Abstract
Despite the potential for deleterious (even fatal) effects on cardiac physiology, 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) abuse abounds driven mainly by its euphoric effects. Acute exposure to MDMA has profound cardiovascular effects on blood pressure and heart rate in humans and animals. To determine the effects of MDMA on cardiac metabolites in rats, MDMA (0, 5, or 10 mg/kg) was injected every 2 h for a total of four injections; animals were sacrificed 2 h after the last injection (8 h drug exposure), and their hearts removed and tissue samples from left ventricular wall dissected. High resolution magic angle spinning proton magnetic resonance spectroscopy (1H-MRS) at 11.7 T, a specialized version of MRS aptly suited for analysis of semi-solid materials such as intact tissue samples, was used to measure the cardiac metabolomic profile, including alanine, lactate, succinate, creatine, and carnitine, in heart tissue from rats treated with MDMA. MDMA effects on MR-visible choline, glutamate, glutamine, and taurine were also determined. Body temperature was measured following each MDMA administration and serotonin and norepinephrine (NE) levels were measured by high pressure liquid chromatography (HPLC) in heart tissue from treated animals. MDMA significantly and dose-dependently increased body temperature, a hallmark of amphetamines. Serotonin, but not NE, levels were significantly and dose-dependently decreased by MDMA in the heart wall. MDMA significantly altered the MR-visible profile with an increase in carnitine and no change in other key compounds involved in cardiomyocyte energy metabolomics. Finally, choline levels were significantly decreased by MDMA in heart. The results are consistent with the notion that MDMA has significant effects on cardiovascular serotonergic tone and disrupts the metabolic homeostasis of energy regulation in cardiac tissue, potentially increasing utilization of fatty acid metabolism. The contributions of serotonergic signaling on MDMA-induced changes in cardiac metabolism remain to be determined.
Keywords: magnetic resonance spectroscopy, carnitine, serotonin, MDMA, heart, rat
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA; ecstasy) is a recreational drug that has stimulant and hallucinogen-like properties. MDMA abuse remains a societal problem in developed nations despite two decades of research indicating that the acute positive effects of MDMA (e.g. euphoria, intense empathy for others, and extreme relaxation) coincide with numerous negative effects such as tachycardia, hypertension, and indirect cardiac stressors associated with suppression of appetite, thirst, and sleep. In fact, numerous studies show that MDMA increases heart rate and blood pressure in rats (1,2) as well as humans (3–5). Despite research elucidating the mechanism by which MDMA negatively affects central and peripheral physiology, MDMA is still used in alarming quantities by teenagers and adults (6,7). The perception by the general public and advocacy groups (e.g. http://www.maps.org/mission.html) that MDMA is a relatively benign drug with mystical, empathogenic properties contribute to the problem. In reality MDMA and related amphetamines produce profound acute effects on cardiovascular physiology that may lead to cardiotoxicity and increased susceptibility for heart-related fatalities, in addition to producing neurocognitive and neurobehavioral deficits.
MDMA is a ring-substituted amphetamine that acutely raises extracellular levels of serotonin (5HT), and to a lesser extent dopamine, and norepinephrine (NE) by reversing the serotonin transporter (SERT), dopamine transporter, NE transporter, or vesicular monoamine transporters (8–10). MDMA is also a substrate for monoamine oxidase (11) and has been shown to directly interact with 5HT receptors (12). The persistent and potentially neurotoxic effects of MDMA on central 5HT neurons (as measured by decreased 5HT levels, SERT, or tryptophan hydroxylase) in non-human experimental subjects is well established (13–15).
The effects of MDMA on cardiac tissue are not as well characterized as they are on brain tissue, however 5HT receptors (16) and SERT (17) are expressed in cardiovascular tissues including the heart. Thus, MDMA effects on the cardiovascular system and heart may occur via release of 5HT from platelets (16) or a direct interaction with 5HT2B receptors (12) on cardiac myocytes. MDMA causes fenfluramine-like proliferation of valvular interstitial cells in vitro (18) by a mechanism that involves 5HT2B receptors (19). In fact, fenfluramine-based appetite suppressants (Fen-Phen) were recalled shortly after introduction due to reports of valvular heart disease, pulmonary hypertension, and cardiac fibrosis (20,21). Roth proposed that fenfluramine and its metabolite norfenfluramine directly activate 5HT2B receptors, a common receptor in the heart. These receptors are partially responsible for regulating myocyte growth and development; therefore, inappropriate overstimulation of serotonergic activity would damage the valves due to uncontrolled replication of the valvular cells (19).
Given the critical energy requirements of cardiac tissue and the potential for MDMA-induced oxidative stress (22), we used proton magnetic spectroscopy (1H-MRS) to determine the biochemical profile of the heart tissue from the ventricular wall of rats treated with either saline or MDMA. In particular, levels of alanine, carnitine, choline, creatine, glutamate, glutamine, lactate, and succinate were measured. Additionally, MDMA-induced changes in body temperature as well as monoamines (5HT and NE) were measured. We hypothesized that MDMA would increase cardiac metabolic activity as reflected by differential changes in MR-visible metabolites associated with intermediary metabolism. We found that acute exposure to MDMA decreased 5HT and choline while increasing levels of total carnitine, supporting the notion that MDMA does not shift the balance of energy use to other pathways, but rather increases the utilization of fatty acid catabolism. Moreover, the results demonstrate the application of 1H-MRS to cardiac metabolism and provide a potential basis for clinical investigations of MDMA-induced alterations in cardiac metabolism.
METHODS
Animals, drug administration, and body temperature measurement
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were housed in a temperature (~24°C), humidity (35–40%), and light (12 h light/dark cycle; on 7 am off 7 pm) controlled room and grouped 4 per cage (bedding changed twice weekly) with food and water available ad libitum. Animals were acclimated for 5 days before testing during which time they were handled and weighed daily (~300 g). Wayne State University maintains AAALAC accredited animal facilities under the Division of Laboratory Animal Resources, and animals are cared for in accordance with applicable portions of the Animal Welfare Act and “Guide for the Care and Use of Laboratory Animals.”
MDMA (Sigma, St Louis, MO, USA) was dissolved in normal saline (0.9% NaCl) at a stock dose and diluted with saline to the appropriate final dose. Racemic (±)MDMA (5 or 10 mg/kg) or saline (1 mL/kg) was injected intraperitoneally (IP) every 2 h for a total of four injections using an established dosing regimen (23–25). Unanesthetized animals were euthanized by rapid decapitation 2 h after the last injection (8 h MDMA exposure) to obtain heart tissue samples.
Rectal body temperature measurements were taken 30 min following the first three drug administrations using a rectal temperature probe (Physitemp Instruments Inc., BAT-12, Clifton, NJ, USA). Temperature data were averaged for each animal and the mean of those means used for analysis.
Heart dissections
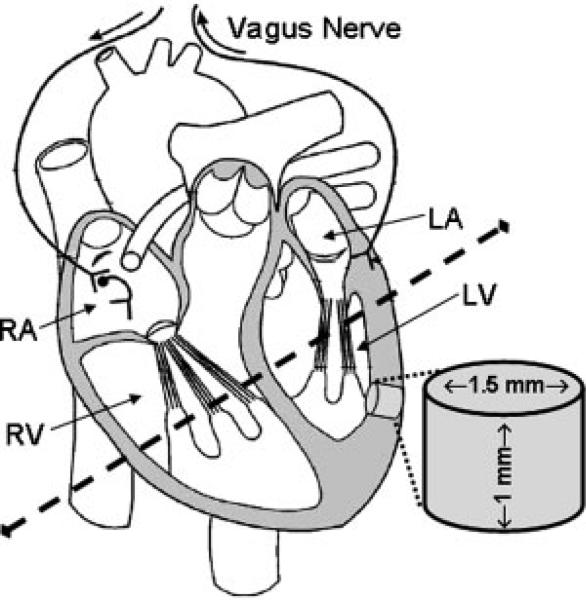
Figure 1 shows a schematic of the rat heart and illustrates the tissue dissections. Hearts were rapidly removed, placed onto an ice-cold Petri dish, and the ventricles were bisected horizontally with a razor blade. The left ventricular wall of the heart was rinsed with ice-cold saline (0.9% NaCl) and placed, exterior side down, on a block of dry ice (solid CO2). Two intact tissue punches were obtained from the heart left ventricular wall (near the septum) of each rat using a 1.5 mm diameter tissue biopsy-punch. Tissue samples (~3 mg) were stored at –80°C for later analysis. One sample was used for 1H-MRS and the other used for high pressure liquid chromatography (HPLC) analysis; sample sizes (N) are given in figure legends and represent the number of animals per group (one sample per animal).
Figure 1.
Schematic of rat heart. Tissue punches were taken from left ventricular wall (near the septum) dissected from MDMA (or saline) treated rats. Intact untreated tissue samples (~3 mg) were used for 1H-MRS and HPLC analysis.
1H-MRS
Frozen intact tissue samples were weighed and placed into a 10 μL Bruker zirconium rotor containing buffer [pH = 7.4; 100 mM K2HPO4/KH2PO4, 200 mM HCOONa, 1 g/L NaN3 diluted 50% with D2O containing 3 mM trimethylsilyl-propionate (TSP Sigma). TSP served as an internal chemical shift reference (0.00 ppm), formate (8.44 ppm) was used for autophasing, and D2O was used to lock on the center frequency. The rotor (with sample) was placed into a Bruker magic angle spinning probe maintained at 4°C in a vertical wide-bore (8.9 cm) Bruker 11.7 T magnet, which was controlled with an Avance DRX-500 console (Bruker Biospin Corp., Billerica, MA, USA). Rotors were spun at 4.2 ± 0.002 kHz at 54.7° relative to the static magnetic field B0. High resolution magic angle spinning 1H-MRS, originally developed for solid state NMR, provides a highly resolved spectrum amenable to quantification in intact tissue samples. Field inhomogeneities were compensated for using a semi-automated shimming procedure (using Bruker Smart Magnet Systems, BSMS). After a pre-saturation pulse for water suppression, spectra were acquired with a 1-D Carr–Purcell–Meiboom–Gill pulse sequence [90-(τ-180-τ)n-acquisition] (26) with TR = 6210 ms, τ = 0.15 ms, and n = 12 for total echo-time (TE) of 3.6 ms; spectral bandwidth = 7.0 kHz (14 ppm), 16 k complex data points, and 32 averages for total acquisition time of 3 min 48 s. Acquisition of data was done using Bruker-XWINNMR version 3.6 software. Spectra were analyzed with a custom LCModel version 6.1–4 (27), established with a basis set of metabolites over a spectral range of 1.0–4.2 ppm. The precision of the LCModel fit to the spectral data was estimated with Cramér–Rao bounds, which are typically less than 10% for the metabolites reported herein. Spectral data were corrected for tissue weight and are presented as a percent of saline control. A typical MR spectrum from rat left ventricular wall tissue of a saline-control treated rat is shown in Fig. 2.
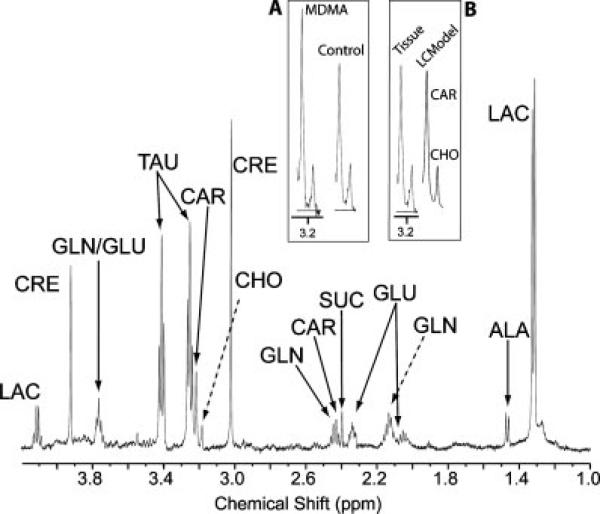
Figure 2.
Representative 1H-MR spectrum from rat heart tissue of saline treated animals. The spectral trace around 3.2 ppm is expanded in the insets (carnitine is the leftmost peak, next to choline). Inset A is an expanded spectral trace from an MDMA-treated animal (left) compared to a saline-treated animal (right). Inset B is the expanded spectral trace from an intact cardiac tissue sample (left) compared to the LCModel trace (right) used for quantitation. Abbreviations: alanine = ALA, carnitine = CAR, choline = CHO, creatine = CRE, glutamate = GLU, glutamine = GLN, lactate = LAC, succinate = SUC, taurine = TAU.
HPLC
Frozen tissues were weighed, sonically disrupted in 200 μL of 200 mM HClO4 then centrifuged for 5 min at 4°C to remove cellular debris. A 100 μL aliquot of the supernatant was placed in an ESA 542 autoinjector maintained at 4°C and 10 μL injected onto a C18-RP column (30°C) with ESA MD-TM mobile phase running at a flow rate of 0.6 mL/min. Coulometric detection was accomplished with an ESA 5011A dual electrode cell and the signal analyzed on an EZChrome Elite data processing platform. Absolute tissue values (ng/mg) for NE and 5HT were determined by comparison with external standard curves and corrected for tissue weight. Data are presented as a percent of saline control.
Statistics
All statistical tests were conducted using Prism 4 (GraphPad) or SPSS v16.0 software with a 95% confidence interval and statistical significance set at p < 0.05 (95% confidence interval). To compare saline-treated rats with the two doses (5 or 10 mg/kg) of MDMA used in this study all data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni's or Dunnet's Multiple Comparison test.
RESULTS
MDMA increases body temperature in rats
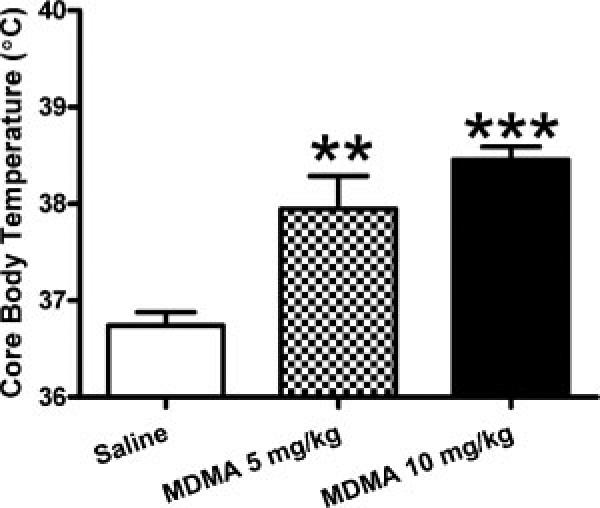
Results presented in Fig. 3 illustrate a hallmark effect of acute MDMA (hyperthermia) in that MDMA dose-dependently and statistically increased rectal body temperature (ANOVA: F2,23 = 19.60, p < 0.001; Bonferroni posthoc: saline vs. 5 mg/kg p < 0.01; saline vs. 10 mg/kg p < 0.001).
Figure 3.
MDMA increases temperature of Sprague-Dawley rats. MDMA (5 or 10 mg/kg 4× every 2 h) dose-dependently increases rectal body temperature. (**p < 0.01, ***p < 0.001 compared to control; mean ± s.e.m.; N ≥ 6/group).
Differential effect of MDMA on tissue levels of serotonin and norepinephrine
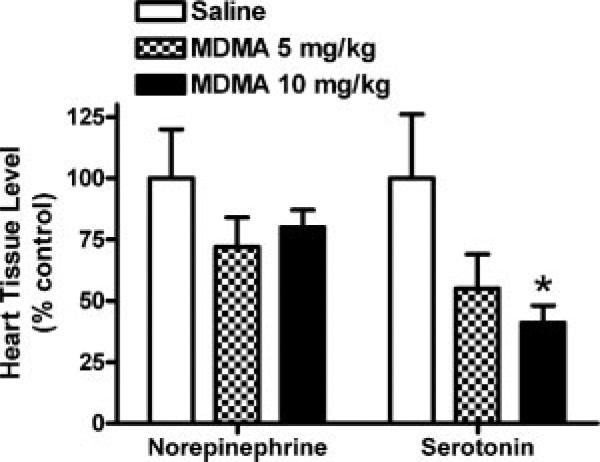
HPLC analysis of monoamine levels in the cardiac ventricular wall revealed (Fig. 4) that MDMA significantly and dose-dependently decreased whole tissue 5HT levels compared to saline (ANOVA: F2,14 = 4.360, p = 0.034; Dunnett's posthoc: saline vs. 10 mg/kg p < 0.05). NE levels were not significantly different between MDMA and control (p > 0.05). Whole tissue NE levels averaged 0.51 ± 0.04 ng/mg tissue and 5HT levels averaged 0.26 ± 0.04 ng/mg tissue in saline-treated rats. Dopamine and 5HIAA (5HT metabolite) levels were below the level of reliable detection.
Figure 4.
MDMA affects serotonin, but not norepinephrine, levels in heart wall tissue. Whole tissues (left ventricular wall) were assayed for 5HT and NE after repeated administration of MDMA or saline-control in rats. Serotonin levels were significantly decreased after MDMA (*p < 0.05 compared to control; mean ± s.e.m.; N = 4–8/group).
MDMA effects on the MR-visible metabolic profile in heart tissue
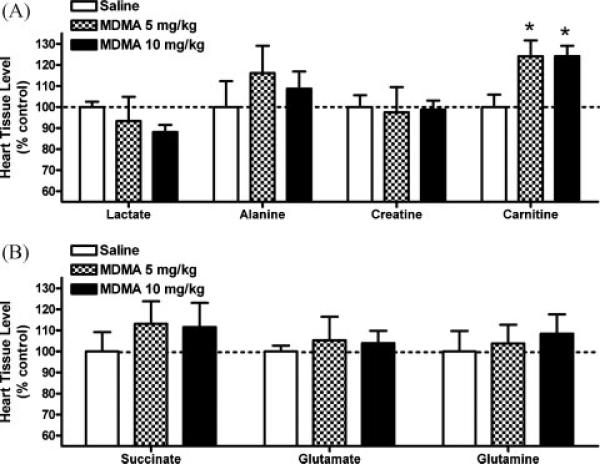
Results presented in Fig. 5A and B show the dose-effects of MDMA 2 h after repeated administration (4 × q 2 h) on MR-visible metabolites involved in cardiomyocyte metabolic energy pathways in the rat heart. In the left ventricular wall, ANOVA of saline, 5 and 10 mg/kg MDMA revealed carnitine levels were significantly increased after 5 mg/kg (124 ± 8%) or 10 mg/kg (124 ± 5%) MDMA (ANOVA: F2,11 = 4.495 p = 0.037; Dunnett's posthoc: saline vs. 5 or 10 mg/kg p < 0.05). Levels of lactate, alanine, and creatine (Fig. 5A) and glutamate, glutamine, and succinate (Fig. 5B) were not significantly different between MDMA and control groups (p > 0.05).
Figure 5.
MDMA affects select 1H-MR-visible metabolites involved in cardiomyocyte energy regulation in rats. In tissue samples obtained from the left ventricular wall of MDMA-treated rats (8 h), a significant increase in carnitine (see inset A, Fig. 2) was determined by proton-magnetic resonance spectroscopy. (*p < 0.05 compared to control; mean ± s.e.m.; N = 4–5/group).
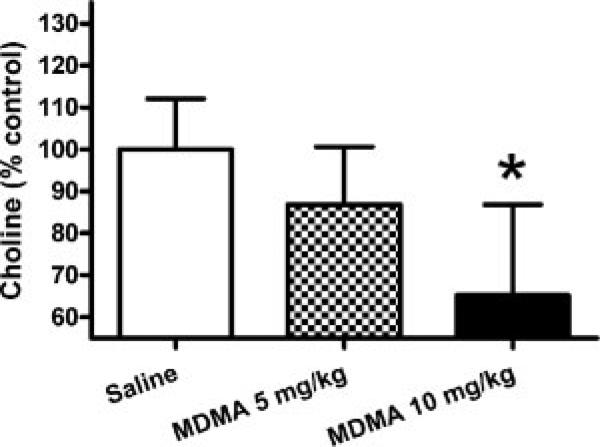
Choline levels were significantly decreased (Fig. 6) after 10 mg/kg MDMA (ANOVA: F2,10 = 4.769 p = 0.035; Dunnett's posthoc: saline vs. 10 mg/kg p < 0.05). Levels of taurine were not significantly different between MDMA and control groups (p > 0.05; data not shown). Concentrations of MR-visible cardiac metabolites in control animals were (nmol/mg tissue; mean ± s.e.m.): alanine 1.05 ± 0.13, carnitine 1.42 ± 0.08, choline 0.37 ± 0.02, creatine 7.59 ± 0.43, glutamate 3.47 ± 0.34, glutamine 3.52 ± 0.10, lactate 20.94 ± 0.54, succinate 0.52 ± 0.05, and taurine 22.45 ± 0.49.
Figure 6.
MDMA decreases 1H-MR-visible choline in left ventricular heart wall tissue. HRMAS 1H-MRS analysis of choline in rat heart tissue (left ventricular wall) revealed a significant decrease after 10 mg/kg MDMA (*p < 0.05 compared to control; mean ± s.e.m.; N = 4–5/group).
DISCUSSION
Recent reviews of 5HT receptor function in the human cardiovascular system detail the molecular biology, anatomy, physiology, pharmacology, and pathology related to serotonergic signaling (16,17,28). MDMA, like fenfluramine, is an indirect serotonergic agonist that elevates 5HT levels via SERT reversal, and therefore the drug effects are broad and not selective with respect to any one 5HT receptor subtype. The heart has a high population of 5HT receptors with both atria, both ventricles, the SA node, and the pulmonary veins containing primarily 5HT4 receptors, 5HT2B receptors in the valves (pulmonary, right AV, aortic, and left AV), 5HT3 receptors in the afferent vagus nerve, and 5HT1B and 5HT2A receptors in the pulmonary and coronary arteries (16). Since 5HT is a powerful vasoconstrictor (29), a smooth muscle mitogen (30), and cardiovascular instability is associated with acute MDMA lethality (31), we used 1H-MRS and HPLC to determine the effect of MDMA on the cardiac metabolic profile and monoamine levels in rats treated with MDMA.
After 8 h of MDMA exposure, tissue levels of 5HT, but not NE, were substantially decreased (Fig. 4). Decreased 5HT tissue levels after 8 h MDMA exposure likely reflects sustained reversal of SERT combined with inhibition of tryptophan hydroxylase (32). In neuronal tissue, SERT reversal leads to a transient elevation of synaptic 5HT and potentiation of 5HT signaling. However, since peripheral 5HT may be more hormonal than neuronal, the precise mechanism (or consequences) of decreased 5HT in cardiac tissue remains to be determined. In fact, the MDMA-induced decrease in 5HT may reflect depletion of 5HT stores in platelets from blood within the tissue since the heart tissue was not perfused before isolation of samples. MDMA-induced serotonergic activation in cardiomyocytes has been shown to regulate cardiovascular function acutely and has long-lasting effects on cardiac myocyte biology (1,18,33); however, it remains to be determined if the MDMA-induced changes in serotonin levels contribute to the changes in MR-visible metabolites.
Along with the acute (positive) subjective effects of euphoria, empathy, and relaxation, MDMA causes several acute (negative) physiological effects including exhaustion, tachycardia, hypertension, dehydration, and hyperthermia. Data in Fig. 3 confirm that MDMA-treated animals displayed dose-dependent hyperthermia.
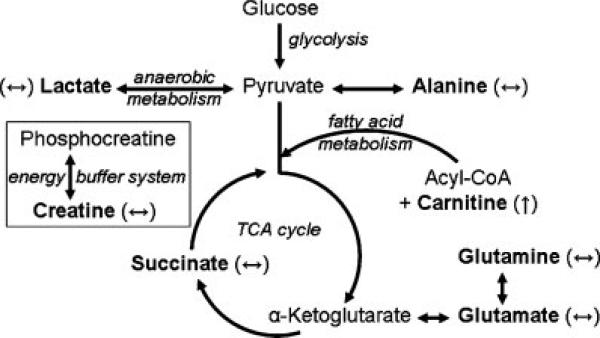
The increased cardiac workload subsequent to MDMA-induced tachycardia requires more energy, which can come from multiple biochemical pathways (see Fig. 7 for review). The heart produces ATP via the citric acid cycle and oxidative phosphorylation, using either glucose (glycolysis) or fatty acids (β-oxidation; fatty acid metabolism) as the source of acetate consumption by the citric acid cycle. In cardiac tissue, fatty acid metabolism is the predominant source of energy since fatty acids are efficiently oxidized to yield large quantities of ATP-energy. Carnitine availability controls the rate of mitochondrial β-oxidation because cytosolic acyl groups can only cross the mitochondrial membrane as an acyl-carnitine conjugate in the presence of carnitine palmitoyltransferase-I (34,35). Besides oxidative phosphorylation based on carbohydrates or fatty acid substrates, cardiac tissue also has the ability to obtain energy from two other sources. In situations of compromised oxygen, lactate dehydrogenase-1 converts pyruvate to lactate and NAD+; in the reverse reaction, cardiac tissue can utilize circulating lactate as a source for pyruvate (and subsequent entry into the citric acid cycle). Additionally, dephosphorylation of high-energy phosphocreatine buffers fluctuations in the ATP/ADP ratio and free energy provided by ATP-energy.
Figure 7.
Energy pathways in cardiomyocytes. Multiple metabolic pathways provide adequate energy sources for cardiac requirements: Glycolysis, anaerobic metabolism, fatty acid metabolism, and the creatine energy buffer system (compartmentalization not shown). MR-visible metabolites are shown in bold font and include lactate, alanine, succinate, glutamate, glutamine, carnitine, and creatine. MDMA-induced changes in metabolite levels are shown in parentheses: ↑ = significant increase and ↔ = no change.
Here we show that MDMA has multiple effects on intermediate metabolites in cardiac energy pathways (Fig. 7). The lack of effect on lactate (or decreased trend) suggests that anaerobic conditions are not driving the conversion of pyruvate to lactate and that glycolysis is likely not compromised. Unchanged glutamate and glutamine levels suggest that the equilibrium with α-ketoglutarate is intact. Unchanged glutamate or glutamine is consistent with a properly functioning citric acid cycle because the enzymes involved in α-ketoglutarate metabolism (isocitrate dehydrogenase and α-ketoglutarate dehydrogenase complex) are key pathway regulators and therefore a disruption in the cycle would be reflected in changes in α-ketoglutarate and its equilibrium with glutamate–glutamine. Furthermore, the levels of succinate, another intermediate of the citric acid cycle, are not significantly affected, which also suggests that the citric acid cycle is not compromised. The significant increase in carnitine following 8 h of MDMA exposure suggests that fatty acid metabolism has increased to sustain its role in the production of ATP beyond normal capacity. Finally, our data show that levels of total creatine are not changed suggesting that the ATP/ADP ratio may be regulated adequately.
Circulating carnitine, obtained from dietary sources or synthesized de novo in the liver, is transported into cardiac tissue by the organic cation transporter-2 (OCTN2) (36,37). Cellular carnitine is primarily cytosolic and exists either as the free entity, an acyl conjugate, or acetylated; 70% of rat cardiac carnitine exists in the free form (38). Acetylated carnitine is localized within mitochondria where it acts as a sink for excess acetate and therefore maintains adequate levels of free coenzyme A (CoA-SH) (39). The proton MR signal at 3.24 ppm represents the N-trimethyl moiety and therefore is a composite of a total cellular carnitine; acetyl carnitine is distinguished by an acetyl-methyl group ~2.1 ppm (40). The absence of a prominent peak at 2.1 ppm (Fig. 2) in the MR-visible cardiac profile suggests that acetyl-carnitine is not a major constituent in cardiac mitochondria, in contrast to skeletal muscle where acetyl-carnitine may represent 40% of the total carnitine. The relatively low concentration of acetyl-carnitine in heart provides an adequate supply of free carnitine to support fatty acid transport into cardiac mitochondria. Increasing muscle carnitine levels by exogenous carnitine has been difficult to demonstrate despite repeated attempts to enhance fatty acid oxidation (relevant to athletic performance and type II diabetes) by supplemental carnitine (41). However, high levels of plasma insulin appear to augment carnitine clearance (into muscle via OCTN2) (39). Our observation that acute MDMA exposure increases cardiac carnitine is the first report of an MDMA-induced alteration in this key mediator of substrate availability for energy homeostasis.
Carnitine has also been suggested to play a protective role in compromised cardiac muscle; as such it is recommended as a supplement for the treatment of congestive heart failure, heart attack, and angina (42). Investigation of carnitine for treatment of ischemia revealed that it enhances the efficiency of heart tissue, rather than decreasing the work on the heart (43). Carnitine has also been shown to effectively prevent mitochondrial injury induced by oxidative stress (44,45) by decreasing free long-chain fatty acids that damage mitochondrial membranes (46). The putative roles of carnitine in cardiac function and protection suggest that the MDMA-induced increase in carnitine may reflect an allostatic compensatory mechanism to regain balance in energy regulation, allow the heart to respond to the increased workload by increasing fatty acid metabolism, and act as an antiradical agent (47).
In addition to the effects on levels of metabolites involved in cardiomyocyte energy utilization, MDMA decreased choline (Fig. 6). Decreased choline may impact a variety of cellular functions including synthesis of phosphatidylcholine membrane constituents or synthesis of acetylcholine. Acetylcholine is a key regulator of parasympathetic activity in the heart where it decreases heart contractility. However, the choline that contributes to the formation of acetylcholine in parasympathetic neurons of the heart is small relative to total choline and unlikely to affect the total MR-visible pool of choline measured in heart tissue, which is comprised primarily of cardiomyocytes. On the other hand, a decrease in choline may affect membrane levels of phosphatidylcholine, which in addition to disrupting the lipid composition of the membrane could influence its role in membrane-associated cell signaling.
In summary, MDMA produces effects on cardiovascular function and energy metabolomics. The increase in carnitine as shown here is consistent with an increase in fatty acid metabolism to maintain energy homeostasis. The relationship between increases in MDMA-induced body temperature and 1H-MRS metabolite levels suggest that the hyperthermia associated with amphetamines may contribute to the changes in energy regulation. Future experiments are focused on determining the involvement of MDMA-induced hyperthermia and locomotor stimulant effects, as well as, determining if these effects are mediated by sympathetic activation or serotonergic signaling.
Acknowledgements
The authors thank Dr Shonagh O'Leary-Moore for technical assistance and Drs Donal O'Leary and Donald Kuhn for experimental advice. This work was funded by Joe Young Research Fund to Psychiatry, NIH grant R01DA016736 to M. P. G., and NIH grant 1K01DA024760 to S. A. P.
Contract/grant sponsor: Joe Young Research Fund to Psychiatry, NIH; contract/grant numbers: R01DA016736; 1K01DA024760.
Abbreviations used
- HPLC
high pressure liquid chromatography
- HR-MAS 1H-MRS
high resolution-magic angle spinning proton magnetic resonance spectroscopy; 5HT
- MDMA
3,4-methylenedioxymethamphetamine
- NE
norepinephrine
- SERT
serotonin transporter
Footnotes
The authors have no conflicts to disclose related to this study.
REFERENCES
- 1.Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ. Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of Ecstasy. J. Pharmacol. Exp. Ther. 2002;302(3):898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- 2.O'Cain PA, Hletko SB, Ogden BA, Varner KJ. Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol. Behav. 2000;70(1–2):141–148. doi: 10.1016/s0031-9384(00)00235-3. [DOI] [PubMed] [Google Scholar]
- 3.Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, Mendelson J. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A. double-blind, placebo-controlled trial. Ann. Int. Med. 2000;133(12):969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- 4.Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J. Pharmacol. Exp. Ther. 1999;290(1):136–145. [PubMed] [Google Scholar]
- 5.Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl.) 2007;189(4):565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- 6.NIDA . Monitoring the Future: National Results on Adolescent Drug Use. National Institute on Drug Abuse; Bethesda, Maryland 20892: 2004. [Google Scholar]
- 7.SAMHSA . Interim National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration. Office of Applied Studies; Rockville, MD: 2004. Drug Abuse Warning Network 2003. [Google Scholar]
- 8.Hansen JP, Riddle EL, Sandoval V, Brown JM, Gibb JW, Hanson GR, Fleckenstein AE. Methylenedioxymethamphetamine decreases plasmalemmal and vesicular dopamine transport: mechanisms and implications for neurotoxicity. J. Pharmacol. Exp. Ther. 2002;300(3):1093–1100. doi: 10.1124/jpet.300.3.1093. [DOI] [PubMed] [Google Scholar]
- 9.Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 2006;319(1):237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- 10.Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol. Pharmacol. 1993;44(6):1227–1231. [PubMed] [Google Scholar]
- 11.Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology. 1994;10(4):231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- 12.Lyon RA, Glennon RA, Titeler M. 3,4-Methylenedioxymethamphetamine (MDMA): stereoselective interactions at brain 5-HT1 and 5-HT2 receptors. Psychopharmacology (Berl.) 1986;88(4):525–526. doi: 10.1007/BF00178519. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1987;240(1):1–7. [PubMed] [Google Scholar]
- 14.Ricaurte GA. Studies of MDMA-induced neurotoxicity in nonhuman primates: a basis for evaluating long-term effects in humans. NIDA Res. Monogr. 1989;94:306–322. [PubMed] [Google Scholar]
- 15.McCann UD, Eligulashvili V, Ricaurte GA. (+/-)3,4-Methylenedioxymethamphetamine (’Ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology. 2000;42(1):11–16. doi: 10.1159/000026665. [DOI] [PubMed] [Google Scholar]
- 16.Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006;111(3):674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Watts SW. 5-Hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin. Exp. Pharmacol. Physiol. 2006;33(7):575–583. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 18.Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3,4-Methylenedioxymethamphetamine (MDMA, ‘‘Ecstasy’’) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol. Pharmacol. 2003;63(6):1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- 19.Roth BL. Drugs and valvular heart disease. N. Engl. J. Med. 2007;356(1):6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 20.Loke YK, Derry S, Pritchard-Copley A. Appetite suppressants and valvular heart disease – a systematic review. BMC Clin. Pharmacol. 2002;2:6. doi: 10.1186/1472-6904-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonneau G, Fartoukh M, Sitbon O, Humbert M, Jagot JL, Herve P. Primary pulmonary hypertension associated with the use of fenfluramine derivatives. Chest. 1998;114(3 Suppl):195S–199S. doi: 10.1378/chest.114.3_supplement.195s. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit. Rev. Neurobiol. 2005;17(2):87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci. Lett. 2004;367(3):349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 24.Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, II, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151(2):533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur. J. Pharmacol. 2000;398(1):11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 26.Cheng LL, Anthony DC, Comite AR, Black PM, Tzika AA, Gonzalez RG. Quantification of microheterogeneity in glioblastoma multiforme with ex vivo high-resolution magic-angle spinning (HRMAS) proton magnetic resonance spectroscopy. Neuro-oncology. 2000;2(2):87–95. doi: 10.1093/neuonc/2.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 28.Cote F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol. Med. 2004;10(5):232–238. doi: 10.1016/j.molmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J. Biol. Chem. 1948;176(3):1243–1251. [PubMed] [Google Scholar]
- 30.Nemecek GM, Coughlin SR, Handley DA, Moskowitz MA. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc. Natl. Acad. Sci. USA. 1986;83(3):674–678. doi: 10.1073/pnas.83.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AP, Henry JA. Acute toxic effects of ’Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br. J. Anaesth. 2006;96(6):678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 32.Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 1986;128(1–2):41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho M, Remiao F, Milhazes N, Borges F, Fernandes E, Monteiro Mdo C, Goncalves MJ, Seabra V, Amado F, Carvalho F, Bastos ML. Metabolism is required for the expression of ecstasy-induced cardiotoxicity in vitro. Chem. Res. Toxicol. 2004;17(5):623–632. doi: 10.1021/tx049960f. [DOI] [PubMed] [Google Scholar]
- 34.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin. Med. Genet. 2006;142(2):77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvajal K, Moreno-Sanchez R. Heart metabolic disturbances in cardiovascular diseases. Arch. Med. Res. 2003;34(2):89–99. doi: 10.1016/S0188-4409(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 36.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J. Pharmacol. Exp. Ther. 1999;290(3):1482–1492. [PubMed] [Google Scholar]
- 38.Negrao CE, Ji LL, Schauer JE, Nagle FJ, Lardy HA. Carnitine supplementation and depletion: tissue carnitines and enzymes in fatty acid oxidation. J. Appl. Physiol. 1987;63(1):315–321. doi: 10.1152/jappl.1987.63.1.315. [DOI] [PubMed] [Google Scholar]
- 39.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. A threshold exists for the stimulatory effect of insulin on plasma L-carnitine clearance in humans. Am. J. Physiol. Endocrinol. Metab. 2007;292(2):E637–641. doi: 10.1152/ajpendo.00508.2006. [DOI] [PubMed] [Google Scholar]
- 40.Kagawa M, Machida Y, Nishi H, Haginaka J. Enantiomeric purity determination of acetyl-L-carnitine by NMR with chiral lanthanide shift reagents. J. Pharm. Biomed. Anal. 2005;38(5):918–923. doi: 10.1016/j.jpba.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007;581(Pt 2):431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allard ML, Jeejeebhoy KN, Sole MJ. The management of conditioned nutritional requirements in heart failure. Heart Fail. Rev. 2006;11(1):75–82. doi: 10.1007/s10741-006-9195-3. [DOI] [PubMed] [Google Scholar]
- 43.Parang P, Singh B, Arora R. Metabolic modulators for chronic cardiac ischemia. J. Cardiovasc. Pharmacol. Ther. 2005;10(4):217–223. doi: 10.1177/107424840501000402. [DOI] [PubMed] [Google Scholar]
- 44.Chang B, Nishikawa M, Sato E, Utsumi K, Inoue M. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch. Biochem. Biophys. 2002;405(1):55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 45.Furuno T, Kanno T, Arita K, Asami M, Utsumi T, Doi Y, Inoue M, Utsumi K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem. Pharmacol. 2001;62(8):1037–1046. doi: 10.1016/s0006-2952(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 46.Chang B, Nishikawa M, Nishiguchi S, Inoue M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int. J. Cancer. 2005;113(5):719–729. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- 47.Reznick AZ, Kagan VE, Ramsey R, Tsuchiya M, Khwaja S, Serbinova EA, Packer L. Antiradical effects in L-propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch. Biochem. Biophys. 1992;296(2):394–401. doi: 10.1016/0003-9861(92)90589-o. [DOI] [PubMed] [Google Scholar]