] [Google Scholar]
- 7.Sukhova G. K., Shi G. P., Simon D. I., Chapman H. A., Libby P. 1998. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Invest. 102: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakala J. K., Oksjoki R., Laine P., Du H., Grabowski G. A., Kovanen P. T., Pentikäinen M. O. 2003. Lysosomal enzymes are released from cultured human macrophages, hydrolyze LDL in vitro, and are present extracellularly in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 23: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 9.Öörni K., Sneck M., Brömme D., Pentikäinen M. O., Lindstedt K. A., Mäyränpää M., Aitio H., Kovanen P. T. 2004. Cysteine protease cathepsin F is expressed in human atherosclerotic lesions, is secreted by cultured macrophages, and modifies low density lipoprotein particles in vitro. J. Biol. Chem. 279: 34776–34784. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I., Williams K. J., Boren J. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116: 1832–1844. [DOI] [PubMed] [Google Scholar]
- 11.Menschikowski M., Kasper M., Lattke P., Schiering A., Schiefer S., Stockinger H., Jaross W. 1995. Secretory group II phospholipase A2 in human atherosclerotic plaques. Atherosclerosis. 118: 173–181. [DOI] [PubMed] [Google Scholar]
- 12.Hurt-Camejo E., Andersen S., Standal R., Rosengren B., Sartipy P., Stadberg E., Johansen B. 1997. Localization of nonpancreatic secretory phospholipase A2 in normal and atherosclerotic arteries. Activity of the isolated enzyme on low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 17: 300–309. [DOI] [PubMed] [Google Scholar]
- 13.Romano M., Romano E., Bjorkerud S., Hurt-Camejo E. 1998. Ultrastructural localization of secretory type II phospholipase A2 in atherosclerotic and nonatherosclerotic regions of human arteries. Arterioscler. Thromb. Vasc. Biol. 18: 519–525. [DOI] [PubMed] [Google Scholar]
- 14.Wooton-Kee C. R., Boyanovsky B. B., Nasser M. S., de Villiers W. J., Webb N. R. 2004. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 24: 762–767. [DOI] [PubMed] [Google Scholar]
- 15.Hanasaki K., Yamada K., Yamamoto S., Ishimoto Y., Saiga A., Ono T., Ikeda M., Notoya M., Kamitani S., Arita H. 2002. Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. J. Biol. Chem. 277: 29116–29124. [DOI] [PubMed] [Google Scholar]
- 16.Karabina S. A., Brocheriou I., Le Naour G., Agrapart M., Durand H., Gelb M., Lambeau G., Ninio E. 2006. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 20: 2547–2549. [DOI] [PubMed] [Google Scholar]
- 17.Marathe S., Kuriakose G., Williams K. J., Tabas I. 1999. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 19: 2648–2658. [DOI] [PubMed] [Google Scholar]
- 18.Schissel S. L., Jiang X., Tweedie-Hardman J., Jeong T., Camejo E. H., Najib J., Rapp J. H., Williams K. J., Tabas I. 1998. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 273: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 19.Xu X. X., Tabas I. 1991. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J. Biol. Chem. 266: 24849–24858. [PubMed] [Google Scholar]
- 20.Schissel S. L., Tweedie-Hardman J., Rapp J. H., Graham G., Williams K. J., Tabas I. 1996. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 98: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havel R.J., Eder H.A., Bragdon J.H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radding C. M., Steinberg D. 1960. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J. Clin. Invest. 39: 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 24.Bolton A. E., Hunter W. M. 1973. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem. J. 133: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosengren B., Peilot H., Umaerus M., Jonsson-Rylander A. C., Mattsson-Hulten L., Hallberg C., Cronet P., Rodriguez-Lee M., Hurt-Camejo E. 2006. Secretory phospholipase A2 group V: lesion distribution, activation by arterial proteoglycans, and induction in aorta by a Western diet. Arterioscler. Thromb. Vasc. Biol. 26: 1579–1585. [DOI] [PubMed] [Google Scholar]
- 26.Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. 1983. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 3: 215–222. [DOI] [PubMed] [Google Scholar]
- 27.Hurt-Camejo E., Camejo G., Rosengren B., Lopez F., Wiklund O., Bondjers G. 1990. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J. Lipid Res. 31: 1387–1398. [PubMed] [Google Scholar]
- 28.Öörni K., Pentikäinen M. O., Annila A., Kovanen P. T. 1997. Oxidation of low density lipoprotein particles decreases their ability to bind to human aortic proteoglycans. Dependence on oxidative modification of the lysine residues. J. Biol. Chem. 272: 21303–21311. [DOI] [PubMed] [Google Scholar]
- 29.Bartold P. M., Page R. C. 1985. A microdetermination method for assaying glycosaminoglycans and proteoglycans. Anal. Biochem. 150: 320–324. [DOI] [PubMed] [Google Scholar]
- 30.Sneck M., Kovanen P. T., Oorni K. 2005. Decrease in pH strongly enhances binding of native, proteolyzed, lipolyzed, and oxidized low density lipoprotein particles to human aortic proteoglycans. J. Biol. Chem. 280: 37449–37454. [DOI] [PubMed] [Google Scholar]
- 31.Hevonoja T., Pentikäinen M. O., Hyvönen M. T., Kovanen P. T., Ala-Korpela M. 2000. Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim. Biophys. Acta. 1488: 189–210. [DOI] [PubMed] [Google Scholar]
- 32.Goni F. M., Alonso A. 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531: 38–46. [DOI] [PubMed] [Google Scholar]
- 33.Winget J. M., Pan Y. H., Bahnson B. J. 2006. The interfacial binding surface of phospholipase A2s. Biochim. Biophys. Acta. 1761: 1260–1269. [DOI] [PubMed] [Google Scholar]
- 34.Linke T., Wilkening G., Lansmann S., Moczall H., Bartelsen O., Weisgerber J., Sandhoff K. 2001. Stimulation of acid sphingomyelinase activity by lysosomal lipids and sphingolipid activator proteins. Biol. Chem. 382: 283–290. [DOI] [PubMed] [Google Scholar]
- 35.Skålen K., Gustafsson M., Rydberg E. K., Hulten L. M., Wiklund O., Innerarity T. L., Boren J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 417: 750–754. [DOI] [PubMed] [Google Scholar]
- 36.Paananen K., Kovanen P. T. 1994. Proteolysis and fusion of low density lipoprotein particles independently strengthen their binding to exocytosed mast cell granules. J. Biol. Chem. 269: 2023–2031. [PubMed] [Google Scholar]
- 37.Hoff H. F., Heideman C. L., Gaubatz J. W., Scott D. W., Titus J. L., Gotto A. M., Jr 1978. Correlation of apolipoprotein B retention with the structure of atherosclerotic plaques from human aortas. Lab. Invest. 38: 560–567. [PubMed] [Google Scholar]
- 38.Smith E. B. 1977. Molecular interactions in human atherosclerotic plaques. Am. J. Pathol. 86: 665–674. [PMC free article] [PubMed] [Google Scholar]
- 39.Camejo G., Hurt E., Romano M. 1985. Properties of lipoprotein complexes isolated by affinity chromatography from human aorta. Biomed. Biochim. Acta. 44: 389–401. [PubMed] [Google Scholar]