Abstract
An intact genotoxic stress response appears to be atheroprotective and insulin sensitizing. ATM, mutated in ataxia telangiectasia, is critical for the genotoxic stress response, and its deficiency is associated with accelerated atherosclerosis and insulin resistance in humans and mice. The antimalarial drug chloroquine activates ATM signaling and improves metabolic phenotypes in mice. p53 is a major effector of ATM signaling, but it is unknown if p53 is required for the beneficial effects of chloroquine. We tested the hypothesis that the cardiometabolic effects of chloroquine are p53-dependent. ApoE-null mice with or without p53 were treated with low-dose chloroquine or saline in the setting of a Western diet. After 8 weeks, there was no p53-dependent or chloroquine-specific effect on serum lipids or body weight. Chloroquine reduced plaque burden in mice wild-type for p53, but it did not decrease lesion extent in p53-null mice. However, chloroquine improved glucose tolerance, enhanced insulin sensitivity, and increased hepatic Akt signaling regardless of the p53 genotype. These results indicate that atheroprotection induced by chloroquine is p53-dependent but the insulin-sensitizing effects of this agent are not. Discrete components of the genotoxic stress response might be targeted to treat lipid-driven disorders, such as diabetes and atherosclerosis.
Keywords: atherosclerosis, insulin resistance, type 2 diabetes mellitus, genotoxic stress response, metabolic syndrome
About 13% of American adults have diabetes (∼40% are undiagnosed) and an additional 30% have prediabetes (impaired fasting glucose or impaired glucose tolerance) (1). These prevalence numbers, which have increased substantially over the past 20 years, predict more cardiovascular disease given the link between diabetes, the insulin resistance that accompanies diabetes, and atherosclerotic burden. Most people with diabetes die of cardiovascular disease, and cardiovascular death is increased in those with the metabolic syndrome (2), a prediabetes condition characterized by insulin resistance. This looming epidemic of insulin resistance–associated vascular disease presents a challenge to develop novel therapies. One potential therapeutic strategy exploits the observation that defects in critical processes required for maintaining cellular homeostasis produce a metabolic milieu characterized by insulin resistance and atherosclerosis.
The genotoxic stress response appears to be one such process. A coordinated series of events that includes cell cycle checkpoints and DNA repair mechanisms to maintain genomic stability (3), this response is triggered by some of the same stressors implicated in insulin resistance and atherosclerosis, such as reactive oxygen species (4, 5). DNA damage disorders caused by mutations in genotoxic stress-response genes are characterized by insulin resistance and atherosclerosis (6). One of these diseases is ataxia telangiectasia, caused by bi-allelic mutations in ataxia telangiectasia mutated (ATM), a serine/threonine kinase canonically activated by double-strand DNA breaks (typically induced by ionizing radiation) (3). Patients with ataxia telangiectasia (AT) have malignancies; they are also insulin-resistant and may develop frank diabetes (7). AT heterozygosity, with a prevalence of 0.5%–2.0% in the general population, increases diabetes and atherosclerosis risk (8).
Like humans with ATM mutations, ATM-deficient mice also have glucose intolerance, elevated blood pressure, atherosclerosis, and insulin resistance (9–11), components of the metabolic syndrome. We recently demonstrated that the ATM activator chloroquine, safely used in humans for more than a century to treat malaria as well as rheumatoid arthritis and systemic lupus erythematosus, decreases atherosclerosis and improves insulin sensitivity in several diet-induced and genetic murine models (9). The chloroquine effect appears to involve Jun N-terminal kinase (JNK), a major mediator of inflammation, insulin resistance, and vascular disease, because chloroquine suppresses macrophage JNK activation in an ATM-dependent manner (9).
p53, dubbed “guardian of the genome,” (12) is a major downstream effector of ATM signaling (3). Because much of the genotoxic stress response flows through the ATM/p53 axis, p53 might be expected to play an important role in the cardiometabolic consequences of insulin resistance and diabetes. Aside from the observation that p53-null mice are susceptible to atherosclerosis (13), it is not known if p53 mediates the relationship between insulin resistance and vascular disease. To test the hypothesis that the cardiometabolic effects of the ATM activator chloroquine are p53-dependent, we compared the effects of chronic administration of chloroquine in apoE-null mice that were wild-type for p53 and apoE-null mice that were p53-deficient.
MATERIALS AND METHODS
Animals
The Washington University Animal Studies Committee approved protocols, and this research was conducted in conformity with the PHS Policy on Humane Care and Use of Laboratory Animals. p53+/− mice (Taconic Laboratories) were backcrossed with apoE−/− mice (C57BL/6 background) to generate p53−/−apoE null and p53+/+apoE null littermates used for experiments that were >N7 C57BL/6. p53 genotyping utilized forward and reverse wild-type primers (5′-GTGTTTCATTAGTTCCCCACCTTGAC-3′ and 5′-ATGGGAGGCTGC CAGTCCTAACCC-3′, respectively) and forward and reverse knockout primers (5′-GTGGGAGGGACAAAAGT TCGAGGCC-3′ and 5′-TTTACGGAGCCCTGGCGCTCGATGT-3′, respectively). All experiments were performed with male mice, in part due to a low yield of p53−/−apoE−/− female births. ATM−/− and ATM+/+ mice (in the apoE-null background) were genotyped as described (9). Mice housed in a specific pathogen-free barrier facility were weaned at 3 weeks of age to a standard mouse chow providing 6% calories as fat, then started on a Western-type diet containing 0.15% cholesterol providing 42% calories as fat (TD 88137, Harlan, Madison, WI) at ∼8 weeks of age. At the time of initiation of the experimental diet, mice were treated with either 0.9% saline (control) or chloroquine diphosphate (Sigma, St. Louis, MO) in saline at 3.5 mg/kg body weight 2 times per week after a loading dose of 7 mg/kg. Chloroquine or saline was continued with the diet intervention for a total of 8 weeks.
Analytical procedures and lesion quantification
Cholesterol, triglycerides, glucose, and free (nonesterified) fatty acids (FFA) were assayed in serum following a 4-h fast as described (14). Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were separated by at least a week using fasted mice that were injected with 10% D-glucose (1 g/kg) or human regular insulin (0.75 units/kg body weight) (Eli Lilly and Co., Indianapolis, IN). Tail vein blood for these tests was assayed at 0, 30, 60, and 120 min using a glucose meter (Contour, Bayer Healthcare, Mishawaka, IN). A published formula [0.25 (fasting value) + 0.5 (1/2-h value) + 0.75 (1-h value) + 0.5 (2-h value)] (15) was used to calculate area under the curve (AUC) for each GTT and ITT. The en face and aortic root cross-section techniques previously described (16) were used to quantify atherosclerosis throughout the aorta as well as at the vessel origin. En face regions were analyzed as the aortic arch (from the aortic valve to the left subclavian artery), thoracic aorta (from the left subclavian artery to the final intercostal arteries), and abdominal aorta (from the intercostals arteries to the bifurcation of the iliac arteries).
Macrophage culture
Macrophages were isolated essentially as described (17) by injecting mice intraperitoneally with a 4% solution of thioglycollate media (Sigma, St. Louis, MO) on day 1. On day 5, peritoneal macrophages were isolated, washed, counted, and plated in RPMI with 10% FBS. Macrophages were subsequently harvested at various times to isolate protein using standard techniques.
Western blotting
Western blotting was performed by standard techniques and used the following antibodies purchased from Cell Signaling (Beverly, MA): total Akt (#9272), phospho-Akt (Ser473) (#9271), phospho-GSK3β (#9336), total p53 (#9282), phospho-p53 (Ser15) (#9284), total JNK (#9252), and phospho-JNK (Thr183/Tyr185) (#9251). Blots are representative of at least three experiments showing the same results.
Statistical analyses
Statistical significance of differences was calculated using the Student t-test for parametric data (Western blot densitometric quantification, serum metabolites, GTT, and ITT results) or the Mann-Whitney test for nonparametric data (atherosclerosis quantification).
RESULTS
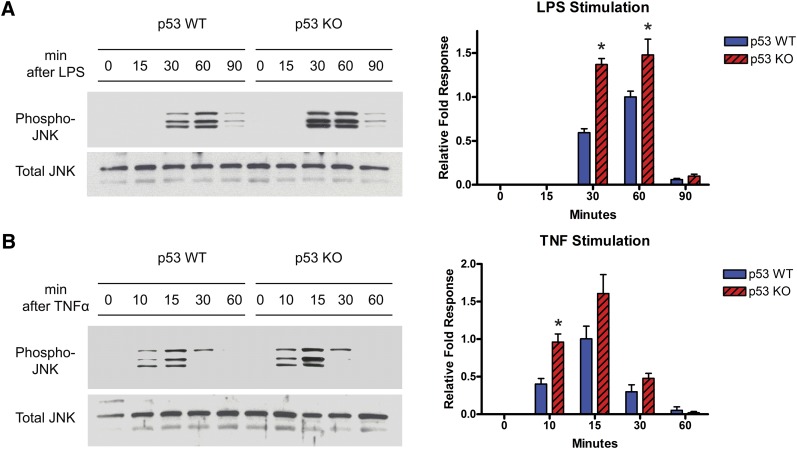
Because macrophages are involved in atherosclerosis as well as insulin resistance (6), the stress kinase JNK has been shown to promote atherosclerosis (18, 19), and the increased JNK signaling in ATM-deficient macrophages is suppressed by chloroquine in an ATM-dependent manner (9), the absence of p53 might be expected to be associated with hyperactive JNK signaling in macrophages like that seen with ATM deficiency. We assessed this possibility by comparing Thr183/Tyr185 JNK phosphorylation, known JNK-activation sites (20), in thioglycollate-elicited peritoneal macrophages from our wild-type and p53-deficient mice. p53 deficiency was associated with increased JNK phosphorylation following treatment with either of two classic proinflammatory stimuli, LPS and TNFα (Fig. 1). A similar response was seen with ATM-null macrophages (see supplemental Fig. I), suggesting that ATM deficiency and p53 deficiency share proinflammatory and proatherogenic antecedents and raising the possibility that the presence of p53 is critical for propagating a chloroquine-initiated ATM signal that is atheroprotective.
Fig. 1.
Assessment of JNK signaling in the presence and absence of p53. Western blot analyses of phospho-JNK and total-JNK were performed using thioglycollate-elicited peritoneal macrophages derived from p53+/+ and p53−/− (on apoE−/− background) mice. JNK activation was achieved by treatment with (A) LPS (10 ng/ml) or (B) TNFα (10 ng/ml) for the indicated times. Densitometric analyses of Western blots normalized to total JNK are shown in the right panels for the indicated times (*P < 0.05). The discrepancy between the number of bands seen on phospho-JNK blots and the total JNK blots likely reflects the variability in the relative abundance of the three JNK subtypes (JNK1, 2, and 3) and their phosphorylation status. JNK, Jun N-terminal kinase; KO, knockout; LPS, lipopolysaccharide; TNF, tumor necrosis factor; WT, wild type.
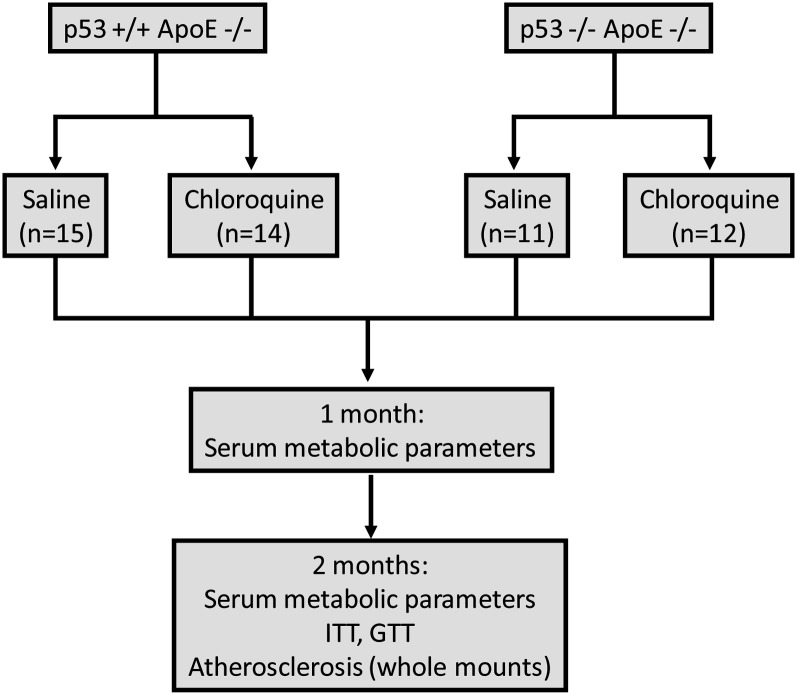
To directly address the potential p53-dependence of chloroquine on atherosclerosis as well as insulin resistance, we used the 2 × 2 study design shown in Fig. 2. ApoE-null mice, roughly half that were p53+/+ (Fig. 2, top left) and half that were p53−/− (Fig. 2, top right), were started on a Western-type diet and randomized to receive biweekly injections of placebo versus a low dose of chloroquine previously shown to decrease atherosclerosis and cause insulin sensitization in apoE-null mice (9). Serum, collected at baseline and after 1 and 2 months of the experimental diet, was analyzed for several metabolic parameters. The primary endpoints of this study were insulin sensitivity (determined by insulin tolerance/glucose tolerance testing and confirmed by Akt signaling) and the extent of aortic atherosclerosis (determined by assessment of both en face and aortic root lesions).
Fig. 2.
Schematic of the 2 × 2 experimental design. p53+/+ apoE−/− mice (n = 29) and p53−/− apoE−/− mice (n = 23) were placed on Western diet (42% calories from fat) at 6–8 weeks of age. They were divided into chloroquine (loading injection of 7 mg/kg, then biweekly injections of 3.5 mg/kg) versus saline injections. Interventions were performed at 1- and 2-month intervals as indicated. GTT, glucose tolerance test; ITT, insulin tolerance test.
Chloroquine activates the ATM/p53 axis in vivo
Chronic administration of low-dose chloroquine to mice activates the ATM/p53 axis in many tissues, including adipose, liver, and the vasculature (9). To confirm that mice in these experiments were achieving the same effects, we immunoblotted tissues for phosphorylation of p53 on its Serine-15 residue (a specific phosphorylation site for ATM) (21). Supplemental Fig. II shows representative blots of increased p53-phosphorylation in the adipose tissue of chloroquine-treated wild-type mice compared with saline-treated mice, indicating that active treatment mice were indeed receiving the drug and that there was an impact on the ATM/p53 axis in these animals.
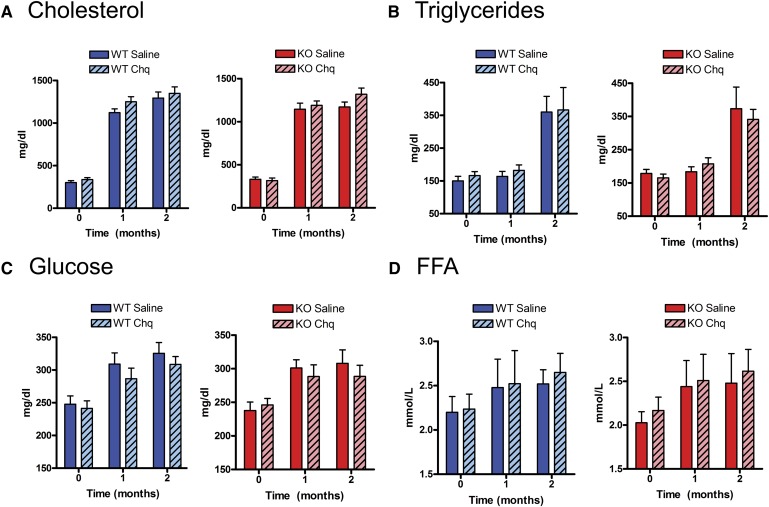
p53-deficiency and chloroquine do not affect weight gain or serum metabolites
Over the 8 weeks of high-fat diet feeding, all of the mice were overtly normal in appearance, and each experimental group manifested similar weight gain (see supplemental Fig. III). There were no significant differences between experimental groups for fasting levels of common metabolites (cholesterol, triglycerides, glucose, and FFA) at baseline (just before initiation of the diet and chloroquine injections, Time 0 of Fig. 3). As shown in Fig. 3A, Western-diet feeding increased fasting cholesterol levels to >1,000 mg/dl in all cohorts. Fasting levels of triglycerides, glucose, and FFA also increased with diet intervention and resultant weight gain (Fig. 3B–D). There was no p53 genotype effect and no chloroquine effect on serum metabolites at baseline or with the high-fat diet. Fasting glucose levels tended to be lower with chloroquine treatment for both wild-type and p53-null cohorts, but these results were not statistically significant (Fig. 3C). This is an expected observation in mice as, in the absence of severe defects in insulin sensitivity (e.g., ob/ob mice), chloroquine effects on fasting glucose are modest while effects on glucose tolerance and insulin tolerance are more pronounced (9).
Fig. 3.
Effects of p53 deficiency and chloroquine treatment on common serum metabolites. Measurements of serum metabolites for (A) cholesterol, (B) triglycerides, (C) glucose, and (D) free fatty acids were performed at baseline then after 1 and 2 months of Western-diet feeding. Diet-induced increases for these parameters were not significantly different among the four groups. Chq, chloroquine; FFA, free fatty acids; KO, knockout; WT, wild type.
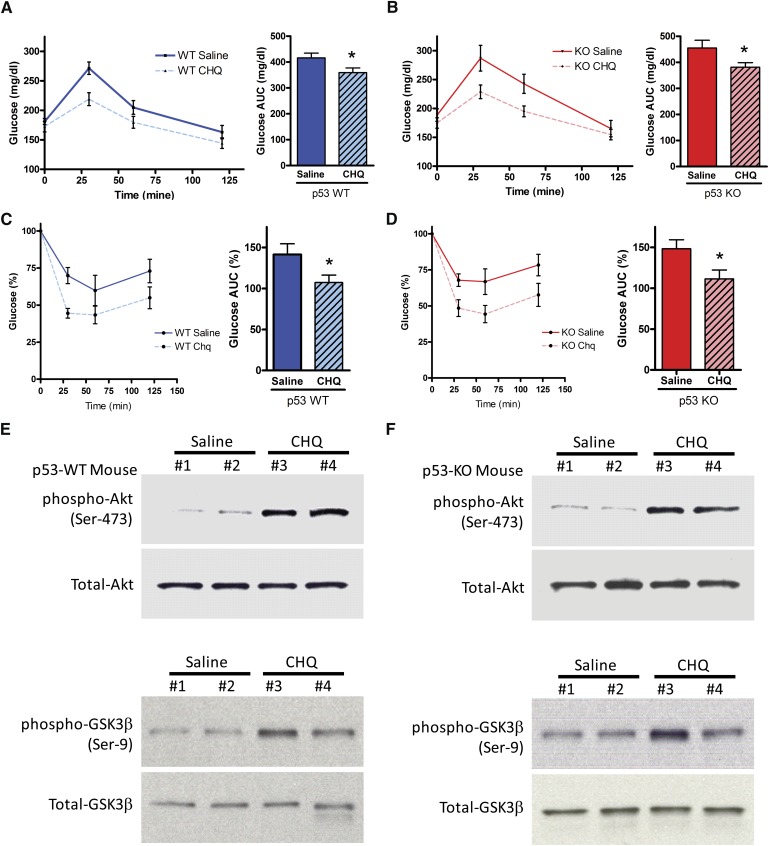
p53 is not required for chloroquine-induced insulin sensitization
Chloroquine is an ATM-dependent insulin sensitizer in mice as the insulin-sensitizing response in glucose tolerance tests is abrogated in ATM-null animals (9). To determine if p53 is involved in this response, glucose tolerance and insulin tolerance testing was performed after 8 weeks of high-fat feeding. Glucose excursions were similarly lower during glucose tolerance testing in both wild-type and p53-null cohorts treated with chloroquine, and area under the curve values were significantly lower in the chloroquine groups (Fig. 4A, B). Hypoglycemic responses were similarly lower during insulin tolerance testing in both wild-type and p53-null cohorts treated with chloroquine, and area under the curve values were significantly lower in the chloroquine groups (Fig. 4C, D). Because the liver is the major insulin-sensitizing site with activation of ATM, we assayed hepatic Akt, a major mediator of insulin sensitivity in vivo, and one of its downstream targets, GSK3β. As determined by phosphorylation status, Akt was activated (phosphorylated Akt is active), and GSK3β was inhibited (phosphorylated GSK3β is inactive) to the same extent in the livers of chloroquine-treated wild-type and p53-null mice (Fig. 4E, F). There were no significant differences in glucose tolerance testing or insulin tolerance testing between saline-injected p53-null mice compared with their wild-type counterparts [see supplemental Fig. IV (A), (B))].
Fig. 4.
Effects of chloroquine on insulin sensitivity in the presence and absence of p53. p53+/+ and p53−/− (on apoE−/− background) are denoted as WT and KO, respectively. (A and B) Glucose tolerance tests (GTT) for WT and KO mice. AUC is the incremental area under the glucose curve. (C and D) Insulin tolerance tests (ITT) for WT and KO mice. Glucose values are expressed as percent of the baseline (time 0) value. AUC is the incremental area under the glucose% curve. (E and F) Western blot analyses of phospho-Akt (Ser-473), total-Akt, phospho-GSK3β (Ser-9), and total-GSK3β from liver tissue homogenates of WT and KO mice after 2 months of chloroquine versus saline injections. *P < 0.05 for AUC data. All results are presented as mean ± SEM. AUC, area under the curve; CHQ, chloroquine; KO, knockout; WT, wild type.
p53 is required for chloroquine-induced atheroprotection
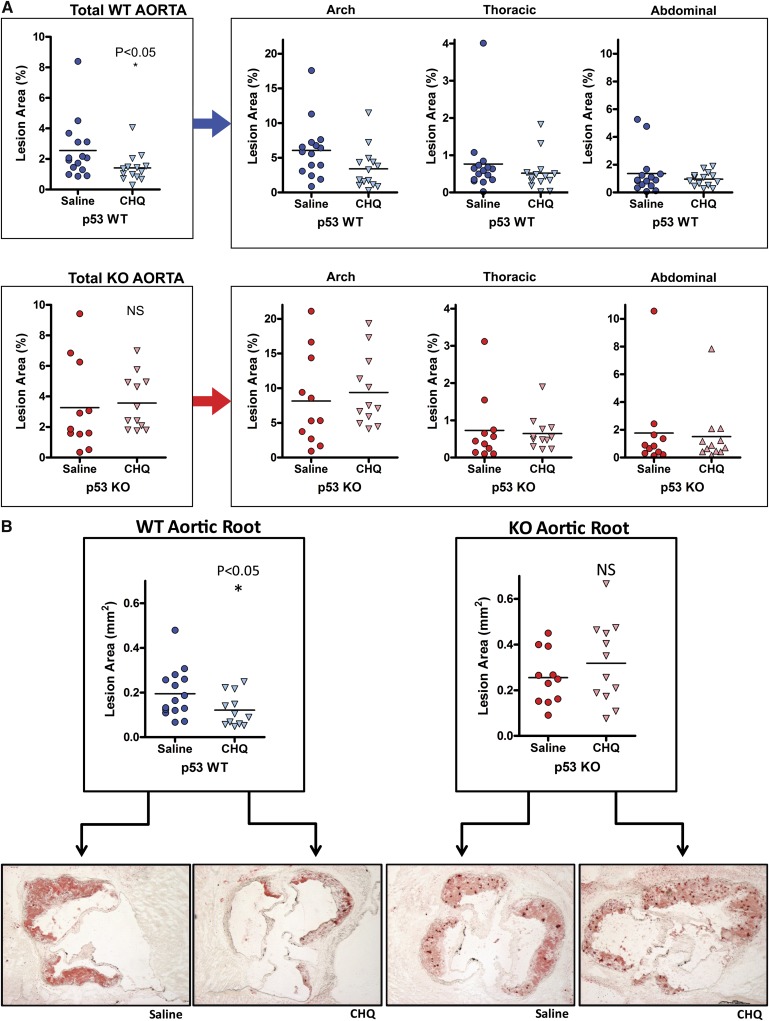
Both ATM- and p53-null mice have been shown to have increased atherosclerosis (9, 10, 22, 23), and activation of ATM with chloroquine decreases atherosclerotic burden in mice (9). To determine if the latter effect is p53-dependent, we quantified atherosclerosis in whole mount aortas as well as the aortic roots of our wild-type and p53-null cohorts after 8 weeks of high-fat feeding. Plaque burden expressed as a percentage of intimal involvement is shown for the total aorta (Fig. 5A, left panels) and for each of three, separate aortic regions (Fig. 5A, right panels). The degree of atherosclerosis present in the aortic root of the same cohorts is also shown (Fig. 5B, top panels), along with representative aortic root sections (Fig. 5B, bottom panels). Chloroquine significantly decreased total aortic and aortic root atherosclerosis in wild-type mice but not in p53-null mice.
Fig. 5.
Effects of chloroquine on atherosclerosis in the presence and absence of p53. Atherosclerotic area involvement was determined by computer image analysis of (A) en face pinned aortas for the total aorta (p53 WT, upper-left panel; p53 KO, lower-left panel), and regions of the aorta (aortic arch, thoracic aorta, and abdominal aorta, right panels), and (B) Oil-Red-O–stained sections of the aortic roots (p53 WT, left panel; p53 KO, right panel). Representative aortic root sections are shown below the data sets. Horizontal lines within the data sets represent medians. *P < 0.05. CHQ, chloroquine; KO, knockout; NS, not significant; WT, wild type.
DISCUSSION
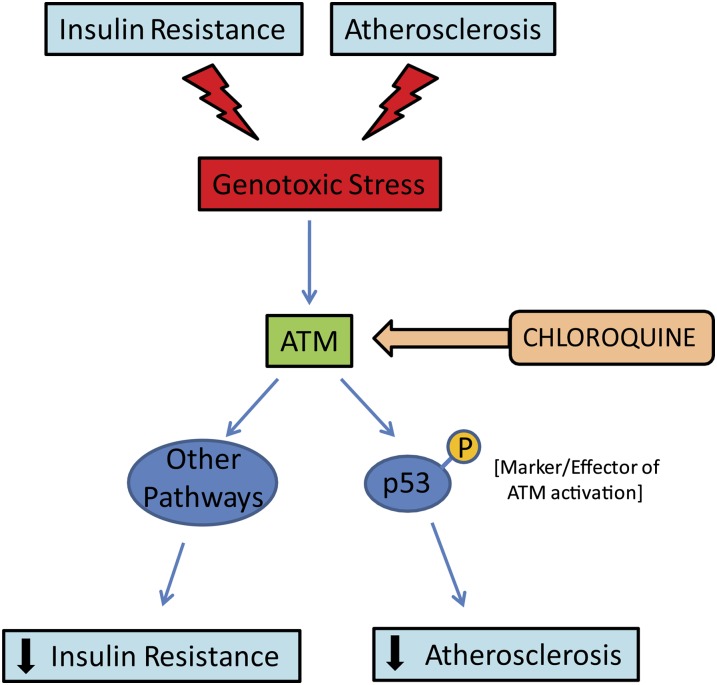
By virtue of its rapid activation after genomic insults and its ability to regulate numerous downstream targets, ATM plays a central role in mediating the genotoxic stress response (24). In addition to suppressing cancer susceptibility, ATM and the genotoxic stress response affect signaling networks relevant to metabolism and vascular disease (9, 10, 25). Chloroquine, an antimalarial compound with poorly understood anti-inflammatory effects, activates ATM signaling (26) and has been shown to have both insulin-sensitizing and antiatherogenic effects in vivo (9). The current work confirms that low-dose chloroquine treatment decreases atherosclerosis and improves glucose tolerance in high-fat–fed mice. It extends these observations by demonstrating that beneficial effects of chloroquine on atherosclerosis require the presence of p53, but the effects on glucose metabolism do not. These data, representing an initial dissection of ATM and its effectors in the context of insulin resistance and atherosclerosis, indicate that the ATM/p53 axis is important for suppressing atherosclerotic plaque formation, but that other components of the genotoxic stress response downstream of ATM must be critical for salutary effects on glucose metabolism (Fig. 6).
Fig. 6.
Model depicting the potential roles of the ATM-p53 genotoxic stress response in insulin resistance and atherosclerosis. ATM, ataxia telangiectasia mutated.
Demonstrating the importance of ATM-p53 signaling induced by chloroquine in the suppression of atherosclerosis is novel but consistent with previous work with mouse models (9, 10, 22, 23). Functional p53 signaling in macrophages, as shown in bone marrow transplantation experiments, seems to be important for atheroma suppression (27, 28). p53 has amazingly diverse effects that can be categorized as stimulating either of two complex responses depending on the biological context: (1) apoptosis and cellular senescence (generally following high-level stress or damage), or (2) cell cycle arrest, metabolic regulation, and DNA repair (generally after low-level stress) (29). Both types of responses could be involved in atheroprotection (22, 23, 27, 28). The precise downstream mediators of the p53 atheroprotective effect are unknown. One obvious putative mediator is p21, which prevents phosphorylation of the retinoblastoma protein (phosphorylated Rb is inactive), an effect implicated in decreasing vascular disease (30). However, p21 deficiency appears to be atheroprotective in mice, perhaps due to effects on macrophage development (31). Further, while the current data suggest that p53 activation is beneficial in atherosclerosis, similar benefits may not accrue in other contexts. The opposite intervention, p53 inhibition, limits ischemia/reperfusion injury in heart and brain (32, 33). Given the broad spectrum of downstream targets of p53, identifying potentially useful distal arms of the genotoxic stress response, such as MAP kinase pathways, could be more likely to have therapeutic utility. In nonmacrophage tissues, p53 is thought to suppress MAP kinase activity by inducing certain phosphatases (34, 35). Our results in macrophages suggest that JNK signaling might at least partly explain the increased vascular disease in both ATM- and p53-deficient settings.
Showing that chloroquine was insulin sensitizing in the absence of p53 was somewhat unexpected. p53 activates key mediators of glucose metabolism and insulin signaling, such as AMP kinase and the Akt-mTOR pathways (36), as well as several proteins, such as 14-3-3, known to interact with the phosphatidylinositol 3-kinase/Akt metabolic arms of insulin signaling (37). p53 is also important for orchestrating complex effects on metabolism that include the maintenance of mitochondrial respiration, mediated in part by induction of synthesis of cytochrome C oxidase 2 (SCO2) (38), and the limitation of glycolysis, mediated in part by TP53-induced glycolysis and apoptosis regulator (TIGAR) (39–41). While this article was in preparation, a study was published addressing the potential role of adipose tissue p53 in metabolism (42). Using adipose tissue–specific knockout and transgenic mice, the authors concluded that p53 promotes adipose tissue senescence, leading to an insulin resistance phenotype (42). Despite these reports linking p53 and glucose metabolism/insulin signaling, our results show that p53 is not required for chloroquine-induced insulin sensitization in apoE-null mice fed a high-fat diet. Chloroquine stimulates ATM signaling, which is complex. Nearly 700 proteins, many of which are not limited to the DNA damage response, have been shown to be directly phosphorylated by ATM (43). These include several proteins involved in cellular metabolism and insulin signaling, such as members of the insulin receptor/phosphatidylinositol 3-kinase/Akt cascade (43). Since ATM has also been shown to be required for full insulin-mediated activation of Akt (43), it is likely that a critical ATM target, independent of p53, regulates insulin sensitivity in vivo.
The notion of modulating the genotoxic stress response through the use of chloroquine to activate ATM (26) has been proposed as a therapeutic strategy in some forms of cancer (44, 45). Such an approach is logical because cancer is a disease characterized by abnormal DNA. Curiously, DNA strand breaks are also common in atherosclerotic lesions, and one of the pleiotropic effects of statin drugs limiting clinical events may be the induction of ATM in the vasculature (25). Ongoing clinical trials will determine whether induction of ATM by low-dose chloroquine will have utility in humans (ClinicalTrials.gov identifiers NCT00455403 and NCT00455325). Regardless of the results of these trials, insulin resistance and atherosclerosis are likely to dominate clinical practice for decades, and further dissection of ATM-dependent pathways might lead to new treatments for diabetes and vascular disease.
Supplementary Material
Acknowledgments
The authors thank Trey Coleman for technical support.
Footnotes
Abbreviations:
- AT
- ataxia telangiectasia
- ATM
- ataxia telangiectasia mutated
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- JNK
- Jun N-terminal kinase
This work was supported by National Institutes of Health Grants P50-HL-083762, DK-076729, DK-56341 (Clinical Nutrition Research Unit, Washington University) and DK-20579 (Diabetes Research and Training Center, Washington University); and by the Physician Scientist Training Program and Cardiovascular Training Grant at Washington University. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures.
REFERENCES
- 1.Cowie C. C., Rust K. F., Ford E. S., Eberhardt M. S., Byrd-Holt D. D., Li C., Williams D. E., Gregg E. W., Bainbridge K. E., Saydah S. H., et al. 2009. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 32: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik S., Wong N. D., Franklin S. S., Kamath T. V., L'Italien G. J., Pio J. R., Williams G. R. 2004. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 110: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 3.Kastan M. B. 2008. DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol. Cancer Res. 6: 517–524. [DOI] [PubMed] [Google Scholar]
- 4.Houstis N., Rosen E. D., Lander E. S. 2006. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 440: 944–948. [DOI] [PubMed] [Google Scholar]
- 5.Van Gaal L. F., Mertens I. L., De Block C. E. 2006. Mechanisms linking obesity with cardiovascular disease. Nature. 444: 875–880. [DOI] [PubMed] [Google Scholar]
- 6.Razani B., Chakravarthy M. V., Semenkovich C. F. 2008. Insulin resistance and atherosclerosis. Endocrinol. Metab. Clin. North Am. 37: 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar R. S., Levis W. R., Rechler M. M., Harrison L. C., Siebert C., Podskalny J., Roth J., Muggeo M. 1978. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N. Engl. J. Med. 298: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 8.Su Y., Swift M. 2000. Mortality rates among carriers of ataxia-telangiectasia mutant alleles. Ann. Intern. Med. 133: 770–778. [DOI] [PubMed] [Google Scholar]
- 9.Schneider J. G., Finck B. N., Ren J., Standley K. N., Takagi M., Maclean K. H., Bernal-Mizrachi C., Muslin A. J., Kastan M. B., Semenkovich C. F. 2006. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 4: 377–389. [DOI] [PubMed] [Google Scholar]
- 10.Wu D., Yang H., Xiang W., Zhou L., Shi M., Julies G., Laplante J. M., Ballard B. R., Guo Z. 2005. Heterozygous mutation of ataxia-telangiectasia mutated gene aggravates hypercholesterolemia in apoE-deficient mice. J. Lipid Res. 46: 1380–1387. [DOI] [PubMed] [Google Scholar]
- 11.Miles P. D., Treuner K., Latronica M., Olefsky J. M., Barlow C. 2007. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am. J. Physiol. Endocrinol. Metab. 293: E70–E74. [DOI] [PubMed] [Google Scholar]
- 12.Lane D. P. 1992. Cancer. p53, guardian of the genome. Nature. 358: 15–16. [DOI] [PubMed] [Google Scholar]
- 13.Mercer J., Bennett M. 2006. The role of p53 in atherosclerosis. Cell Cycle. 5: 1907–1909. [DOI] [PubMed] [Google Scholar]
- 14.Li B., Nolte L. A., Ju J. S., Han D. H., Coleman T., Holloszy J. O., Semenkovich C. F. 2000. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat. Med. 6: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 15.Haffner S. M., Stern M. P., Hazuda H. P., Pugh J. A., Patterson J. K. 1986. Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 315: 220–224. [DOI] [PubMed] [Google Scholar]
- 16.Semenkovich C. F., Coleman T., Daugherty A. 1998. Effects of heterozygous lipoprotein lipase deficiency on diet-induced atherosclerosis in mice. J. Lipid Res. 39: 1141–1151. [PubMed] [Google Scholar]
- 17.Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci R., Sumara G., Sumara I., Rozenberg I., Kurrer M., Akhmedov A., Hersberger M., Eriksson U., Eberli F. R., Becher B., et al. 2004. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 306: 1558–1561. [DOI] [PubMed] [Google Scholar]
- 19.Sumara G., Belwal M., Ricci R. 2005. “Jnking” atherosclerosis. Cell. Mol. Life Sci. 62: 2487–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyriakis J. M., Avruch J. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81: 807–869. [DOI] [PubMed] [Google Scholar]
- 21.Canman C. E., Lim D. S., Cimprich K. A., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan M. B., Siliciano J. D. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 281: 1677–1679. [DOI] [PubMed] [Google Scholar]
- 22.Guevara N. V., Kim H. S., Antonova E. I., Chan L. 1999. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat. Med. 5: 335–339. [DOI] [PubMed] [Google Scholar]
- 23.Mercer J., Figg N., Stoneman V., Braganza D., Bennett M. R. 2005. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ. Res. 96: 667–674. [DOI] [PubMed] [Google Scholar]
- 24.Kastan M. B., Lim D. S. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1: 179–186. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoudi M., Gorenne I., Mercer J., Figg N., Littlewood T., Bennett M. 2008. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ. Res. 103: 717–725. [DOI] [PubMed] [Google Scholar]
- 26.Bakkenist C. J., Kastan M. B. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 421: 499–506. [DOI] [PubMed] [Google Scholar]
- 27.van Vlijmen B. J., Gerritsen G., Franken A. L., Boesten L. S., Kockx M. M., Gijbels M. J., Vierboom M. P., van Eck M., van De Water B., van Berkel T. J., et al. 2001. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ. Res. 88: 780–786. [DOI] [PubMed] [Google Scholar]
- 28.Merched A. J., Williams E., Chan L. 2003. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler. Thromb. Vasc. Biol. 23: 1608–1614. [DOI] [PubMed] [Google Scholar]
- 29.Vousden K. H., Prives C. 2009. Blinded by the light: the growing complexity of p53. Cell. 137: 413–431. [DOI] [PubMed] [Google Scholar]
- 30.Chang M. W., Barr E., Seltzer J., Jiang Y. Q., Nabel G. J., Nabel E. G., Parmacek M. S., Leiden J. M. 1995. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 267: 518–522. [DOI] [PubMed] [Google Scholar]
- 31.Merched A. J., Chan L. 2004. Absence of p21Waf1/Cip1/Sdi1 modulates macrophage differentiation and inflammatory response and protects against atherosclerosis. Circulation. 110: 3830–3841. [DOI] [PubMed] [Google Scholar]
- 32.Liu P., Xu B., Cavalieri T. A., Hock C. E. 2008. Inhibition of p53 by pifithrin-alpha reduces myocyte apoptosis and leukocyte transmigration in aged rat hearts following 24 hours of reperfusion. Shock. 30: 545–551. [DOI] [PubMed] [Google Scholar]
- 33.Leker R. R., Aharonowiz M., Greig N. H., Ovadia H. 2004. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp. Neurol. 187: 478–486. [DOI] [PubMed] [Google Scholar]
- 34.Wu G. S. 2004. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 3: 156–161. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Ouyang W., Li J., Wei L., Ma Q., Zhang Z., Tong Q., He J., Huang C. 2005. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 65: 6601–6611. [DOI] [PubMed] [Google Scholar]
- 36.Feng Z., Hu W., de Stanchina E., Teresky A. K., Jin S., Lowe S., Levine A. J. 2007. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 67: 3043–3053. [DOI] [PubMed] [Google Scholar]
- 37.Barry E. F., Felquer F. A., Powell J. A., Biggs L., Stomski F. C., Urbani A., Ramshaw H., Hoffmann P., Wilce M. C., Grimbaldeston M. A., et al. 2009. 14–3-3:Shc scaffolds integrate phosphoserine and phosphotyrosine signaling to regulate phosphatidylinositol 3-kinase activation and cell survival. J. Biol. Chem. 284: 12080–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matoba S., Kang J. G., Patino W. D., Wragg A., Boehm M., Gavrilova O., Hurley P. J., Bunz F., Hwang P. M. 2006. p53 regulates mitochondrial respiration. Science. 312: 1650–1653. [DOI] [PubMed] [Google Scholar]
- 39.Bensaad K., Vousden K. H. 2007. p53: new roles in metabolism. Trends Cell Biol. 17: 286–291. [DOI] [PubMed] [Google Scholar]
- 40.Corcoran C. A., Huang Y., Sheikh M. S. 2006. The regulation of energy generating metabolic pathways by p53. Cancer Biol. Ther. 5: 1610–1613. [DOI] [PubMed] [Google Scholar]
- 41.Green D. R., Chipuk J. E. 2006. p53 and metabolism: inside the TIGAR. Cell. 126: 30–32. [DOI] [PubMed] [Google Scholar]
- 42.Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., Nojima A., Nabetani A., Oike Y., Matsubara H., et al. 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 43.Matsuoka S., Ballif B. A., Smogorzewska A., McDonald E. R., 3rd, Hurov K. E., Luo J., Bakalarski C. E., Zhao Z., Solimini N., Lerenthal Y., et al. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 316: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 44.Loehberg C. R., Thompson T., Kastan M. B., Maclean K. H., Edwards D. G., Kittrell F. S., Medina D., Conneely O. M., O'Malley B. W. 2007. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Res. 67: 12026–12033. [DOI] [PubMed] [Google Scholar]
- 45.Maclean K. H., Dorsey F. C., Cleveland J. L., Kastan M. B. 2008. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J. Clin. Invest. 118: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.