Abstract
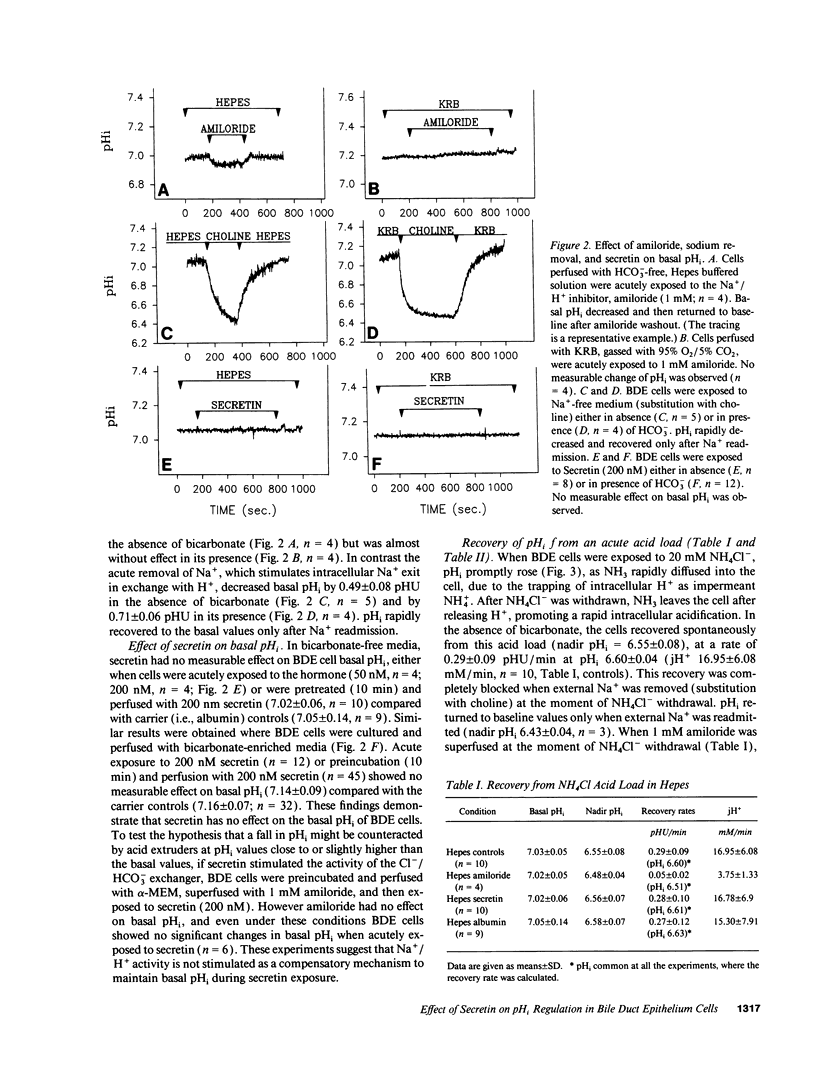
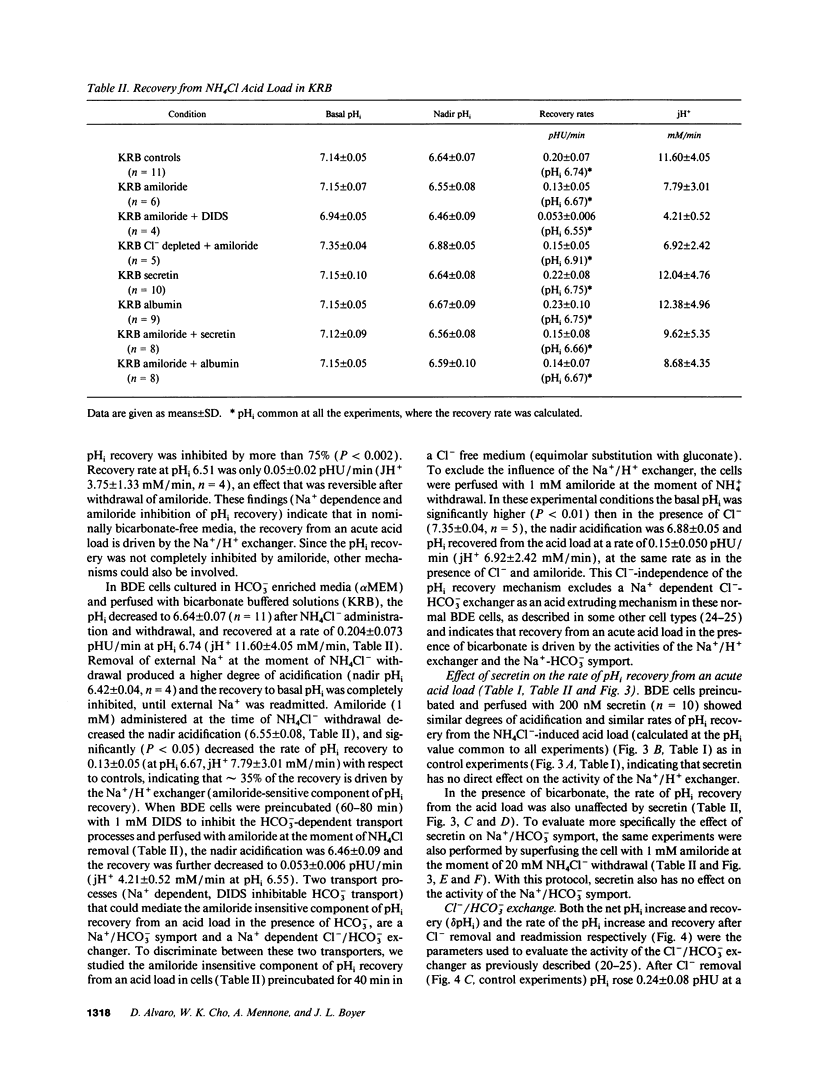
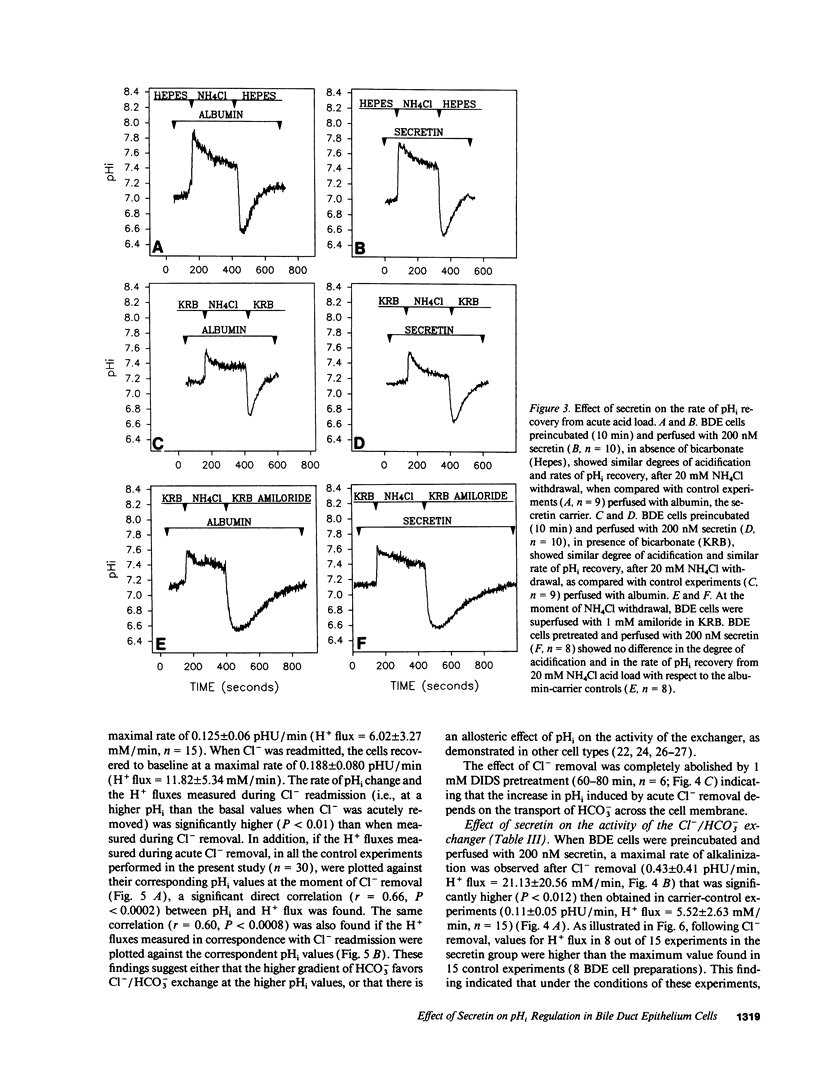
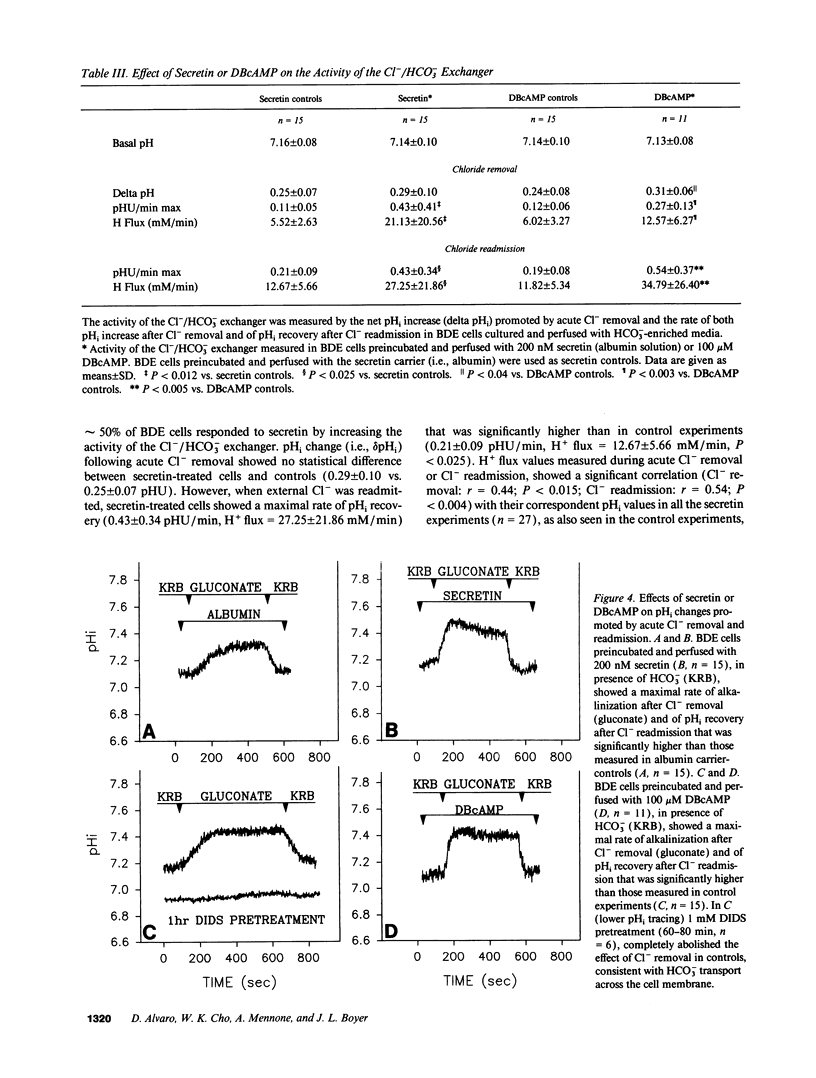
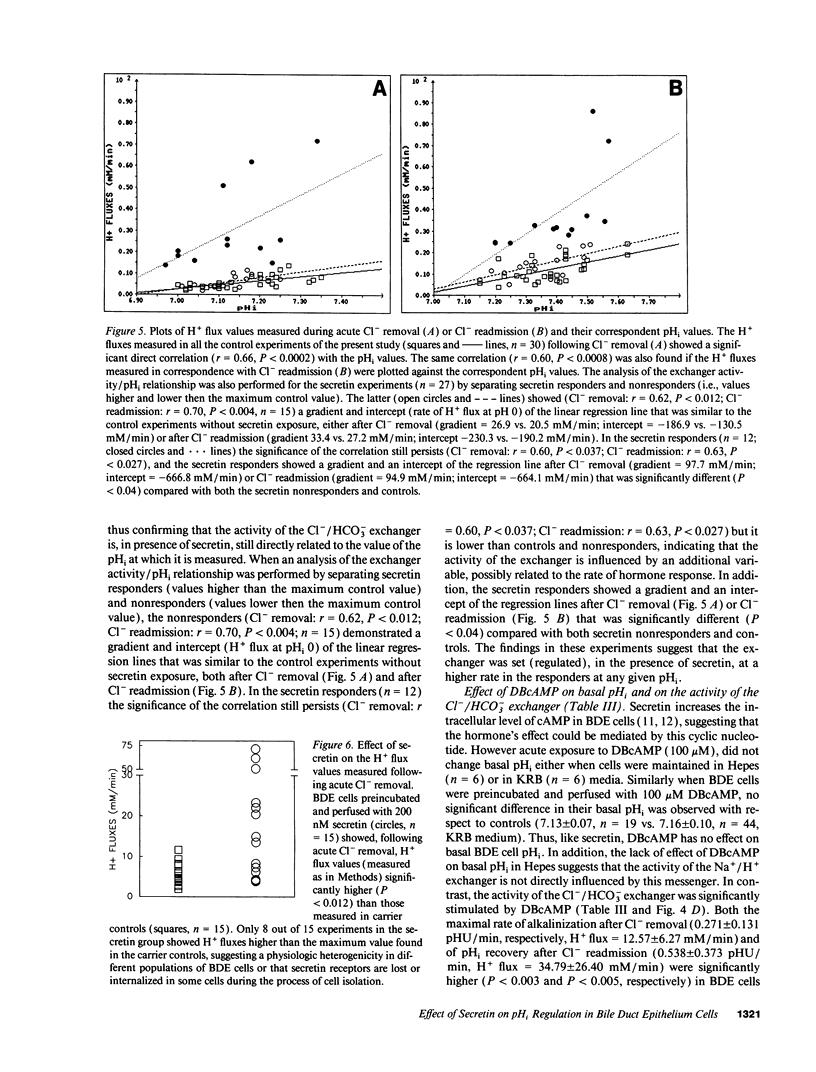
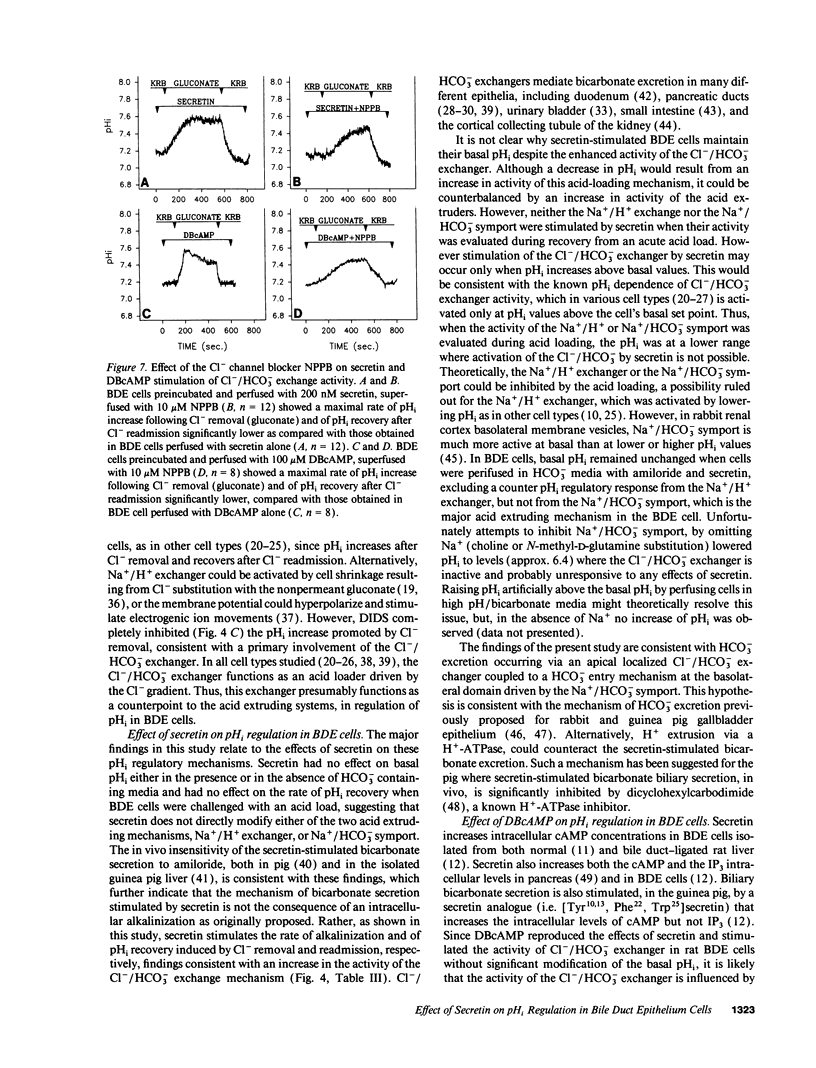
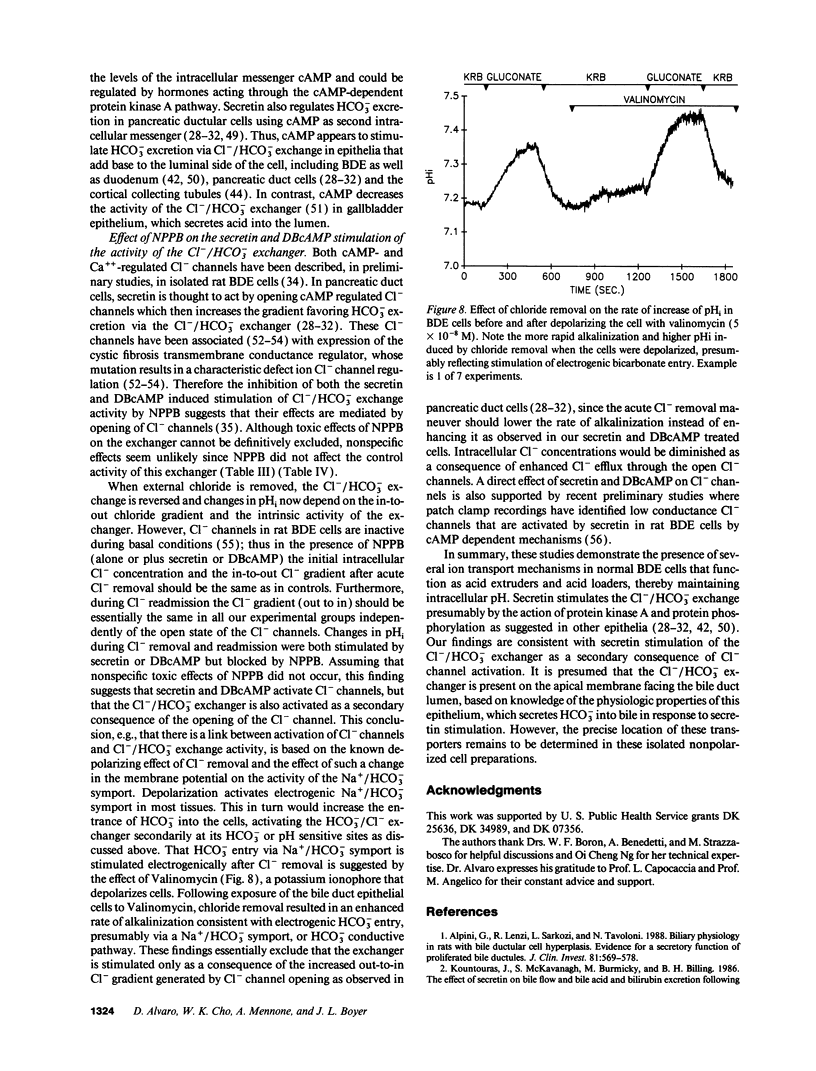
The effects of secretin on ion transport mechanisms involved in regulation of intracellular pH (pHi) and HCO3- excretion were characterized in bile duct epithelial (BDE) cells isolated from normal rat liver. pHi was measured with 2,7-bis(carboxy-ethyl)-5(6)-carboxy-fluorescein-acetomethylester (BCECF-AM) using a microfluorimetric method. Basal pHi of BDE was 7.04 +/- 0.06 in Hepes and 7.16 +/- 0.10 in KRB and was unaffected by secretin (50-200 nM). Recovery rates from an acid load in Hepes or in KRB media (with and without amiloride) were also not altered by secretin, indicating that Na+/H+ exchange and Na+/HCO3- cotransport were not affected by this hormone. After acute Cl- removal, pHi rose 0.24 +/- 0.08 pHU at a maximal rate of 0.125 +/- 0.06 pHU/min (H+ flux rates = 6.02 +/- 3.27 mM/min) and recovered after Cl- readmission (0.188 +/- 0.08 pHU/min; H+ flux rates = 11.82 +/- 5.34 mM/min). Pretreatment with 1 mM DIDS inhibited the effects of Cl- removal, while valinomycin, which induces cell depolarization, enhanced these effects, probably by stimulating electrogenic HCO3- influx. Secretin significantly increased both the maximal rate of alkalinization after Cl- removal (P < 0.012) and of pHi recovery after Cl- readmission (P < 0.025), indicating stimulation of Cl-/HCO3- exchange activity. These findings were reproduced with N6,2'-O-Dibutyryladenosine-3',5'-cyclic monophosphate (DBcAMP). The Cl- channel blocker 5-nitro-2'-(3-phenylpropylamino)-benzoate (NPPB, 10 microM) significantly decreased the effects of secretin and DBcAMP on the pHi changes promoted by acute Cl- removal/readmission. These findings establish that secretin stimulates the activity of the Cl-/HCO3- exchanger in BDE cells, probably by activating Cl- channels via the intracellular messenger cAMP. This in turn depolarizes the cell, stimulating electrogenic Na+/HCO3- symport. The cell depolarization induced by Cl- channel activation should enhance HCO3- entrance through electrogenic Na+/HCO3- symport, which in turn stimulates the Cl-/HCO3- exchange. These mechanisms could account for secretin stimulated bicarbonate secretion in bile.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988 Feb;81(2):569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Zhai W. R., Liu M. H., Slott P. A., Paronetto F., Tavoloni N. Isolation of a nonparenchymal liver cell fraction enriched in cells with biliary epithelial phenotypes. Gastroenterology. 1989 Nov;97(5):1248–1260. doi: 10.1016/0016-5085(89)91696-x. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Strazzabosco M., Corasanti J. G., Haddad P., Graf J., Boyer J. L. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Liver sinusoidal cells. Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972 Jul;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot-Chabaud M., Dumont M., Corbic M., Erlinger S. Effect of acid-base balance on biliary bicarbonate secretion in the isolated perfused guinea pig liver. Am J Physiol. 1990 Jun;258(6 Pt 1):G863–G872. doi: 10.1152/ajpgi.1990.258.6.G863. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Buanes T., Grotmol T., Landsverk T., Raeder M. G. Secretin empties bile duct cell cytoplasm of vesicles when it initiates ductular HCO3- secretion in the pig. Gastroenterology. 1988 Aug;95(2):417–424. doi: 10.1016/0016-5085(88)90499-4. [DOI] [PubMed] [Google Scholar]
- Corasanti J. G., Gleeson D., Boyer J. L. Effects of osmotic stresses on isolated rat hepatocytes. I. Ionic mechanisms of cell volume regulation. Am J Physiol. 1990 Feb;258(2 Pt 1):G290–G298. doi: 10.1152/ajpgi.1990.258.2.G290. [DOI] [PubMed] [Google Scholar]
- Dunk C. R., Brown C. D., Turnberg L. A. Stimulation of Cl/HCO3 exchange in rat duodenal brush border membrane vesicles by cAMP. Pflugers Arch. 1989 Sep;414(6):701–705. doi: 10.1007/BF00582138. [DOI] [PubMed] [Google Scholar]
- Farouk M., Vigna S. R., McVey D. C., Meyers W. C. Localization and characterization of secretin binding sites expressed by rat bile duct epithelium. Gastroenterology. 1992 Mar;102(3):963–968. doi: 10.1016/0016-5085(92)90183-y. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Basavappa S., McGill J., Melhus O., Cohn J. A. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993 Jan;91(1):319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Henderson R. M., Krumpholz B., Boyer J. L. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J Membr Biol. 1987;95(3):241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Grotmol T., Buanes T., Raeder M. G. DCCD (N,N'-dicyclohexylcarbodiimide) inhibits biliary secretion of HCO-3. Scand J Gastroenterol. 1987 Mar;22(2):207–213. doi: 10.3109/00365528708991881. [DOI] [PubMed] [Google Scholar]
- Grotmol T., Buanes T., Raeder M. G. The effect of amiloride on biliary HCO3- secretion in the anaesthetized pig. Acta Physiol Scand. 1987 Jul;130(3):447–455. doi: 10.1111/j.1748-1716.1987.tb08161.x. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Knuchel J., Krähenbühl S., Zimmermann A., Reichen J. Effect of secretin on bile formation in rats with cirrhosis of the liver: structure-function relationship. Gastroenterology. 1989 Oct;97(4):950–957. doi: 10.1016/0016-5085(89)91503-5. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Marino C. R., Matovcik L. M., Gorelick F. S., Cohn J. A. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991 Aug;88(2):712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. J., Smith J. D., Garcia-Soto J. J., Grinstein S. Internal pH-sensitive site couples Cl-(-)HCO3- exchange to Na+-H+ antiport in lymphocytes. Am J Physiol. 1989 Feb;256(2 Pt 1):C428–C433. doi: 10.1152/ajpcell.1989.256.2.C428. [DOI] [PubMed] [Google Scholar]
- McGill J. M., Basavappa S., Fitz J. G. Characterization of high-conductance anion channels in rat bile duct epithelial cells. Am J Physiol. 1992 Apr;262(4 Pt 1):G703–G710. doi: 10.1152/ajpgi.1992.262.4.G703. [DOI] [PubMed] [Google Scholar]
- Mugharbil A., Knickelbein R. G., Aronson P. S., Dobbins J. W. Rabbit ileal brush-border membrane Cl-HCO3 exchanger is activated by an internal pH-sensitive modifier site. Am J Physiol. 1990 Oct;259(4 Pt 1):G666–G670. doi: 10.1152/ajpgi.1990.259.4.G666. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Wehner F., Winterhager J. M. Transcellular bicarbonate transport in rabbit gallbladder epithelium: mechanisms and effects of cyclic AMP. Pflugers Arch. 1990 May;416(3):312–321. doi: 10.1007/BF00392068. [DOI] [PubMed] [Google Scholar]
- Reuss L. Cyclic AMP inhibits Cl-/HCO3- exchange at the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1987 Aug;90(2):173–196. doi: 10.1085/jgp.90.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M., Lesoine G. A., Bergman J. A., McKinney T. D. A pH modifier site regulates activity of the Na+:HCO3- cotransporter in basolateral membranes of kidney proximal tubules. J Clin Invest. 1991 Oct;88(4):1135–1140. doi: 10.1172/JCI115413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D. L., Beauwens R., Palmisano J., Mitchell P. P., Steinmetz P. R. A double-membrane model for urinary bicarbonate secretion. Am J Physiol. 1985 Oct;249(4 Pt 2):F546–F552. doi: 10.1152/ajprenal.1985.249.4.F546. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M., Mennone A., Boyer J. L. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991 May;87(5):1503–1512. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuenkel E. L., Machen T. E., Williams J. A. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- Sundaram U., Knickelbein R. G., Dobbins J. W. pH regulation in ileum: Na(+)-H+ and Cl(-)-HCO3- exchange in isolated crypt and villus cells. Am J Physiol. 1991 Mar;260(3 Pt 1):G440–G449. doi: 10.1152/ajpgi.1991.260.3.G440. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Biden T. J., Meehan C. J., Andreu D., Merrifield R. B. Secretin stimulates cyclic AMP and inositol trisphosphate production in rat pancreatic acinar tissue by two fully independent mechanisms. Proc Natl Acad Sci U S A. 1987 May;84(10):3146–3150. doi: 10.1073/pnas.84.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Weintraub W. H., Machen T. E. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989 Sep;257(3 Pt 1):G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]
- Winterhager J. M., Stewart C. P., Heintze K., Petersen K. U. Electroneutral secretion of bicarbonate by guinea pig gallbladder epithelium. Am J Physiol. 1986 Apr;250(4 Pt 1):C617–C628. doi: 10.1152/ajpcell.1986.250.4.C617. [DOI] [PubMed] [Google Scholar]