Abstract
Enhanced production of monounsaturated fatty acids (FA) derived from carbohydrate-enriched diets has been implicated in the development of obesity and insulin resistance. The FA elongases Elovl-5 and Elovl-6 are regulated by nutrient and hormone status, and have been shown using intact yeast and mammalian microsome fractions to be involved in the synthesis of monounsaturated FAs (MUFA). Herein, targeted knockdown and overexpression of Elovl-5 or Elovl-6 was used to determine their roles in de novo synthesis of specific MUFA species in mammalian cells. Treatment of rat insulinoma (INS)-1 cells with elevated glucose increased de novo FA synthesis and the ratio of MUFAs to saturated FAs. Elovl-5 knockdown decreased elongation of 16:1,n-7. Elovl-5 overexpression increased synthesis of 18:1,n-7; however, this increase was dependent on stearoyl-CoA desaturase–driven 16:1,n-7 availability. Knockdown of Elovl-6 decreased elongation of 16:0 and 16:1,n-7, resulting in accumulation of 16:1,n-7. Elovl-6 overexpression preferentially drove synthesis of 16:0 elongation products 18:0 and 18:1,n-9 but not 18:1,n-7. These findings demonstrate that coordinated induction of FA elongase and desaturase activity is required for balanced synthesis of specific n-7 versus n-9 MUFA species. Given the relative abundance of 16:0 to 16:1,n-7 and the specificity of Elovl-6 for 16:0, Elovl-6 is a major elongase for 18:1,n-9 production.
Keywords: fatty acid synthesis, stearoyl-CoA desaturase, palmitoleate, oleate, vaccinate, palmitate
Diets enriched in carbohydrates and saturated fat are well established to cause altered fatty acid (FA) metabolism and elevated triglyceride accumulation, contributing to the development of obesity and type 2 diabetes. Elevated levels of carbohydrates specifically enriched in mono- and disaccharides induce the transcription of genes that increase glucose metabolism and lipogenesis in the liver (1–3), diverting excess catabolic metabolites into FAs for storage as triglycerides and cholesterol esters. FA elongase and desaturase enzymes catalyze the conversion of saturated FAs (SFA) synthesized de novo from glucose into monounsaturated FAs (MUFA), such as palmitoleate (16:1,n-7) or oleate (18:1,n-9). The accumulation of MUFAs has been associated with hypertriglyceridemia and adiposity (4–6), and inhibition of MUFA synthesis decreases triglyceride levels and protects from diet-induced obesity and insulin resistance (7–9). Interestingly, 16:1,n-7 was recently identified as an adipose tissue-derived lipid hormone capable of enhancing muscle insulin sensitivity (10). These findings emphasize the importance of understanding the mechanisms regulating the production of MUFAs.
Synthesis of FAs de novo involves the enzymes acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) that carboxylate cytosolic acetyl-CoA to malonyl-CoA and covalently bond malonyl-CoA C2 units to produce the C16 FA palmitate (16:0), respectively (11). After activation to palmitoyl-CoA, a FA elongase adds an additional malonyl-CoA to make stearoyl-CoA (18:0). MUFAs not derived from exogenous sources are synthesized by stearoyl-CoA desaturases (SCD), delta-9 desaturases that are rate-limiting for MUFA synthesis (12). SCD-mediated addition of a cis-double bond to palmitoyl-CoA and stearoyl-CoA form palmitoleoyl-CoA (16:1,n-7) and oleoyl-CoA (18:1,n-9), respectively. Palmitoleoyl-CoA may also be elongated to vaccenyl-CoA (18:1,n-7). Synthesis of C18 MUFAs de novo from glucose requires chain elongation of C16 FAs by a FA elongase (Elovl), yet it remains uncertain which elongases are involved in synthesis of specific MUFA species (e.g., 18:1,n-7 versus 18:1,n-9).
Substrate specificity analyses using yeast and in vitro microsomal preparations identified the elongases Elovl-5 (FAE1, Relo1, Helo1) and Elovl-6 (LCE, FACE, rElo2) to be involved in MUFA synthesis (13–16). Elovl-6 and SCD gene expression are induced by insulin through activation of sterol regulatory element binding protein-1 (SREBP-1) and by glucose through activation of the carbohydrate-regulatory element binding protein/MAX-like factor X heterodimer (17). In mice, hepatic Elovl-5, Elovl-6, and SCDs are induced by activation of peroxisome proliferator–activated receptor α and leptin deficiency, whereas they are suppressed by long-term feeding of diets high in SFAs (17). Coordinated expression of elongases and desaturases by transcription factors that regulate lipogenic pathways thus control the levels of MUFAs. In vitro assays have shown that Elovl-5 elongates unsaturated FAs, including 16:1,n-7, γ-linolenate (18:3,n-6), and stearidonate (18:4,n-3) (13, 14, 16). Relative to Elovl-5, Elovl-6 more effectively elongates C12-16 saturated FAs and 16:1,n-7 (13, 15). Because oleate (18:1,n-9) is the predominate MUFA in cells, Elovl-6 may play a larger role in MUFA synthesis than Elovl-5 by converting 16:0 to stearate (18:0), the saturated precursor of 18:1,n-9.
Modulating the expression of FA elongase and desaturase genes has physiological significance, as mice with knockdowns of either Elovl-6 or SCD1 are protected from diet-induced obesity (7–9). The role of FA elongases in regulating the determination of specific de novo–derived MUFA end products, however, remains to be defined. This study presents a comprehensive analysis of the effects of both decreased and increased expression of Elovl-5, Elovl-6, and SCDs on FAs synthesized de novo from glucose in a mammalian cell line. The results demonstrate that altering the expression of each enzyme causes significant changes in de novo synthesis of specific MUFA species.
MATERIALS AND METHODS
Cell culture
INS-1 cells, a rat insulinoma cell line (18), were maintained in RPMI-1640 media containing 11.1 mM glucose and supplemented with 10% fetal bovine serum, 1 mM pyruvate, 10 mM HEPES, 50 μM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin (INS-1 medium). For FA synthesis from glucose, cells were cultured for 48 h in INS-1 media containing 4 or 16.7 mM glucose and treated, during the last 24 h, with either 10 or 41.75 μCi [U-14C]glucose (264 mCi/mmol, ICN Pharmaceuticals, Costa Mesa, CA), respectively.
RNA extraction and quantitative real-time PCR
Total RNA was extracted with TRIZOL (Invitrogen, Carlsbad, CA) according to manufacturer's directions. First-stand cDNA was synthesized using the iScript cDNA Synthesis kit (BioRad, Hercules, CA). Quantitative real-time (qRT) PCR was conducted using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen), synthesized cDNA and sets of gene-specific forward and reverse primers (supplemental Table I) (Integrated DNA Technologies, Coralville, IA). qRT-PCR reactions were carried out using a Mx3000P quantitative PCR system (Stratagene, La Jolla, CA). All samples were analyzed in triplicate. The relative amounts of mRNA were analyzed by the comparative cycle threshold method. Ribosomal protein L32 (RPL32) mRNA levels, which were unaffected by treatments, were used to normalize mRNA levels.
Modulation of FA elongase and desaturase activities with siRNA
Control, Elovl-5, Elovl-6, and SCD siRNA were from Thermo Fisher Scientific. INS-1 cells (2 × 106) were electroporated in 100 µl electroporation buffer (7 mM ATP, 11.6 mM MgCl2-6H2O, 68 mM K2HPO4, 14 mM NaHCO3, and 2.2 mM glucose) containing 0.1 nmol siRNA using a Amaxa Nucleofector (program D-026). Cells were cultured for 24 h in 5.5 mM glucose and subsequently cultured for 24 h in INS-1 media containing 11.1 mM glucose. Cells were then harvested for mRNA analysis or continued culturing overnight with 1 μCi [2-14C]acetic acid (51 mCi/mmol, ICN Pharmaceuticals, Inc., Costa Mesa, CA), after which lipids were extracted and analyzed for 14C incorporation (see below).
Overexpression of FA elongases and desaturase with adenoviruses
Elovl-5 and Elovl-6 cDNA were cloned and used for construction of recombinant adenoviruses as previously described (19). SCD2 cDNA was cloned in the same manner using cDNA synthesized from INS-1 cell mRNA and the following primers: sense 5′-ATAGTCGACATGCCGGCTCACATACTGCAAGAG-3′; antisense 5′-ATACTCGAGTCAGCCACTCTTGCAGCTCTCCTCCCC-3′ (Acc. No. AB032243). Adenoviruses overexpressing SCD2 were constructed using the AdEasy Adenoviral Vector System (Stratagene) as previously described (17). An adenovirus overexpressing β-galactosidase (B-gal) was obtained from Dr. Newgard, Duke University, NC. For gene transduction, 90% confluent cell cultures were infected for 2 h with 5 or 10 pfu per cell and then cultured for an additional 24 h in 5.5 mM glucose medium to allow for gene expression. Cells were then cultured in INS-1 media containing 11.1 mM glucose with 1 μCi [2-14C]acetic acid for 24 h prior to lipid extraction.
Lipid extraction and fatty acid analysis
Cells were harvested and lipids extracted as described by Wang et al. (19). Total lipid extracts from the [2-14C]acetic acid–labeling studies were saponified, extracted, and fatty acids were then fractionated by reverse phase-HPLC using a J'sphere ODS-H80 (YMC-Waters) column and quantified by flow-through scintillation counting (IN/US).
Elongation and desaturation indexes
Indexes were determined using the 14C counts incorporated into each specific FA species and calculating the ratios of product(s) to substrate. The elongation index for 16:0 was defined as the ratio of 18:0 plus 18:1,n-9 to 16:0, while the elongation index for 16:1,n-7 was defined as the ratio of 18:1,n-7 to 16:1,n-7. The total desaturation index was defined as the ratio of MUFAs (16:1,n-7, 18:1,n-7, 18:1,n-9) to SFAs (14:0, 16:0, 18:0).
Statistical analysis
All experiments represent the average of three independent experiments done in duplicate. Data was analyzed by Student's t-test. P < 0.05 was considered significant.
RESULTS
De novo FA synthesis in INS-1 cells cultured in elevated glucose
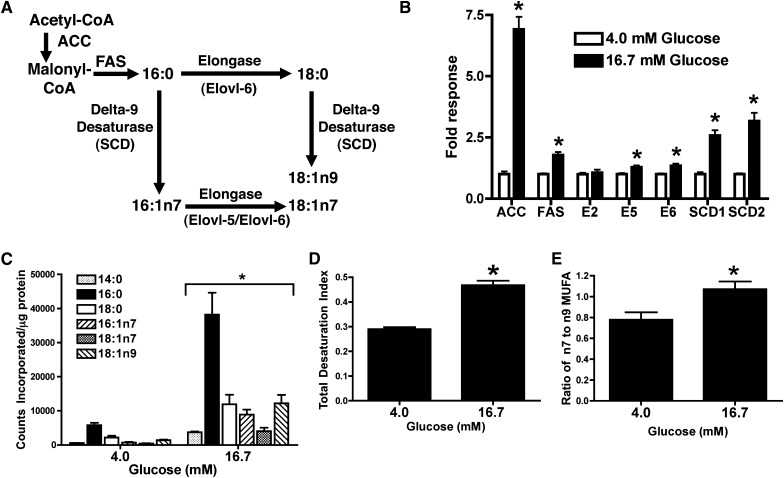
INS-1 cells were used to study the roles of Elovl-5 and Elovl-6 in de novo synthesis of specific MUFA species: 16:1,n-7; 18:1,n-7; and 18:1,n-9 (Fig. 1A). INS-1 cells were selected as a model because they exhibit increased lipogenic gene expression and triacylglyceride storage in response to elevated glucose or liver X receptor (LXR) activation (20–22) and are currently being used to study the impact of FA elongases and desaturases on palmitate-induced endoplasmic reticulum (ER) stress and apoptosis. Culturing INS-1 cells for 48 h in 16.7 mM glucose markedly increased mRNA levels of ACCα, FAS, SCD1, and SCD2 compared with cells cultured in 4 mM glucose (Fig. 1B). In contrast, Elovl-5 and Elovl-6 mRNA levels were only modestly induced by glucose. Overall, the changes in lipogenic gene expression were associated with a 6- and 10-fold increase in de novo synthesized SFAs and MUFAs, respectively, as assessed by incorporation of 14C-glucose into FAs (Fig. 1C). Elevated glucose thus led to a 1.6-fold increase in the total desaturation index (Fig. 1D). Of these newly synthesized MUFAs, there was a significant increase in the n-7 relative to n-9 species (Fig. 1E). In subsequent experiments, INS-1 cells cultured in elevated glucose were used to examine the role of Elovl-5 and Elovl-6 on synthesis of specific MUFA species.
Fig. 1.
Increased lipogenic gene expression and de novo FA synthesis in INS-1 cells cultured in elevated glucose. mRNA levels of lipogenic genes and de novo FA synthesis were determined in INS-1 cells cultured for 48 h in 4 mM or 16.7 mM glucose. For de novo FA synthesis, culture media was supplemented with [U-14C]glucose for the last 24 h. Total lipids were extracted, saponified, and 14C incorporation into FAs was quantified. A: Diagram of enzymes regulating end products of de novo FA synthesis. B: Levels of ACCα, FAS, Elovl-2 (E2), Elovl-5 (E5), Elovl-6 (E6), SCD1, and SCD2 mRNA. Data are relative to RPL32 expression and normalized to cells cultured in 4 mM glucose. C: Incorporation of 14C into specific FA species. Data are counts incorporated into specific FA species normalized to total protein. D: Total desaturation index. E: Ratio of 14C-labeled n-7 (16:1,n-7 + 18:1,n-7) to n-9 (18:1,n-9) MUFA. Values are the mean ± SEM for three independent experiments. *P < 0.05 compared with low glucose. ACC, acetyl-CoA carboxylase; Elovl, fatty acid elongase; FA, fatty acid; INS, rat insulinoma; MUFA, monounsaturated fatty acid; SCD, stearoyl-CoA desaturase.
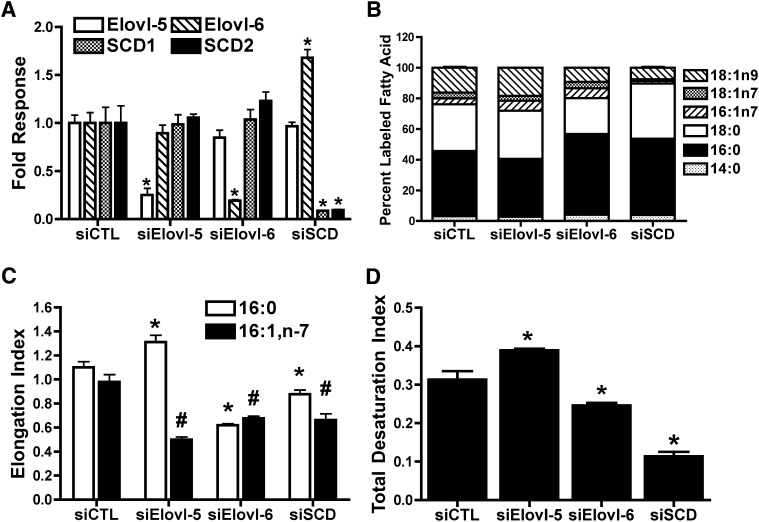
Selective knockdown of Elovl-5 or Elovl-6 alters synthesis of specific MUFA species
siRNAs were used to determine the relative contribution of Elovl-5, Elovl-6, or SCD1/2 on de novo synthesis of specific FAs species in INS-1 cells cultured in elevated glucose. siRNAs selective against Elovl-5 or Elovl-6 decreased expression of the target mRNA by 75% and 80%, respectively, with no significant effect on nontarget mRNA levels (Fig. 2A). SCD siRNA reduced SCD1 and SCD2 by 90% and resulted in a 1.7-fold increase in Elovl-6. Next, de novo FA synthesis was assessed in siRNA-treated cells using 14C-acetic acid. Decreased expression of Elovl-5 mRNA led to increased 14C-labeling of 16:1,n-7 and decreased labeling of 16:0 (Fig. 2B; supplemental Fig. I). There was no significant change in 14C-labeled 18:0 or 18:1,n-7, but there was a trend for increased 14C-labeled 18:1,n-9. Elovl-5 siRNA decreased the elongation index for 16:1,n-7 by ∼50%, whereas the elongation index for 16:0 and the total desaturation index were modestly increased (Fig. 2C, D). These data are consistent with Elovl-5 principally elongating 16:1,n-7 to 18:1,n-7 (Fig. 1A).
Fig. 2.
Effect of siRNA-mediated knockdown of Elovl-5, Elovl-6, or SCD on gene expression and de novo FA synthesis. INS-1 cells electroporated with CTL, Elovl-5, Elovl-6, or SCD siRNA were cultured in 11.1 mM glucose. After 24-h, cells were harvested for mRNA analysis or incubated an additional 12 h in 11.1 mM glucose plus [2-14C]acetic acid to label de novo synthesized lipid. Total lipids were extracted, saponified, and 14C incorporation into FAs was quantified. A: Levels of Elovl-5, Elovl-6, SCD1, and SCD2 mRNA. Data are relative to RPL32 expression and normalized to siCTL-treated cells. *P < 0.02 compared with siCTL. B: Incorporation of 14C into FA species. Data are reported as percentage of total labeled FA species. C: Elongation index for 16:0 and 16:1,n-7. *P < 0.05 for 16:0; #P < 0.03 for 16:1,n-7 compared with siCTL. D: Total desaturation index. *P < 0.05 compared with siCTL. Values are mean ± SEM for three individual experiments. CTL, control; Elovl, fatty acid elongase; FA, fatty acid; INS, rat insulinoma; SCD, stearoyl-CoA desaturase.
Treatment of INS-1 cells with Elovl-6 siRNA decreased 14C-labeling of 18:0 and 18:1,n-9, and it increased 14C-labeling of 16:0 and 16:1,n-7 (Fig. 2B; supplemental Fig. I). There was no change in labeled 18:1,n-7. Reduced Elovl-6 expression decreased the elongation indexes for both 16:0 and 16:1n7 and modestly decreased the total desaturation index (Fig. 2C, D). These data are consistent with Elovl-6 mediating elongation of 16:0 to 18:0 and 16:1n-7 to 18:1,n-7 (Fig. 1A). INS-1 cells treated with SCD siRNA decreased 14C-labeling of 16:1,n-7, 18:1,n-7 and 18:1,n-9 and increased 14C-labeling of 16:0 and 18:0 (Fig. 2B; supplemental Fig. I). Loss of SCD expression decreased the elongation indexes for 16:0 and 16:1,n-7 and the total desaturation index (Fig. 2C, D).
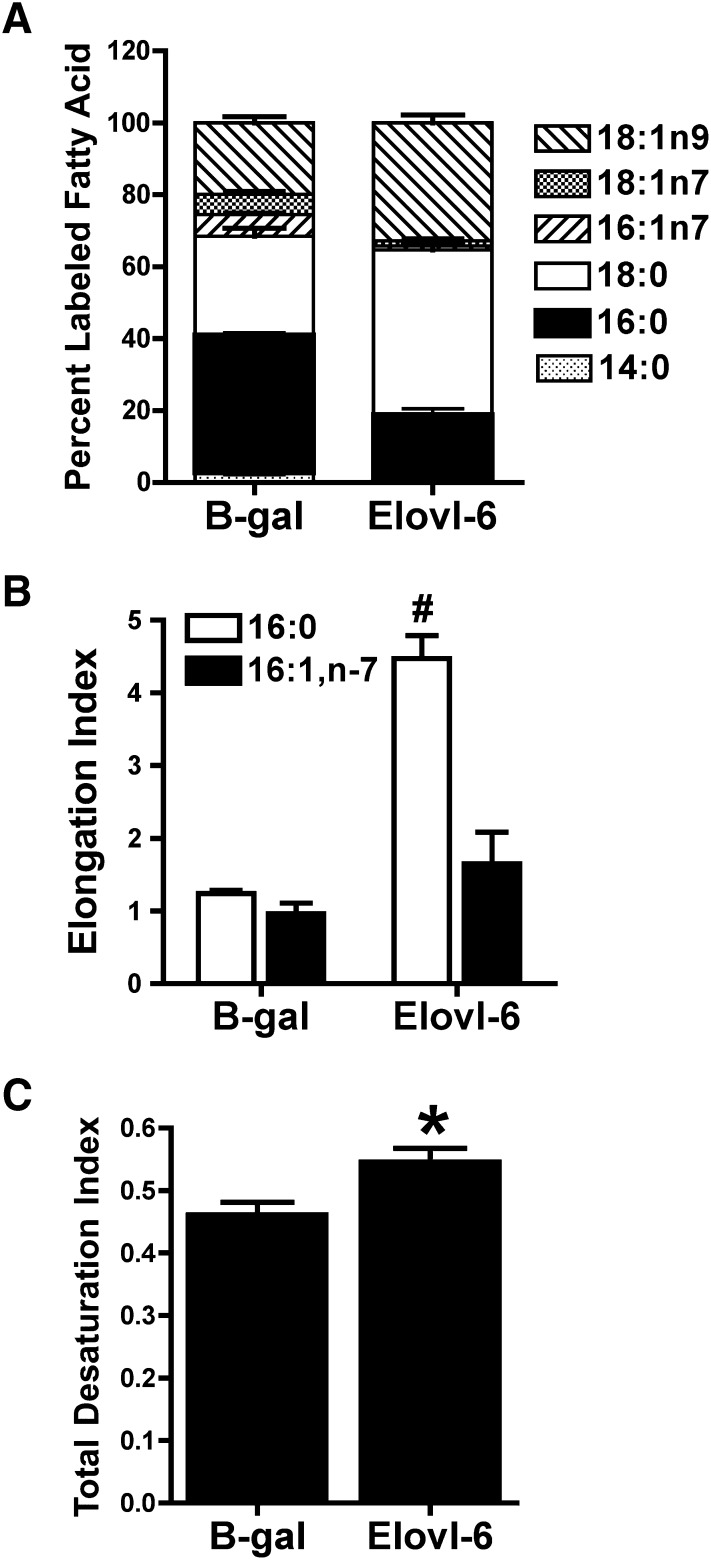
Overexpression of Elovl-6 increases synthesis of 18:0 and 18:1,n-9
To further examine the selectivity of Elovl-5 and Elovl-6 in de novo FA synthesis, adenoviruses were constructed to overexpress each individual gene and then examined for their effect on de novo FA end products. Compared with a control adenovirus containing β-galactosidase, overexpression of Elovl-6 resulted in a large increase in 14C-labeled 18:0 and 18:1,n-9 and decreased labeling of 16:0, 16:1,n-7 and 18:1,n-7 (Fig. 3A; supplemental Fig. II). The elongation index for 16:0 increased 3.6-fold, whereas the elongation index for 16:1,n-7 was unaffected (Fig. 3B). Overexpression of Elovl-6 increased the total desaturation index (Fig. 3C). These results are consistent with Elovl-6 elongating 16:0 to 18:0 for synthesis of 18:1,n-9 and this occurs at the expense of 16:1,n-7 and 18:1,n-7 production.
Fig. 3.
Effect of increased Elovl-6 expression on de novo FA synthesis. INS-1 cells infected with adenoviruses expressing B-gal or Elovl-6 were cultured for 24 h in 11.1 mM glucose and [2-14C]acetic acid. Total lipids were extracted, saponified, and 14C incorporation into FAs was quantified. A: Incorporation of 14C into FA species. Data are reported as percentage of total labeled FA species. B: Elongation index for 16:0 and 16:1,n-7. #P < 0.01 for 16:0 compared with B-gal. C: Total desaturation index. *P < 0.05 compared with B-gal. Values are the mean ± SEM for three individual experiments. B-gal, β-galactosidase; Elovl, fatty acid elongase; FA, fatty acid; INS, rat insulinoma.
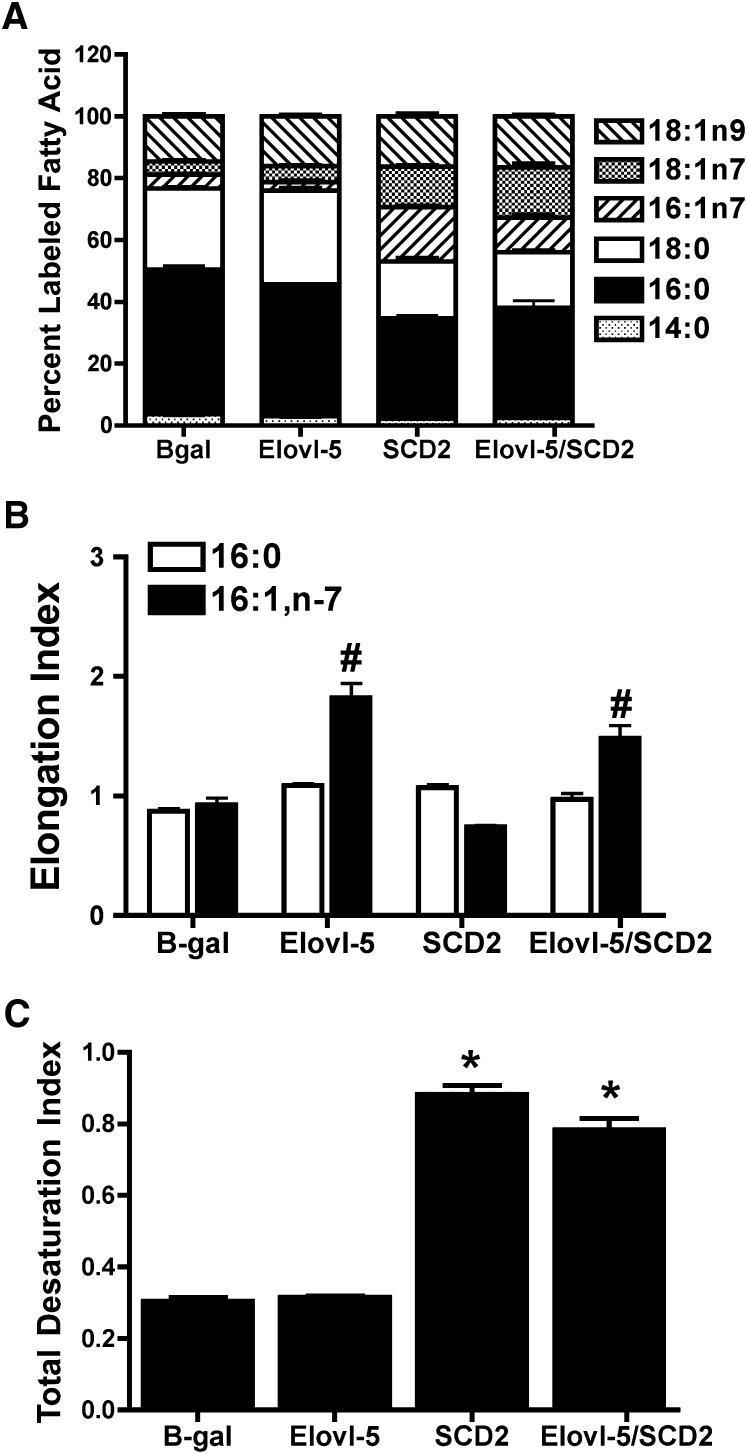
Overexpression of Elovl-5 increases synthesis of 18:1,n-7
Elovl-5 overexpression had a limited effect on 14C-acetic acid incorporation into FAs compared with Elovl-6 overexpression. Nonetheless, elevated Elovl-5 expression led to decreased 14C-labeled 16:0 and 16:1,n-7, and increased 14C-labeled 18:0 and 18:1,n-7 (Fig. 4A; supplemental Fig. III). The effect of Elovl-5 on 16:1,n-7– and 18:1,n-7–labeling corresponded with a significant increase in the 16:1,n-7 elongation index (Fig. 4B). The minimal effect of Elovl-5 might have been due to low 16:1,n-7 substrate availability. To test this possibility, cells were treated with an adenovirus overexpressing SCD2 alone or in combination with Elovl-5 overexpression. SCD2 overexpression caused a decrease in 14C-labeled 16:0 and 18:0 and a marked increase in 14C-labeled 16:1,n-7 and 18:1,n-7 (Fig. 4B). This resulted in a ∼3-fold increase in the total desaturation index (Fig. 4C). Compared with SCD2 alone, the combination of Elovl-5 and SCD2 further increased 18:1,n-7 and reduced 16:1,n-7 (Fig. 4B), thus demonstrating that Elovl-5 elongates de novo synthesized 16:1,n-7.
Fig. 4.
Effect of increased Elovl-5 and/or SCD2 expression on de novo FA synthesis. INS-1 cells infected with adenoviruses that express B-gal, Elovl-5, and/or SCD2 were cultured for 24 h in 11.1 mM glucose and [2-14C]acetic acid. Total lipids were extracted, saponified, and 14C incorporation into FAs was quantified. A: Incorporation of 14C into FA species. Data are reported as percentage of total labeled FA species. B: Elongation index for 16:0 and 16:1,n-7. #P < 0.02 for 16:1,n-7 compared with B-gal. C: Total desaturation index. *P < 0.0001 compared with B-gal. Values are the mean ± SEM for three individual experiments. B-gal, β-galactosidase; Elovl, fatty acid elongase; FA, fatty acid; INS, rat insulinoma; SCD, stearoyl-CoA desaturase.
DISCUSSION
The role of FA elongases in determining end products of de novo FA synthesis from glucose has been largely speculative. Although substrate specificities of Elovl-5 and Elovl-6 in vitro using yeast and microsomal preparations indicated elongation of 16 carbon FAs (13, 15), the effect of altered expression of these enzymes on the specific species of FA synthesized from glucose has not been addressed. This study is the first to characterize the effects of both reduced and enhanced expression of Elovl-5 and Elovl-6 on the intracellular end products of de novo–derived FAs in mammalian cells. The findings reveal a significant role for FA elongase activity in regulating the synthesis of de novo–derived MUFAs and establishing the balance among 16:1,n-7, 18:1,n-7, and 18:1,n-9.
Elongation of FAs by Elovl-5 is essential for control of hepatic lipid homeostasis, as overexpression in liver decreases triglyceride content and knockdown leads to activation of SREBP-1c, increased lipogenic gene expression, and hepatic steatosis (23, 24). Elovl-5 substrates include 16:1,n-7 and polyunsaturated FAs, such as 18:2,n-6 and 18:4,n-3, which are precursors for 20:4,n-6 and 20:5,n-3 synthesis, respectively (13, 14, 16, 17). Elevated levels of carbohydrate leads to increased synthesis of both 18:1,n-7 and 18:1,n-9 FA in INS-1 cells and liver (8). Targeted reduction of Elovl-5 expression in INS-1 cells decreases the elongation of 16:1,n-7 to 18:1,n-7 and increases elongation of 16:0 to 18:0 and the synthesis of 18:1,n-9, illustrating an ex vivo role for Elovl-5 in the elongation of 16:1,n-7. Findings in INS-1 cells are in stark contrast to Elovl-5–null mice, which have increased rather than decreased hepatic levels of 18:1,n-7 (23). Although 18:1,n-7 levels were unexpectedly elevated in Elovl-5–null mice, polyunsaturated fatty acid (PUFA) levels were reduced as expected. The increased levels of hepatic 18:1,n-7 may be associated with reduced synthesis of 22:6,n-3, an inhibitor of SREBP-1 processing (11, 23, 25). Indeed Elovl-5–null mice had increased SREBP-1c levels, de novo FA synthesis, and Elovl-6 expression, which can elongate 16:1,n-7 to 18:1,n-7 (23).
Overexpression of Elovl-5 in INS-1 cells decreased the amount of de novo–derived 16:1,n-7 but only had a minimal effect on 18:1,n-7. The minimal effect of increased Elovl-5 expression was likely due to limited substrate availability, as INS-1 cells have very low concentrations of 16:1,n-7 relative to 16:0. Consistent with this possibility, overexpression of SCD2 significantly increased 16:1,n-7 synthesis, which was available for Elovl-5 to elongate to 18:1,n-7. Under many physiologic states, increased SCD expression occurs with increased expression of Elovl-5 (and Elovl-6) (17). Elovl-5 might function to keep cellular concentrations of 16:1,n-7 low, thereby preventing accumulation of 16:1,n-7 that can serve as a cell-signaling molecule (10). Although the mechanism is unknown, exposure of cells to 16:1,n-7 enhances insulin signaling, and its accumulation in the blood has been shown to correlate with increased muscle insulin sensitivity and protection from hepatic steatosis (7, 10). Despite the ability of Elovl-5 to elongate 18:3,n-6, 18:4,n-3, and 20:5,n-3 in vitro, overexpression or knockout of Elovl-5 (or Elovl-6) did not affect PUFA production in INS-1 cells (data not shown). The lack of evidence of PUFA elongation might be due to insufficient substrate (e.g., 18:2,n-6, 18:3,n-3) as INS-1 cell phospholipids are deficient in PUFA (26).
The preferred substrate for triglyceride storage of excess FAs is the MUFA oleate (18:1,n-9) (27). Synthesis of 18:1,n-9 de novo requires elongation of 16:0 to 18:0 before desaturation. Elovl-6 overexpression in INS-1 cells largely drove the elongation of 16:0 to 18:0 and promoted synthesis of 18:1,n-9 rather than elongation of 16:1,n-7 to 18:1,n-7. Conversely, reduced expression of Elovl-6 significantly decreased the products of 16:0 elongation (i.e., 18:0 and 18:1,n-9) while increasing 16:1,n-7. Elongation of 16:1,n-7 to 18:1,n-7 was also decreased with siElovl-6. A role for Elovl-6 in 16:0 elongation is supported by Elovl-6–null mice, which displayed decreased hepatic accumulation of 18:0 and 18:1,n-9 and increased 16:0 and 16:1,n-7 (7). The effect of decreased elongation of both 16:0 and 16:1,n-7 by reduced Elovl-6 expression and activity is a shuttling of de novo–synthesized FA toward the production of 16:1,n-7. Our data show that expression and activity of Elovl-6 is mostly involved in elongation of de novo–synthesized 16:0 to produce n-9 MUFA.
Although Elovl-5 and Elovl-6 activities can influence synthesis of specific MUFA species, SCD activity clearly plays the predominate role in total MUFA synthesis. This was exemplified by the large reduction in 16:1,n-7, 18:1,n-7, and 18:1,n-9 in INS-1 cells with reduced SCD1 and SCD2. Interestingly, Elovl-6 mRNA, but not Elovl-5 mRNA, was induced in SCD-deficient INS-1 cells. This finding supports the unique role of Elovl-6 in synthesis of 18:1,n-9, the predominate MUFA in cells, and suggests that MUFAs provide negative-feedback control on Elovl-6 expression. Overexpression of SCD2 in INS-1 cells, in the absence of increased Elovl-5/Elovl-6, led to increased 16:1,n-7 and 18:1,n-7 but had little impact on 18:1,n-9 synthesis. In a similar manner, culturing INS-1 cells in elevated glucose induced SCD1 and SCD2 mRNA levels to a greater extent than Elovl-5 or Elovl-6, which in turn shifted the ratio of n-7 to n-9 MUFA synthesis. These data suggest that without coordinate regulation of FA elongases, elevated SCD activity will disrupt the balance of 16:1,n-7, 18:1,n-7 and 18:1,n-9.
CONCLUSION
This study presents a comprehensive analysis of the effects of altered expression of Elovl-5 and Elovl-6 on de novo–synthesized MUFAs. Our results demonstrate that Elovl-5 preferentially converts 16:1,n-7 to 18:1,n-7, whereas Elovl-6 preferentially elongates 16:0 to 18:0, which can be further desaturated to 18:1,n-9. Loss of coordinate control of Elovl-5, Elovl-6, and SCD can disrupt production of specific MUFA species, which might influence cell function. As Elovl-5 and Elovl-6 can be differentially expressed under various physiologic and pharmacologic conditions and are also capable of elongating exogenous lipids (17, 19), it is likely that FA elongases play a broader role in determining the balance of MUFA species.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- B-gal
- β-galactosidase
- Elovl
- fatty acid elongase
- FA
- fatty acid
- FAS
- fatty acid synthase
- INS
- rat insulinoma
- MUFA
- monounsaturated fatty acid
- PUFA
- polyunsaturated fatty acid
- SFA
- saturated fatty acid
- SCD
- stearoyl-CoA desaturase
- SREBP
- sterol-regulatory element binding protein
This work was supported by the National Institutes of Health Grant DK-43220; the US Department of Agriculture Cooperative State Research, Education and Extension Service Grant 2003-35200-13400; the Michigan Agriculture Experiment Station (D.B.J.); the Michigan Life Science Corridor Grant GR352; the American Diabetes Association Grant 7-06-RA-103 (L.K.O.); and the Scientific and Technological Research Council of Turkey (C.G.O.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the US Department of Agriculture, or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and one table.
REFERENCES
- 1.Yamashita H., Takenoshita M., Sakurai M., Bruick R. K., Henzel W. J., Shillinglaw W., Arnot D., Uyeda K. 2001. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA. 98: 9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foufelle F., Ferre P. 2002. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 366: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai Y., Nishio Y., Nakamura T., Maegawa H., Kikkawa R., Kashiwagi A. 2002. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 282: E1180–E1190. [DOI] [PubMed] [Google Scholar]
- 4.Attie A. D., Krauss R. M., Gray-Keller M. P., Brownlie A., Miyazaki M., Kastelein J. J., Lusis A. J., Stalenhoef A. F., Stoehr J. P., Hayden M. R., et al. 2002. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J. Lipid Res. 43: 1899–1907. [DOI] [PubMed] [Google Scholar]
- 5.Okada T., Furuhashi N., Kuromori Y., Miyashita M., Iwata F., Harada K. 2005. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 82: 747–750. [DOI] [PubMed] [Google Scholar]
- 6.Paillard F., Catheline D., Duff F. L., Bouriel M., Deugnier Y., Pouchard M., Daubert J. C., Legrand P. 2008. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 18: 436–440. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaka T., Shimano H., Yahagi N., Kato T., Atsumi A., Yamamoto T., Inoue N., Ishikawa M., Okada S., Ishigaki N., et al. 2007. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 13: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. 2007. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 6: 484–496. [DOI] [PubMed] [Google Scholar]
- 9.Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 99: 11482–11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jump D. B. 2004. Fatty acid regulation of gene transcription. Crit. Rev. Clin. Lab. Sci. 41: 41–78. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M., Bruggink S. M., Ntambi J. M. 2006. Identification of mouse palmitoyl-coenzyme A Delta9-desaturase. J. Lipid Res. 47: 700–704. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki K., Aki T., Fukuda Y., Kawamoto S., Shigeta S., Ono K., Suzuki O. 2002. Identification and expression of a rat fatty acid elongase involved in the biosynthesis of C18 fatty acids. Biosci. Biotechnol. Biochem. 66: 613–621. [DOI] [PubMed] [Google Scholar]
- 14.Leonard A. E., Bobik E. G., Dorado J., Kroeger P. E., Chuang L. T., Thurmond J. M., Parker-Barnes J. M., Das T., Huang Y. S., Mukerji P. 2000. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem. J. 350: 765–770. [PMC free article] [PubMed] [Google Scholar]
- 15.Moon Y. A., Shah N. A., Mohapatra S., Warrington J. A., Horton J. D. 2001. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 276: 45358–45366. [DOI] [PubMed] [Google Scholar]
- 16.Parker-Barnes J. M., Das T., Bobik E., Leonard A. E., Thurmond J. M., Chaung L. T., Huang Y. S., Mukerji P. 2000. Identification and characterization of an enzyme involved in the elongation of n-6 and n-3 polyunsaturated fatty acids. Proc. Natl. Acad. Sci. USA. 97: 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Botolin D., Xu J., Christian B., Mitchell E., Jayaprakasam B., Nair M. G., Peters J. M., Busik J. V., Olson L. K., et al. 2006. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 47: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asfari M., Janjic D., Meda P., Li G., Halban P. A., Wollheim C. B. 1992. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 130: 167–178. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Botolin D., Christian B., Busik J., Xu J., Jump D. B. 2005. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 46: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva Xavier G., Rutter G. A., Diraison F., Andreolas C., Leclerc I. 2006. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J. Lipid Res. 47: 2482–2491. [DOI] [PubMed] [Google Scholar]
- 21.Diraison F., Ravier M. A., Richards S. K., Smith R. M., Shimano H., Rutter G. A. 2008. SREBP1 is required for the induction by glucose of pancreatic beta-cell genes involved in glucose sensing. J. Lipid Res. 49: 814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green C. D., Jump D. B., Olson L. K. 2009. Elevated insulin secretion from liver X receptor-activated pancreatic beta-cells involves increased de novo lipid synthesis and triacylglyceride turnover. Endocrinology. 150: 2637–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon Y. A., Hammer R. E., Horton J. D. 2009. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 50: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Torres-Gonzalez M., Tripathy S., Botolin D., Christian B., Jump D. B. 2008. Elevated hepatic fatty acid elongase-5 activity affects multiple pathways controlling hepatic lipid and carbohydrate composition. J. Lipid Res. 49: 1538–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botolin D., Wang Y., Christian B., Jump D. B. 2006. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J. Lipid Res. 47: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanadham S., Hsu F., Zhang S., Bohrer A., Ma Z., Turk J. 2000. Electrospray ionization mass spectrometric analyses of phospholipids from INS-1 insulinoma cells: comparison to pancreatic islets and effects of fatty acid supplementation on phospholipid composition and insulin secretion. Biochim. Biophys. Acta. 1484: 251–266. [DOI] [PubMed] [Google Scholar]
- 27.Ntambi J. M., Miyazaki M. 2004. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 43: 91–104. [DOI] [PubMed] [Google Scholar]