PrTX-I, a noncatalytic and myotoxic Lys49-phospholipase A2 from B. pirajai venom, was cocrystallized with the inhibitor rosmarinic acid from C. verbenacea. The crystals diffracted X-rays to 1.8 Å resolution and the structure was solved, indicating a remarkable electronic density for the ligand at the entrance to the hydrophobic channel.
Keywords: phospholipase A2, Bothrops pirajai venom, myotoxins, rosmarinic acid, Cordia verbenacea
Abstract
PrTX-I, a noncatalytic and myotoxic Lys49-phospholipase A2 from Bothrops pirajai venom, was crystallized in the presence of the inhibitor rosmarinic acid (RA). This is the active compound in the methanolic extract of Cordia verbenacea, a plant that is largely used in Brazilian folk medicine. The crystals diffracted X-rays to 1.8 Å resolution and the structure was solved by molecular-replacement techniques, showing electron density that corresponds to RA molecules at the entrance to the hydrophobic channel. The crystals belong to space group P212121, indicating conformational changes in the structure after ligand binding: the crystals of all apo Lys49-phospholipase A2 structures belong to space group P3121, while the crystals of complexed structures belong to space groups P21 or P212121.
1. Introduction
Envenomation by snakes belonging to the Bothrops genus is economically and socially important in Latin America as it is responsible for more than 85% of all ophidian accidents reported in the area (Ferreira et al., 1992 ▶; Ribeiro et al., 1998 ▶; de Oliveira, 2009 ▶). These envenomations are characterized by prominent local tissue damage arising from myonecrosis, haemorrhage and oedema (Rosenberg, 1990 ▶). These drastic local effects are not efficiently neutralized by serum therapy and may cause permanent tissue loss and amputation of the affected limb (Gutierrez & Lomonte, 1995 ▶).
Phospholipases A2 (PLA2s) are the main components of these venoms and in addition to their catalytic role show a broad spectrum of pharmacological activities such as neurotoxicity, myotoxicity and cardiotoxicity. Some of these activities are correlated with the enzymatic activity, while others are completely independent (Kini & Evans, 1989 ▶; Soares & Giglio, 2003 ▶). PLA2s also affect the coagulation cascade, platelet aggregation and the inflammatory response (Kini, 1997 ▶; Andriao-Escarso et al., 2002 ▶).
PLA2 homologues with skeletal muscle-damaging activity (myotoxicity) are widely distributed in venomous snakes, with Lys49-PLA2s being the most studied and best characterized subgroup. The loss of catalytic activity in PLA2 homologues was initially attributed to the natural substitution D49K, as a lysine in this position impairs Ca2+ coordination (Maraganore et al., 1984 ▶; Arni & Ward, 1996 ▶; Ward et al., 1998 ▶), but other peculiarities have subsequently also been demonstrated to be involved in this phenomenon (Ward et al., 2002 ▶; Lee et al., 2001 ▶; dos Santos, Fernandes et al., 2009 ▶). Synthetic peptides and site-directed mutagenesis studies have strongly suggested that the C-terminal region of these proteins contains the sequence that is responsible for the expression of this activity in Lys49-PLA2s (Chioato et al., 2002 ▶, 2007 ▶; Lomonte, Angulo & Calderon, 2003 ▶; Lomonte, Angulo & Santamaria, 2003 ▶; Nunez et al., 2001 ▶; Ward et al., 1998 ▶, 2002 ▶; Cintra-Francischinelli et al., 2010 ▶).
In recent years, great efforts have been made with the aim of understanding the mechanism of action of snake-venom myotoxins in order to find efficient inhibitors of these proteins. One of these attempts has focused on the scientific study of plant species that are of general use in folk medicine (Mors et al., 2000 ▶; Borges et al., 2000 ▶, 2001 ▶; Biondo et al., 2003 ▶, 2004 ▶; Januario et al., 2004 ▶; Veronese et al., 2005 ▶; Esmeraldino et al., 2005 ▶; Oliveira et al., 2005 ▶). Ticli and coworkers studied a methanolic extract of Cordia verbenacea and demonstrated that this extract was able to neutralize paw oedema induced by B. jararacussu snake venom and by its main basic Lys49-PLA2s (Ticli et al., 2005 ▶). After the isolation of rosmarinic acid (RA) as the active component of the abovementioned extract, it was shown that RA is able to significantly inhibit the myotoxic effect induced by two basic bothropic PLA2s (BthTX-I and BthTX-II; Ticli et al., 2005 ▶). Electrophoretic analysis showed that the basis of such inhibition could not be attributed to proteolytic degradation of the myotoxic PLA2s (Ticli et al., 2005 ▶), while circular-dichroism studies showed that no significant secondary-structural changes were observed on ligand binding (Ticli et al., 2005 ▶).
In this work, we report the crystallization and X-ray diffraction data collection of PrTX-I, a basic myotoxic Lys49-PLA2 isolated from B. pirajai snake venom, complexed with RA. The final crystallographic model of the complex may provide insight into the mechanism(s) that lead to inhibition of the myotoxicity of snake-venom PLA2s.
2. Materials and methods
2.1. Protein purification and crystallization
PrTX-I was isolated from B. pirajai snake venom as described previously (Mancuso et al., 1995 ▶), while RA was purchased from Sigma–Aldrich. The lyophilized sample of PrTX-I was dissolved to a concentration of 12 mg ml−1 in Tris–HCl pH 7.5 buffer. The same buffer was used to dissolve the commercial RA to a 10:1 molar ratio of inhibitor:protein. The sparse-matrix method (Jancarik & Kim, 1991 ▶) was used to perform initial screening of the crystallization conditions (Crystal Screens I and II, Hampton Research). Crystals were obtained by the sitting-drop vapour-diffusion method (McPherson, 1982 ▶), combining 1 µl protein solution, 0.5 µl RA solution and 1 µl reservoir solution, and were equilibrated against 0.5 ml of the same precipitant solution (Fig. 1 ▶). After the optimization process, the best crystals were obtained at 291 K in a reservoir solution containing 20% PEG 4000, sodium citrate pH 5.6 and 20% propanol. These crystals measured approximately 0.6 × 0.1 × 0.05 mm after one month.
Figure 1.
Crystal of PrTX-I complexed with rosmarinic acid.
2.2. X-ray data collection and processing
X-ray diffraction data were collected at a wavelength of 1.427 Å (at 100 K) using a synchrotron-radiation source [the MX2 station at Laboratório Nacional de Luz Síncrotron (LNLS), Campinas, Brazil] using a MAR CCD imaging-plate detector (MAR Research). A crystal was mounted in a nylon loop and flash-cooled in a stream of nitrogen at 100 K without using any cryoprotectant. The data were processed using the HKL program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
The data-collection statistics are given in Table 1 ▶. The crystals belong to the orthorhombic system and the data set was 95.0% complete at 1.8 Å resolution, with an R merge of 6.9%.
Table 1. X-ray diffraction data-collection and processing statistics.
Values in parentheses are for the highest resolution shell. Data were processed using the HKL suite (Otwinowski & Minor, 1997 ▶).
| Unit-cell parameters (Å) | a = 49.4, b = 67.0, c = 85.5 |
| Space group | P212121 |
| Resolution (Å) | 40.0–1.80 (1.89–1.80) |
| Unique reflections | 25698 (3766) |
| Completeness (%) | 95.0 (98.8) |
| Rmerge† (%) | 6.9 (37.3) |
| Radiation source | Synchrotron (MX2 station, LNLS) |
| Data-collection temperature (K) | 100 |
| Average I/σ(I) | 18.7 (2.6) |
| Redundancy | 4.0 (3.7) |
| Matthews coefficient VM (Å3 Da−1) | 2.6 |
| Molecules in the asymmetric unit | 2 |
| Solvent content (%) | 53.1 |
R
merge = 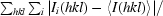
 , where Ii(hkl) is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈I(hkl)〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
, where Ii(hkl) is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈I(hkl)〉 is the mean intensity of that reflection. Calculated for I > −3σ(I).
Calculations based on the molecular weight of the protein indicated the presence of two molecules in the asymmetric unit. This corresponds to a Matthews coefficient (V M; Matthews, 1968 ▶) of 2.6 Å3 Da−1 and a calculated solvent content of the crystals of 53.1%. These values are within the expected range for typical protein crystals, assuming a value of 0.74 cm3 g−1 for the partial specific volume of the protein.
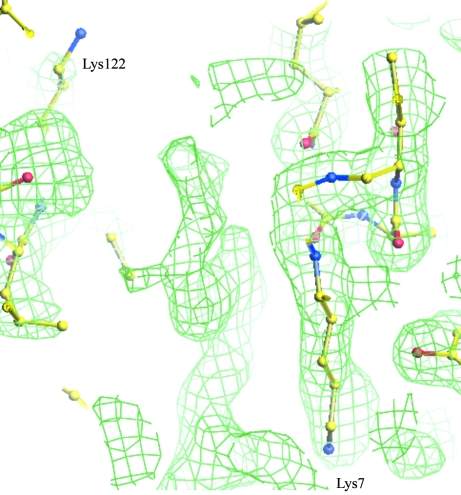
The crystal structure was solved by the molecular-replacement technique as implemented in the program AMoRe (Navaza, 1994 ▶) using the coordinates of native PrTX-I (dos Santos, Soares et al., 2009 ▶) as a model and confirmed the presence of a dimer in the asymmetric unit. Electron-density maps that unambiguously correspond to RA molecules were observed at the entrance to the hydrophobic channels (Fig. 2 ▶).
Figure 2.
2|F obs| − |F calc| electron-density map contoured at 1.0 standard deviation at the entrance to the hydrophobic channel, where electron density that corresponds to rosmarinic acid was found.
A recent review of Lys49-PLA2 crystallographic structures indicated that the apo structures belong to space group P3121, while the complexed forms belong to space groups P21 and P212121 (dos Santos, Soares et al., 2009 ▶). The space-group change is a consequence of conformational changes that occur when a ligand is bound to Lys49-PLA2s (dos Santos, Soares et al., 2009 ▶). Since space group P212121 was observed for the PrTX-I–RA complex, it is possible to suggest that inhibitor binding has led to changes in the quaternary structure of PrTX-I. After refinement of the structure, we will be able to confirm the presence of the inhibitor and the interactions that are established between RA and PrTX-I.
It has been demonstrated that RA is able to potentiate the ability of commercial equine polyvalent antivenom to neutralize the lethal and myotoxic effects of crude B. jararacussu venom (Ticli et al., 2005 ▶). RA was also capable of neutralizing two isolated Lys49-PLA2s from B. jararacussu snake venom in experimental models (Ticli et al., 2005 ▶). Therefore, detailed structural studies of the PrTX-I–RA complex may provide new and important insights into how structural changes in the quaternary structure of Lys49-PLA2s after ligand binding can lead to toxin inhibition.
Acknowledgments
The authors gratefully acknowledge financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Laboratório Nacional de Luz Síncrontron (LNLS, Campinas-SP).
References
- Andriao-Escarso, S. H., Soares, A. M., Fontes, M. R., Fuly, A. L., Correa, F. M., Rosa, J. C., Greene, L. J. & Giglio, J. R. (2002). Biochem. Pharmacol.64, 723–732. [DOI] [PubMed]
- Arni, R. K. & Ward, R. J. (1996). Toxicon, 34, 827–841. [DOI] [PubMed]
- Biondo, R., Pereira, A. M. S., Marcussi, S., Pereira, P. S., Franca, S. C. & Soares, A. M. (2003). Biochimie, 85, 1017–1025. [DOI] [PubMed]
- Biondo, R., Soares, A. M., Bertoni, B. W., Franca, S. C. & Pereira, A. M. S. (2004). Plant Cell Rep.22, 549–552. [DOI] [PubMed]
- Borges, M. H., Soares, A. M., Rodrigues, V. M., Andriao-Escarso, S. H., Diniz, H., Hamaguchi, A., Quintero, A., Lizano, S., Gutierrez, J. M., Giglio, J. R. & Homsi-Brandeburgo, M. I. (2000). Comp. Biochem. Physiol. B Biochem. Mol. Biol.127, 21–30. [DOI] [PubMed]
- Borges, M. H., Soares, A. M., Rodrigues, V. M., Oliveira, F., Fransheschi, A. M., Rucavado, A., Giglio, J. R. & Homsi-Brandeburgo, M. I. (2001). Toxicon, 39, 1863–1869. [DOI] [PubMed]
- Chioato, L., Aragao, E. A., Ferreira, T. L., de Medeiros, A. I., Faccioli, L. H. & Ward, R. J. (2007). Biochim. Biophys. Acta, 1768, 1247–1257. [DOI] [PubMed]
- Chioato, L., De Oliveira, A. H., Ruller, R., Sa, J. M. & Ward, R. J. (2002). Biochem. J.366, 971–976. [DOI] [PMC free article] [PubMed]
- Cintra-Francischinelli, M., Pizzo, P., Angulo, Y., Gutierrez, J. M., Montecucco, C. & Lomonte, B. (2010). Toxicon, 55, 590–596. [DOI] [PubMed]
- dos Santos, J. I., Fernandes, C. A., Magro, A. J. & Fontes, M. R. (2009). Protein Pept. Lett.16, 887–893. [DOI] [PubMed]
- dos Santos, J. I., Soares, A. M. & Fontes, M. R. (2009). J. Struct. Biol.167, 106–116. [DOI] [PubMed]
- Esmeraldino, L. E., Souza, A. M. & Sampaio, S. V. (2005). Phytomedicine, 12, 570–576. [DOI] [PubMed]
- Ferreira, M. L., Moura-da-Silva, A. M., Franca, F. O., Cardoso, J. L. & Mota, I. (1992). Toxicon, 30, 1603–1608. [DOI] [PubMed]
- Gutierrez, J. M. & Lomonte, B. (1995). Toxicon, 33, 1405–1424. [DOI] [PubMed]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411.
- Januario, A. H., Santos, S. L., Marcussi, S., Mazzi, M. V., Pietro, R. C., Sato, D. N., Ellena, J., Sampaio, S. V., Franca, S. C. & Soares, A. M. (2004). Chem. Biol. Interact.150, 243–251. [DOI] [PubMed]
- Kini, R. M. (1997). Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism, pp. 1–28. Chichester: Wiley.
- Kini, R. M. & Evans, H. J. (1989). Toxicon, 27, 613–635. [DOI] [PubMed]
- Lee, W. H., da Silva Giotto, M. T., Marangoni, S., Toyama, M. H., Polikarpov, I. & Garratt, R. C. (2001). Biochemistry, 40, 28–36. [DOI] [PubMed]
- Lomonte, B., Angulo, Y. & Calderon, L. (2003). Toxicon, 42, 885–901. [DOI] [PubMed]
- Lomonte, B., Angulo, Y. & Santamaria, C. (2003). Toxicon, 42, 307–312. [DOI] [PubMed]
- Mancuso, L. C., Correa, M. M., Vieira, C. A., Cunha, O. A., Lachat, J. J., de Araujo, H. S., Ownby, C. L. & Giglio, J. R. (1995). Toxicon, 33, 615–626. [DOI] [PubMed]
- Maraganore, J. M., Merutka, G., Cho, W., Welches, W., Kezdy, F. J. & Heinrikson, R. L. (1984). J. Biol. Chem.259, 13839–13843. [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- McPherson, A. (1982). Preparation and Analysis of Protein Crystals. New York: Wiley.
- Mors, W. B., Nascimento, M. C., Pereira, B. M. & Pereira, N. A. (2000). Phytochemistry, 55, 627–642. [DOI] [PubMed]
- Navaza, J. (1994). Acta Cryst. A50, 157–163.
- Nunez, C. E., Angulo, Y. & Lomonte, B. (2001). Toxicon, 39, 1587–1594. [DOI] [PubMed]
- Oliveira, C. Z., Maiorano, V. A., Marcussi, S., Sant’ana, C. D., Januario, A. H., Lourenco, M. V., Sampaio, S. V., Franca, S. C., Pereira, P. S. & Soares, A. M. (2005). J. Ethnopharmacol.98, 213–216. [DOI] [PubMed]
- Oliveira, R. C. W. de (2009). Animais Peçonhentos do Brasil: Biologia, Clínica e Terapêutica dos Envenenamentos. São Paulo: Sarvier.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Ribeiro, L. A., Albuquerque, M. J., de Campos, V. A., Katz, G., Takaoka, N. Y., Lebrao, M. L. & Jorge, M. T. (1998). Rev. Assoc. Med. Bras.44, 312–318. [DOI] [PubMed]
- Rosenberg, P. (1990). Handbook of Toxinology, edited by W. T. Shyer & D. Mebs, pp. 68–223. New York: Dekker.
- Soares, A. M. & Giglio, J. R. (2003). Toxicon, 42, 855–868. [DOI] [PubMed]
- Ticli, F. K., Hage, L. I., Cambraia, R. S., Pereira, P. S., Magro, A. J., Fontes, M. R., Stabeli, R. G., Giglio, J. R., Franca, S. C., Soares, A. M. & Sampaio, S. V. (2005). Toxicon, 46, 318–327. [DOI] [PubMed]
- Veronese, E. L., Esmeraldino, L. E., Trombone, A. P., Santana, A. E., Bechara, G. H., Kettelhut, I., Cintra, A. C., Giglio, J. R. & Sampaio, S. V. (2005). Phytomedicine, 12, 123–130. [DOI] [PubMed]
- Ward, R. J., Alves, A. R., Ruggiero Neto, J., Arni, R. K. & Casari, G. (1998). Protein Eng.11, 285–294. [DOI] [PubMed]
- Ward, R. J., Chioato, L., de Oliveira, A. H., Ruller, R. & Sa, J. M. (2002). Biochem. J.362, 89–96. [DOI] [PMC free article] [PubMed]