Crystals of the complex between the Fab fragment of a human anti-EphA2 antibody and the N-terminal domain of human EphA2 have been obtained. Diffraction data were collected to 2.55 Å resolution.
Keywords: antibodies, ephrin receptors, Fab fragments, ephrin type-A receptor 2
Abstract
The recombinant N-terminal domain of human ephrin type-A receptor 2 (rEphA2) has been crystallized in complex with the recombinantly produced Fab fragment of a fully human antibody (1C1; IgG1/κ). These are the first reported crystals of an ephrin receptor bound to an antibody. The orthorhombic crystals belonged to space group C2221 (the 00l reflections obey the l = 2n rule), with unit-cell parameters a = 78.93, b = 120.79, c = 286.20 Å. The diffraction of the crystals extended to 2.0 Å resolution. However, only data to 2.55 Å resolution were considered to be useful owing to spot overlap caused by the long unit-cell parameter. The asymmetric unit is most likely to contain two 1C1 Fab–rEphA2 complexes. This corresponds to a crystal volume per protein weight (V M) of 2.4 Å3 Da−1 and a solvent content of 49.5%. The three-dimensional structure of this complex will shed light on the molecular basis of 1C1 specificity. This will also contribute to a better understanding of the mechanism of action of this antibody, the current evaluation of which as an antibody–drug conjugate in cancer therapy makes it a particularly interesting case study.
1. Introduction
Ephrin receptors constitute the largest family of receptor tyrosine kinases (RTKs; Zhang & Hughes, 2006 ▶). Interaction with their ephrin ligands plays a critical role in modulating cell–cell interactions and cell migration during development (Aoto & Chen, 2007 ▶; Kuijper et al., 2007 ▶; Zhang & Hughes, 2006 ▶). Specifically, the ephrin type-A receptor 2 (EphA2) belongs to one of the two known classes of ephrin receptors (A and B; Aoto & Chen, 2007 ▶; Gale et al., 1996 ▶). Overexpression of EphA2 is observed in several types of cancer (Surawska et al., 2004 ▶) and is linked to decreased survival rates in ovarian and esophageal cancers (Meade-Tollin & Martinez, 2007 ▶; Miyazaki et al., 2003 ▶). Together with the limited normal expression of EphA2, this makes this receptor an attractive target for antibody–drug conjugate therapy. In particular, the anti-human EphA2 fully human monoclonal antibody 1C1 was conjugated to the microtubule inhibitor monomethyl auristatin phenylalanine (MMAF) and shown to inhibit the growth of EphA2-expressing tumors through its ability to induce rapid internalization and deliver the cytotoxic payload (Jackson et al., 2008 ▶).
In an effort to better understand the molecular basis of the recognition of human EphA2 by 1C1 and decipher its internalization-dependent mechanism of action, we set out to solve the X-ray crystal structure of the corresponding complex. Here, the first reported diffracting crystals of a human ephrin receptor bound to an antibody are described and characterized.
2. Materials and methods
2.1. Cloning, expression and purification of 1C1 Fab and human EphA2
The heavy and light chains of the Fab portion of 1C1 were cloned into a mammalian expression vector essentially as described previously (Oganesyan et al., 2008 ▶, 2009 ▶). More precisely, each chain was placed under the control of its own human cytomegalovirus major immediate early (hCMVie) promoter and enhancer (described in Boshart et al., 1985 ▶). Each of these two genes also incorporated an SV40 poly-A sequence to allow proper processing of its 3′ end. In this system, a human γ1 chain truncated at position Lys235 (according to the Kabat numbering convention; Kabat et al., 1991 ▶) is secreted along with a human κ chain (Oganesyan et al., 2008 ▶, 2009 ▶). The construct was then transiently transfected into human embryonic kidney (HEK) 293 cells using 293fectin (Invitrogen, Carlsbad, California, USA) and standard protocols. 1C1 Fab was typically harvested 144 and 216 h post-transfection. Owing to the nature of its heavy-chain subfamily (VH3), 1C1 Fab can bind to protein A (Potter et al., 1996 ▶). Therefore, in the first purification step conditioned medium containing the soluble Fab fragment was applied onto a HiTrap Protein A HP column (GE Healthcare, Piscataway, New Jersey, USA) and eluted using the commercially available Mild Elution Buffer according to the manufacturer’s instructions (Thermo Scientific, Rockford, Illinois, USA). The eluted material was then dialyzed against 50 mM sodium acetate pH 5.0 and further purified using a HiTrap SP HP column in an NaCl gradient according to the manufacturer’s instructions (GE Healthcare). Purified 1C1 Fab, which was typically >95% homogeneous as judged by SDS–PAGE, was then concentrated to approximately 30 mg ml−1 (as measured by the absorbance at 280 nm).
1C1 Fab has previously been shown to bind to the N-terminal portion of the 510-residue-long extracellular domain of human EphA2 (Dr Li Peng, personal communication). Therefore, Gln25 (the first residue of the mature protein) to Lys200 of the extracellular domain of human EphA2 (numbering as defined in entry No. P29317 of the Swiss-Prot protein database) were PCR-amplified from a plasmid containing the entire extracellular part and cloned into a mammalian expression vector under the control of the hCMVie promoter. More precisely, the recombinant gene was cloned in frame with the signal sequence of human CD33 followed by a His6 tag. The construct also incorporated an SV40 poly-A sequence to allow proper processing of its 3′ end. The resulting construct was transiently transfected into HEK 293 cells using 293fectin (Invitrogen) and standard protocols. Cells were grown at 310 K under 8% CO2 for 3–4 d before human rEphA2 was harvested. The soluble human rEphA2 contained in the conditioned medium was purified using a HisTrap FF column in an imidazole gradient at pH 7.5 according to the manufacturer’s instructions (GE Healthcare). The eluted protein was directly applied onto a HiTrap Q HP column equilibrated with 50 mM Tris–HCl pH 7.5 and eluted in an NaCl gradient according to the manufacturer’s instructions (GE Healthcare). This procedure allowed us to obtain over 95% homogeneous human rEphA2 as judged by SDS–PAGE. Purified rEphA2 was then concentrated to approximately 6 mg ml−1 (as measured by the absorbance at 280 nm).
2.2. Complex preparation and crystallization
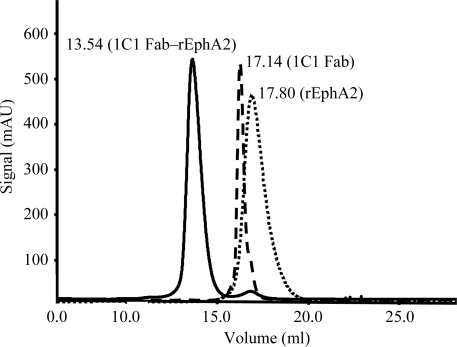
Previously purified 1C1 Fab and rEphA2 were mixed in a 1:1 molar ratio. The resulting mixture was adjusted to a total protein concentration of approximately 10 mg ml−1 (as measured by the absorbance at 280 nm) using a Vivaspin 2 concentrator (30 kDa cutoff, Sartorius AG, Edgewood, New York, USA). Further purification was carried out by size-exclusion chromatography using a Superdex S200 column (GE Healthcare) equilibrated with 50 mM Tris–HCl pH 7.5, 100 mM NaCl. A representative chromatogram of 1C1 Fab alone, rEphA2 alone and the 1C1 Fab–rEphA2 complex is shown in Fig. 1 ▶. The purified complex was then concentrated to 10 mg ml−1 using a Vivaspin 2 concentrator and submitted to crystallization trials as described below.
Figure 1.
Superimposition of the size-exclusion chromatograms of 1C1 Fab, rEphA2 and the 1C1 Fab–rEphA2 complex.
Sitting-drop crystallization experiments were initially set up in 96-well plates with conical flat-bottomed drop compartments (Corning 3785; VWR, West Chester, Pennsylvania, USA) using a Phoenix crystallization robot (Art Robbins, Sunnyvale, California, USA). Favorable conditions were first identified using the following commercially available crystallization screens: Crystal Screen HT, Index (Hampton Research, Aliso Viejo, California, USA), Wizard I and II (Emerald BioSystems, Bainbridge Island, Washington, USA) and ProPlex (Molecular Dimensions, Apopka, Florida, USA). In screening mode, the reservoir and drop compartments of the 96-well plates were filled with 50 and 0.3 µl, respectively, of the various screen solutions using the Phoenix robot. 0.3 µl of the 1C1 Fab–rEphA2 complex at a concentration of 10 mg ml−1 in 50 mM Tris–HCl pH 7.5, 100 mM NaCl was then added to the drop compartment.
Based on initial screening results, the C3 screen solution from ProPlex (20% PEG 4000, 0.1 M sodium acetate pH 5.0, 0.2 M ammonium acetate) was selected for further optimization in hanging drops using the protein-complex solution at a concentration of 10 mg ml−1 in 50 mM Tris–HCl pH 7.5, 100 mM NaCl. More precisely, the reservoirs were first filled with 200 µl of a 50–100% solution of ProPlex C3. Drop volumes ranging from 2.5 to 4 µl were then prepared at the following protein:reservoir ratios (v:v): 1.5:1, 2:1 and 3:1. Diffraction-quality crystals were grown once optimal conditions had been identified (see §3).
2.3. X-ray diffraction data collection and processing
Diffraction data were collected from a single crystal on beamline 31-ID-D of the Advanced Photon Source at Argonne National Laboratory (University of Chicago, Chicago, Illinois, USA) equipped with a Rayonix 225 HE detector (Rayonix LLC, Evanston, Illinois, USA). The crystal shown in Fig. 2 ▶(a) was harvested from the corresponding drop using a loop and stacked plates were carefully separated from one another. Cryoprotection was achieved by soaking the crystal in 12.8% PEG 4000, 0.06 M sodium acetate pH 5.0, 0.13 M ammonium acetate, 20% glycerol. The selected crystal plate was then flash-cooled in liquid nitrogen. 360 consecutive images were collected using an oscillation range of 0.5°, a crystal-to-detector distance of 290 mm and an exposure time of 0.7 s. The diffraction images were integrated and scaled using HKL-2000 (Otwinowski & Minor, 1997 ▶).
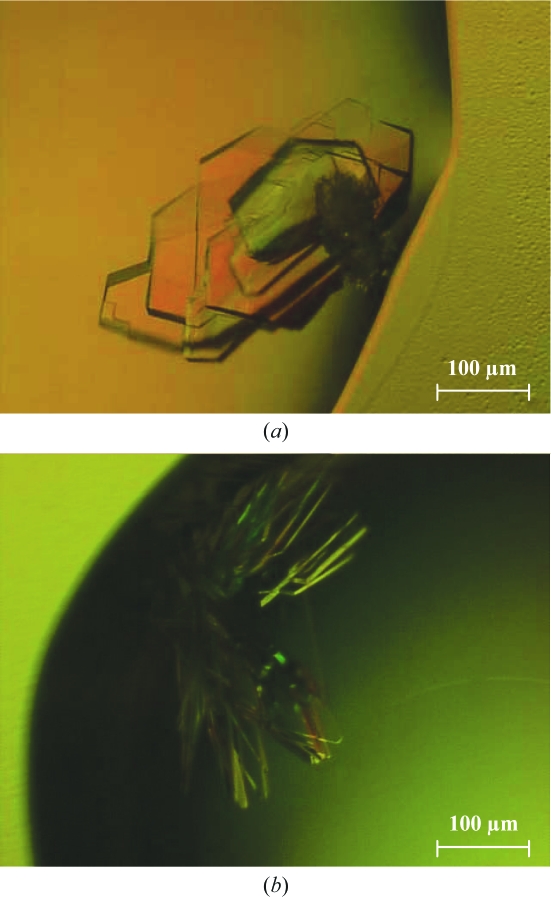
Figure 2.
Crystals of the 1C1 Fab–rEphA2 complex. The crystals shown in (a) and (b) were grown in hanging drops during the optimization phase. Reservoirs were filled with 80% ProPlex C3 screen solution as described in §3. Crystals grew to dimensions of up to 0.3 × 0.3 × 0.02 mm in hanging drops, in which 1.5 µl 10 mg ml−1 protein complex solution in 50 mM Tris–HCl pH 7.5, 100 mM NaCl was mixed with 1 µl reservoir solution (16% PEG 4000, 0.08 M sodium acetate pH 5.0, 0.16 M ammonium acetate). Under these and similar conditions, only crystals of two different morphologies could be obtained (often in the same drop), namely the so-called ‘stacked plates’ (a) and ‘book pages’ (b).
3. Results and discussion
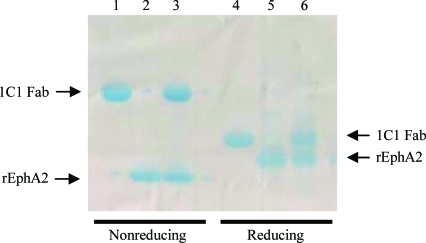
Diffraction-quality plate-like crystals grew in about a week in hanging drops in which 1.5 µl of the 1C1 Fab–rEphA2 complex at a concentration of 10 mg ml−1 in 50 mM Tris–HCl pH 7.5, 100 mM NaCl was mixed with 1 µl reservoir solution (80% ProPlex C3; 16% PEG 4000, 0.08 M sodium acetate pH 5.0, 0.16 M ammonium acetate). They reached dimensions of up to 0.3 × 0.3 × 0.02 mm as seen in Fig. 2 ▶. SDS–PAGE analysis of these crystals confirmed that they indeed contained the expected complex formed by 1C1 Fab and rEphA2 (Fig. 3 ▶).
Figure 3.
SDS–PAGE profile of 1C1 Fab (lanes 1 and 4), rEphA2 (lanes 2 and 5) and a dissolved crystal of the 1C1 Fab–rEphA2 crystal complex (lanes 3 and 6) under reducing and nonreducing conditions. The 4–20% gradient gel (Bio-Rad, Hercules, California, USA) was stained with Bio-Safe Coomassie Stain (Bio-Rad). It shows rEphA2 migrating at around 15 and 19 kDa under nonreducing and reducing conditions, respectively. This difference is likely to be attributable to the presence of two internal disulfide bonds, the reduction of which alters the compactness (and thus the migration) of the molecule. The same argument can be applied to the 1C1 Fab fragment, which migrated slightly under its expected molecular weight of 50 kDa at around 45 kDa under nonreducing conditions. As expected, its heavy and light chains both migrated at 25 kDa under reducing conditions.
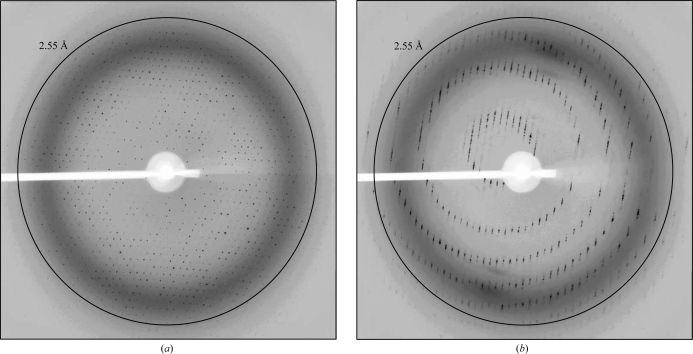
The diffraction limit of the crystal was 2.0 Å. Because of the long unit-cell parameter, spot separation was not achievable in resolution shells beyond 2.55 Å (Fig. 4 ▶). Microseeding approaches were explored but only produced smaller and thinner crystals. The space group was determined to be C2221 (the l = 2n rule applies for 00l reflections), with unit-cell parameters a = 78.93, b = 120.79, c = 286.20 Å and a crystal mosaicity in the range 0.4–0.6°. The asymmetric unit is likely to contain two 1C1 Fab–rEphA2 complexes. This corresponds to a V M of 2.4 Å3 Da−1 and a solvent content of 49.5%. Data statistics are shown in Table 1 ▶. Structure determination using molecular replacement is currently under way.
Figure 4.
Diffraction images of the 1C1 Fab–rEphA2 crystal 70° apart are shown at ω angles of 0 or 70° in (a) and (b), respectively. These particular frames were chosen to show the two extremes of the crystal’s diffraction quality. Although the diffraction limit of the crystal was 2.0 Å, a long unit-cell parameter of ∼290 Å only allowed data processing to 2.55 Å resolution using a crystal-to-detector distance of 290 mm.
Table 1. X-ray data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Wavelength (Å) | 0.9793 |
| Resolution (Å) | 50.0–2.55 (2.60–2.55) |
| Space group | C2221 |
| Unit-cell parameters (Å) | a = 78.93, b = 120.79, c = 286.20 |
| Total reflections | 223275 |
| Unique reflections | 40691 |
| Mosaicity (°) | 0.4–0.6 |
| Average redundancy | 5.5 (4.2) |
| Completeness (%) | 93.5 (88.9) |
| Rmerge | 0.117 (0.396) |
| Mean I/σ(I) | 16.5 (3.5) |
Acknowledgments
We thank Dr Ann Boriack-Sjodin for facilitating our data-collection process. We are also grateful to the staff of beamline 31-ID-D of the Advanced Photon Source, Argonne National Laboratory for assistance during X-ray data collection.
References
- Aoto, J. & Chen, L. (2007). Brain Res.1184, 72–80. [DOI] [PMC free article] [PubMed]
- Boshart, M., Weber, F., Jahn, G., Dorsch-Hasler, K., Fleckenstein, B. & Schaffner, W. (1985). Cell, 41, 521–530. [DOI] [PubMed]
- Gale, N. W., Holland, S. J., Valenzuela, D. M., Flenniken, A., Pan, L., Ryan, T. E., Henkemeyer, M., Strebhardt, K., Hirai, H., Wilkinson, D. G., Pawson, T., Davis, S. & Yancopoulos, G. D. (1996). Neuron, 17, 9–19. [DOI] [PubMed]
- Jackson, D. et al. (2008). Cancer Res.68, 9367–9374. [DOI] [PubMed]
- Kabat, E. A., Wu, T. T., Perry, H. M., Gottesman, K. S. & Foeller, C. (1991). Sequences of Proteins of Immunological Interest. Washington, DC: National Institutes of Health.
- Kuijper, S., Turner, C. J. & Adams, R. H. (2007). Trends Cardiovasc. Med.17, 145–151. [DOI] [PubMed]
- Meade-Tollin, L. & Martinez, J. D. (2007). Cancer Biol. Ther.6, 288–289. [DOI] [PubMed]
- Miyazaki, T., Kato, H., Fukuchi, M., Nakajima, M. & Kuwano, H. (2003). Int. J. Cancer, 103, 657–663. [DOI] [PubMed]
- Oganesyan, V., Damschroder, M. M., Cook, K. E., Wu, H. & Dall’Acqua, W. F. (2009). Acta Cryst. F65, 14–16. [DOI] [PMC free article] [PubMed]
- Oganesyan, V., Damschroder, M. M., Leach, W., Wu, H. & Dall’Acqua, W. F. (2008). Mol. Immunol.45, 1872–1882. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Potter, K. N., Li, Y. & Capra, J. D. (1996). J. Immunol.157, 2982–2988. [PubMed]
- Surawska, H., Ma, P. C. & Salgia, R. (2004). Cytokine Growth Factor Rev.15, 419–433. [DOI] [PubMed]
- Zhang, J. & Hughes, S. (2006). J. Pathol.208, 453–461. [DOI] [PubMed]