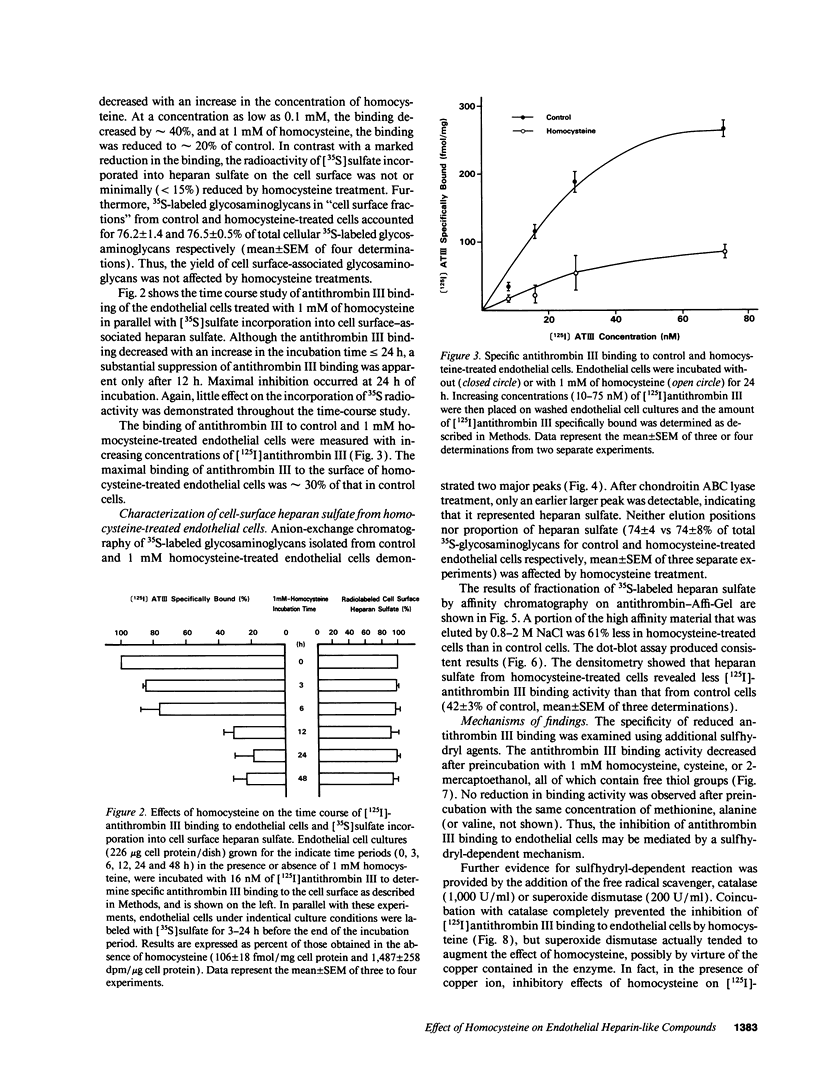
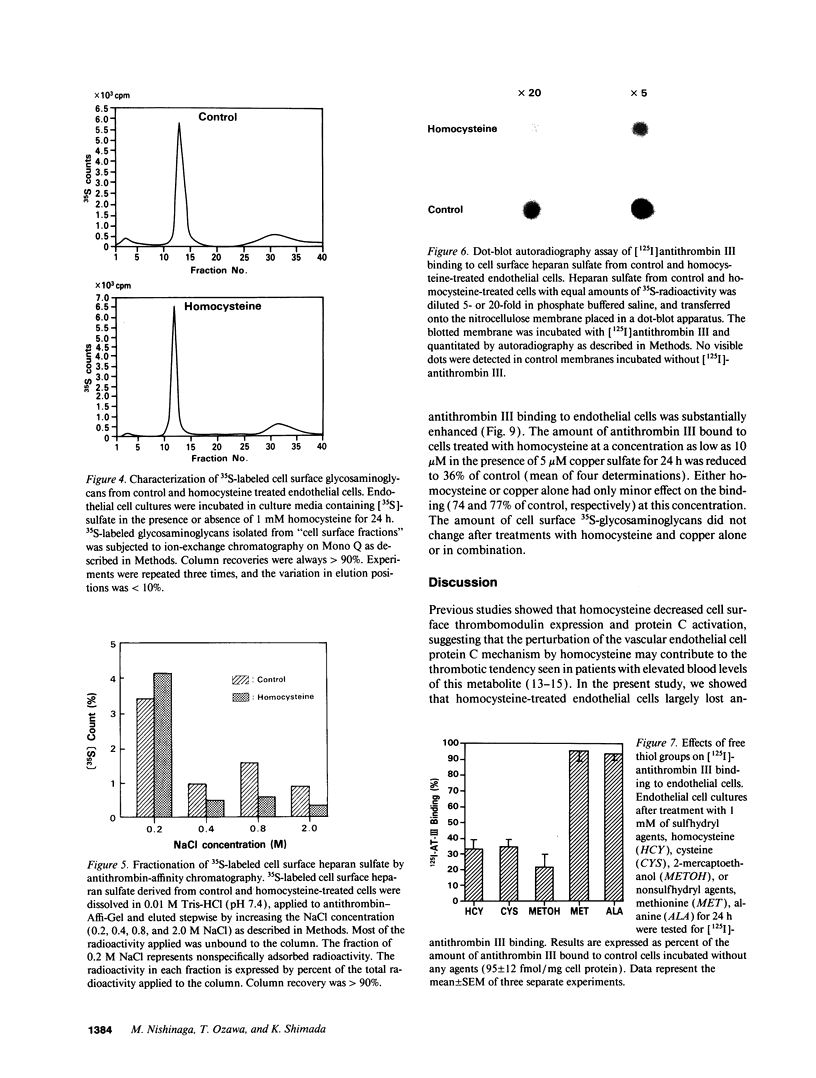
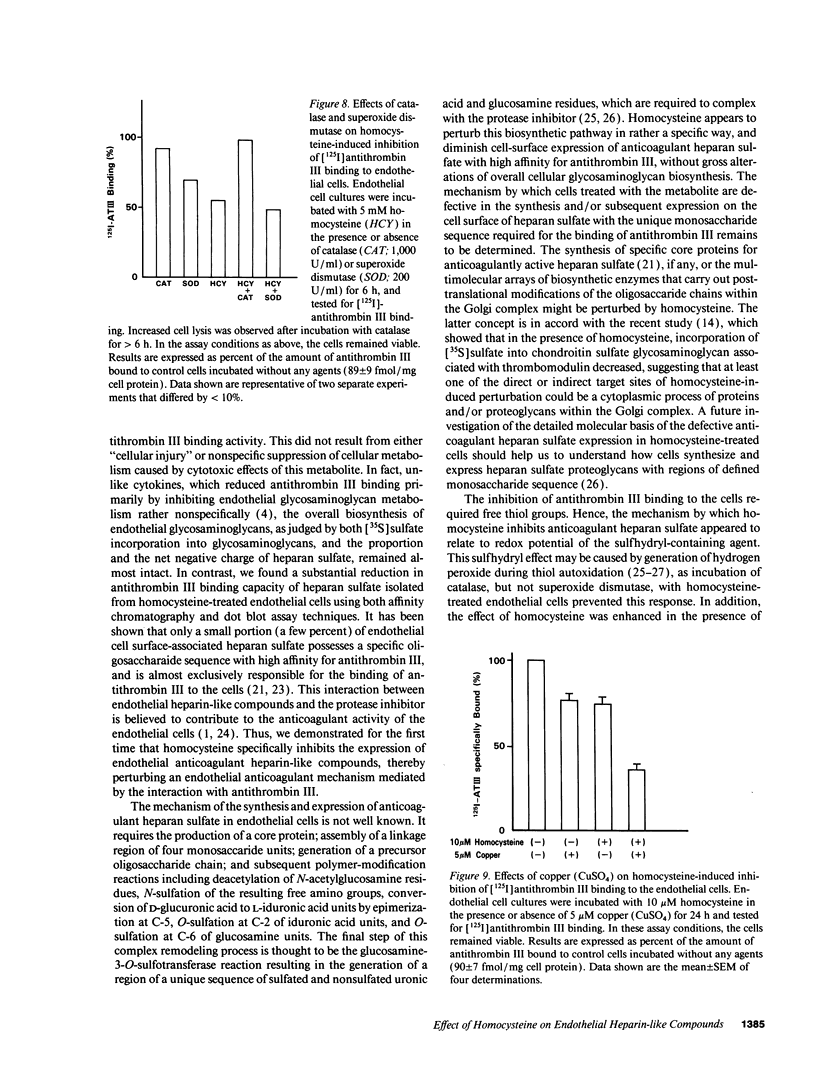
Abstract
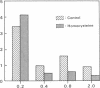
Previous studies showed that homocysteine, a thrombo-atherogenic and atherogenic agent, inhibits an endothelial thrombomodulin-protein C anticoagulant pathway. We examined whether homocysteine might affect another endothelial anticoagulant mechanism; i.e., heparin-like glycosaminoglycan-antithrombin III interactions. Incubations of porcine aortic endothelial cell cultures with homocysteine reduced the amount of antithrombin III bound to the cell surface in a dose- and time-dependent fashion. The inhibitory effect was observed at a homocysteine concentration as low as 0.1 mM, and the maximal suppression occurred at 1 mM of homocysteine after 24 h. In contrast with a marked reduction in the maximal antithrombin III binding capacity (approximately 30% of control), the radioactivity of [35S]sulfate incorporated into heparan sulfate on the cell surface was minimally (< 15%) reduced. The cells remained viable after homocysteine treatment. Although neither net negative charge nor proportion in total glycosaminoglycans of cell surface heparan sulfate was altered by homocysteine treatment, a substantial reduction in antithrombin III binding capacity of heparan sulfate isolated from homocysteine-treated endothelial cells was found using both affinity chromatography and dot blot assay techniques. The antithrombin III binding activity of endothelial cells decreased after preincubation with 1 mM homocysteine, cysteine, or 2-mercaptoethanol; no reduction in binding activity was observed after preincubation with the same concentration of methionine, alanine, or valine. This sulfhydryl effect may be caused by generation of hydrogen peroxide, as incubation of catalase, but not superoxide dismutase, with homocysteine-treated endothelial cells prevented this reduction, whereas copper augmented the inhibitory effects of the metabolite. Thus, our data suggest that the inhibited expression of anticoagulant heparan sulfate may contribute to the thrombogenic property resulting from the homocysteine-induced endothelial cell perturbation, mediated by generation of hydrogen peroxide through alteration of the redox potential.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991 Apr 25;324(17):1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- De Agostini A. L., Lau H. K., Leone C., Youssoufian H., Rosenberg R. D. Cell mutants defective in synthesizing a heparan sulfate proteoglycan with regions of defined monosaccharide sequence. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9784–9788. doi: 10.1073/pnas.87.24.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman N. P., Hicks C., Wang J., Wilcken D. E. Human arterial endothelial cell detachment in vitro: its promotion by homocysteine and cysteine. Atherosclerosis. 1991 Nov;91(1-2):77–83. doi: 10.1016/0021-9150(91)90189-a. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The protein C anticoagulant pathway. Arterioscler Thromb. 1992 Feb;12(2):135–145. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Honda G., Suzuki K. An atherogenic stimulus homocysteine inhibits cofactor activity of thrombomodulin and enhances thrombomodulin expression in human umbilical vein endothelial cells. Blood. 1992 Jun 1;79(11):2930–2936. [PubMed] [Google Scholar]
- Kobayashi M., Shimada K., Ozawa T. Human recombinant interleukin-1 beta- and tumor necrosis factor alpha-mediated suppression of heparin-like compounds on cultured porcine aortic endothelial cells. J Cell Physiol. 1990 Sep;144(3):383–390. doi: 10.1002/jcp.1041440304. [DOI] [PubMed] [Google Scholar]
- Kojima T., Leone C. W., Marchildon G. A., Marcum J. A., Rosenberg R. D. Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J Biol Chem. 1992 Mar 5;267(7):4859–4869. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lentz S. R., Sadler J. E. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J Clin Invest. 1991 Dec;88(6):1906–1914. doi: 10.1172/JCI115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Kusche M., Lidholt K., Oscarsson L. G. Biosynthesis of heparin and heparan sulfate. Ann N Y Acad Sci. 1989;556:36–50. doi: 10.1111/j.1749-6632.1989.tb22488.x. [DOI] [PubMed] [Google Scholar]
- Malinow M. R. Hyperhomocyst(e)inemia. A common and easily reversible risk factor for occlusive atherosclerosis. Circulation. 1990 Jun;81(6):2004–2006. doi: 10.1161/01.cir.81.6.2004. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Mudd S. H., Skovby F., Levy H. L., Pettigrew K. D., Wilcken B., Pyeritz R. E., Andria G., Boers G. H., Bromberg I. L., Cerone R. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985 Jan;37(1):1–31. [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers G. M., Conn M. T. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood. 1990 Feb 15;75(4):895–901. [PubMed] [Google Scholar]
- Rodgers G. M., Kane W. H. Activation of endogenous factor V by a homocysteine-induced vascular endothelial cell activator. J Clin Invest. 1986 Jun;77(6):1909–1916. doi: 10.1172/JCI112519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Rosenberg J. S. Natural anticoagulant mechanisms. J Clin Invest. 1984 Jul;74(1):1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez G., Thornalley P. J., Hill H. A., Hems R., Bannister J. V. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim Biophys Acta. 1982 Oct 28;719(1):24–31. doi: 10.1016/0304-4165(82)90302-6. [DOI] [PubMed] [Google Scholar]
- Shimada K., Kawamoto A., Matsubayashi K., Ozawa T. Histidine-rich glycoprotein does not interfere with interactions between antithrombin III and heparin-like compounds on vascular endothelial cells. Blood. 1989 Jan;73(1):191–193. [PubMed] [Google Scholar]
- Shimada K., Ozawa T. Evidence that cell surface heparan sulfate is involved in the high affinity thrombin binding to cultured porcine aortic endothelial cells. J Clin Invest. 1985 Apr;75(4):1308–1316. doi: 10.1172/JCI111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Ozawa T. Modulation of glycosaminoglycan production and antithrombin III binding by cultured aortic endothelial cells treated with 4-methylumbelliferyl-beta-D-xyloside. Arteriosclerosis. 1987 Nov-Dec;7(6):627–636. doi: 10.1161/01.atv.7.6.627. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Malinow M. R., Willett W. C., Newcomer L. M., Upson B., Ullmann D., Tishler P. V., Hennekens C. H. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992 Aug 19;268(7):877–881. [PubMed] [Google Scholar]
- Starkebaum G., Harlan J. M. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986 Apr;77(4):1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harlan J. M., Harker L. A., Striker G. E. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980 Apr 1;18(1-2):113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb. 1980;5:1–39. [PubMed] [Google Scholar]