Abstract
Fat is delivered to tissues by apoB-containing lipoproteins synthesized in the liver and intestine with the help of an intracellular chaperone, microsomal triglyceride transfer protein (MTP). Leptin, a hormone secreted by adipose tissue, acts in the brain and on peripheral tissues to regulate fat storage and metabolism. Our aim was to identify the role of leptin signaling in MTP regulation and lipid absorption using several mouse models deficient in leptin receptor (LEPR) signaling and downstream effectors. Mice with spontaneous LEPR B mutations or targeted ablation of LEPR B in proopiomelanocortin (POMC) or agouti gene related peptide (AGRP) expressing cells had increased triglyceride in plasma, liver, and intestine. Furthermore, melanocortin 4 receptor (MC4R) knockout mice expressed a similar triglyceride phenotype, suggesting that leptin might regulate intestinal MTP expression through the melanocortin pathway. Mechanistic studies revealed that the accumulation of triglyceride in the intestine might be secondary to decreased expression of MTP and lipid absorption in these mice. Surgical and chemical blockade of vagal efferent outflow to the intestine in wild-type mice failed to alter the triglyceride phenotype, demonstrating that central neural control mechanisms were likely not involved in the observed regulation of intestinal MTP. Instead, we found that enterocytes express LEPR, POMC, AGRP, and MC4R. We propose that a peripheral, local gut signaling mechanism involving LEPR B and MC4R regulates intestinal MTP and controls intestinal lipid absorption.
Keywords: MTP, apoB, fat absorption, leptin receptor, POMC, AGRP
Dietary fat is a major source of energy. Biosynthesis of apolipoprotein B (apoB)-containing lipoproteins in the gut is required for the absorption of fat into the body. Biosynthesis of apoB-containing lipoproteins is critically dependent on the activity of microsomal triglyceride transfer protein (MTP) that mainly resides in the lumen of the endoplasmic reticulum. MTP physically binds to apoB and transfers lipids from membranes/lipid droplets to nascent apoB. These activities are believed to play critical roles in the lipidation of nascent apoB and in the formation of primordial lipoprotein particles (1). ApoB and MTP are also expressed in the liver to mobilize hepatic triacylglycerol by synthesizing very low density lipoproteins (2–4). Regulation of MTP expression at transcriptional and posttranscriptional levels is a major determinant of hepatic and intestinal fat mobilization (5), and recent evidence indicates that MTP expression is differentially regulated in the liver and intestine (6). For example, hepatic, but not intestinal, MTP is increased by peroxisome proliferator-activated receptor α (PPARα) agonists (7). In the intestine, but not in the liver, inositol-requiring enzyme 1β (IRE1β) has been suggested to posttranscriptionally degrade MTP mRNA (6). Specific intestinal inhibition of MTP is considered a viable therapeutic approach to lower plasma lipids (8), as hepatic MTP inhibition might promote hepatosteatosis and increased plasma transaminases. Therefore, new pharmacologic interventions to curb intestinal MTP activity are underway (9). Identification of novel tissue-specific regulatory mechanisms might be beneficial in targeting intestinal MTP to lower plasma lipids and avoid deleterious hepatic side effects associated with its inhibition.
Leptin is released by adipose tissue into the circulation in proportion to the amount of stored lipid, and it plays an important role in the control of metabolism (10, 11). Leptin acts on the central nervous system (12–14) by binding to its receptor (LEPR-B) to decrease food intake and increase energy expenditure (15), as well as to regulate body weight and adipose tissue mass (10, 12, 15, 16). Two types of leptin-responsive neurons, orexigenic neuropeptide Y/agouti gene related peptide (NPY/AGRP) neurons and anorexigenic proopiomelanocortin/cocaine amphetamine regulated transcript (POMC/CART) neurons, modulate ingestive behavior and metabolism (17, 18), and they are regulated in a reciprocal manner by leptin (17, 19, 20). Leptin reduces body weight by inhibiting AGRP neurons and stimulating anorexigenic POMC neurons. In the hypothalamic arcuate nucleus, leptin induces production and release of POMC, a polypeptide precursor that is cleaved to generate α- and γ-melanocyte-stimulating hormone (MSH). Interaction of MSH with melanocortin 4 receptor (MC4R) in the hypothalamus decreases food intake and increases energy expenditure (21). In contrast, the MC4R antagonist AGRP increases food intake and decreases energy expenditure (22).
Mice bearing mutations either in the leptin (Lepob/ob) or leptin receptor (Leprdb/db) genes show high AGRP and low POMC neuronal activities; the combination of these changes leads to increased food intake and reduced energy expenditure, causing profound obesity (23–25). Decreased hypothalamic Pomc and increased Agrp mRNA levels in Lepob/ob mice can be normalized by exogenous leptin administration (26–29). Furthermore, transgenic complementation of the LEPR-B in neurons of Leprdb/db mice (30), viral-mediated expression of LEPRs into the arcuate nucleus of LEPR-deficient rodents (31, 32), or central leptin administration into the cerebral ventricles in Lepob/ob and normal mice (33) reduces body weight and food intake. Similarly, a critical role for melanocortin signaling in the regulation of body weight has been established (34). Targeted deletion of Mc4r in mice results in obesity, hyperphagia, and reduced energy expenditure (35), and a similar phenotype is seen in humans with inactivating MC4R mutations (36).
Leptin also has a wide repertoire of peripheral effects, mediated indirectly through the central nervous system as well as via direct actions on target tissues. Direct leptin action suppresses insulin expression and secretion in pancreatic β-cells (37, 38); stimulates lipolysis and fatty acid oxidation in adipose tissue, skeletal muscle, and pancreas (39–41); decreases triglyceride content and secretion rates in liver (42); and inhibits apolipoprotein AIV transcription in the jejunum (43). The apolipoprotein AIV plays an important role in the transport of triacylglycerol with chylomicrons (44). Chylomicrons are the major apoB-containing lipoproteins synthesized and secreted with the help of MTP by enterocytes to transport dietary fat and fat-soluble vitamins into the circulation. Consequently, we studied the role of LEPR in the regulation of lipid absorption by the intestine. Our studies identified local gut LEPR and MC4R signaling mechanisms in enterocytes that control MTP expression and lipid absorption.
MATERIALS AND METHODS
Animals and diets
Pomc-CRE Leprfl/fl, Agrp-CRE Leprfl/fl, and Leprfl/fl mice have been previously described (45). LEPR mutant Leprdb/db) mice on the FVB strain (46) and melanocortin 4 receptor (MC4R) knockout (Mc4r−/−) mice on a mixed 129 × B6 background (35) were maintained by the New York Obesity Research Center (Molecular Genetics/Molecular Biology Core, SC). Mice (12–14 weeks old) were fed PicoLab Rodent Diet 20 (an irradiated mouse breeder chow with 55% carbohydrate, 20% protein, and 9% fat; LabDiet; PMI Nutrition International, St. Louis, MO) and water ad libitum unless otherwise stated. Mttpfl/fl mice (47) were crossed with villin-CRE to generate villin-CRE-mttpfl/+ mice. They were maintained on rodent chow diet. Food was removed the night before the experiments. All the mice used in the study were 10–12 weeks old. Blood was collected from tail veins. All procedures were reviewed and approved by the Animal Care and Use Committees of Albert Einstein College of Medicine and SUNY Downstate Medical Center, conforming to accepted standards of humane animal care.
Hepatic and celiac vagotomies
Hepatic and celiac vagotomies were performed as previously described (48). Briefly, animals were anesthetized and laparotomy was performed. The stomach was exposed and hepatic and celiac branches of the abdominal vagus were identified. Sham vagotomy mice were then sutured and treated with antibodies. The hepatic and celiac nerve bundles of mice in the vagotomy groups were isolated on a fine wire loop and rapidly heated until the loop cut through the nerve. Mice were then closed up and treated with antibiotics. All the mice were euthanized two weeks after the vagotomies.
Plasma lipid measurements
Total plasma cholesterol and triglyceride levels were measured using kits (Thermo Fisher Scientific). HDL lipid levels were measured after precipitating apoB lipoproteins (49). Lipids in apoB lipoproteins were determined by subtracting HDL lipids from total lipids. Plasma was pooled from all the animals in each group, and lipoproteins were separated by gel filtration (flow rate of 0.2 ml/min) using a Superose 6-column, and 200-µL fractions were collected.
Short-term lipid absorption and hepatic lipoprotein production studies
Villin-CRE Mttpfl/+, Mttpfl/fl, Pomc-CRE Leprfl/fl, and Leprfl/fl mice (3 per group) were fasted overnight and injected intraperitoneally with poloxamer 407. After 1 h, mice were gavaged with 1 µCi of [3H]triolein in 15 µL of olive oil (49). Two h after the gavage, plasma was collected and used to measure radioactivity and plasma lipids. In a separate experiment to study hepatic lipoprotein production, overnight fasted Pomc-CRE Leprfl/fl, and Leprfl/fl mice were injected intraperitoneally with poloxamer 407 and plasma was collected after 3 h.
Determination of MTP activity
Samples from the liver (0.1 g) and proximal small intestine (∼1–2 cm) were homogenized in low-salt buffer, centrifuged, and supernatants were used in triplicate for protein determination and the MTP assay (50) using a kit (Chylos, Inc).
Western blot analyses
Tissues were homogenized in PBS containing 1% Triton-X 100. Proteins (50 μg) were resolved on 4%–20% gradient gels (Biorad). A mouse IgG2a monoclonal anti-MTP antibody (BD), and a mouse anti-GAPDH (Santa Cruz) were used as primary antibodies to detect endogenous proteins. Alexa Fluor 633 (Invitrogen) anti-mouse or anti-rabbit secondary antibodies were used to visualize and quantify the blots using STORM 860 (Amersham).
Immunohistochemistry
For imaging of POMC and LEPR in the intestine, we performed immunohistochemistry on formaldehyde-fixed tissues. LEPR was imaged using a Lepr-CRE Rosa26-floxed eGFP mouse line with GFP as the proxy for LEPR expression (51). Mice were initially perfused with saline followed by 10% formaldehyde. Tissues were embedded in Tissue-TEK OCT compound for cryostat sectioning. Sections (10 µm) were incubated with two primary antibodies (rabbit anti-ACTH [Phoenix Pharmaceuticals] and chicken anti-GFP [Aves Labs]) overnight. After washing, sections were incubated for two h with two secondary antibodies (Cy3 labeled goat anti-rabbit IgG and FITC labeled goat anti-chicken IgY; both from Invitrogen) and mounted in Vectashield (Vector Laboratories), a mounting medium containing DAPI. Antibody dilutions were empirically determined for optimal staining. Images were observed on a Zeiss Axioplan 2 fluorescence microscope.
Semiquantitative and quantitative RT-PCR
Total RNA from tissues and cells were isolated using TriZol (Invitrogen). The purity was assessed by the A260/A280 ratio and RNAs with ratios more than 1.7 were used for cDNA synthesis. The first strand cDNA was synthesized using Omniscript RT (Qiagen) kit, and then used for semiquantitative and quantitative RT-PCR. For semiquantitative RT-PCR, 50 ng of cDNA was used per 25 µl reaction volume. Transcripts were amplified using the primers designed by using PrimerExpress 3.0 (Applied Biosystems). PCR was performed at 93°C for 3 min and then 35 cycles of 93°C for 30 s, 57°C for 30 s, and 72°C for 45 s. Final PCR amplification products were subjected to agarose gel electrophoresis and ethidium bromide staining to confirm product size. Quantitative RT-PCR was performed using SYBR Green to quantify changes in mRNA. The data were analyzed using the ΔΔCT method and presented as arbitrary units.
Statistics
Data are presented as mean ± SD. Statistical significance (P < 0.05) was determined using the Student's t-test (GraphPad Prism).
RESULTS
Mutations in leptin receptor decrease intestinal MTP and increase tissue lipids
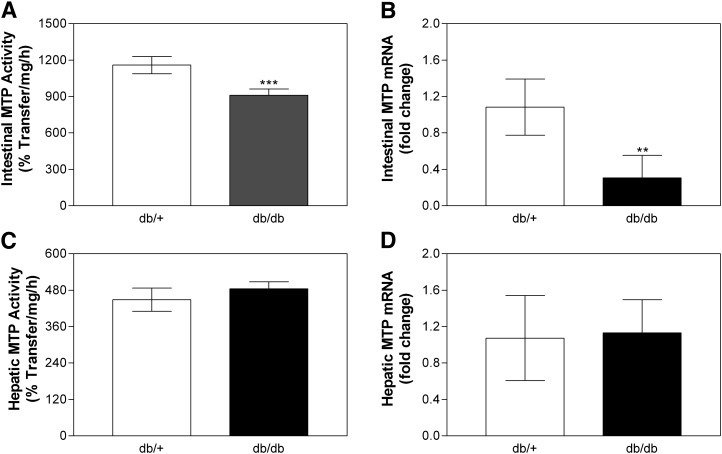
To evaluate the role of LEPR in lipid absorption, we first measured steady-state plasma lipids in homozygous LEPR mutant (Leprdb/db) and heterozygous (Leprdb/+) mice (Table 1). Total plasma cholesterol and triglyceride were elevated significantly in db/db mice relative to heterozygotes. Increased plasma cholesterol and triglycerides in db/db mice were due to 33% and 80% increases in HDL and non-HDL apoB lipoproteins (Table 1), respectively. These augmentations in plasma lipoproteins are consistent with other studies and might be related to lower lipoprotein lipase activity in these mice (52). Besides increases in plasma lipids, we also observed that db/db mice accumulated significant amounts of triglycerides in the intestine and liver, without any significant change in tissue cholesterol (Table 2). Because mutations in Lepr increased intestinal and hepatic triglycerides, we wondered whether this increase was related to defects in apoB-lipoprotein assembly and secretion. MTP is an essential chaperone for the biosynthesis of these lipoproteins and is known to be modulated by various stimuli, such as dietary fat and carbohydrate (53), free fatty acids (54), and insulin (55). db/db mice had decreased intestinal MTP activity and mRNA (Fig. 1A, B), while these were unchanged in the liver compared with control Leprdb/+ mice (Fig. 1C, D). Therefore, the loss of LEPR signaling in Leprdb/db mice increased plasma apoB lipoproteins and tissue triglyceride while reducing intestinal MTP expression.
TABLE 1.
Effect of leptin receptor or MC4R receptor deficiency on plasma lipid levels
| Cholesterol (mg/dl) |
Triglycerides (mg/dl) |
||||||
|---|---|---|---|---|---|---|---|
| n | Total | HDL | non-HDL | Total | HDL | non-HDL | |
| Leprdb/+ | 5 | 237.5 ± 8.2 | 189.7 ± 14.9 | 47.8 ± 15.7 | 195.1 ± 23.3 | 120.7 ± 7.7 | 67.7 ± 18.4 |
| Leprdb/db | 5 | 302.4 ± 26.4 | 252.5 ± 28.0 | 49.9 ± 21.4 | 242.1 ± 33.3 | 127.5 ± 14.6 | 121.5 ± 40.3 |
| (+27.3)b | (+33.1)b | (+4.4) | (+24.1)a | (+5.6) | (+79.5)a | ||
| WT | 7 | 176.0 ± 26.8 | 130.2 ± 14.9 | 45.8 ± 18.3 | 307.6 ± 24.2 | 128.5 ± 26.5 | 179.1 ± 38.0 |
| Pomc-Cre | 7 | 248.3 ± 26.7 | 185.1 ± 31.9 | 63.1 ± 24.4 | 411.2 ± 40.3 | 159.7 ± 21.2 | 251.4 ± 49.4 |
| (+41.1)c | (+42.2)b | (+37.8) | (+33.7)c | (+24.3) | (+40.4)b | ||
| Agrp-Cre | 7 | 283.3 ± 37.5 | 204.9 ± 49.3 | 78.4 ± 14.4 | 466.2 ± 62.7 | 151.5 ± 34.6 | 314.7 ± 49.5 |
| (+61.0)c | (+57.4)b | (+71.2)b | (+51.6)c | (+17.9) | (+75.7)c | ||
| Mc4r+/+ | 6 | 161.4 ± 13.7 | 114.9 ± 7.8 | 46.5 ± 7.8 | 114.6 ± 10.7 | 69.0 ± 7.7 | 45.7 ± 12.8 |
| Mc4r−/− | 6 | 417.2 ± 51.0 | 307.8 ± 26.2 | 109.4 ± 29.3 | 148.6 ± 30.7 | 81.2 ± 8.2 | 67.3 ± 23.3 |
| (+158.5)c | (+167.9)c | (+135.3)c | (+29.7)a | (+17.7) | (+47.3) | ||
Plasma from Leprdb/+, Leprdb/db, Pomc-CRE Leprfl/fl (Pomc-Cre), Agrp-CRE Leprfl/fl (Agrp-Cre), Leprfl/fl (WT), MC4R wild-type (Mc4r+/+), MC4R knockout (Mc4r−/−) mice were used to measure total, HDL, and non-HDL cholesterol as well as triglyceride. Numbers in parentheses refer to percentage change from controls. Values are mean ± SD.
Abbreviations: AGRP, agouti gene related peptide; LEPR-B, leptin receptor B; MC4R, melanocortin 4 receptor; POMC, proopiomelanocortin; WT, wild-type.
P < 0.05 compared with control.
P < 0.01 compared with control.
P < 0.001 compared with control.
TABLE 2.
Effect of leptin receptor or MC4R receptor deficiency on tissue lipids
| Intestine |
Liver |
||||
|---|---|---|---|---|---|
| n | Cholesterol (µg/mg protein) | Triglycerides (µg/mg protein) | Cholesterol (µg/mg protein) | Triglycerides (µg/mg protein) | |
| Leprdb/+ | 5 | 40.9 ± 3.4 | 109.4 ± 29.7 | 18.5 ± 2.4 | 198.0 ± 44.1 |
| Leprdb/db | 5 | 39.5 ± 6.4 | 168.2 ± 36.2 | 21.4 ± 2.0 | 664.2 ± 185.9 |
| (-3.4) | (+53.8)a | (+15.7) | (+235.5)c | ||
| WT | 7 | 32.6 ± 5.3 | 83.4 ± 24.1 | 12.4 ± 1.7 | 111.5 ± 46.7 |
| Pomc-Cre | 7 | 30.6 ± 4.9 | 135.9 ± 17.3 | 13.7 ± 2.3 | 157.2 ± 30.0 |
| (-6.1) | (+62.9)c | (+10.5) | (+41.0)a | ||
| Agrp-Cre | 7 | 32.7 ± 8.0 | 196.3 ± 21.2 | 11.6 ± 1.3 | 162.4 ± 33.8 |
| (+0.3) | (+135.4)c | (-6.5) | (+45.7)a | ||
| Mc4r+/+ | 6 | 42.0 ± 2.2 | 70.3 ± 16.7 | 817.0 ± 171.6 | 70.3 ± 16.7 |
| Mc4r−/− | 6 | 35.7 ± 3.2 | 101.5 ± 15.1 | 1248.7 ± 223.2 | 101.5 ± 15.1 |
| (-15.0)b | (+44.4)b | (+52.8)b | (+44.4)b | ||
Intestinal and hepatic tissues from Leprdb/+, Leprdb/db, Pomc-CRE Leprfl/fl (Pomc-Cre), Agrp-CREfl/fl (Agrp-Cre), Leprfl/fl (WT), MC4R wild-type (Mc4r+/+), MC4R knockout (Mc4r−/−) mice were used to measure cholesterol and triglyceride mass. Values are mean ± SD. Abbreviations: AGRP, agouti gene related peptide; LEPR-B, leptin receptor B; MC4R, melanocortin 4 receptor; POMC, proopiomelanocortin; WT, wild-type.
P < 0.05 compared with control.
P < 0.01 compared with control.
P < 0.001 compared with control.
Fig. 1.
Effect of leptin receptor mutations on MTP activity and mRNA levels. Intestinal and liver samples from Leprdb/+ and db/db female mice (n = 5 per group) were used for MTP activity assays (Panels A and C). RNA was used to measure in triplicate MTP (Panels B and D) and ARPp0 mRNA. The ratio between MTP and ARPp0 in db/+ was used to normalize mRNA levels in all samples. Values are mean ± SD. **P < 0.01, ***P < 0.001 compared with db/+. LEPR, leptin receptor; MTP, microsomal triglyceride transfer protein.
Ablation of leptin receptors in either POMC- or AGRP-expressing cells reduces intestinal MTP and increases tissue lipids
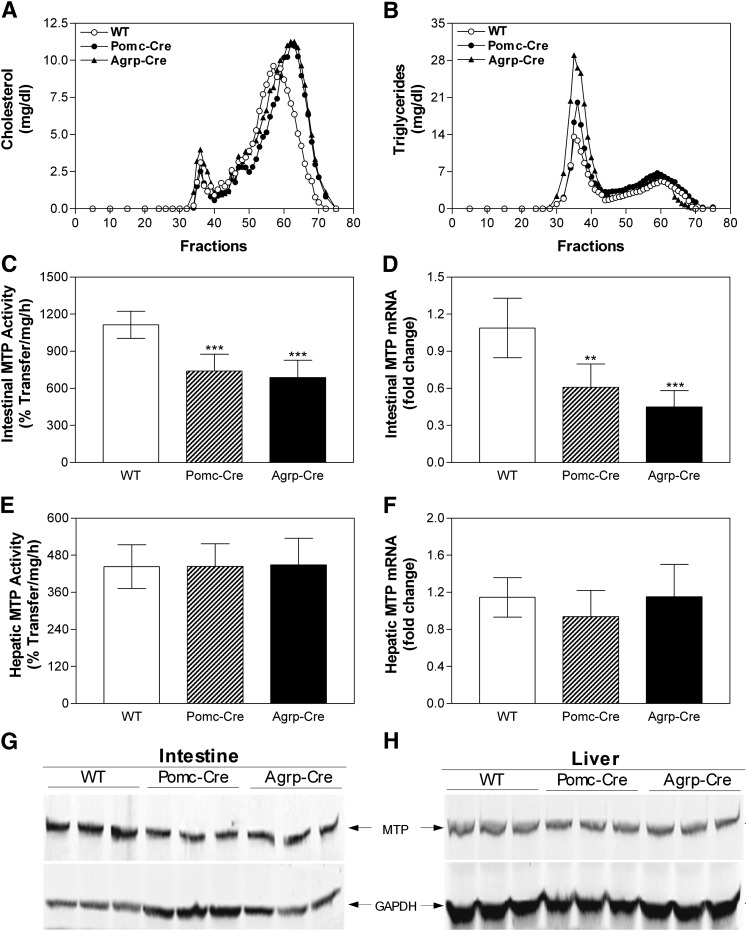
Leptin exerts its central effects on energy homeostasis in part by binding to its receptors expressed in POMC and AGRP neurons of the hypothalamus (56). Therefore, we hypothesized that decreases in intestinal MTP in db/db mice might be due to a defect in LEPR signaling in these neurons. To examine this, we used Pomc-CRE Leprfl/fl (Pomc-Cre) and Agrp-CRE Leprfl/fl (Agrp-Cre) mice with specific deficiency of LEPRs in POMC and AGRP cells (45), respectively. Ablation of LEPRs in POMC or AGRP cells recapitulated several aspects of the db/db phenotype, including significantly increased plasma cholesterol and triglyceride compared with control Leprfl/fl (WT) mice (Table 1). Augmentations in plasma cholesterol of these mice were due to increases in HDL (Table 1). On the other hand, elevations in plasma triglyceride levels were mainly due to increases in non-HDL apoB lipoproteins (Table 1). Gel filtration also revealed that cholesterol in HDL lipoproteins (Fig. 2A) and triglycerides in non-HDL apoB lipoproteins (Fig. 2B) were increased in Pomc-Cre Leprfl/fl and Agrp-Cre Leprfl/fl mice. These cell type-specific LEPR knockout mice also accumulated more triglycerides in the intestine and liver compared with wild-type controls, but there were no significant differences in intestinal and hepatic cholesterol between these groups (Table 2). Determination of MTP activity (Fig. 2C), mRNA (Fig. 2D), and protein (Fig. 2G) in the intestines of Pomc-Cre Leprfl/fl and Agrp-Cre Leprfl/fl mice revealed significant decreases compared with control mice. In contrast, hepatic MTP activity (Fig. 2E, F), mRNA (Fig. 2F), and protein (Fig. 2H) did not differ as a function of genotype. Thus, deletion of LEPR in POMC and AGRP cells increases plasma lipoproteins, elevates intestinal and hepatic triglyceride, and reduces intestinal MTP.
Fig. 2.
Plasma lipids are elevated but intestinal MTP activity, mRNA, and protein levels are decreased in cell-specific leptin receptor knockout mice. Plasma from Pomc-CRE Leprfl/fl (Pomc-Cre), Agrp-CRE Leprfl/fl (Agrp-Cre) and Leprfl/fl (WT) female mice on breeder chow diet (n = 5 per group) were separated by gel filtration to measure lipids in different lipoproteins (Panels A and B). Representative data from one mouse in each group are shown. Intestinal and hepatic samples were used to measure MTP activity (Panels C and E, respectively) and to isolate total RNA for the measurement in triplicate of MTP (Panels D and F, respectively) and ARPp0 mRNA. The ratio between gene of interest and ARPp0 in WT mice was used to normalize mRNA levels in all samples. Intestinal and hepatic samples were also used to measure MTP protein by western blotting (Panels G and H, respectively). Values are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT. AGRP, agouti gene related peptide; LEPR, leptin receptor; MTP, microsomal triglyceride transfer protein; POMC, proopiomelanocortin; WT, wild-type.
Leptin receptor ablation in POMC neurons results in reduced fat absorption
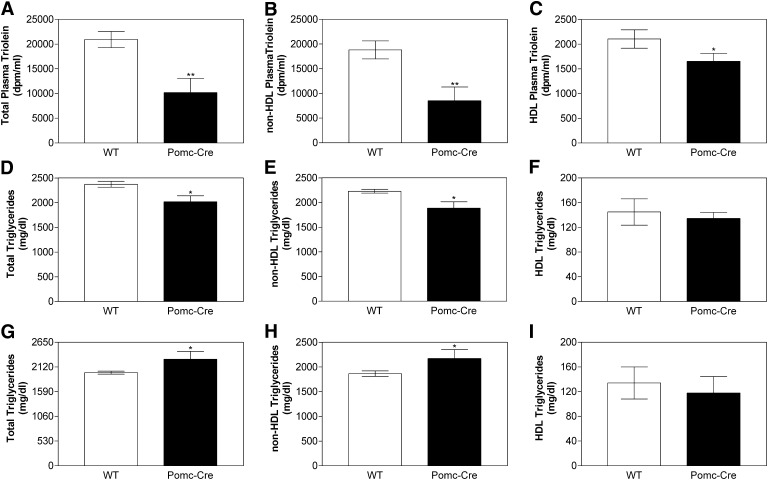
Experiments were then performed to determine the consequences of reduced intestinal MTP expression on lipoprotein production and tissue triglyceride. Pomc-Cre Leprfl/fl mice were fasted overnight and injected with poloxamer 407, a lipoprotein lipase inhibitor that blocks lipoprotein catabolism leading to accumulation of lipids in the plasma (57), and gavaged with radiolabeled triolein along with olive oil. Intestinal absorption of radiolabeled triolein was reduced in these mice, mainly due to a significant reduction (∼55%) in triglycerides present in apoB lipoproteins (Fig. 3A–C). Absorption of total lipids was also measured by quantifying plasma triglyceride and cholesterol mass. Total triglycerides were significantly reduced (∼15%) in plasma and apoB lipoproteins (Fig. 3D, E) of Pomc-Cre Leprfl/fl mice with no significant difference in HDL triglycerides (Fig. 3F). Lesser percent reduction in total mass compared with those seen in radiolabeled triglycerides might be due to higher amounts of plasma triglycerides in these mice and secretion of apoB lipoproteins from the liver. No significant differences were observed in plasma cholesterol (data not shown). These studies indicate that reduced expression of intestinal MTP in Pomc-Cre Leprfl/fl mice is associated with reduced absorption of dietary triglycerides and reduced intestinal lipoprotein production.
Fig. 3.
Intestines from Pomc-Cre Leprfl/fl mice secrete fewer lipids with apoB-containing lipoproteins. Pomc-Cre and WT mice (n = 3) fasted overnight were injected intraperitoneally with poloxamer 407 (45 mg/mouse). After 1 h, mice were fed 1 µCi [3H]triolein in 15 µL of olive oil. Plasma was collected after 2 h and used to measure radioactivity (Panels A–C) as well as triglyceride (Panels D–F) mass in total plasma, non-HDL, and HDL lipoproteins. In another experiment, overnight fasted Pomc-Cre and WT mice were injected with poloxamer 407 and plasma was collected after 3 h to determine hepatic lipoprotein production. Triglyceride was measured in total plasma (Panel G), non-HDL (Panel H), and HDL (Panel I) lipoproteins. Each measurement was done in triplicate with three mice per group. Values are mean ± SD, *P < 0.05, **P < 0.01 compared with WT. LEPR, leptin receptor; MTP, microsomal triglyceride transfer protein; POMC, proopiomelanocortin; WT, wild-type.
We also studied lipoprotein production by the liver in wild-type and Pomc-Cre Leprfl/fl mice after inhibiting lipoprotein lipase by poloxamer 407. There was a significant increase (14.5%) in total plasma triglyceride (Fig. 3G) due to an increase in apoB lipoproteins (Fig. 3H) but not HDL lipoproteins (Fig. 3I), indicating that the Pomc-Cre Leprfl/fl liver assembles and secretes higher amounts of lipoproteins than wild-type liver. Since MTP levels are not changed in Pomc-Cre Leprfl/fl liver, we suggest that these increases in lipoprotein production might be secondary to increased lipid accumulation in the liver. In short, these studies demonstrate significantly lower intestinal and higher hepatic lipoprotein production in LEPR-deficient mice.
Partial intestinal deficiency reduces triglyceride absorption
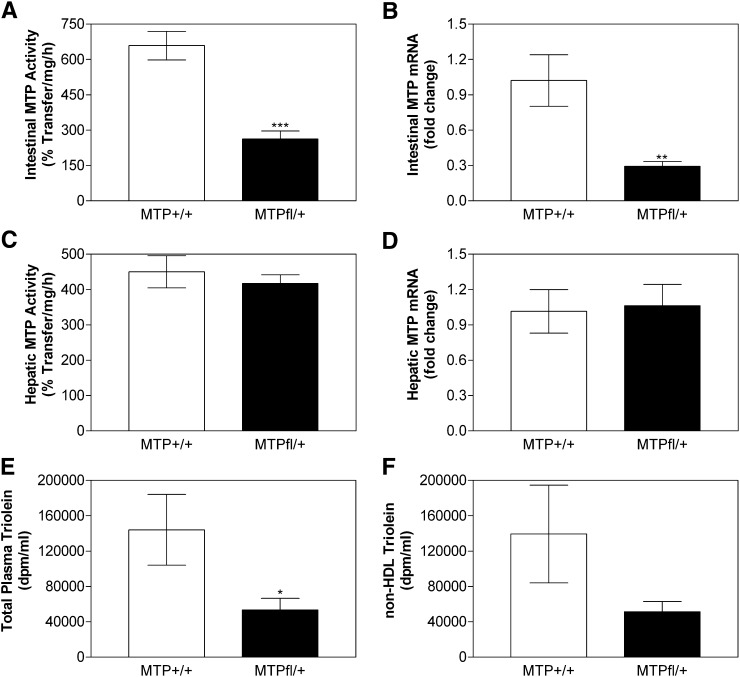
To test the hypothesis that reduced MTP leads to decreased intestinal lipid absorption and lipoprotein production, we obtained partial intestine-specific Mttp gene deletion by crossing Mttpfl/fl mice with villin-Cre mice. Analysis of MTP activity and mRNA in the intestine of villin-CRE Mttpfl/+ showed a decrease of 60% and 71%, respectively, compared with Mttpfl/fl (Fig. 4A, B), indicating partial gene ablation. There was no change in the MTP activity and mRNA levels in the livers of these mice (Fig. 4C, D). These studies indicate partial intestine-specific Mttp gene ablation. Next, we performed acute [3H]triolein absorption experiment in villin-CRE mttpfl/+ and Mttpfl/fl mice. Intestinal absorption of radiolabel triolein was decreased by 63% in villin-CRE mttpfl/+ compared with Mttpfl/fl mice (Fig. 4E); this decrease was mainly due to decrease in non-HDL lipoprotein radiolabel triolein counts (Fig. 4F). Thus, partial intestine-specific MTP deficiency leads to substantial decrease in acute triglyceride absorption.
Fig. 4.
Partial intestinal deficiency of MTP decreases lipid absorption. Intestinal (Panels A and B) and hepatic (Panels C and D) MTP activity (Panels A and C) and mRNA (Panels B and D) were determined in overnight fasted Mttpfl/fl and villin-CRE mttpfl/+ mice (n = 3). In another independent experiment, Mttpfl/fl and Villin-CRE mttpfl/+ mice (n = 3) fasted overnight were injected intraperitoneally with poloxamer 407 (45 mg/mouse). After 1 h, mice were fed 1 µCi [3H]triolein in 15 µL of olive oil. Plasma was collected after 2 h and used to measure radioactivity (Panels E and F) in total plasma and non-HDL lipoproteins. Each measurement was done in triplicate with three mice per group. Values are mean ± SD, *P < 0.05, **P < 0.01 compared with Villin-CRE mttp+/+. MTP, microsomal triglyceride transfer protein.
MC4R deletion increases plasma lipids and decreases intestinal MTP
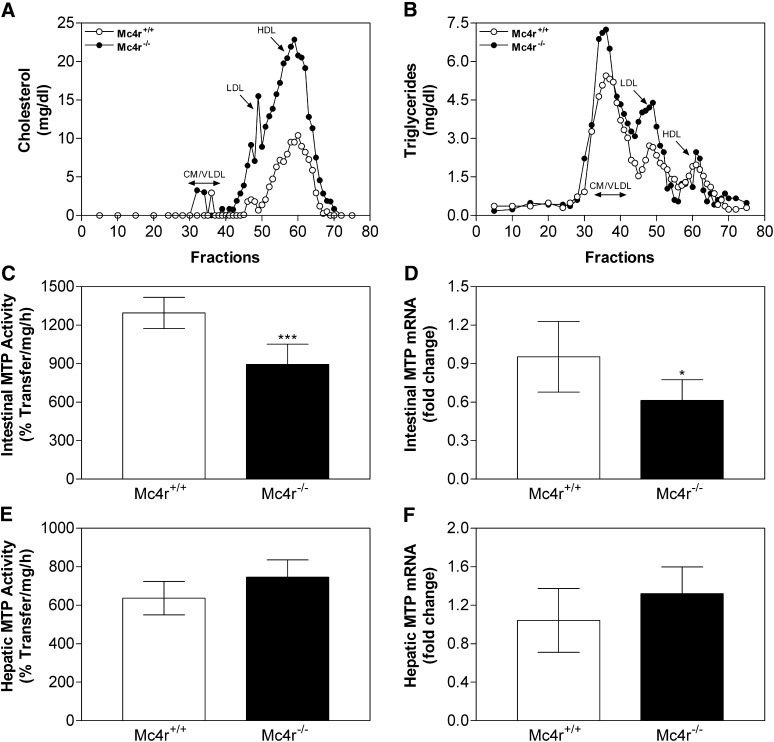
Since many of leptin's actions are mediated by stimulation of the melanocortin system (26, 58–60), we wondered whether MC4R knockout (Mc4r−/−) mice would have intestinal and hepatic lipid profiles similar to LEPR knockout mice. Mc4r−/− mice showed a significant increase in total plasma cholesterol and triglyceride levels (Table 1). The increase in cholesterol was due to increases in both HDL and non-HDL lipoproteins. Fast-protein liquid chromatography (FPLC) analysis showed a broad distribution of cholesterol across different lipoproteins (Fig. 5A).Triglycerides were high in apoB lipoproteins but not in HDL (Fig. 5B). In Mc4r−/− mice intestinal and hepatic triglycerides were increased, intestinal cholesterol was reduced, and hepatic cholesterol was elevated (Table 2). Further analyses revealed decreased MTP activity (Fig. 5C) and MTP mRNA levels in the intestine (Fig. 5D) but no significant change in the liver (Fig. 5E, F). Hence, Mc4r−/− mice showed decreased intestinal MTP expression and increased intestinal and plasma triglyceride, similar to LEPR-deficient mice. These studies indicate that reductions in leptin and melanocortin signaling diminish intestinal MTP expression.
Fig. 5.
Deletion of MC4R decreases intestinal MTP activity and mRNA levels. Plasma samples from wild-type (Mc4r+/+) and MC4R knockout (Mc4r−/−) mice were subjected to gel filtration and fractions were analyzed for cholesterol (Panel A) and triglycerides (Panel B). Intestinal and liver samples from these animals (n = 6 per group) were used for MTP activity assays (Panels C and E). RNA was used to measure in triplicate MTP (Panels D and F) and ARPp0 mRNA. The ratio between MTP and ARPp0 in Mc4r+/+ mice was used to normalize mRNA levels. Values are mean ± SD. *P < 0.05, ***P < 0.001 compared with wild-type mice. MC4R, melanocortin 4 receptor; MTP, microsomal triglyceride transfer protein.
The central nervous system is not required to regulate intestinal MTP
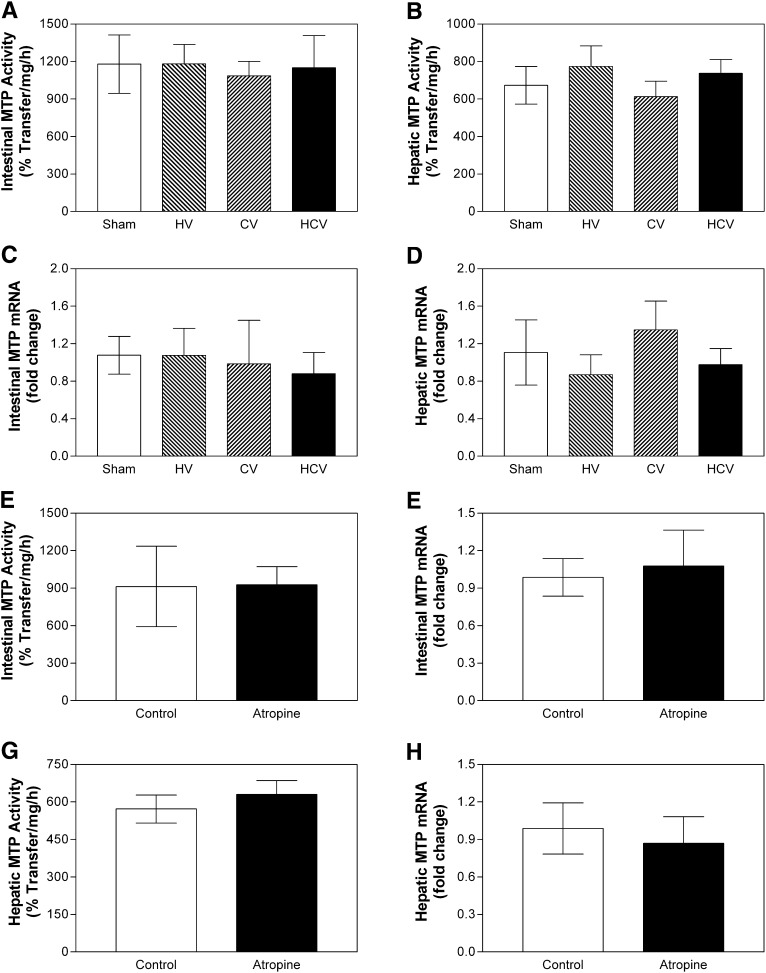
As central leptin signaling has been implicated in the control of multiple determinants of energy balance, including the control of adipose tissue, hepatic glucose production, and food intake, we next evaluated the role of neural communication between brain and gut in the regulation of intestinal MTP levels and activity. Mice were subjected to sham (Sham) surgical operations, hepatic vagotomy (HV), celiac vagotomy (CV), or combined hepatic and celiac vagotomy (HCV). Celiac branch vagotomy alone interrupts a large portion of the vagal efferent innervation of the small intestine, while hepatic vagotomy eliminates vagal hepatic efferent innervation as well as a small portion of the intestinal nerve supply. Therefore, the combined hepatic and celiac vagotomy condition produces a larger degree of denervation of the intestine than either celiac or hepatic vagotomy alone. Both MTP activity as well as mRNA levels remained unchanged in the intestine and liver of these mice compared with sham (Fig. 6A–D). These data indicate that vagal innervation is not required for the regulation of intestinal MTP. To further validate these observations, we used chemical inhibition to interfere with neuronal communication. Follow-up studies using peripheral atropine to block all vagal cholinergic outflow to the entire intestine also failed to affect intestinal and hepatic MTP activity (Fig. 6E, G) and mRNA (Fig. 6F, H). Taken together, these data suggest that our observations of reduced intestinal MTP in LEPR signaling-deficient mice are not due to altered leptinergic brain-gut signaling but may instead reflect the local actions of leptin at the level of enterocytes.
Fig. 6.
Surgical transection and chemical inhibition of extrinsic vagal gut neuronal communication has no effect on intestinal MTP. Panels A–D: Hepatic and celiac vagotomies in male C57BL6/J mice were performed as previously described (48). Mice were allowed to recover for 2 weeks and fasted overnight before euthanizing. Intestinal and hepatic tissues from hepatic vagotomy (HV, n = 13), celiac vagotomy (CV, n = 9), hepatic and celiac double vagotomy (HCV, n = 7), and sham (Sham, n = 21) operated mice were used for MTP activity assays (Panels A and C). RNA was used to measure in triplicate MTP (Panels B and D), and ARPp0 mRNA. The ratio between gene of interest and ARPp0 in sham mice was used to normalize mRNA levels in all samples. Panels E–H: Male C57BL6/J mice (5 per group) were injected intraperitoneally with atropine methyl nitrate (10 mg/kg). After 3 h of fasting, intestinal and liver samples were used to measure MTP activity (Panels E and G, respectively) and mRNA levels (Panels F and H, respectively). Values are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 compared with Sham. HCV, hepatic and celiac vagotomy; HV, hepatic vagotomy; MTP, microsomal triglyceride transfer protein.
Expression of LEPR, POMC, AGRP, and MC4R in enterocytes
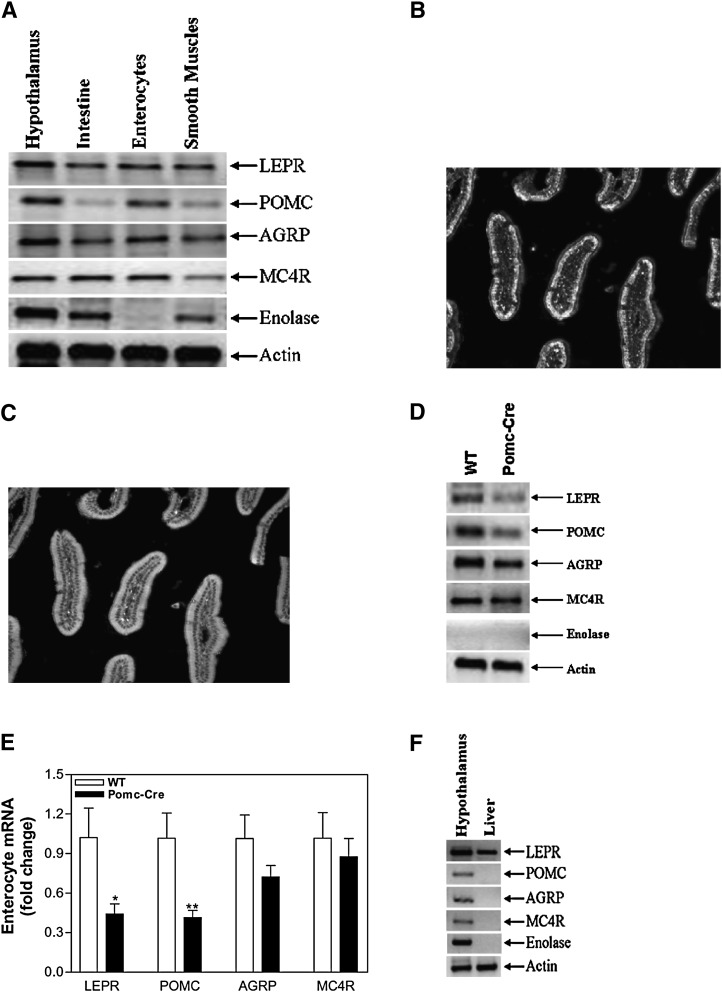
Although complete or tissue-specific deletions of LEPRs modulate intestinal lipid and MTP levels, the above data suggested that extrinsic innervation of the intestine is not required for this modulation. Hence, we hypothesized that leptin might control intestinal lipid levels and MTP expression via local signaling mechanisms intrinsic to the gut. Consequently, we examined the expression of different genes involved in leptin signaling (Fig. 7).Semiquantitative PCR analysis showed that LEPR, POMC, AGRP, and MC4R were expressed in the intestine as well as in the hypothalamus (Fig. 7A). In the intestine, LEPRs might be expressed at nerve endings or in enterocytes. To identify the site of expression of these molecules, we isolated enterocytes from the intestine and evaluated the remaining tissue as a source of smooth muscle harboring nerve endings. We used neuron-specific enolase (61) to document that isolated enterocytes were not contaminated with nerve cells (Fig. 7A). Neuronal enolase was not present in enterocyte preparations, indicating successful separation of enterocytes from nerve cells. Semiquantitative analysis suggested that LEPR, POMC, AGRP, and MC4R are expressed in the enterocytes (Fig. 7A). To further study the expression of these genes, we used Lepr-CRE Rosa26-floxed eGFP mouse line with GFP as the proxy for LEPR expression (51). Expression of POMC and LEPR was studied by immunohistochemistry using specific antibodies. Immunopositive labeling for POMC indicate that enterocytes express POMC (Fig. 7B). Furthermore, expression of GFP in the enterocytes also suggests that these cells express LEPR-B (Fig. 7C).
Fig. 7.
Enterocytes express LEPR, POMC, AGRP, and MC4R. Semiquantitative RT-PCR was used to determine the expression of LEPR-B, POMC, AGRP, and MC4R in the hypothalamus, intestine, enterocytes, and smooth muscle cells (Panel A). Immunohistochemistry to visualize expression of POMC (Panel B) and LEPR (Panel C) in Lepr-CRE Rosa26-floxed GFP mice was performed as described in “Materials and Methods.” Expression of LEPR, POMC, AGRP, and MC4R was also determined by semiquantitative RT-PCR in the enterocytes isolated from WT and Pomc-Cre Leprfl/fl mice (Panel D). Quantitative RT-PCR was used to measure changes in the expression of these genes in enterocytes (Panel E). Expression of LEPR, POMC, AGRP, and MC4R was also determined by semiquantitative RT-PCR in the liver isolated from WT mouse (Panel F). AGRP, agouti gene related peptide; LEPR-B, leptin receptor B; MC4R, melanocortin 4 receptor; POMC, proopiomelanocortin; WT, wild-type.
Next, we looked at the expression of LEPRs and other signaling molecules in the enterocytes of Pomc-CRE Leprfl/fl mice. Semiquantitative analyses showed reduced expression of LEPR and POMC with no effect on AGRP and MC4R in these mice (Fig. 7D). Quantitative analyses (Fig. 7E) showed 57% and 59% reductions in LEPR and POMC, respectively. These studies indicate that enterocytes express several components of the LEPR signaling and that expression of Cre recombinase under the control of the POMC promoter results in partial ablation of POMC and LEPR-B. To begin to address why hepatic MTP expression was intact in LEPR-deficient animals, we examined expression of leptin signaling pathway genes in the liver. While LEPR-B was expressed in liver, POMC, AGRP, and MC4R were not detectable (Fig. 7F). Therefore, ablation of LEPR had no significant effect on hepatic MTP expression due to the absence or low-level expression of POMC, AGRP, and MC4R.
DISCUSSION
These studies demonstrate that LEPR regulates intestinal MTP in mouse enterocytes independently of extrinsic vagal innervation of the gut. Furthermore, melanocortins and MC4 receptors localized to enterocytes likely participate in this regulation. Such an enterocyte-specific regulatory mechanism could provide an additional peripheral control of fat mobilization independently of that determined by central nervous system leptin signaling.
Regulation of intestinal MTP and lipid absorption by leptin receptor signaling
Leptin is an adipokine secreted when adipose tissue receives dietary lipids to inhibit food intake. We had anticipated that high leptin concentrations would decrease intestinal MTP expression and thereby limit lipid absorption as part of an energy regulatory response to lower energy intake. Hence, we had predicted that LEPR deficiency would increase MTP expression and enhance lipid absorption. Instead, we observed significant decreases in intestinal MTP expression and lipid absorption. It is unclear whether the observed reductions in intestinal MTP and lipoprotein production are causally related to LEPR deficiency or are secondary to enhanced hyperlipidemia. Most likely, these changes represent a long-term adaptation to chronic LEPR deficiency and plasma lipid accumulation. Such an adaptation would help lower exogenous lipid absorption and thereby attenuate hyperlipidemia and obesity. Alternatively, these observations point to novel mechanisms that might exist in the intestine to lower intestinal lipoprotein production during fasting to facilitate endogenous lipid mobilization by the liver. Another possibility is that intestinal MTP is regulated by gastric leptin. It is known that stomach is another major organ, besides adipocytes, that synthesizes and secretes leptin. Some stomach-derived leptin is not proteolytically degraded in the gastric juice (43, 62) and may reach the intestine in an active form (63–65). Therefore, gut leptin, which is rapidly secreted in the lumen after a meal (in contrast to slow secretion of leptin by adipocytes as a satiety signal), may be a key molecule controlling intestinal nutrient absorption. Leptinergic regulation of intestinal MTP might represent a postprandial response of jejunal enterocytes to promote the absorption of dietary fat.
In addition to its well-established role in the hypothalamic control of food intake and energy balance (10, 12, 15, 16, 66), leptin has also been reported to play a role in peripheral tissue biology (38, 67, 68). Our study shows that MTP expression in the intestine is not affected by vagal efferent outflow, as both selective and combined hepatic and intestinal vagotomies had no effect on intestinal MTP mRNA or activity levels (Fig. 6). Furthermore, atropine blockade of all cholinergic vagal efferent transmission (69) failed to affect intestinal MTP expression. These data support the interpretation that parasympathetic neuronal communication between the brain and the intestine is not important in the regulation of intestinal MTP by leptin. Instead, our data point to a direct role of leptin receptors in enterocytes.
It has previously been shown that enterocytes express LEPRs and exhibit increased signal transducer and activator of transcription 3 (STAT3) phosphorylation in response to leptin (43). We extend these studies and show for the first time that enterocytes also express MC4R, POMC, and AGRP. Furthermore, we show that expression of the Cre recombinase under POMC promoter results in partial ablation of floxed LEPR. These studies highlight the presence of a local, autocrine, regulatory loop where LEPRs regulate the expression of POMC and AGRP in enterocytes. The POMC products bind to MC4R and regulate MTP. It is tempting to speculate that LEPR-signaling mechanisms might have evolved in the gut and later diversified into two different sets of neurons to regulate various aspects of energy metabolism.
POMC and AGRP are expressed in distinct hypothalamic neuronal populations that exert opposing effects on melanocortin receptor signaling and energy balance. Therefore, similar decreases in intestinal MTP activity in mice subjected to ablation of LEPRs after the expression of Cre-recombinase using either POMC or AGRP promoters were perplexing (Fig. 2). Subsequent analyses of POMC and AGRP, however, revealed that enterocytes express both of these genes. Furthermore, activation of Cre-recombinase by POMC promoter resulted in a partial deletion of LEPR in enterocytes. We interpret these data to suggest that LEPR expression in enterocytes is influenced by both POMC and AGRP. Thus, deficient LEPR signaling in both Pomc-CRE Leprfl/fl and Agrp-CRE Leprfl/fl mice leads to comparable intestinal MTP deficiencies in these strains.
Most of the mouse models we tested in this study are also hyperinsulinemic. Although we did not rule out the effect of hyperinsulinemia on the intestinal MTP activity and mRNA levels, hyperinsulinemic mice have higher intestinal (70) and hepatic (71) MTP expression and plasma triglyceride. Similarly, diabetic men and rabbits have been shown to express significantly higher intestinal MTP mRNA levels compared with nondiabetic subjects (72, 73). Contrary to these results, we observed decreased intestinal expression of MTP, and this decreased activity of MTP may have led to increased accumulation of triglycerides in the intestine.
Differential regulation of intestinal and hepatic MTP by leptin receptor signaling
The data presented in this study highlight differences in the control of intestinal and hepatic MTP expression in distinct mouse models of LEPR deficiency. LEPR deficiency decreases MTP expression in the intestine but has no effect on hepatic MTP expression. Both enterocytes and hepatocytes express LEPR and respond to leptin (43, 74, 75). Therefore, it was surprising that intestinal cells respond to LEPR deficiency and regulate MTP expression and lipid absorption, but hepatocytes do not. This dichotomy could be explained by our observations that enterocytes express POMC, AGRP, and MC4R, while hepatocytes do not express these genes. It has previously been shown that liver does not express MC4R (76); we are unaware of studies describing POMC and AGRP expression in the liver. Therefore, we speculate that differential regulation of hepatic and intestinal MTP by LEPR might be secondary to the expression melanocortins and MC4R in these cells.
Leptin receptor deficiency affects plasma and tissue lipid metabolism
LEPR deficiency is known to cause hyperlipidemia in db/db mice. Here, we report for the first time that ablation of LEPR in either POMC- or AGRP-expressing cells also causes hyperlipidemia. Furthermore, we observed that whole body or POMC/AGRP cell-specific deficiency in LEPR results in significant accumulation of hepatic and intestinal triglycerides. Therefore, LEPR deficiency results in lipid accumulation in the intestine, liver, and plasma. Experiments to delineate molecular mechanisms for the accumulation of triglycerides in the intestine revealed that deficiency in LEPR signaling decreases intestinal MTP expression, reduces assembly and secretion of lipoproteins, and elevates triglyceride accumulation. However, there were no significant reductions in hepatic MTP activity and mRNA; therefore, the hepatic steatosis observed in LEPR-deficient mice might not be due to MTP deficiency. Instead, we speculate that excess delivery of lipoproteins secondary to enhanced accumulation of plasma lipoproteins might contribute to hepatic triglyceride accumulation in LEPR-deficient mice. Another possibility is that these mice have reduced oxidation of triglycerides due to decreased leptin signaling (45).
Similarly, our proposed mechanisms for intestinal triglyceride accumulation do not explain enhanced assimilation of lipoproteins in the plasma; reduced lipid absorption and lipoprotein secretion by the intestine is expected to lower plasma lipoproteins. Three possibilities may account for the hyperlipidemias in these mice. First db/db mice have reduced lipoprotein lipase activity (52), thereby reducing lipoprotein catabolism, while promoting hyperlipidemia. Second, total lipid oxidation is reduced in db/db mice and other cell-specific LEPR deficiencies, causing an accumulation of fat (45). Overall decreases in lipid utilization would promote lipid accumulations in serum and tissue. Third, we observed increases in hepatic lipoprotein production, suggesting that enhanced hepatic lipoprotein production might also contribute to hyperlipidemia. Therefore, different mechanisms might lead to lipid accumulation in plasma, liver, and intestine in LEPR-deficient mice.
CONCLUSION
These studies identify intestine-specific regulatory mechanisms involving LEPR, POMC, and AGRP that control MTP expression and lipid absorption. Understanding signaling pathways that lead to intestine-specific MTP regulation might be potentially useful in achieving tissue-specific MTP inhibition.
Footnotes
Abbreviations:
- AGRP
- agouti gene related peptide
- ApoB
- apolipoprotein B
- CART
- cocaine amphetamine regulated transcript
- CV
- celiac vagotomy
- HCV
- hepatic and celiac vagotomy
- HDL
- high density lipoprotein
- HV
- hepatic vagotomy
- LEPR
- leptin receptor
- MC4R
- melanocortin 4 receptor
- MSH
- melanocyte-stimulating hormone
- MTP
- microsomal triglyceride transfer protein
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
This study was partially supported by National Institutes of Health Grants RO1 DK-46900 (M.M.H.); RO1 DK-57621 and PO1 DK-26687 (S.C.); RO1 DK-47208 (G.J.S.); and RO1 HL-51586 (L.C); and by Skirball Institute for Nutrient Sensing, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 2.White D. A., Bennett A. J., Billett M. A., Salter A. M. 1998. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 80: 219–229. [PubMed] [Google Scholar]
- 3.Hussain M. M. 2000. A proposed model for the assembly of chylomicrons. Atherosclerosis. 148: 1–15. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M. M., Iqbal J., Anwar K., Rava P., Dai K. 2003. Microsomal triglyceride transfer protein: a multifunctional protein. Front. Biosci. 8: s500–s506. [DOI] [PubMed] [Google Scholar]
- 5.Hussain M. M., Rava P., Pan X., Dai K., Dougan S. K., Iqbal J., Lazare F., Khatun I. 2008. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr. Opin. Lipidol. 19: 277–284. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J., Dai K., Seimon T., Jungreis R., Oyadomari M., Kuriakose G., Ron D., Tabas I., Hussain M. M. 2008. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 7: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameen C., Edvardsson U., Ljungberg A., Asp L., Akerblad P., Tuneld A., Olofsson S. O., Linden D., Oscarsson J. 2005. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal D., West K. L., Zern T. L., Shrestha S., Vergara-Jimenez M., Fernandez M. L. 2005. JTT-130, a microsomal triglyceride transfer protein (MTP) inhibitor lowers plasma triglycerides and LDL cholesterol concentrations without increasing hepatic triglycerides in guinea pigs. BMC Cardiovasc. Disord. 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain M. M., Bakillah A. 2008. New approaches to target microsomal triglyceride transfer protein. Curr. Opin. Lipidol. 19: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahima R. S., Flier J. S. 2000. Leptin. Annu. Rev. Physiol. 62: 413–437. [DOI] [PubMed] [Google Scholar]
- 11.Himms-Hagen J. 1999. Physiological roles of the leptin endocrine system: differences between mice and humans. Crit. Rev. Clin. Lab. Sci. 36: 575–655. [DOI] [PubMed] [Google Scholar]
- 12.Spiegelman B. M., Flier J. S. 2001. Obesity and the regulation of energy balance. Cell. 104: 531–543. [DOI] [PubMed] [Google Scholar]
- 13.Cowley M. A., Cone R. D., Enriori P., Louiselle I., Williams S. M., Evans A. E. 2003. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann. N. Y. Acad. Sci. 994: 175–186. [DOI] [PubMed] [Google Scholar]
- 14.Prieur X., Tung Y. C., Griffin J. L., Farooqi I. S., O'Rahilly S., Coll A. P. 2008. Leptin regulates peripheral lipid metabolism primarily through central effects on food intake. Endocrinology. 149: 5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman J. M., Halaas J. L. 1998. Leptin and the regulation of body weight in mammals. Nature. 395: 763–770. [DOI] [PubMed] [Google Scholar]
- 16.Leibel R. L., Chung W. K., Chua S. C., Jr 1997. The molecular genetics of rodent single gene obesities. J. Biol. Chem. 272: 31937–31940. [DOI] [PubMed] [Google Scholar]
- 17.Cowley M. A., Smart J. L., Rubinstein M., Cerdan M. G., Diano S., Horvath T. L., Cone R. D., Low M. J. 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 411: 480–484. [DOI] [PubMed] [Google Scholar]
- 18.Elias C. F., Aschkenasi C., Lee C., Kelly J., Ahima R. S., Bjorbaek C., Flier J. S., Saper C. B., Elmquist J. K. 1999. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 23: 775–786. [DOI] [PubMed] [Google Scholar]
- 19.Balthasar N., Coppari R., McMinn J., Liu S. M., Lee C. E., Tang V., Kenny C. D., McGovern R. A., Chua S. C., Jr., Elmquist J. K., et al. 2004. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 42: 983–991. [DOI] [PubMed] [Google Scholar]
- 20.Korner J., Savontaus E., Chua S. C., Jr., Leibel R. L., Wardlaw S. L. 2001. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J. Neuroendocrinol. 13: 959–966. [DOI] [PubMed] [Google Scholar]
- 21.Ellacott K. L., Cone R. D. 2004. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog. Horm. Res. 59: 395–408. [DOI] [PubMed] [Google Scholar]
- 22.Small C. J., Liu Y. L., Stanley S. A., Connoley I. P., Kennedy A., Stock M. J., Bloom S. R. 2003. Chronic CNS administration of Agouti-related protein (Agrp) reduces energy expenditure. Int. J. Obes. Relat. Metab. Disord. 27: 530–533. [DOI] [PubMed] [Google Scholar]
- 23.Chen H., Charlat O., Tartaglia L. A., Woolf E. A., Weng X., Ellis S. J., Lakey N. D., Culpepper J., Moore K. J., Breitbart R. E., et al. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 84: 491–495. [DOI] [PubMed] [Google Scholar]
- 24.Chua S. C., Jr., Chung W. K., Wu-Peng X. S., Zhang Y., Liu S. M., Tartaglia L., Leibel R. L. 1996. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 271: 994–996. [DOI] [PubMed] [Google Scholar]
- 25.Cohen P., Zhao C., Cai X., Montez J. M., Rohani S. C., Feinstein P., Mombaerts P., Friedman J. M. 2001. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno T. M., Kleopoulos S. P., Bergen H. T., Roberts J. L., Priest C. A., Mobbs C. V. 1998. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 47: 294–297. [DOI] [PubMed] [Google Scholar]
- 27.Thornton J. E., Cheung C. C., Clifton D. K., Steiner R. A. 1997. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 138: 5063–5066. [DOI] [PubMed] [Google Scholar]
- 28.Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J. S. 1996. Role of leptin in the neuroendocrine response to fasting. Nature. 382: 250–252. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz M. W., Baskin D. G., Bukowski T. R., Kuijper J. L., Foster D., Lasser G., Prunkard D. E., Porte D., Jr., Woods S. C., Seeley R. J., et al. 1996. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 45: 531–535. [DOI] [PubMed] [Google Scholar]
- 30.Kowalski T. J., Liu S. M., Leibel R. L., Chua S. C., Jr 2001. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 50: 425–435. [DOI] [PubMed] [Google Scholar]
- 31.Coppari R., Ichinose M., Lee C. E., Pullen A. E., Kenny C. D., McGovern R. A., Tang V., Liu S. M., Ludwig T., Chua S. C., Jr, et al. 2005. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 1: 63–72. [DOI] [PubMed] [Google Scholar]
- 32.Morton G. J., Niswender K. D., Rhodes C. J., Myers M. G., Jr., Blevins J. E., Baskin D. G., Schwartz M. W. 2003. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 144: 2016–2024. [DOI] [PubMed] [Google Scholar]
- 33.Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. 1995. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 269: 546–549. [DOI] [PubMed] [Google Scholar]
- 34.Garfield A. S., Lam D. D., Marston O. J., Przydzial M. J., Heisler L. K. 2009. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol. Metab. 20: 203–215. [DOI] [PubMed] [Google Scholar]
- 35.Huszar D., Lynch C. A., Fairchild-Huntress V., Dunmore J. H., Fang Q., Berkemeier L. R., Gu W., Kesterson R. A., Boston B. A., Cone R. D., et al. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 88: 131–141. [DOI] [PubMed] [Google Scholar]
- 36.Cone R. D. 2000. Haploinsufficiency of the melanocortin-4 receptor: part of a thrifty genotype? J. Clin. Invest. 106: 185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni R. N., Wang Z. L., Wang R. M., Hurley J. D., Smith D. M., Ghatei M. A., Withers D. J., Gardiner J. V., Bailey C. J., Bloom S. R. 1997. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Invest. 100: 2729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emilsson V., Liu Y. L., Cawthorne M. A., Morton N. M., Davenport M. 1997. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 46: 313–316. [DOI] [PubMed] [Google Scholar]
- 39.Siegrist-Kaiser C. A., Pauli V., Juge-Aubry C. E., Boss O., Pernin A., Chin W. W., Cusin I., Rohner-Jeanrenaud F., Burger A. G., Zapf J., et al. 1997. Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest. 100: 2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y. T., Shimabukuro M., Koyama K., Lee Y., Wang M. Y., Trieu F., Newgard C. B., Unger R. H. 1997. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc. Natl. Acad. Sci. USA. 94: 6386–6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 415: 339–343. [DOI] [PubMed] [Google Scholar]
- 42.Huang W., Dedousis N., Bandi A., Lopaschuk G. D., O'Doherty R. M. 2006. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 147: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 43.Morton N. M., Emilsson V., Liu Y. L., Cawthorne M. A. 1998. Leptin action in intestinal cells. J. Biol. Chem. 273: 26194–26201. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher J. W., Weinberg R. B., Shelness G. S. 2004. apoA-IV tagged with the ER retention signal KDEL perturbs the intracellular trafficking and secretion of apoB. J. Lipid Res. 45: 1826–1834. [DOI] [PubMed] [Google Scholar]
- 45.van deWall E., Leshan R., Xu A.W., Balthasar N., Coppari R., Liu S. M., Jo Y. H., MacKenzie R. G., Allison D. B., Dun N. J., et al. 2008. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 149: 1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chua S., Jr., Liu S. M., Li Q., Yang L., Thassanapaff V. T., Fisher P. 2002. Differential beta cell responses to hyperglycaemia and insulin resistance in two novel congenic strains of diabetes (FVB- Lepr (db)) and obese (DBA-Lep (ob)) mice. Diabetologia. 45: 976–990. [DOI] [PubMed] [Google Scholar]
- 47.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274: 6051–6055. [DOI] [PubMed] [Google Scholar]
- 48.Powley T. L., Fox E. A., Berthoud H. R. 1987. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am. J. Physiol. 253: R361–R370. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J., Hussain M. M. 2005. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 50.Athar H., Iqbal J., Jiang X. C., Hussain M. M. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 51.Leinninger G. M., Jo Y. H., Leshan R. L., Louis G. W., Yang H., Barrera J. G., Wilson H., Opland D. M., Faouzi M. A., Gong Y., et al. 2009. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi K., Forte T. M., Taniguchi S., Ishida B. Y., Oka K., Chan L. 2000. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 49: 22–31. [DOI] [PubMed] [Google Scholar]
- 53.Lin M. C., Arbeeny C., Bergquist K., Kienzle B., Gordon D. A., Wetterau J. R. 1994. Cloning and regulation of hamster microsomal triglyceride transfer protein. The regulation is independent from that of other hepatic and intestinal proteins which participate in the transport of fatty acids and triglycerides. J. Biol. Chem. 269: 29138–29145. [PubMed] [Google Scholar]
- 54.Qiu W., Taghibiglou C., Avramoglu R. K., Van Iderstine S. C., Naples M., Ashrafpour H., Mhapsekar S., Sato R., Adeli K. 2005. Oleate-mediated stimulation of microsomal triglyceride transfer protein (MTP) gene promoter: implications for hepatic MTP overexpression in insulin resistance. Biochemistry. 44: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 55.Lin M. C., Gordon D., Wetterau J. R. 1995. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J. Lipid Res. 36: 1073–1081. [PubMed] [Google Scholar]
- 56.Myers M. G., Jr., Munzberg H., Leinninger G. M., Leshan R. L. 2009. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 9: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz M. W., Seeley R. J., Campfield L. A., Burn P., Baskin D. G. 1996. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 98: 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrison C. D., Morton G. J., Niswender K. D., Gelling R. W., Schwartz M. W. 2005. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am. J. Physiol. Endocrinol. Metab. 289: E1051–E1057. [DOI] [PubMed] [Google Scholar]
- 60.Kitamura T., Feng Y., Kitamura Y. I., Chua S. C., Jr., Xu A. W., Barsh G. S., Rossetti L., Accili D. 2006. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 12: 534–540. [DOI] [PubMed] [Google Scholar]
- 61.Marangos P. J., Schmechel D. E. 1987. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu. Rev. Neurosci. 10: 269–295. [DOI] [PubMed] [Google Scholar]
- 62.Sobhani I., Bado A., Vissuzaine C., Buyse M., Kermorgant S., Laigneau J. P., Attoub S., Lehy T., Henin D., Mignon M., et al. 2000. Leptin secretion and leptin receptor in the human stomach. Gut. 47: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lostao M. P., Urdaneta E., Martinez-Anso E., Barber A., Martinez J. A. 1998. Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett. 423: 302–306. [DOI] [PubMed] [Google Scholar]
- 64.Pearson P. Y., O'Connor D. M., Schwartz M. Z. 2001. Novel effect of leptin on small intestine adaptation. J. Surg. Res. 97: 192–195. [DOI] [PubMed] [Google Scholar]
- 65.Ducroc R., Guilmeau S., Akasbi K., Devaud H., Buyse M., Bado A. 2005. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 54: 348–354. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature. 372: 425–432. [DOI] [PubMed] [Google Scholar]
- 67.Kieffer T. J., Heller R. S., Leech C. A., Holz G. G., Habener J. F. 1997. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 46: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cioffi J. A., Shafer A. W., Zupancic T. J., Smith-Gbur J., Mikhail A., Platika D., Snodgrass H. R. 1996. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat. Med. 2: 585–589. [DOI] [PubMed] [Google Scholar]
- 69.Berstein G., Haga K., Haga T., Ichiyama A. 1988. Agonist and antagonist binding of muscarinic acetylcholine receptors purified from porcine brain: interconversion of high- and low-affinity sites by sulfhydryl reagents. J. Neurochem. 50: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 70.Qin B., Qiu W., Avramoglu R. K., Adeli K. 2007. Tumor necrosis factor-alpha induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes. 56: 450–461. [DOI] [PubMed] [Google Scholar]
- 71.Bartels E. D., Lauritsen M., Nielsen L. B. 2002. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes. 51: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 72.Phillips C., Mullan K., Owens D., Tomkin G. H. 2006. Intestinal microsomal triglyceride transfer protein in type 2 diabetic and non-diabetic subjects: the relationship to triglyceride-rich postprandial lipoprotein composition. Atherosclerosis. 187: 57–64. [DOI] [PubMed] [Google Scholar]
- 73.Phillips C., Bennett A., Anderton K., Owens D., Collins P., White D., Tomkin G. H. 2002. Intestinal rather than hepatic microsomal triglyceride transfer protein as a cause of postprandial dyslipidemia in diabetes. Metabolism. 51: 847–852. [DOI] [PubMed] [Google Scholar]
- 74.Brabant G., Muller G., Horn R., Anderwald C., Roden M., Nave H. 2005. Hepatic leptin signaling in obesity. FASEB J. 19: 1048–1050. [DOI] [PubMed] [Google Scholar]
- 75.Cohen P., Yang G., Yu X., Soukas A. A., Wolfish C. S., Friedman J. M., Li C. 2005. Induction of leptin receptor expression in the liver by leptin and food deprivation. J. Biol. Chem. 280: 10034–10039. [DOI] [PubMed] [Google Scholar]
- 76.Mountjoy K. G., Jenny Wu C. S., Dumont L. M., Wild J. M. 2003. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology. 144: 5488–5496. [DOI] [PubMed] [Google Scholar]