Abstract
Abstract Childhood adrenoleukodystrophy (cALD) is a metabolic disorder in which very long-chain fatty acids (VLCFA) accumulate due to ALD protein gene defects, ultimately leading to lipotoxicity-induced neuroinflammatory demyelinating disease. Therefore, we examined VLCFA-mediated alterations in the metabolism of lipoxidative enzymes and inflammatory mediators in the cALD brain. 5-Lipoxygenase (5-LOX)-derived leukotrienes were significantly elevated in all the areas of white matter in the cALD brain. Unlike cyclooxygenase-2 expression, which was moderately high only in the plaque area, expression of 5-LOX and cytosolic phospholipase A2 was prominent in all the areas. This lipoxidative burden in the cALD brain was further shown by reduced levels of glutathione and enhanced expression of heat shock protein-70/manganese superoxide dismutase. These pathological observations were confirmed through in vitro mechanistic investigation. After increasing VLCFA through silencing Abcd1+Abcd2 in mouse primary astrocytes, enhanced expression of 5-LOX was observed, and this increased expression was blocked by treatment with monoenoic fatty acids. These results link the previously observed accumulation of VLCFA in cALD to the 5-LOX enzyme pathway. A similar increase in 5-LOX expression in astrocytes was also detected following treatment with exogenous VLCFA (C26:0). In sum, through 5-LOX activation, VLCFA accumulation causes a lipotoxic response consistent with cALD brain pathology.
Keywords: phospholipase A2, leukotrienes, phosphatidylcholine, plasmenylethanolamine
Childhood cerebral adrenoleukodystrophy (cALD), a multifactorial neuroinflammatory demyelinating disease, is the most common variant of X-linked ALD (X-ALD) (1–3). X-ALD is caused by defects in the ABCD1 gene [ALD protein (ALDP)], resulting in a significant inhibition of peroxisomal β-oxidation and a consequent accumulation of saturated straight chain very long-chain fatty acids (VLCFA). However, the exact role of ALDP in the metabolism of VLCFA is still not understood (1, 4). The VLCFA C26:0 has been documented to cause metabolic alterations leading to membrane perturbation, redox imbalance, and changes in membrane lipid composition (5–8), as well as the induction of inflammatory mediators in cultured astrocytes (9). Thus, an appropriate composition of lipids in the cellular membrane is critical for the normal function of metabolic enzymes. Subtle alterations in myelin lipids have been reported to contribute to membrane disruption and myelin loss in multiple sclerosis patients (10).
In cALD, the metabolism of phospholipids and sphingolipids is drastically altered. While the levels of sphingolipids, including sphingomyelin (SM), are reduced (11, 12), the levels of phospholipids are increased, even in active plaque areas (13). However, some phospholipid species, including plasmenyethanolamine, are lost even within the normal-looking areas of white matter in the cALD brain (14). At the same time, other phospholipid levels, such as phosphatidylcholine (PC), are increased (13). PC is the major substrate of cytosolic phospholipase A2 (cPLA2), which release arachidonic acid (AA) from PC. AA is a major substrate for lipoxygenases and cyclooxygenases (COX) (15, 16). Originating from the oxidative metabolism of AA, lipid mediators such as malondialdehyde, 4-hydroxynonenal, acrolein, and protein carbonyl have been documented in both histologically normal and inflammatory areas of the cALD brain (14, 17). Levels of the potent chemoattractant leukotriene B4 (LTB4) and blood brain barrier toxic cysteinyl leukotrienes (Cys LTs) are also elevated in the cerebrospinal fluid (CSF) of demyelinating and predemyelinating cALD patients, indicating the involvement of 5-lipoxygenase (5-LOX) activity in the neuropathology of cALD disease. However, the role of lipoxygenase- and COX-derived bioactive lipids, including LTs and prostaglandins (PGs), has not previously been investigated in the cALD brain.
Biosynthesis of LTs and PGs requires AA released by the activation of cPLA2 (phospho-cPLA2; p-cPLA2). AA is then metabolized by 5-LOX and COX-2, leading to formation of LTs and PGs, respectively. Both cPLA2 and 5-LOX concomitantly translocate to the nuclear envelope, where cPLA2 presents AA to the 5-LOX/5-LOX activating protein complex (18). Subsequently, this complex converts AA into 5-HpETE, which is unstable and gets converted to LTA4 by 5-LOX. LTA4 is the common substrate for the biosynthesis of LTB4 and Cys LTs (18, 19). The amount of free AA available as a substrate for 5-LOX and the levels of PC determine the overall activity of the 5-LOX enzyme (20). PC selectively directs 5-LOX to the nuclear membrane, and calcium facilitates this 5-LOX membrane translocation (21). 5-LOX products have diversified functions. Whereas LTB4 is a potent chemoattractant, Cys LTs induce cell death or proliferation via their receptors in a cell-type-specific manner (22, 23). Because LTs modify cysteine residues of proteins/peptides, including glutathione (GSH), they are implicated in oxidative/lipoxidative stress and subsequent inflammation (23–25).
In the present study, we investigated the expression and activity of phospholipid/AA metabolizing inflammatory enzymes, including cPLA2, 5-LOX, and COX-2, in various areas of the cALD brain and evaluated potential causal relationships between VLCFA and those enzymes involved in the metabolism of AA. We observed not only increased expression of 5-LOX and high levels of LTs but also decreased levels of GSH, even in the histologically normal areas away from the plaque in the cALD brain. Similar to 5-LOX, the expression levels of cPLA2 and p-cPLA2 were also increased. Enrichment of C26:0, either by gene interference (silencing of Abcd1+Abcd2) in mouse primary astrocytes or by treatment of glial cells with exogenous C26:0, resulted in an increased expression of 5-LOX, indicating a causal relationship with the accumulation of VLCFA. This relationship was further supported by the decreased expression of 5-LOX when the levels of C26:0 were decreased by treatment with a combination of oleic and erucic acids.
METHODS
Human cALD brain and normal brain tissues
Frozen (−80°C) postmortem cALD brain tissue (cerebrum section slices from four patients) and age-matched frozen control brain tissue (cerebrum section slices from three subjects) were obtained from the Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, USA. Another sample of frozen cALD brain tissue was harvested within 2 h of death (age 9 years) at our hospital at the Medical University of South Carolina (MUSC) and was used in this study as described earlier (11, 14). All brain samples were received after the submission of research design to the Brain and Tissue Bank. MUSC Institutional Review Board recognizes such samples as “non human subject research” and allowed us to use them without its approval. All five cALD brains had elevated levels of VLCFA, measured as levels of C26:0 (μg/mg protein) and increased levels of cholesterol ester compared with age-matched control brains. The different areas of white matter (plaque, plaque shadow, away from plaque) from each cALD brain were identified and sliced by an experienced pathologist (Dr. Avtar K. Singh, M.D., Department of Pediatrics and Department of Pathology and Laboratory Medicine, MUSC, Charleston, SC). Three different white matter samples of each area from each cALD brain and three white matter brain samples from each normal control brain were dissected on a cold plate and weighed after removal of meninges, blood vessels, and gray matter. Gray matter samples were also analyzed and compared with white matter. Gray matter from the cALD brain showed no significant alterations in the levels of VLCFA and cholesterol ester compared with gray matter from the normal control brain tissue.
Lipid extraction and analysis from brain tissue
Total lipids from brain tissue were extracted and resolved as described earlier (12, 14, 26, 27). Levels of two phospholipids, PC and phosphatidylethanolamine (PE), and the sphingolipid SM were measured by high performance TLC and quantitated by densitometry as described earlier (27, 28).
Preparation of mouse primary astrocytes
C57BL6 breeding pairs were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained at the institution's animal facility. Animal procedures were approved by the Institutional Animal Care and Use Committee. Animals received humane care in compliance with MUSC's experimental guidelines and the NRC's criteria for humane care in the Guide for Care and Use of Laboratory Animals.
Primary astrocyte-enriched cultures were prepared from the whole cortex of 1-day-old C57BL/6 mice as described earlier in rats and mice (29, 30). Briefly, the cortex was rapidly dissected in ice-cold calcium/magnesium-free Hanks’ balanced salt solution (pH 7.4) as described previously (30). The tissue was then minced, incubated in Hanks’ balanced salt solution containing trypsin (2 mg/ml) for 20 min, washed twice with culture medium DMEM containing 10% FBS and 10 μg/ml gentamicin, and then disrupted by triturating through a Pasteur pipette, following which cells were seeded in 75 cm2 culture flasks (Falcon, Franklin, NJ). After incubation at 37°C in 5% CO2 for 1 day, the medium was completely changed. The cultures received half exchanges with fresh medium twice per week. After 10 days, the cells were shaken for at least 30 min on an orbital shaker to remove the microglia, and flasks were incubated for 1 day and then shaken again for 8 h to remove the oligodendrocytes. The remaining population was used for astrocyte cultures [approximately 95–99% pure as determined by immunostaining for glial fibrillary acidic protein (GFAP)] (9). All the cultured cells were maintained at 37°C in 5% CO2.
Gene silencing by siRNA of Abcd1 and Abcd2 in primary astrocytes
The silencing of Abcd1 and Abcd2 in mouse primary astrocytes has been described earlier (9, 14). Briefly, the Silencer siRNA (Ambion, Austin, TX) was used for Abcd1 and/or Abcd2 silencing in primary mouse astrocytes. Mouse astrocytes cultured in DMEM with 10% FBS and in the presence of antibiotic were transfected with siRNA for Abcd1 and/or Abcd2 using the siPORT NeoFX transfection agent (Ambion). Three siRNA for each Abcd1 and Abcd2 (Ambion) were used (Abcd1: siRNA 1, ID 162218, 5′-CCUCUACAACCUAAUUUAUtt-3′, 5′-AUAAAUUAGGUUGUAGAGGtg-3′; siRNA 2, ID 60153, 5′-GGUAUUUGAAGAUGUCAAAtt-3′, 5′-UUUGACAUCUUCAAAUACCtg-3′; siRNA 3, ID 60064, 5′-GGAAAUUGCCUUCUACGGGtt-3′, 5′-CCCGUAGAAGGCAAUUUCCtc-3′. Abcd2: SiRNA1, ID 188185, 5′-GGCUUUAGCUUACCAGAUGtt-3′, 5′-CAUCUGGUAAGCUAAAGCCtt-3′; siRNA 2, ID 214996, 5′-GGUAAAUGUCUAGAAAUGGtt-3′, 5′-CCAUUUCUAGACAUUUACCtg-3′; siRNA 3, ID 214997, 5′-GCUGUAGAGAUCAAUAGAGtt-3′, 5′-CUCUAUUGAUCUCUACAGCtc-3′). The siRNAs were mixed and diluted in OPTI-MEM1 medium to a final concentration of 30 nM/well. The siRNA transfection agent was dispensed into culture plates as directed by the manufacturer. Included also were a positive control using GAPDH siRNA (Ambion) and a negative control having sequence similarity to no known human, mouse, or rat gene. Cells were maintained in DMEM with reduced serum (2%). Silencing was observed by Western blot and mRNA quantification. For protein analysis of the transfected cells, two wells per plate were lysed and used for immunoblots (Western blot). In some experiments, cells were maintained either in the presence of oleic acid sodium salt (40 μM) and erucic acid sodium salt (40 μM) in a ratio of 1:1 for 4 days in DMEM with 2% fetal bovine serum before harvesting for analysis. Sodium salts of both oleic and erucic acids were solubilized in DMEM.
Enrichment of fatty acids and detection of 5-LOX expression in mouse primary astrocytes and in rat mixed glial cells
Rat cortical mixed glial cell and mouse primary astrocyte cultures were generated as previously described (9). After reaching confluency, cells were trypsinized and counted. 5 × 104 cells were added to each chamber of several 4-chamber slides (LabTEK). After allowing cells to adhere for 24 h, they were incubated for 6 days with 0–1 μM C16:0 CoA, C26:0 CoA, or CoA (CoAs were solubilized in DMEM). Media were changed every day, with fresh fatty acids added. Slides were fixed and then stained using standard immunofluorescence protocols for 1 h with the following antibodies: anti-GFAP (rabbit polyclonal, 1:200; DAKO, Carpinteria, CA) and anti-5-LOX goat polyclonal (1:100; SantaCruz Biotech.). Bound primary antibodies were detected with fluorophore-conjugated secondary antibodies (Alexa Fluor 488 and 555, 1:500 Molecular Probes; Invitrogen, Carlsbad, CA). Hoechst 33342 (Molecular Probes, Invitrogen) was used to visualize nuclei. Slides were analyzed for epifluorescence using an Olympus BX-60 research microscope. Images were acquired using a 12.5 megapixel cooled digital color camera (DP70, Olympus, Center Valley, PA) and DP Controller software. Image processing and compilation were performed using Adobe Photoshop CS2 Software (Adobe Systems Inc., San Jose, CA). Minimal adjustments to image brightness, contrast, and levels were made on intact figures to enhance image clarity.
For the lipids and Western studies, the cells were treated similarly in 6-well plates under identical conditions as described above for immunohistochemical studies. Lipids and fatty acids were analyzed as previously described (14).
Preparation and analysis of fatty acid methyl ester by GC and quantitation of VLCFA and PUFA
Fatty acid methyl esters (FAMEs) from the brain-extracted lipids or cultured cells or lipid fraction PC were prepared according to the procedure of Lepage and Roy (31) with modification as previously described (32). Heptacosanoic acid (C27:0; 2 μg) and heneicosanoic acid (C21:0; 2 μg) were used as internal standards. The purified samples of FAME were analyzed on a fused silica capillary column 25 M 007 Series methyl silicone, 0.25 mm internal diameter from Quadrex Corp. (Woodbridge, CT) in a gas chromatograph GC-17A connected with a flame ionization detector from Shimadzu Corporation. The peaks of individual FAMEs were identified by comparison of their relative retention times with those of known standards, and they were later verified by the addition of standards to prepared samples. The individual FAMEs were measured as absolute quantities by comparison with an internal standard and also as an area percent, as described earlier (33). The measurement of VLCFA (C26:0) is represented either as ratio of C26:0/C22:0 or as mass of 26:0 (nmol/mg protein) as described earlier (14, 26, 32, 33).
Expression of cPLA2, 5-LOX, p-5-LOX and COX-2 by Western blot of brain areas from cALD and control (normal) brains
Brain tissues obtained from control and ALD patients and cultured cells were homogenized on ice in a sucrose buffer (0.25 mM sucrose, 1 mM ethylenediaminetetraacetic acid, 3 mM imidazole protease inhibitor cocktail, pH 7.4). After centrifugation at 10,000 g for 15 min at 4°C, the total protein content in the supernatant was determined. Equal amounts of supernatant protein were subjected to SDS-PAGE (4–20% Tris-HCl Criterion gradient Gel; Bio-Rad Laboratories, Hercules, CA) and electrophoretically transferred to 0.2 μm nitrocellulose membrane (Bio-Rad Laboratories). Blots were probed as described earlier using primary antibodies against cPLA2, 5-LOX, COX-2, heat shock protein-70 (HSP-70), β-actin (Santa Cruz Biotech, Santa Cruz, CA), manganese superoxide dismutase (MnSOD) (Sigma-Aldrich, St Louis, MO), phospho-cPLA2, and phospho-5-LOX (Cell Signaling Technology, Inc. Danvers, MA).
Expression of 5-LOX, COX-2, p-cPLA2, ED1, and GFAP by immunohistochemistry of brain section from cALD and control (normal) brains
Immunoreactive 5-LOX, COX-2, p-cPLA2, ED1, and GFAP were detected using specific antibodies. Paraffin-embedded sections from formalin-fixed brain tissues were stained for 5-LOX (goat polyclonal from Santa Cruz Biotech, Santa Cruz, CA), COX-2 (goat polyclonal, from Santa Cruz Biotech), p-cPLA2 (rabbit polyclonal, from Cell Signaling Technology), ED1 (mouse monoclonal from Biosource International, Camarillo, CA), and anti-GFAP (rabbit polyclonal, 1:200; DAKO) as described earlier (11, 14). Deparaffinized tissue sections were subjected to an antigen unmasking protocol with citrate buffer (Vector Labs, Burlingame, CA). Nonspecific antibody binding was blocked by incubating sections in TBS containing 5% normal donkey serum and 3% BSA (blocking buffer) for 1 h. Primary antibodies were diluted in blocking buffer and applied to sections overnight at 4°C. Controls included replacement of primary antibodies with surrogate immunoglobulins or no primary antibody. Slides were washed 3 × 5 min in TBS with 0.05% Tween 20. Bound primary antibodies were detected with a fluorophore-conjugated secondary antibody (AlexaFluor 488 and 555, Molecular Probes; Invitrogen) at a concentration of 10 μg/ml and diluted in blocking solution for 1 h at room temperature. Slides were washed 3 × 5 min in TBS with 0.05% Tween 20 followed by an additional wash for 5 min in distilled water. For double labeling, the second primary antibody was added prior to incubation with fluorescently labeled secondary antibodies. Nuclei were labeled with Hoechst dye. Slides were mounted with GelMount mounting medium under No. 1.5 cover slips. All sections were analyzed using an Olympus BX-60 microscope, and images were captured using a digital video camera controlled by Adobe Photoshop 7.0 (Adobe Systems).
Measurement of leukotrienes in brain tissue
Samples of white matter from different areas of cALD and control brains were quickly isolated on ice. The samples were prepared as described (34). In brief, the tissues were weighed and homogenized in ice-cold absolute ethanol. After centrifugation of the homogenates at 15,000 g at 4°C for 30 min, the supernatant was collected and filtered through a 0.2 μm filter. The filtration was dried under nitrogen and resuspended in an ELISA buffer. The tissue levels of LTB4 and Cys LT were measured according to the protocol of the enzyme immunoassay kit (Assay Designs). All measurements were carried out three times.
Measurement of GSH in brain
Levels of reduced GSH in the white matter from different areas of cALD and control brains were measured using a BIOXYTECH® GSH/GSSG-412™ colorimetric assay kit from Oxis Research. The kit uses 5, 5′-dithiobis-2-nitrobenzoic acid and GSH reductase as described by Tietze (35) with some modification, using 1-methyl-2-vinylpyridinium trifluoromethanesulfonate instead of N-ethylmaleimide. Brain tissues were minced and homogenized (g/10 ml) in 5% m-phosphoric acid. The procedure was followed per the manufacturer's instructions, and the GSH levels were recorded as micromolar GSH based on standards supplied with the kit. Data are expressed as percent change.
Statistical evaluation
Statistical analysis was performed using Graphpad Prism 3.0 software (San Diego, CA). All values were expressed as mean ± SD of n determinations or as indicated. The results were examined by an unpaired t-test. Multiple comparisons were performed using ANOVA followed by the Bonferroni test as appropriate. A P-value <0.05 was considered significant.
RESULTS
Alterations in white matter of cALD brain characterized by histopathological and biochemical studies
The neuroinflammatory demyelinating disease in cALD is secondary to ALDP gene mutation/deletion and subsequent VLCFA accumulation. To understand the events involved in VLCFA/lipid-mediated membrane perturbation and the transformation to a neuroinflammatory disease, we examined the lipid alterations and lipid-derived inflammatory mediators in histologically normal as well as inflammatory areas of the cALD brain as compared with the age-matched control brain.
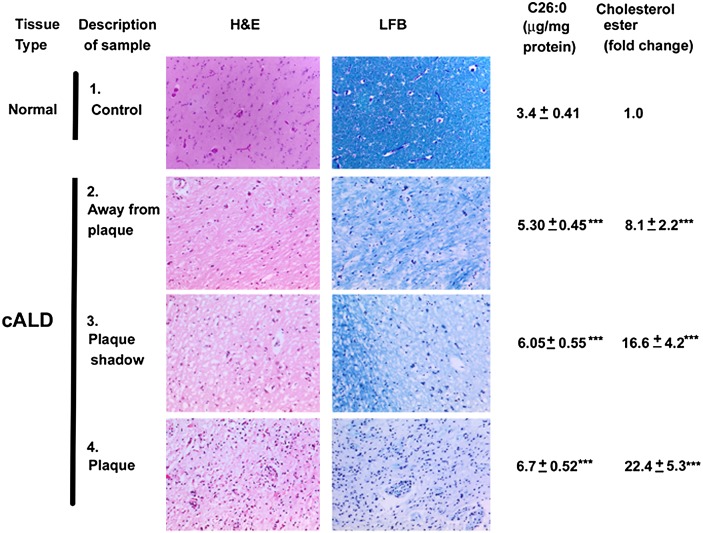
Brain tissue sections/areas from white matter in cerebrum, as shown in Fig. 1, were studied by hematoxylin and eosin and Luxol Fast Blue-PAS staining to determine levels of infiltration and myelin. Both the plaque and plaque shadow areas had significant infiltration as well as demyelination. Infiltration and demyelination in the cALD plaque area was biochemically supported by the accumulation of excessive VLCFA and cholesterol ester (Fig. 1). Surprisingly, the histologically normal area away from the plaque also showed demyelination and cellular infiltration, albeit significantly less than the plaque and the plaque shadow areas. The plaque shadow area had significantly higher levels of VLCFA and cholesterol ester compared with the area away from the plaque. Nonetheless, all areas of the cALD brain had greater accumulations of VLCFA and cholesterol ester, higher infiltration, and greater demyelination compared with the normal control white matter (Fig. 1). This finding is consistent with reduced levels of PE and SM in all areas of cALD white matter, as reported previously (11, 14). However, compared with PE, the levels of PC did not change significantly in these areas of the cALD brain, indicating specific alteration of PE (Table 1). An earlier work by Theda et al. (13) reported increased levels of other phospholipids as well as PC in all areas of the cALD brain. However, this study was limited to only one patient. Our study in five cALD brains showed remarkable alterations in the ratio of PC/PE and PC/SM in all three areas of the cALD brain (Table 1). In addition to the altered levels, PC showed a significantly increased accumulation of C26:0 in the plaque area compared with other areas. Still, the plaque shadow and the area away from the plaque also had significantly higher levels of C26:0 compared with the normal control. Remarkably, the levels of total AA (C20:4) were also increased in the plaque and the plaque shadow areas of the cALD brain compared with the normal control brain (Table 1). These lipid alterations indicate a functional abnormality of membrane-associated enzymes involved in lipid homeostasis and metabolism in the cALD brain.
Fig. 1.
A photomicrographic representation and molecular identification of cALD brain showing areas from plaque, plaque shadow, and away from plaque in white matter. The samples were collected from the white matter brain regions in and around the plaque from cALD patients (2–4) and white matter from age-matched control (normal) subjects (1) as described (14). The normal-looking area from cALD brain is away from plaque (2) and the plaque shadow region is the peripheral area (3) of the plaque (4). All the areas stained with hematoxylin and eosin demonstrated the accumulation of infiltrates (macrophages and lymphocytes), apparently highest in the plaque area followed by plaque shadow and away from plaque areas in cALD brain. Similarly, loss of myelin (Luxol Fast Blue-PAS staining) was greater in plaque than in areas of plaque shadow and away from plaque. Control/normal brain had no significant infiltration or loss of myelin. Photomicrographs are representative of three different brain samples from cALD and three different normal brains. Lipid analysis showed that accumulation of both VLCFA (C26:0) measured as absolute concentration by GC and cholesterol ester measured as fold change by high performance TLC was maximum in plaque compared with the plaque shadow and away from plaque areas. Data from biochemical analysis are expressed as mean ± SD. (n = 5, cALD; and n = 3, normal control brains). ***P < 0.001 versus control.
TABLE 1.
Levels of phospholipids, VLCFA, and AA in normal control and cALD white matter brain tissues
| Normal (Ct) | cALD-Away from Plaque | cALD-Plaque Shadow | cALD-Plaque | |
|---|---|---|---|---|
| PC (μg/mg protein) | 90.82 ± 10.9 | 74.94 ± 10.4 | 73.63 ± 11.8 | 83.27 ± 10.6 |
| PC/PE (ratio) | 0.90 ± 0.10 | 1.70 ± 0.12*** | 2.51 ± 0.21***+ | 2.65 ± 0.20***+ |
| PC/SM (ratio) | 1.35±.15 | 2.03 ± 0.18*** | 2.51 ± 0.21*** | 2.34 ± 0.25*** |
| C26:0 (%) in PC | 0.25 ± 0.02 | 2.29 ± 0.28*** | 4.5 ± 0.36***+++ | 7.20 ± 0.58***+++ |
| C20:4 (%) in brain tissue | 7.10 ± 1.01 | 9.27 ± 1.10* | 12.27 ± 1.10** | 10.63 ± 1.12** |
Levels of PC, PE, and SM were measured by high performance TLC after extraction of the lipids from different areas of cALD and normal control (Ct) brains (see Fig. 1). Data are expressed as mean ± SD (n = 3) and were measured as μg lipid/mg protein or as ratio. Levels of hexacosanoic acid (VLCFA; C26:0) in PC and AA (C20:4) were measured by GC in different areas of cALD and normal Ct brains. Data are expressed as mean ± SD (n = 3) and are presented as area percent change of the total identified fatty acids. ***P < 0.001, **P < 0.01, *P < 0.05 versus normal Ct; +++P < 0.001, +P < 0.05 versus cALD-away from plaque.
Expression of PLA2, 5-LOX, and COX-2 in white matter of cALD brain analyzed by Western and immunohistochemical studies
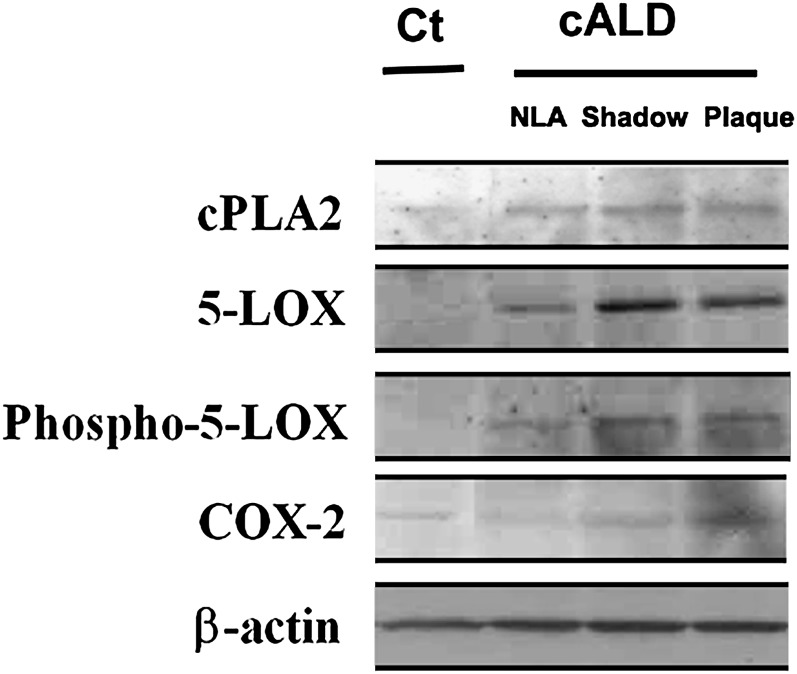
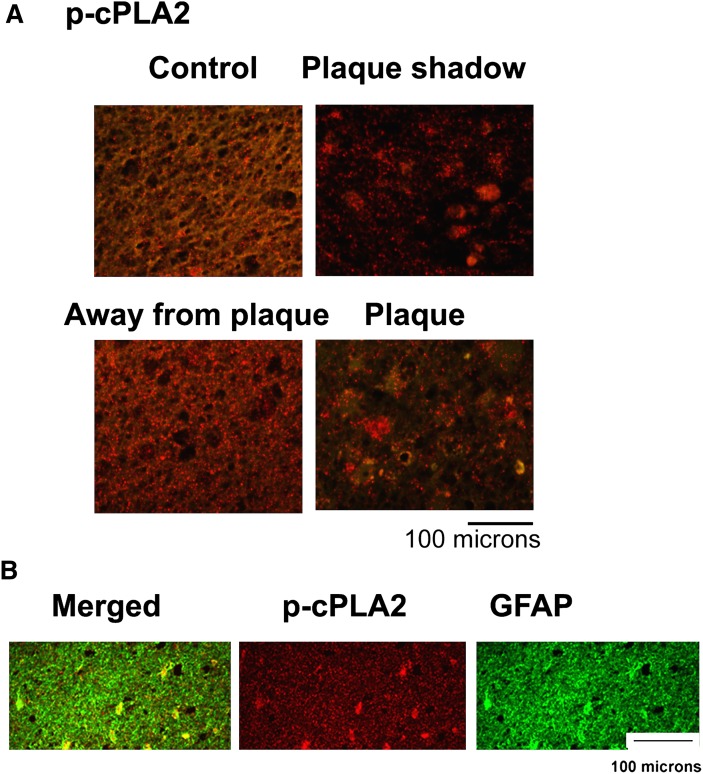
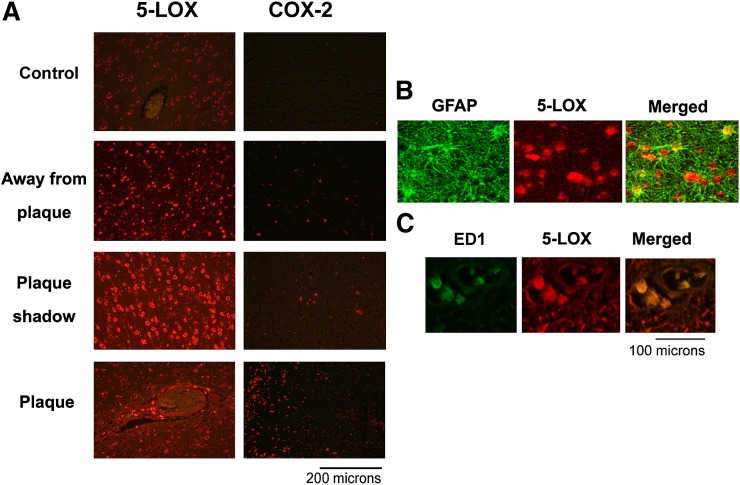
Alterations in phospholipid homeostasis (Table 1) led us to examine the expression of the lipid metabolizing lipolytic enzyme cPLA2/p-cPLA2 as well as the major lipoxidative enzymes 5-LOX and COX-2 (Figs. 2–4).All areas from the cALD brain exhibited increased immunoreactivities, not only for cPLA2 and 5-LOX but also for phospho-cPLA2 (Fig. 4) and phospho-5-LOX (Fig. 2). In contrast to the expression of cPLA2 and 5-LOX, however, COX-2 was not overexpresssed except in the plaque area (Fig. 2, lane 4) of the cALD brain. Compared with the cALD brain, the normal control brain had mild or undetectable expression of all three enzymes. Data indicating expression of 5-LOX studied by Western analysis in all three areas from the cALD white matter was supported by immunohistochemical study of 5-LOX in the same areas (Fig. 3A). Both studies documented higher expression of 5-LOX in the plaque shadow than the other areas of the brain. A double labeling study revealed that the 5-LOX expression colocalized with astrocytes (Fig. 3B; GFAP positive cells) as well as with activated microglia and infiltrated macrophages (Fig. 3C; ED1 positive cells). The expression of p-cPLA2 was predominantly localized with astrocytes (GFAP positive cells) (Fig. 4B).
Fig. 2.
Expression of cPLA2, 5-LOX, p-5-LOX, and COX-2 by immunoblot in different areas of cALD and normal control brains. Control (Ct) normal brain (1) and different areas (2; normal looking away from plaque), (3; plaque shadow), (4; plaque) from white matter of the cALD brain were analyzed for the expression of cPLA2, 5-LOX, p-5-LOX, and COX-2 proteins by immunoblot using specific antibodies. While the expression of cPLA2 as well as 5-LOX/p-5-LOX was increased in all the areas of cALD, COX-2 expression remained localized mainly in the plaque area. Control normal brain had no significant expression of 5-LOX and COX-2 enzymes. Data are representative of five different samples from cALD and three different samples from control normal brains.
Fig. 4.
Photomicrograph of immunohistochemistry of p-cPLA2 and colocalization of expression of 5-LOX with GFAP in different areas from cALD and normal control brains. A: Enhanced immunoreactivity (red staining) showed higher expression of p-cPLA2 in plaque shadow and plaque areas from cALD brain compared with normal control. B: Immunostaining for p-cPLA2 (red) and GFAP (green) colocalized as yellowish fluorescence in the plaque shadow area. 400× magnification.
Fig. 3.
Photomicrograph of immunohistochemistry of 5-LOX and COX-2 and colocalization of expression of 5-LOX with GFAP and ED1 in different areas from cALD and normal control brains. A: Enhanced immunoreactivity (red staining) showed higher expression of 5-LOX in all the areas from cALD brain compared with normal control, though plaque shadow area has greater expression than plaque and area away from plaque. COX-2 was expressed mainly in plaque area. B: Immunostaining for 5-LOX (red) and GFAP (green) and (C) for 5-LOX (red) and ED1 (green) colocalized as yellowish fluorescence in the plaque shadow area. 400× magnification.
5-LOX-derived leukotrienes in white matter of cALD brain
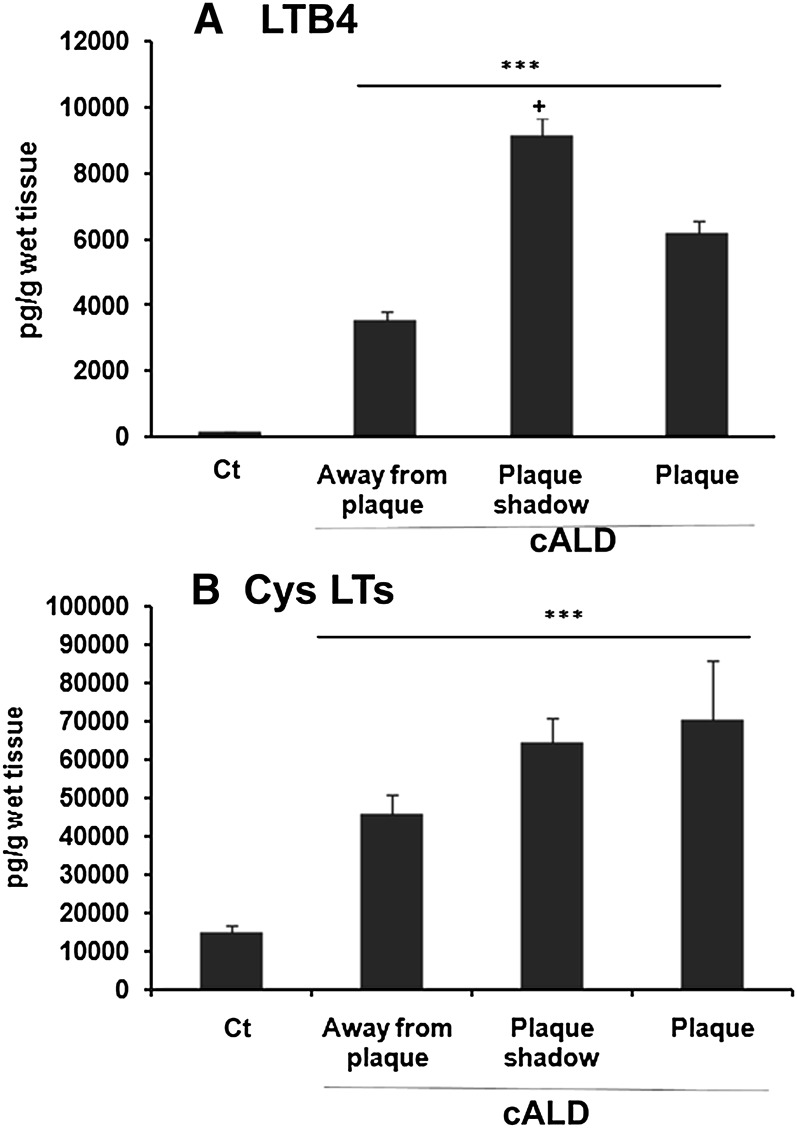
Leukotrienes (LTB4 and Cys LTs) have been reported to participate in neuroinflammation (36), and higher leukotriene levels were also found in the CSF of cALD patients with demyelination or prior to demyelination (37). Therefore, we investigated the levels of these leukotrienes in brain tissues using ELISA and observed several-fold higher levels of both LTB4 (Fig. 5A) and Cys LTs (Fig. 5B) in all areas of the cALD brain compared with the normal control brain. LTB4 levels were higher (P < 0.05) in the plaque shadow area compared with the area away from the plaque and the plaque areas (Fig. 5A). Interestingly, the levels of LTs (Fig. 5) paralleled the expression of 5-LOX (Fig. 3) in the three regions of the cALD brain.
Fig. 5.
Levels of LTB4 and Cys LTs in different areas of cALD and normal control brains. Leukotrienes were measured in brain tissues using ELISA as described in Materials and Methods. The levels of both of the leukotrienes LTB4 (A) and Cys LTs (B) were increased in all the cALD brains. Data are representative of five different samples from cALD and three different samples from control normal brains and are expressed as mean ± SD. Area wise results are presented as pg/g wet tissue. ***P < 0.001 versus normal control (Ct); +P < 0.05 versus plaque and away from plaque.
Redox imbalance in white matter of cALD brain
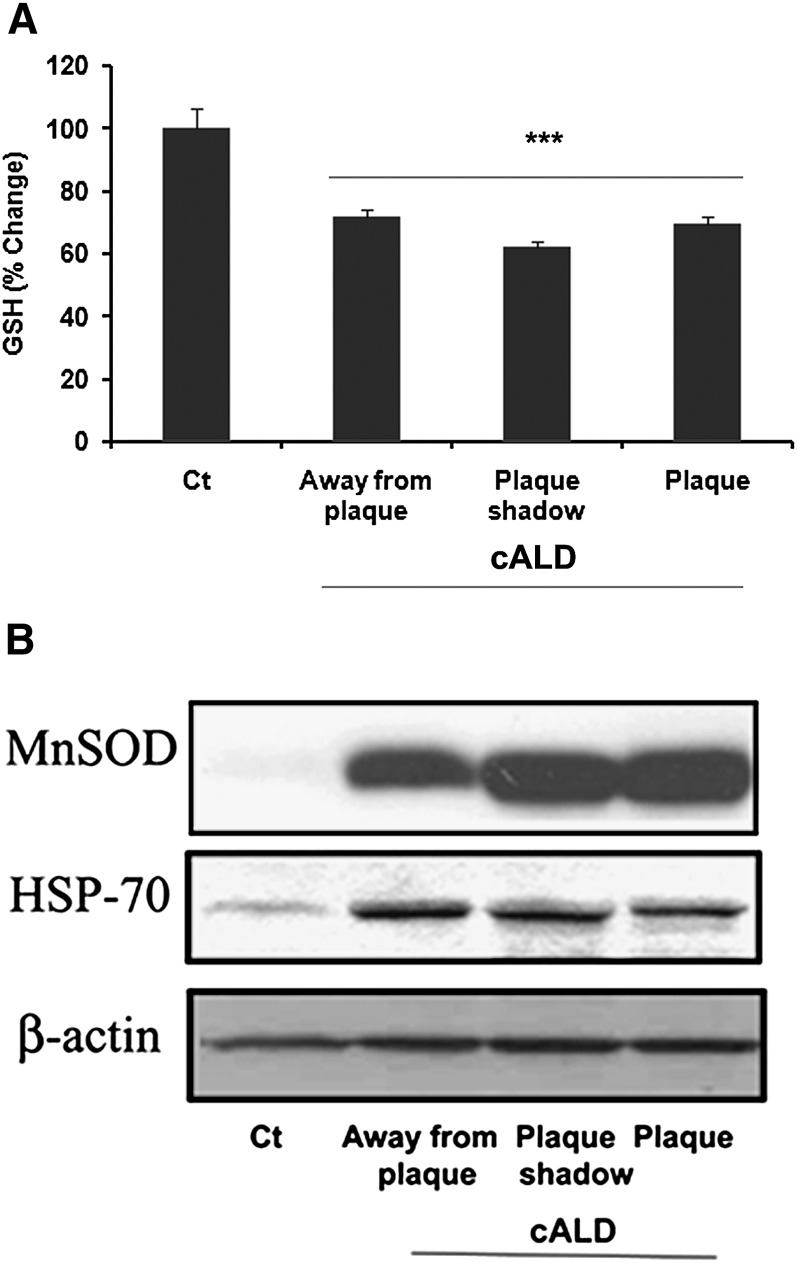
Oxidative burden has been reported to play a role in the pathobiology of X-ALD (7, 8, 14, 17, 38). We previously reported reduced levels of GSH in the plaque area of the cALD brain (39). In this study, we measured the levels of GSH in the different areas of white matter of the cALD brain. The levels of GSH were decreased significantly in all three areas compared with the normal control brain (Fig. 6A). To further examine the status of oxidative stress in the pathobiology of cALD, we measured the expression of stress-related genes such as HSP-70 and MnSOD. Increased expression of MnSOD has been shown earlier in the cALD brain, indicating oxidative stress (17). Here, we observed robust protein expression of MnSOD in all areas of the cALD brain. The normal control brain did not show the expression of MnSOD (Fig. 6B). Similar to MnSOD, expression of HSP-70 was also remarkably increased in all three areas of the cALD brain under investigation (Fig. 6B). Strong immunoreactivities for both MnSOD and HSP-70, even in the histopathologically normal area away from the plaque, are indicative of redox imbalance in the cALD brain.
Fig. 6.
Levels of reduced GSH and the expression of MnSOD and HSP-70 in different areas of cALD and normal control brains. A: GSH was measured in brain tissues using a colorimetric kit. Data are representative of five different samples from cALD and three different samples from normal control brains and are expressed as mean ± SD. Area wise results are presented as percent change of micromolar concentrations of GSH. Normal control brain tissue has 47.3 μM GSH concentration. B: White matter from control normal brain and from different areas (normal looking away from plaque; plaque shadow; plaque) of the same c-ALD brain are analyzed for the expression of MnSOD and HSP-70 by Western blot using specific antibodies. Expression of both MnSOD and HSP-70 was increased in all the areas of cALD. Control normal brain had no significant expression of MnSOD and only mild expression of HSP-70. Data are representative of five different samples from cALD and three different samples from control normal brains. ***P < 0.001 versus normal control (Ct).
Enriching of astrocyte and glial cell cultures with VLCFA results in increased expression of 5-LOX
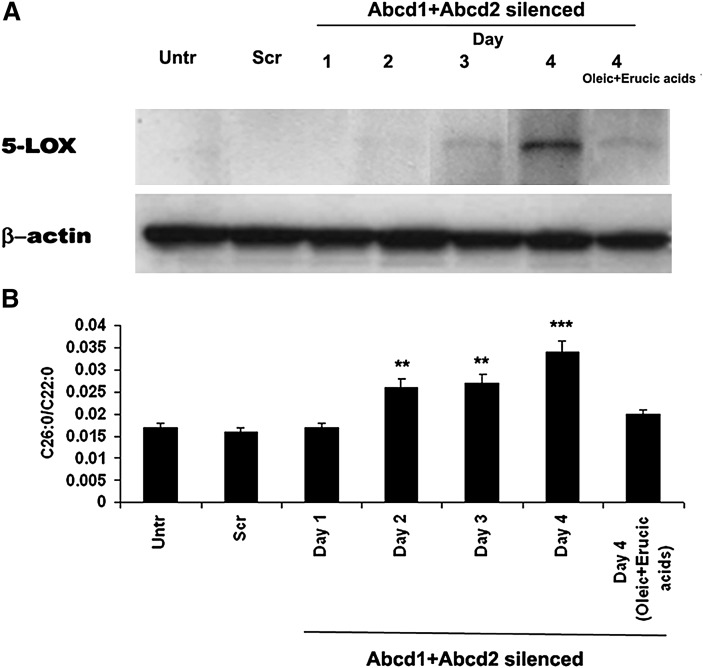
To investigate whether VLCFA-mediated lipid modification is directly responsible for initiating an inflammatory disease process, we examined the status of 5-LOX in cultured primary astrocytes enriched with VLCFA. Gene silencing by SiRNA of Abcd1 and Abcd2 was used to increase the levels of VLCFA as described earlier (9). We observed a linear relationship between the silencing-induced enhanced levels of VLCFA (26:0/22:0) and the expression of 5-LOX with increasing time after silencing (Fig. 7). On day 4 of the silencing, the levels of VLCFA (0.034 + .0025) and the expression of 5-LOX were greatest. Cells treated with Scramble (Scr) sequence showed neither accumulation of VLCFA nor increased expression of 5-LOX measured at day 4 following the nucleotide treatment. The silencing beyond day 4 had no significant effects on either the levels of VLCFA or 5-LOX expression (data not shown). The silencing of Abcd1 or Abcd2 individually resulted in only a moderate increase in the levels of VLCFA as well as the expression of 5-LOX as compared with the silencing of both together (9). Because treatment with a mixture of monoenoic fatty acids (oleic acid + erucic acid) has been documented to decrease levels of VLCFA (9), we investigated and observed that silencing of Abcd1+Abcd2 in the presence of oleic acid + erucic acid significantly reduced the expression of 5-LOX as well as the levels of VLCFA (Fig. 7).
Fig. 7.
Effect of Abcd1+Abcd2 silencing by SiRNA on the expression of 5-LOX and the levels of C26:0/C22:0 in mouse primary astrocytes. Procedure of silencing by SiRNA of Abcd1 and Abcd2 is described in Materials and Methods. Expression of 5-LOX and β-actin was measured day wise by Western blot using specific antibodies (A). Measurement of C26:0 as ratio (26:0/22:0) (B) was carried as described in Materials and Methods. Silencing of the Abcd1+Abcd2 for 4 days resulted in remarkably increased expression of 5-LOX as well as increased levels of C26:0. Scr cells showed neither expression of 5-LOX nor increased levels of C26:0 after 4 days. A daily treatment with oleic acid + erucic acid (40 μM each, solubilized in DMEM) together with SiRNA of Abcd1+Abcd2 decreased not only the increased expression of 5-LOX but also the increased levels of C26:0 measured at day 4 after the silencing. ***P < 0.001 versus treated and Scr, **P < 0.01 versus untreated and Scr.
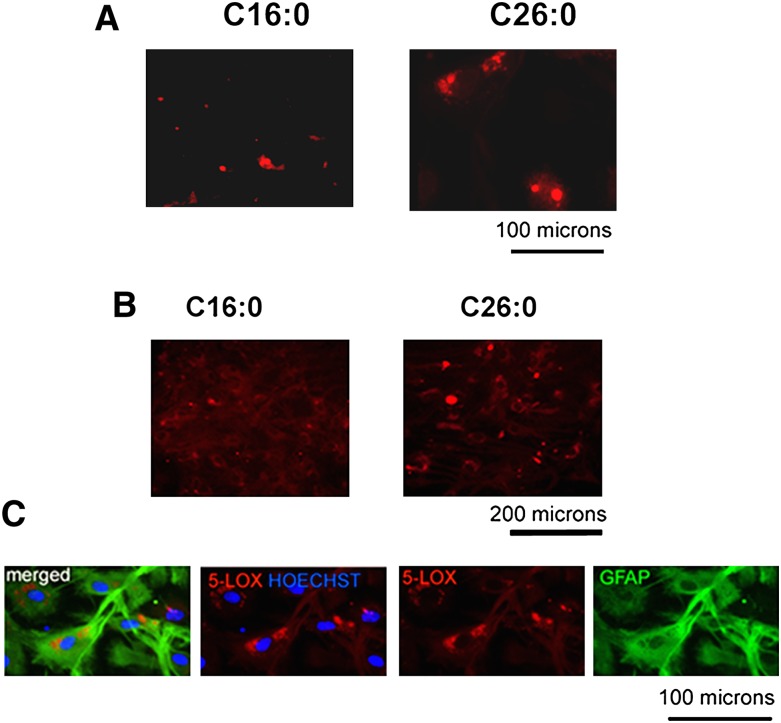
To further elucidate the relationship between excessive accumulation of VLCFA (C26:0) and the expression of 5-LOX, both primary astrocytes and mixed glial cells were treated with different concentrations of C26:0 CoA, C16:0 CoA, and CoA. Treatment for 6 days with C26:0 CoA (1 µM) resulted in a higher expression of 5-LOX than the treatment with C16:0 CoA (1 µM) in both the astrocytes (Fig. 8A) and the mixed glial cells (Fig. 8B). The expression of 5-LOX in mixed glial cells was found to be prominent in astrocytes as determined by coimmunostaining of 5-LOX + ve GFAP (Fig. 8C). The C26:0 CoA-treated cells had 5-LOX expression present mainly in and around the nucleus of the cell (Fig. 8C). Taken together, these observations support a link between the accumulations of VLCFA and the expression of the 5-LOX enzyme.
Fig. 8.
Effect of enrichment of C26:0 and C16:0 on the expression of 5-LOX in mouse primary astrocytes and rat mixed glial cells. Incubation of cells with C26:0 CoA (1 μM, solubilized in DMEM), but not C16:0 CoA (1 μM, solubilized in DMEM), for 6 days induced 5-LOX expression (red) as shown by immunofluorescence in primary astrocytes (A) and in mixed glial cells (B). 5-LOX expression was found to colocalize with GFAP +ve cells (green; astrocytes) in primary rat mixed glial cell culture. 5-LOX was localized around cell nucleus (Hoechst dye, blue) (C).
DISCUSSION
The goal of this study was to investigate the intrinsic nature of VLCFA-mediated production of lipoxidative mediators and the consequent molecular events involved in cALD brain pathology. As the major substrate of cPLA2 and a modulator of 5-LOX activity, abnormally high proportions of PC enriched with C26:0 at the sn-1 position of the glycerol backbone may stabilize the membrane and aid in activating lipolytic enzymes to release AA from the sn-2 position. AA is subsequently metabolized by 5-LOX to produce LTs, which initiate an oxidative cascade. As a consequence, the LTs reduce GSH levels and form toxic adducts capable of inducing an immune response (25). LTB4, the most potent chemoattractant, recruits leukocytes to the site of injury, contributing to inflammation and thus may cause the pathology of cALD.
Data in Table 1 show that the absolute levels of PC in all the areas studied from the cALD brain were not significantly different compared with that from the normal control brain. However, the levels of both PE and SM were significantly low in these areas, rendering the membrane significantly enriched with PC. In fact, PC in the area away from plaque was found to be enriched with C26:0 by at least 8 times compared with the normal control (Table 1). Levels of C26:0 PC from both the plaque and plaque shadow areas were elevated to an even greater degree (16–35 times) (Table 1). Similar accumulations of C26:0 in PC (up to 39 times) have been reported previously (13). However, alongside this increase of PC, we also observed significant accumulations of AA in all areas of the cALD brain (Table 1). These observations document not only the PC abnormality but also its proportionately high level compared with other phospholipids, even in the histologically normal area away from the plaque. The effects of C26:0- and C20:4-enriched PC on its metabolizing enzymes, which include cPLA2 and p-cPLA2, and this enrichment's effects downstream to PLA2 have not been previously examined. Therefore, we investigated whether this cPLA2/5-LOX enzyme system, which relies mainly on PC, is involved in cALD disease pathology. The possible role of leukotriene-producing enzymes in the pathology of inflammatory demyelination in cALD is supported by the recent observation of increased LTs in the CSF of cALD patients (37).
Expression of both cPLA2 and 5-LOX/p-5-LOX was found in all cALD brain areas, including the histologically normal area away from the plaque (Fig. 2). Detection of phosphorylated cPLA2 (Fig. 4) and phosphorylated 5-LOX (Fig. 2) indicates the presence of activated enzymes. Activation of cPLA2 requires low levels of calcium for membrane translocation and activity. Recently, VLCFA have been shown to alter calcium homeostasis (6), which might be responsible for the cPLA2 activity in VLCFA accumulation pathologies. Like cPLA2, 5-LOX requires calcium for membrane translocation. Its activity is dependent on its membrane association, especially its interaction with PC. We hypothesized that enrichment of PC with C26:0 causes a higher affinity for 5-LOX than for COX-2 and thus increases 5-LOX activity/expression. The COX-2 enzyme, which does not require interaction with the membrane, was not induced in the histologically normal area away from the plaque, indicating that alteration in VLCFA does not affect COX-2 expression. Low expression of COX-2 in the inflammatory plaque and plaque shadow areas may be a consequence of the inflammatory cascade subsequent to the PC-induced increase in activity/expression seen with 5-LOX.
The expression of 5-LOX was elevated in astrocytes, not only in the plaque shadow and the plaque areas but also in the histologically normal areas away from the plaque (Fig. 3), suggesting it may be a sentinel marker in the cALD brain. The consequent LTs produced in astrocytes may affect the cells in both autocrine and paracrine fashions. Neural cells, including oligodendrocytes, have LT receptors. LTs modulate not only chemoattraction but also cell proliferation and cell death. Therefore, we hypothesize that LTs are involved in astrocytic proliferation and in the cell death of oligodendrocytes. LTB4 also recruits inflammatory cells via chemokines. We have already reported that the cALD brain shows a robust chemokine involvement in all the areas of white matter (11), which might be modulated by astrocytic 5-LOX/LTs. The expression of 5-LOX was also observed in microglia/macrophages (Fig. 3C) in the cALD brain, indicating its involvement in neuroinflammation. 5-LOX is reported to be expressed in immune cells, including microglia/macrophages under neuroinflammatory conditions (22, 24).
Levels of both LTB4 and Cys LTs were significantly increased in all areas, as shown in Fig. 5. Cys LTs are known to deplete the levels of GSH, and a similar observation of decreased GSH levels in all areas of the cALD brain (Fig. 6A) supports the hypothesis that LTs may contribute to the observed oxidative burden, even in the histologically normal-looking area of the cALD brain. The VLCFA C26:0 has been implicated in reductions of GSH in cultured cells (8), and it also alters calcium homeostasis and mitochondrial potential in neural cells, including oligodendrocytes (6). Redox imbalance is supported by the enhanced expression of MnSOD and HSP-70 in the normal-looking area away from the plaque (Fig. 6B). Increased expression of HSP-70 and MnSOD in the area away from the plaque further supports the notion that oxidative stress participates in the transition from metabolic to neuroinflammatory cALD.
We next investigated a potential causal relationship between the accumulation of VLCFA and the expression of 5-LOX. We measured the expression of 5-LOX as a result of increasing concentrations of C26:0 following gene silencing of both Abcd1 and Abcd2. The accumulation of C26:0 paralleled the expression of 5-LOX (Fig. 7), thus supporting the hypothesis that increased levels of VLCFA are directly responsible for the enhanced expression of 5-LOX. These conclusions were further supported by an in vitro study documenting the expression of 5-LOX following enrichment of astrocytic or mixed glial cell cultures with exogenous C26:0 (Fig. 8). Enhanced expression of 5-LOX correlates with the accumulation of C26:0 in the cells (Table 2) as well as in the PC fraction of phospholipids (Table 3). These observations of 5-LOX expression in cell culture models due to VLCFA-mediated derangement indicate a role of 5-LOX in the pathobiology of cALD.
TABLE 2.
Levels of C26:0 and C16:0 in primary mixed glial cells
| Treatment/Measurements | C26:0 CoA | C16:0 CoA | CoA Ester | Untreated |
|---|---|---|---|---|
| C26:0/C22:0 | 5.32 ± 0.03*** | 0.22 ± 0.19 | 0.13 ± 0.01 | 0.05 ± 0.01 |
| C26:0 (%) | 2.94 ± 0.19*** | 0.14 ± 0.11 | 0.01 ± 0.01 | 0.03 ± 0.01 |
Rat mixed glial cells were treated with 1 µM C26:0 CoA, C16:0 CoA, and CoA ester for 6 days. Levels of fatty acids were measured using capillary GC. Data are expressed as mean ± SD (n = 3) and are presented as ratio as well as area percent change of the total identified fatty acids. ***P < 0.001 versus 16:0 CoA, CoA ester, and untreated.
TABLE 3.
Levels of C26:0 and C16:0 in PC fraction isolated from primary mixed glial cells
| Treatment/Measurements | C26:0 CoA | C16:0 CoA | CoA ester | Untreated |
|---|---|---|---|---|
| C26:0/C22:0 | 0.47 ± 0.02*** | 0.03 ± 0.01 | 0.01 ± 0.009 | 0.01 ± 0.01 |
| C26:0 (%) | 0.41 ± 0.05*** | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
PC fraction of phospholipids was isolated and purified using HPTLC from rat mixed glial cells after treatment with 1 µM C26:0 CoA, C16:0 CoA, and CoA ester for 6 days. Levels of fatty acids were measured using capillary GC. Data are expressed as mean ± SD (n = 3) and are presented as ratio as well as area percent change of the total identified fatty acids. ***P < 0.001 versus 16:0 CoA, CoA ester, and untreated.
Collectively, these results show that VLCFA accumulation directly increases 5-LOX enzyme expression and thus mediates the production of lipoxidative mediators. This identification of 5-LOX-derived lipotoxicity provides a new, mechanism-based target for intervention in cALD.
Acknowledgments
The authors thank Dr. Avtar K. Singh, M.D. (a pathologist) from the Ralph H Johnson VA Medical Center for dissecting and identifying different areas of human brain white matter. The authors are grateful to Dr. Tom Smith from the MUSC Writing Center for his valuable editing and correction of the manuscript. The authors would like to thank Joyce Bryan for procurement of animals and chemicals used in this study. The authors also acknowledge Dr. Shailendra Giri and Dr. Md Nasrul Hoda for their assistance with the analytical work.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ALD
- adrenoleukodystrophy
- ALDP
- adrenoleukodystrophy protein
- cALD
- childhood adrenoleukodystrophy
- COX-2
- cyclooxygenase-2
- cPLA2
- cytosolic phospholipase A2
- CSF
- cerebrospinal fluid
- Cys LT
- cysteinyl leukotriene
- FAME
- fatty acid methyl ester
- GFAP
- glial fibrillary acidic protein
- GSH
- glutathione
- HSP-70
- heat shock protein-70
- 5-LOX
- 5-lipoxygenase
- LTB4
- leukotriene B4
- MnSOD
- manganese superoxide dismutase
- MUSC
- Medical University of South Carolina
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- prostaglandin
- p-cPLA2
- phospho-cPLA2
- PLA2
- phospholipase A2
- Scr
- Scramble
- SM
- sphingomyelin
- VLCFA
- very long-chain fatty acid
- X-ALD
- X-linked adrenoleukodystrophy
These studies were supported by grants (NS-22576, NS-34741, and NS-37766) from the National Institutes of Health, Bethesda, MD. This work was also supported by National Institutes of Health Grants C06 RR-018823 and C06 RR-015455 from the Extramural Research Facilities Program of the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Singh I. 1997. Biochemistry of peroxisomes in health and disease. Mol. Cell. Biochem. 167: 1–29. [DOI] [PubMed] [Google Scholar]
- 2.Singh I. 2002. Peroxisomal fatty acid oxidation and cellular redox. Methods Enzymol. 352: 361–372. [DOI] [PubMed] [Google Scholar]
- 3.Moser H. W., Mahmood A., Raymond G. V. 2007. X-linked adrenoleukodystrophy. Nat. Clin. Pract. Neurol. 3: 140–151. [DOI] [PubMed] [Google Scholar]
- 4.Semmler A., Kohler W., Jung H. H., Weller M., Linnebank M. 2008. Therapy of X-linked adrenoleukodystrophy. Expert Rev. Neurother. 8: 1367–1379. [DOI] [PubMed] [Google Scholar]
- 5.Ho J. K., Moser H., Kishimoto Y., Hamilton J. A. 1995. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J. Clin. Invest. 96: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hein S., Schonfeld P., Kahlert S., Reiser G. 2008. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum. Mol. Genet. 17: 1750–1761. [DOI] [PubMed] [Google Scholar]
- 7.Di Biase A., Di Benedetto R., Fiorentini C., Travaglione S., Salvati S., Attorri L., Pietraforte D. 2004. Free radical release in C6 glial cells enriched in hexacosanoic acid: implication for X-linked adrenoleukodystrophy pathogenesis. Neurochem. Int. 44: 215–221. [DOI] [PubMed] [Google Scholar]
- 8.Fourcade S., Lopez-Erauskin J., Galino J., Duval C., Naudi A., Jove M., Kemp S., Villarroya F., Ferrer I., Pamplona R., et al. 2008. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 17: 1762–1773. [DOI] [PubMed] [Google Scholar]
- 9.Singh J., Khan M., Singh I. 2009. Silencing of Abcd1 and Abcd2 genes sensitizes astrocytes for inflammation: implication for X-adrenoleukodystrophy. J. Lipid Res. 50: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler D., Bandaru V. V., Calabresi P. A., Nath A., Haughey N. J. 2008. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 131: 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paintlia A. S., Gilg A. G., Khan M., Singh A. K., Barbosa E., Singh I. 2003. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies. Neurobiol. Dis. 14: 425–439. [DOI] [PubMed] [Google Scholar]
- 12.Wilson R., Sargent J. R. 1993. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J. Neurochem. 61: 290–297. [DOI] [PubMed] [Google Scholar]
- 13.Theda C., Moser A. B., Powers J. M., Moser H. W. 1992. Phospholipids in X-linked adrenoleukodystrophy white matter: fatty acid abnormalities before the onset of demyelination. J. Neurol. Sci. 110: 195–204. [DOI] [PubMed] [Google Scholar]
- 14.Khan M., Singh J., Singh I. 2008. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J. Neurochem. 106: 1766–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillis J. W., Horrocks L. A., Farooqui A. A. 2006. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Rev. 52: 201–243. [DOI] [PubMed] [Google Scholar]
- 16.Phillis J. W., O'Regan M. H. 2003. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit. Rev. Neurobiol. 15: 61–90. [DOI] [PubMed] [Google Scholar]
- 17.Powers J. M., Pei Z., Heinzer A. K., Deering R., Moser A. B., Moser H. W., Watkins P. A., Smith K. D. 2005. Adreno-leukodystrophy: oxidative stress of mice and men. J. Neuropathol. Exp. Neurol. 64: 1067–1079. [DOI] [PubMed] [Google Scholar]
- 18.Radmark O. 2002. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 68–69: 211–234. [DOI] [PubMed] [Google Scholar]
- 19.Soberman R. J., Christmas P. 2003. The organization and consequences of eicosanoid signaling. J. Clin. Invest. 111: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarini S., Gijon M. A., Folco G., Murphy R. C. 2006. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J. Biol. Chem. 281: 10134–10142. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni S., Das S., Funk C. D., Murray D., Cho W. 2002. Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J. Biol. Chem. 277: 13167–13174. [DOI] [PubMed] [Google Scholar]
- 22.Lewis R. A., Austen K. F., Soberman R. J. 1990. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 323: 645–655. [DOI] [PubMed] [Google Scholar]
- 23.Huang X. J., Zhang W. P., Li C. T., Shi W. Z., Fang S. H., Lu Y. B., Chen Z., Wei E. Q. 2008. Activation of CysLT receptors induces astrocyte proliferation and death after oxygen-glucose deprivation. Glia. 56: 27–37. [DOI] [PubMed] [Google Scholar]
- 24.Radmark O., Werz O., Steinhilber D., Samuelsson B. 2007. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 32: 332–341. [DOI] [PubMed] [Google Scholar]
- 25.Brock T. G. 2005. Regulating leukotriene synthesis: the role of nuclear 5-lipoxygenase. J. Cell. Biochem. 96: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 26.Khan M., Pahan K., Singh A. K., Singh I. 1998. Cytokine-induced accumulation of very long-chain fatty acids in rat C6 glial cells: implication for X-adrenoleukodystrophy. J. Neurochem. 71: 78–87. [DOI] [PubMed] [Google Scholar]
- 27.Khan M., Contreras M., Singh I. 2000. Endotoxin-induced alterations of lipid and fatty acid compositions in rat liver peroxisomes. J. Endotoxin Res. 6: 41–50. [DOI] [PubMed] [Google Scholar]
- 28.Ganser A. L., Kerner A. L., Brown B. J., Davisson M. T., Kirschner D. A. 1988. A survey of neurological mutant mice. I. Lipid composition of myelinated tissue in known myelin mutants. Dev. Neurosci. 10: 99–122. [DOI] [PubMed] [Google Scholar]
- 29.Pahan K., Sheikh F. G., Khan M., Namboodiri A. M., Singh I. 1998. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 273: 2591–2600. [DOI] [PubMed] [Google Scholar]
- 30.Won J. S., Choi M. R., Suh H. W. 2001. Stimulation of astrocyte-enriched culture with C2 ceramide increases proenkephalin mRNA: involvement of cAMP-response element binding protein and mitogen activated protein kinases. Brain Res. 903: 207–215. [DOI] [PubMed] [Google Scholar]
- 31.Lepage G., Roy C. C. 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27: 114–120. [PubMed] [Google Scholar]
- 32.Khan M., Haq E., Giri S., Singh I., Singh A. K. 2005. Peroxisomal participation in psychosine-mediated toxicity: implications for Krabbe's disease. J. Neurosci. Res. 80: 845–854. [DOI] [PubMed] [Google Scholar]
- 33.Pai G. S., Khan M., Barbosa E., Key L. L., Craver J. R., Cure J. K., Betros R., Singh I. 2000. Lovastatin therapy for X-linked adrenoleukodystrophy: clinical and biochemical observations on 12 patients. Mol. Genet. Metab. 69: 312–322. [DOI] [PubMed] [Google Scholar]
- 34.Chu L. S., Fang S. H., Zhou Y., Yu G. L., Wang M. L., Zhang W. P., Wei E. Q. 2007. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 28: 763–772. [DOI] [PubMed] [Google Scholar]
- 35.Tietze F. 1969. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27: 502–522. [DOI] [PubMed] [Google Scholar]
- 36.Klegeris A., McGeer P. L. 2002. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol. Aging. 23: 787–794. [DOI] [PubMed] [Google Scholar]
- 37.Mayatepek E., Baumann M., Meissner T., Hanefeld F., Korenke G. C. 2003. Role of leukotrienes as indicators of the inflammatory demyelinating reaction in x–linked cerebral adrenoleukodystrophy. J. Neurol. 250: 1259–1260. [DOI] [PubMed] [Google Scholar]
- 38.Uto T., Contreras M. A., Gilg A. G., Singh I. 2008. Oxidative imbalance in nonstimulated X-adrenoleukodystrophy-derived lymphoblasts. Dev. Neurosci. 30: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh I., Pahan K., Khan M., Singh A. K. 1998. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J. Biol. Chem. 273: 20354–20362. [DOI] [PubMed] [Google Scholar]