Abstract
Studies in humans and monkeys report widespread multisensory interactions at or near primary visual and auditory areas of neocortex. The range and scale of these effects has prompted increased interest in interconnectivity between the putatively “unisensory” cortices at lower hierarchical levels. Recent anatomical tract-tracing studies have revealed direct projections from auditory cortex to primary visual area (V1) and secondary visual area (V2) that could serve as a substrate for auditory influences over low-level visual processing. To better understand the significance of these connections, we looked for reciprocal projections from visual cortex to caudal auditory cortical areas in macaque monkeys. We found direct projections from area prostriata and the peripheral visual representations of area V2. Projections were more abundant after injections of temporoparietal area and caudal parabelt than after injections of caudal medial belt and the contiguous areas near the fundus of the lateral sulcus. Only one injection was confined to primary auditory cortex (area A1) and did not demonstrate visual connections. The projections from visual areas originated mainly from infragranular layers, suggestive of a “feedback”-type projection. The selective localization of these connections to peripheral visual areas and caudal auditory cortex suggests that they are involved in spatial localization.
Keywords: auditory cortex, connectivity, monkey, multisensory, V1
Introduction
Convergence of neuronal inputs from different sensory modalities is required for multisensory integration. Recent anatomical studies have identified direct “heteromodal” projections between low-level putatively modality-specific auditory, visual, and somatosensory cortices (Falchier et al. 2002; Rockland and Ojima 2003; Cappe and Barone 2005; De La Mothe et al. 2006; Smiley et al. 2007; Hall and Lomber 2008; Wang et al. 2008). These findings are reproduced in a number of species including ferrets (Bizley et al. 2007), gerbils (Budinger et al. 2006, 2008; Budinger and Scheich 2009), rats (Vaudano et al. 1991), and mice (Wang and Burkhalter 2007). They are augmented by a virtual explosion of physiological demonstrations of multisensory interaction in low-level cortices in a number of species, even at the level of koniocortex (reviewed by Ghazanfar and Schroeder 2006; Driver and Noesselt 2008; Schroeder et al. 2008). The wide generality of cross-modal connections between low-level sensory cortices, and their apparent impact on unisensory processing, suggests that they are a fundamental part of neocortical circuitry.
Are low-level cross-modal projections specific to certain functional pathways? Are they reciprocal? Are their anatomical characteristics those of feedforward, feedback, or lateral projections? We addressed these questions at the level of primate primary visual cortex (V1) and secondary visual cortex (V2) areas because they receive direct input from auditory cortex (Falchier et al. 2002; Rockland and Ojima 2003). V2 provides the conduit for the vast majority of V1 outputs to higher order visual cortices, with the exception of a few “level-jumping” projections, like those from V1 to V4 (Felleman and Van Essen 1991).
The auditory cortical projections to V1 appear be functionally specific, in that they arise primarily from the caudal auditory areas, which are proposed to be the auditory analog of the dorsal visual (where) pathway (Romanski, Tian, et al. 1999; Rauschecker and Tian 2000). These projections terminate exclusively in the region of V1 and V2 representing peripheral visual space (Falchier et al. 2002; Rockland and Ojima 2003). Regarding “reciprocity”, there is some indication of a V2 projection to auditory cortex (Rockland and Ojima 2003), but the question is basically open.
The first goal of this study was to investigate if there is a substantial projection from early visual cortex to the different areas of caudal auditory cortex. To our knowledge, this issue has not been addressed systematically in macaque monkeys, although a previous study looked for similar connections in marmosets (Cappe and Barone 2005). Our second goal was to define the type of anatomical pathway linking visual to auditory cortex. That is, we wanted to know whether the visual–auditory projection is a feedforward type that would drive excitatory responses in layer IV neurons or a feedback type that would modulate the excitability of the neurons outside layer IV (Rockland and Pandya 1979; Felleman and Van Essen 1991; Bullier et al. 2001). We used retrograde tracers to investigate the extent and nature of inputs to caudal auditory cortices stemming from V2 and nearby low-level visual areas. Our findings verify this input and outline a type of “feedback loop” that may be a characteristic feature of early cross-modal corticocortical pathways.
Materials and Methods
Animal Subjects
The experiments described in this report used 10 adult macaque monkeys (Rhesus monkeys) and were conducted at the Nathan Kline Institute (Orangeburg, NY) and at the Institute for Psychology, Hungarian Academy of Sciences (Budapest, Hungary). All procedures involving animals followed National Institutes of Health guidelines for the use of laboratory animals. In each animal, injections of one or more neuroanatomical tracers were made into target areas of the lateral fissure. Injections into the caudal auditory areas were guided by prior functional mapping of frequency tuning, tonotopy, and somatosensory responses with linear multiarray electrodes.
General Surgical Procedures
Aseptic techniques were employed during all surgical procedures. Anesthesia was induced by intramuscular injection of ketamine hydrochloride (10 mg/kg), then maintained by intravenous administration of sodium pentobarbital (25.0 mg/kg). Body temperature was kept at 37–38 °C with a heating pad. Heart rate, respiration, and body temperature were continuously monitored throughout the procedure and used to adjust anesthetic depth. The head was held by hollow ear bars affixed to a stereotaxic frame (David Kopf Instruments, Tujunga, CA). With standard aseptic surgical techniques, a midline incision was made to expose the skull, followed by retraction of the skin and left temporal muscle. A craniotomy was performed exposing the intraparietal sulcus and dorsal superior temporal gyrus. After retraction of the dura mater, warm silicone was applied to the brain to prevent desiccation of the cortex. Photographs of the exposed cortical surface were taken for recording the locations of electrode penetrations in relation to the surface vascular pattern.
Tracer Injections
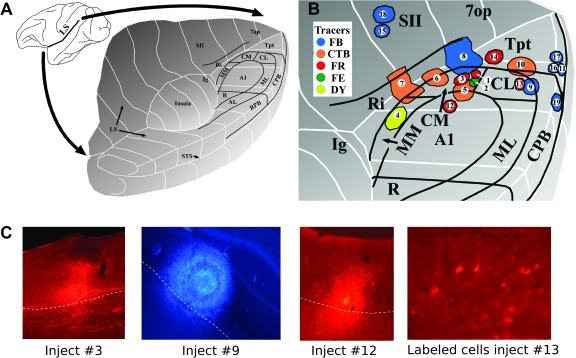
We performed 19 injections of retrograde tracers in 10 rhesus monkeys (Fig. 1 and Table 1). Multiarray electrode recording was used to identify cortical areas prior to the injection (Smiley et al. 2007). Two different methods were used to place neuroanatomical tracers (Table 1). “Cannula injections” were made by lowering a multiarray electrode through the parietal cortex to the level of caudal medial auditory belt area (CM) on the lower bank of the lateral sulcus and injecting the tracer through a fine cannula threaded through the shaft of the electrode array (see above). The cannula opened between channels 8 and 9, permitting precise laminar targeting of the injection by examination of laminar field potential and action potential patterns (Lakatos et al. 2005). In this study, the injections were centered on layer IV. However, especially with larger tracer injections, there was sometimes leakage up and down the shaft of the electrode. For this reason, most cannula injections used submicromolar tracer volumes, and some had contamination of white matter and cortex above and below the targeted site. “Direct pressure injections” were made through a pulled glass pipette affixed to a 1- or 2-μl Hamilton syringe under visual guidance and stereotaxic control, as recently described (Hackett et al. 2005). In this approach, the target area was first localized by using the multiarray electrode, as described above for cannula injections. The electrode was then removed and the sulcus gently retracted to allow injection at the stereotaxic coordinates determined from the electrode recording.
Figure 1.
(A) Three-dimensional schematic of the opened lateral sulcus showing the approximate location of the cortical areas in auditory cortex. (B) Enlarged view showing the locations of the 19 injection sites described in the text (modified from Smiley et al. 2007). The sites are numbered as in Table 1. The different tracers are color coded as shown in the inset. FR, fluoro-ruby; FE, fluoro-emerald; DY, diamidino yellow. (C) Photomicrographs show injection site in areas CM (injection no. 3), CL/CPB (no. 9), and A1 (no. 12). Labeled cells in V2 after injection in area CL (case no. 13).
Table 1.
Injection sites and summary of labeling in early visual areasa
| Group | Monkey | Tracer | Areas injected | Injection no. | Volume (μl) | Injection proc. | N total (N supra) |
Total cells par./temp. | Percent par./temp. | |
| No. of cells V2 | No. of cells prostriata | |||||||||
| 1 | ME | DY | MM/Ri | 4 | 1 | C | 0 | 0 | 1216 | 0.000 |
| MAG | FR | A1 | 12 | 0.4 | C | 0 | 0 | 2189 | 0.000 | |
| 2 | MAB | FR | CM | 1 | 0.4 | C | 1 (0) | 5 (3) | 2436 | 0.246 |
| MAM | FR | CM/SII/Ipa | 3 | 1.2 | C | 0 | 2 (0) | 2097 | 0.095 | |
| MZ | FE | CM/7op | 2 | 0.4 | C | 0 | 0 | 1233 | 0.000 | |
| MY | CTb | CM/A1 | 5 | 0.4 | P | 3 (0) | 0 | 16 316 | 0.018 | |
| MZ | CTb | CM/Ri | 6 | 0.4 | P | 0 | 1 | 46 820 | 0.002 | |
| MC | FB | CM/Ri/Tpt | 8 | 1 | P | 0 | 0 | 3269 | 0.000 | |
| MAG | CTb | CM/Ri/SII/Ipa | 7 | 0.2 | P | 2 (0) | 8 (1) | 75 828 | 0.013 | |
| 3 | MS | FR | CL | 13 | 0.4 | P | 5 (0) | 15 (0) | 286 | 6.993 |
| MAG | FB | CL/CPB | 9 | 0.4 | P | 76 (14) | 23 (0) | 13 973 | 0.709 | |
| MR | FB | CPB | 19 | 0.2 | P | 13 (0) | 7 (1) | 2993 | 0.668 | |
| 4 | MS | FB | Tpt gyral | 11 | 0.2 | P | 16 (0) | 90 (13) | 3550 | 2.986 |
| MT | FB | Tpt gyral | 17 | 0.4 | P | 12 (7) | 0 | 1761 | 0.681 | |
| MAB | FB | Tpt gyral | 16 | 0.2 | P | 7 (0) | 49 (6) | 10 297 | 0.544 | |
| MAB | CTb | Tpt planum | 10 | 0.4 | P | 17 (1) | 34 (6) | 35 398 | 0.144 | |
| MY | FR | Tpt planum | 14 | 0.4 | C | 3 (0) | 0 | 1468 | 0.204 | |
| 5 | MY | FB | SII/A1 | 15 | 0.2 | C | 0 | 0 | 8581 | 0.000 |
| MZ | FB | SII/CL | 18 | 0.2 | C | 0 | 0 | 4522 | 0.000 | |
Injections are organized into groups (Group.) according to the main area injected, as presented in the text. Tracers included cholera toxin subunit B (CTb), diamidino yellow (DY), fast blue (FB), fluoro-ruby (FR) or fluoro-emerald (FE). Injection procedures (Injection proc.) included direct pressured injections (P) or injections through a multiarray electrode cannula (C). (N) Total number of cells labeled in area V2 and prostriata. (N supra) Number of labeled cells in supragranular layers. The total cells number of cells plotted on the lateral surface of the parietal and temporal lobes in the ipsilateral hemisphere (Tot. cells par./temp.) was used as a reference to calculate the relative number of cells in early visual areas (Percent par./temp.).
Note: CL, caudal lateral auditory belt area; CM, caudal medial auditory belt area; CPB, caudal parabelt auditory area; DY, diamidino yellow; FE, fluoro-emerald; FR, fluoro-ruby; Ipa, area Ipa from Cusick et al. (1995); SII, secondary somatosensory area.
With either method of injection, it proved difficult to contain injections completely within a target area. Thus, some injections encroached on adjacent fields (Table 1), as determined by subsequent architectonic analysis. In this study, we used different retrograde tracers: diamidino yellow, fast blue (FB), cholera toxin B (CTb), fluoro-ruby, and fluoro-emerald. The survival times of 12–14 days were sufficient to ensure the labeling of long-distance projections. Monkeys were transcardially perfused and processed for histology as previously described (Smiley et al. 2007).
The distribution of retrograde labeling was recorded by plotting the cell distribution on at least every 12th 80-μm–thick section through both hemispheres of the entire brain. Auditory and visual areas were identified by their cytoarchitectonic features identified in adjacent series of sections that were Nissl stained, myelin stained, or immunolabeled with mouse anti-parvalbumin (Sigma-Aldrich, St Louis, MO). Area prostriata was clearly differentiated from the adjacent areas V1 and V2 by its low density of parvalbumin-positive cells across layers and, especially in the supragranular layers, by its characteristic appearance in Nissl-stained sections (accentuated layer II, incipient layer IV, lightly stained layer VI, poorly defined border between V and VI) and by its previously described topographical location (Morecraft et al. 2000; Rockland and Ojima 2003). In addition, area prostriata was more lightly myelinated than area V2 and nearby area 23 (Palmer and Rosa 2006). Area prostriata spans ∼4 mm in the calcarine sulcus. Caudally, area prostriata is in direct continuity with area V1 and is flanked laterally by visual area V2 (Morecraft et al. 2000).
Results
We examined the projections from low-level visual cortex to caudal auditory cortex. Injections of retrograde tracers were placed into caudal areas of the monkey lateral sulcus via direct pressure injections or through the cannulae of a linear multielectrode recording array (see Materials and Methods).
Individual injections were mainly limited to one cortical area but in most cases also involved adjacent areas in the lateral sulcus and occasionally in the superior temporal sulcus (Fig. 1). Therefore, those injection sites were designated according to the primary/secondary areas involved, for example, CM/primary auditory cortex (A1). In each case, we describe the injections according to the cortical area mainly involved by the injection site and then discuss the potential consequences of involvement of adjacent areas. The injection sites 1 to 13 were previously described in detail in a study of the local cortical and thalamic connections of caudal belt areas (Hackett et al. 2007; Smiley et al. 2007). Table 1 summarizes the characteristics of individual injections. In both Table 1 and the following descriptions, the injections are organized into groups according to the areas injected. These groups include the following: 1) injections into A1 and middle medial area (MM), 2) injections mainly into CM, 3) injections into caudal lateral auditory belt area (CL) and caudal parabelt auditory area (CPB), 4) injections into temporoparietal area (Tpt), and 5) injections mainly into area SII on the dorsal bank of the lateral sulcus.
Labeling in visual areas was plotted on 0.96-mm–spaced histological sections. Although both hemispheres were searched, labeling in visual areas V2 and prostriata was found only in the ipsilateral hemisphere.
Group 1: A1 and Medial Belt
Injection 12 (MAG Fruby) was made through a cannula and was a comparatively small injection clearly confined to area A1 near its caudal medial border. The injection was mainly confined to the middle cortical layers and did not reveal projections from visual cortex.
Injection 4 (ME DY), in medial belt area MM, was performed using a cannula in a location centered between A1 and retroinsular area (Ri). A small amount of tracer leaked into area Ri medially. Electrophysiological recordings performed in the site prior to injection showed response to somatosensory and auditory stimuli (Hackett et al. 2007; Smiley et al. 2007). This injection revealed no projecting cells from visual cortex.
Group 2: Caudal Belt Area CM
We placed 7 injections in area CM. Some of these also involved the adjacent medial areas A1, Ri, and SII, and one also involved area Ipa in the fundus of the superior temporal sulcus. Injection 1 (MAB FR) was made using a multiarray injectrode and was confined to area CM at the caudal and medial border of area A1. This injection labeled 5 cells in area prostriata and only one in area V2, distributed over 2 consecutive histological sections. Of the remaining 6 injections, 2 (nos 2 and 8) produced no labeling in visual areas and 4 (nos 3, 5, 6, and 7) produced weak labeling (Table 1). It is unlikely that the adjacent somatosensory area Ri receives robust visual projections as 2 of these injections (nos 6 and 7) had significant involvement of that area.
Overall, the injections into CM and the adjacent medial areas produced weak and variable projections from areas V2 and prostriata. The labeled cells were located in the dorsal bank of the anterior calcarine sulcus, within ∼3 mm from its rostral end. At this location, the rostral extent of V1 is still present in the fundus of the sulcus and areas prostriata and V2 extend medially across the dorsal bank of the sulcus. Area V2 on the dorsal bank represents the lower visual quadrant (Van Essen et al. 1984, 1986); area V2 at the very anterior part of calcarine sulcus represents the peripheral visual field, beyond 30° of eccentricity, possibly restricted to the monocular field (Van Essen et al. 1984, 1986; Gattass et al. 1997). The organization of area prostriata is less documented, but it is known to be highly connected with the peripheral visual representation of areas V1, V2, and middle temporal visual area (MT) and thus is probably involved in dorsal visual stream processing (Gattass et al. 1997; Palmer and Rosa 2006). Area prostriata was identified by histological criteria as well as its previously described topography (see Materials and Methods section). Fig. 2A shows, on a medial view of the brain, the rostral-to-caudal extent of the location of labeled cells in the visual cortex. This same location was labeled by injections of CM as well as by injections in the other auditory areas described below.
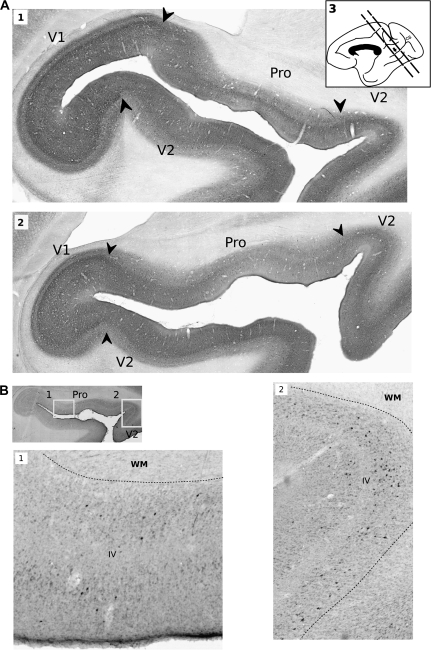
Figure 2.
Histological sections are shown to illustrate the topography of visual cortical areas and labeling in calcarine sulcus (CS), white matter (WM), and layer IV (IV). This example is taken from injection 10, which was located in gyral Tpt (MAB CTb). (A1) and (A2) Parvalbumin immunolabeling shows the limits between cortical areas in CS. (A3) A drawing of the medial view of the monkey brain shows the location of histological sections where labeled cells were found in the CS. (B1) and (B2) The laminar distribution of cells labeled with retrograde tracer is shown at higher magnification, in sections processed for CTb immunolabeling. The inset at left shows the origin of the micrographs in prostriata (B1) and V2 (B2).
Group 3: Caudal Lateral Belt and CPB
Three injections were placed in the CL and CPB areas. These produced labeled cells in the same region of V2 and area prostriata seen with injections in area CM, but there was clearly a larger number of labeled cells. Injection 13 (MS Fruby) was the only injection confined to area CL. This injection was small, and caused comparatively sparse labeling in auditory cortex, but nevertheless labeled a slightly larger number of cells in visual cortex than any of the CM injections (Table 1). In the absence of additional injections confined to CL, it is uncertain if this finding is representative of CL's connections. Injection 9 (MAG FB) was a large injection (0.4 μL) that targeted area CL and encroached on part of CPB (Fig. 1). This injection labeled 76 cells in peripheral area V2, over a region spanning a rostrocaudal distance of approximately 3 consecutive 0.96-mm–spaced sections. In addition, this injection produced relatively dense labeling in area prostriata, with 23 projecting cells distributed over 5 consecutive sections. Injection 19 (MR FB) was placed in the caudal CPB and revealed moderate projections from both areas V2 (13 cells) and prostriata (7 cells) distributed across 3 consecutive sections.
Group 4: Area Tpt
Five injections involved the gyral and medial (planum) parts of area Tpt. In most of these, the number of labeled cells in areas V2 and prostriata was usually at least as great as that produced by CPB injections. The exception was injection 14 (MY FR), located on the medial planum surface, which produced only a few cells in visual areas. Injection 10 (MAB CTb), which was lateral to injection 14 on the planum surface and encroached on caudal belt area CL, produced moderate labeling in both V2 (17 cells) and prostriata (34 cells). Injections 11, 16, and 17 were placed in the gyral portion of Tpt. All 3 showed moderate labeling of area V2 (Fig 3A). Two injections (11 and 16) had strong labeling of prostriata (49 and 90 cells, respectively), but injection 17 did not label the prostriata. In all Tpt injections, the location of labeled cells in the rostral calcarine sulcus was the same as that found with belt and parabelt injections.
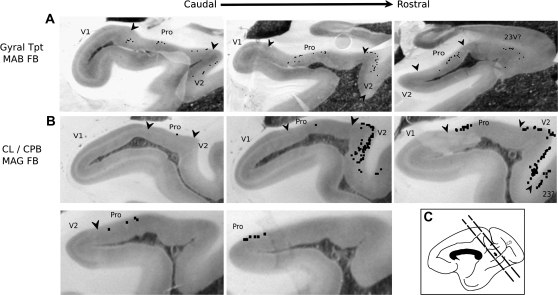
Figure 3.
The distribution of individual labeled cells (black squares) produced by 2 different injections is shown, plotted directly onto photomicrographs of histological sections. In each example, consecutive 0.96-mm–spaced sections are arranged in caudal-to-rostral order. (A) Injection 16 (MAB FB) in gyral Tpt resulted in labeled cells mainly in infragranular layers in areas prostriata and V2, in 3 consecutive sections. (B) Injection 9 (MAG FB) in CL/CPB resulted in labeled cells across 5 consecutive sections, with comparatively more labeling in V2 than prostriata.
Group 5: Area SII
Injections 15 and 18 (MY FB and MZ FB) were centered on somatosensory area SII on the dorsal bank of the lateral sulcus and did not cause labeling of early visual cortex. In both cases, there was some leakage of the tracer into auditory areas A1 or CL on the ventral bank of the sulcus. However, only the very superficial layers of auditory areas were involved, and lack of label in visual cortex may not be representative of their connections. Labeled cells were found in the parietal areas of the postcentral gyrus, intraparietal sulcus, and inferior parietal gyrus, consistent with the known connections of SII (Disbrow et al. 2003).
Quantitative Comparison of Visual Inputs to Different Auditory Areas
As described above, the number of labeled cells in visual areas was typically low after injections in CM and higher after injections of CPB and Tpt (Table 1). This difference is illustrated by statistical analysis of the number of labeled visual cells produced by these injections. For this purpose, we compared the relative number of cells in visual V2/prostriata, expressed as a percent of the total cells plotted on the lateral surface of the temporal and parietal lobes (Smiley et al. 2007). An alternative approach that simply analyzed the total number of plotted cells in these visual areas produced nearly identical statistical differences (not shown). In our previous study (Smiley et al. 2007), an error was made in computing the total number of cells in injections 1–10, and the correct values are presented in Table 1. The extent of underreporting was similar across layers and areas and had little effect on previously reported within-injection comparisons.
Due to the inhomogeneous variance of these samples, a nonparametric test (Kruskal–Wallis, SPSS 12.0) was used to compare differences between injection groups 2 (mainly CM), 3 (CPB/CL), and 4 (Tpt) (Table 1). This demonstrated a significant group difference (chi square = 8.78, degrees of freedom = 2, P < 0.01). Post hoc Mann–Whitney tests showed significant differences only between group 2 and each of the other groups, after Bonferroni correction for 3 comparisons (P values < 0.05/3). The findings indicate that areas CPB and Tpt have a higher density of visual inputs than area CM. This difference is not easily explained by the types of tracers used as CM injections labeled few visual cells even using large injections of the more sensitive tracers CTb and FB (injections 5, 6, and 8). Only one injection (no. 13) was confined to area CL, and more data are needed to evaluate whether the relatively high number of labeled visual cells is representative of the connections of CL.
After most injections, there were roughly 3 times as many cells in prostriata as in V2 (Table 1). However, 2 injections in CPB (nos 9 and 19) had proportionally more cells in V2 than prostriata (Fig 3B). Although statistical comparison of the proportion of cells in V2 compared with prostriata in groups 3 and 4 was not significant (Mann–Whitney tests, P > 0.1), it is possible that larger sample sizes would show preferential connections of V2 and prostriata with different auditory areas.
Laminar Distribution of Projecting Cells in Areas Prostriata and V2
The distribution of labeled cells in areas V2 and prostriata after injections of auditory areas was mainly infragranular. In V2, 86% of the labeled cells were infragranular; only infragranular cells were found in 8 of the 11 injections that labeled V2. In prostriata, 87% of the labeled cells were infragranular; only infragranular cells were found in 7 of the 12 injections that labeled area prostriata (Table 1).
These values are approximate because we did not use a high sampling frequency (0.96 mm spacing), and it is possible that we overlooked small foci of supragranular cells (Barone and Kennedy 2000; Vezoli et al. 2004).
Discussion
Our results indicate that the peripheral visual representations of early visual areas V2 and prostriata project to caudal auditory cortex. This means that the projections between caudal auditory cortex and low-level visual cortex first noted a few years ago (Falchier et al. 2002; Rockland and Ojima 2003) show some level of reciprocity. The present study also showed that the visual to auditory projection, like the reciprocal projection, appears to be denser in CPB and Tpt compared with core and belt areas (Fig. 4). The laminar distribution of the neurons projecting from V2 and prostriata onto auditory cortex corresponds to a feedback-type pathway. In this regard, the visual to auditory cortical pathway is strikingly similar to its reciprocal pathway (Falchier et al. 2002). Thus, auditory and low-level visual cortices are involved in a bidirectional network of feedback loops.
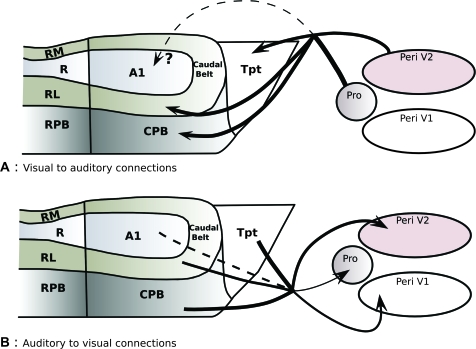
Figure 4.
Summary of cortical pathways described between caudal auditory cortex and low-level visual areas. (A) The present study demonstrated projections from the peripheral visual field representation in V2 (Peri V2) and prostriata (Pro) to caudal auditory areas. (B) The reciprocal projections from caudal auditory areas to early visual areas were previously described by Falchier et al. (2002) and Rockland and Ojima (2003). The thickness of arrows is proportional to their relative abundance. Dashed lines represent weak connections (A1 to visual) or putative pathways (visual cortex to A1, upper panel).
Reciprocity of Visuo-Auditory Convergence Pathways
Previous studies demonstrated that auditory cortex projects to areas V1, V2, and prostriata (Falchier et al. 2002; Rockland and Ojima 2003). The density of the projections was sparser to V1 than to the other areas and involved mainly the peripheral visual fields in both V1 and V2. Only a few retrogradely labeled auditory cells were found after injections of the near-central visual fields of V1. In auditory cortex, the source of the visual projection was mainly from cells in areas CPB and Tpt, but there were also some cells in belt areas and possibly in A1 (Falchier et al. 2002).
In the present study, we demonstrated a reciprocal projection from areas V2 and prostriata. However, we did not find evidence of projections from V1 to auditory cortex, even though we used a number of injections including 7 in CPB and Tpt. Thus, it appears that the projection from auditory cortex to V1 is not reciprocated. This is not surprising as V1 is known to receive sparse, unreciprocated, feedback-type projections from a variety of association and limbic areas of temporal, parietal, and frontal cortex (Felleman and Van Essen 1991; Barone et al. 2000; Clavagnier et al. 2004).
With respect to primary auditory area A1, its involvement in visual cross-modal connections is unclear. Retrograde injections in visual area V1 provided some evidence of a sparse input from A1 (Falchier et al. 2002). The present study did not demonstrate the reciprocal visual projections to A1, but only a single injection was made into A1, and it is possible that a sparse visual projection to A1 would be revealed by additional injections. In marmosets, retrograde tracers in A1 also did not produce labeled cells in early visual areas (Cappe and Barone 2005), but the ferret does have a projection from V1 to A1 (Bizley et al. 2007). At least some visual responses have been identified in A1 (Kayser et al. 2007, 2008), but these could arise from other sources besides early visual cortex, for example, from thalamic nuclei or multisensory association areas (Musacchia and Schroeder 2009; Smiley and Falchier 2009).
Relative Abundance of Visual Projections to Different Auditory Areas
In macaque monkeys, functional magnetic resonance imaging (fMRI) mapping of visual responses in the superior temporal plane showed that the areas surrounding the caudal end of A1 were more robustly activated than the rostral areas near core area R (Kayser et al. 2007). Within the caudal areas, the most robust activation was in lateral areas CL and CPB, whereas lesser activation was seen in areas A1 and CM. Area Tpt was not differentiated in that study but was included as part of CPB. Electrode recordings also showed visual responses across these areas in macaque monkeys, but these did not clearly demonstrate a stronger response in lateral compared with medial areas (Kayser et al. 2008). In humans, fMRI studies have not always differentiated between auditory areas, but at least some showed more robust visual activation in caudal areas (e.g., van Atteveldt et al. 2004; Lehmann et al. 2006; Antal et al. 2008).
Consistent with fMRI activation in monkeys (Kayser et al. 2007), the present study found that injections in lateral areas CL, CPB, and Tpt produced more labeling in visual cortex than injections in CM and the adjacent medial areas. Although only one tracer injection was confined to area CL, the slightly stronger labeling of visual cortex in that case seems to be consistent with the finding of Kayser et al. (2007) that area CL has comparatively greater visual activation. The present study did not place tracers in rostral auditory areas, but previous findings found that auditory projections to visual cortex arise mainly in the more caudal auditory areas (Falchier et al. 2002; Rockland and Ojima 2003). Thus, the relative abundance of visual–auditory anatomical connections appears to mirror the distribution of fMRI activation.
Visual Field Eccentricity and Multimodal Connections
Our results confirm previous observations showing that the peripheral visual field representation seems to be specifically involved in auditory–visual cross-modal convergence. The labeled neurons in area V2 that we observed after retrograde tracer injections in caudal auditory cortex were all located in the far peripheral representation of the lower visual field. This is consistent with the topography of the reciprocal auditory to visual projection, which targets the peripheral visual field representations of areas V1 and V2 auditory (Falchier et al. 2002; Rockland and Ojima 2003). Results from other species demonstrate that the particular involvement of peripheral visual field representation in early multisensory convergence is not specific to primate. In the ferret, for example, projections from visual to auditory cortex originated from peripheral visual field representation of areas 17 and 18 (Bizley et al. 2007).
Segregation of peripheral and central field visual pathways is a common feature of cortical organization. For example, peripheral but not central representations of areas V1 and V2 are connected with numerous dorsal visual stream areas in temporal and parietal cortex (Colby et al. 1988; Gattass et al. 1990; Nakamura et al. 1993; Gattass et al. 1997; Ungerleider et al. 2008). Area prostriata is reported to have visual function (Sousa et al. 1991; Rosa et al. 1997) and appears to preferentially connect with the peripheral representation of areas V1, V2, and MT (Palmer and Rosa 2006). Its projections to peripheral MT suggest an involvement in motion processing in visual periphery. The observation that early peripheral visual representations are connected with caudal auditory cortex is consistent with a “dorsal-pathway” type function of these multisensory connections (Falchier et al. 2002). Indeed, caudal auditory areas are considered to be part of a dorsal auditory stream, based on their connections with spatial domains of the frontal lobe and their role in sound spatial localization (Rauschecker 1998; Romanski, Bates, et al. 1999; Romanski, Tian, et al. 1999; Romanski and Goldman-Rakic 2002; Rauschecker and Tian 2004). Recent findings from fMRI connectivity analysis in humans showed that auditory cortex has a distinct relationship with the peripheral representation of visual field in the calcarine sulcus (Eckert et al. 2008; Cate et al. 2009). Together, the findings from monkey and other species emphasize the involvement of dorsal visual and auditory streams in low-level cross-modal convergence.
Laminar Distribution of Projecting Cells
The results of both anterograde and retrograde tracing experiments in the macaque showed that auditory projections onto peripheral areas V1 and V2 can be characterized as feedback type, according to the mainly infragranular distribution of their parent neurons and extragranular termination of their axons (Falchier et al. 2002; Rockland and Ojima 2003). The present study shows that the reciprocal projections from visual areas to auditory cortex arise from mainly infragranular cells. Although anterograde tracer experiments are still lacking to confirm the features of this projection, this pattern of retrograde labeling is consistent with a feedback laminar organization (Rockland and Pandya 1979; Maunsell and Van Essen 1983; Kennedy and Bullier 1985; Weller and Kaas 1985; Barbas 1986; Boussaoud et al. 1990; Felleman and Van Essen 1991; Distler et al. 1993; Batardiere et al. 1998; Barone and Kennedy 2000; Grant and Hilgetag 2005). Projections from early visual and other sensory modalities to auditory cortex were found to be organized similarly in the gerbil (Budinger et al. 2008). These results suggest a model in which putative unisensory visual and auditory cortices do not interact in a classical feedforward–feedback relationship but rather by way of a feedback loop. A possible implication of this organization is that the dominant effects of these connections between early sensory areas are modulatory. For example, in auditory cortex, the main response to visual stimuli was a modulation of auditory responses, and visual responses by themselves typically did not drive neuron spiking (Schroeder and Foxe 2002; Kayser et al. 2008). Similarly, in primary visual cortex, auditory inputs were detected as current sources in the superficial layers that modulate visual responses but did not by themselves drive action (Lakatos et al. 2008; Schroeder and Lakatos 2009). To our knowledge, this pattern of connectivity has not been described elsewhere in the primate. The possibility that this pattern of connection may be a common feature of connectivity between early stages of distinct hierarchical sensory networks remains to be further investigated.
Cross-species Comparisons
Areas 17, 18, and 19 project directly to auditory cortex in the ferret (Bizley et al. 2007). Electrophysiological recordings in that species also confirmed that the proportion of auditory neurons modulated by nonauditory inputs is higher in association areas than in core areas (Bizley et al. 2007). Similarly, tracing experiments in the gerbil reported a similar direct pathway between secondary visual areas and auditory cortex (Lakatos et al. 2008; Schroeder and Lakatos 2009). In the rat, projections have been documented between auditory core area Te1 and primary/secondary visual areas (Vaudano et al. 1991). The wide generality of cross-modal connections between low-level sensory cortices reinforces the view that they are a fundamental part of neocortical circuitry.
Influence of Visual Inputs on the Physiology of Auditory Cortex
Studies in human and nonhuman species have shown that visual information can influence auditory processing. In humans, fMRI studies demonstrated activation of the caudal superior temporal plane during tasks that required viewing of simple moving visual stimuli (Martuzzi et al. 2007; Antal et al. 2008), visual identification of graphically presented phonemes (van Atteveldt et al. 2004; Bernstein et al. 2008), and viewing speakers’ facial movements during speech perception (Calvert 2001; Olson et al. 2002; Calvert and Campbell 2003; Wright et al. 2003; Pekkola et al. 2006). In monkeys, visual stimuli ranging from naturalistic scenes to flashes effectively modulated auditory responses in auditory cortex (Kayser et al. 2007). Visual responses in monkey auditory cortex included both suppression and enhancement of auditory activity, and these visual effects were found in areas A1, CL, CM, and CPB (Kayser et al. 2007, 2008). These effects were observed both in anesthetized and awake monkeys, suggesting that they are not purely a result of attentional modulation (Kayser et al. 2007). However, there appears to be some effect of attention as the visual–auditory interactions are more robust in nonanesthetized monkeys (Kayser et al. 2007) and in humans during tasks that require attention (Cate et al. 2009). Overall, the findings in humans and monkeys suggest that visual input to auditory cortex may be relevant to several cognitive processes, including enhancement of spatial, temporal, and object-related multisensory perception.
Considering that visual responses are found in several auditory areas and affect multiple processes, it is plausible that they arise from other anatomical sources in addition to areas V2 and prostriata. In particular, auditory areas have robust connections with the nearby superior temporal polysensory areas, which is hypothesized to have a key role in audiovisual integration–relevant speech perception (Calvert 2001; Ghazanfar et al. 2005; Ghazanfar and Turesson 2008). Additional candidate sources of visual input include the pulvinar and multisensory nuclei in the caudal thalamus (De La Mothe et al. 2006; Hackett et al. 2007) and several other areas of association and limbic cortex that are connected to auditory cortex (reviewed in Smiley and Falchier 2009). At present, definitive physiological experiments that establish the sources of visual inputs to auditory areas are lacking.
As discussed above, auditory connections with early visual areas arise mainly in the peripheral visual representations. A possible implication of this arrangement is that these connections may have a role in optimizing head–eyes orientation when sensory sources are in the peripheral visual field, where visual detection is not as efficient (Goldring et al. 1996; Hughes et al. 1998; Giard and Peronnet 1999; McDonald et al. 2000). This is suggested by experiments in ferrets where visual responses in auditory cortex were clearly tuned to visual stimuli located near the contralateral surface of the head (Bizley et al. 2007). Similarly, in humans, attention to the spatial location of auditory stimuli causes activation of visual cortex at the region of peripheral field representation (Cate et al. 2009).
Funding
National Institute of Health (MH-061989, R01 MH 067138-04 to J.F.S., and NIH/NIDCD-04318 to T.A.H.).
Acknowledgments
Conflict of Interest: None declared.
References
- Antal A, Baudewig J, Paulus W, Dechent P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis Neurosci. 2008;25:17–26. doi: 10.1017/S0952523808080024. [DOI] [PubMed] [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- Barone P, Batardiere A, Knoblauch K, Kennedy H. Laminar distribution of neurons in extrastriate areas projecting to visual areas V1 and V4 correlates with the hierarchical rank and indicates the operation of a distance rule. J Neurosci. 2000;20:3263–3281. doi: 10.1523/JNEUROSCI.20-09-03263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Kennedy H. Non-uniformity of neocortex: areal heterogeneity of NADPH-diaphorase reactive neurons in adult macaque monkeys. Cereb Cortex. 2000;10:160–174. doi: 10.1093/cercor/10.2.160. [DOI] [PubMed] [Google Scholar]
- Batardiere A, Barone P, Dehay C, Kennedy H. Area-specific laminar distribution of cortical feedback neurons projecting to cat area 17: quantitative analysis in the adult and during ontogeny. J Comp Neurol. 1998;396:493–510. doi: 10.1002/(sici)1096-9861(19980713)396:4<493::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bernstein LE, Auer ET, Jr., Wagner M, Ponton CW. Spatiotemporal dynamics of audiovisual speech processing. Neuroimage. 2008;39:423–435. doi: 10.1016/j.neuroimage.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Bajo VM, Nelken I, King AJ. Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb Cortex. 2007;17:2172–2189. doi: 10.1093/cercor/bhl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Budinger E, Laszcz A, Lison H, Scheich H, Ohl FW. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res. 2008;1220:2–32. doi: 10.1016/j.brainres.2007.07.084. [DOI] [PubMed] [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009 doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Bullier J, Hupe JM, James AC, Girard P. The role of feedback connections in shaping the responses of visual cortical neurons. Prog Brain Res. 2001;134:193–204. doi: 10.1016/s0079-6123(01)34014-1. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Campbell R. Reading speech from still and moving faces: the neural substrates of visible speech. J Cogn Neurosci. 2003;15:57–70. doi: 10.1162/089892903321107828. [DOI] [PubMed] [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Cate AD, Herron TJ, Yund EW, Stecker GC, Rinne T, Kang X, Petkov CI, Disbrow EA, Woods DL. Auditory attention activates peripheral visual cortex. PLoS One. 2009;4:e4645. doi: 10.1371/journal.pone.0004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn Affect Behav Neurosci. 2004;4:117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Seltzer B, Cola M, Griggs E. Chemoarchitectonics and corticocortical terminations within the superior temporal sulcus of the rhesus monkey: evidence for subdivisions of superior temporal polysensory cortex. J Comp Neurol. 1995;360:513–535. doi: 10.1002/cne.903600312. [DOI] [PubMed] [Google Scholar]
- De La Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Distler C, Boussaoud D, Desimone R, Ungerleider LG. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp. 2008;29:848–857. doi: 10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Gattass R, Rosa MG, Sousa AP, Pinon MC, Fiorani JM, Neuenschwander S. Cortical streams of visual information processing in primates. Braz J Med Biol Res. 1990;23:375–393. [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. Cortical projections of area V2 in the macaque. Cereb Cortex. 1997;7:110–129. doi: 10.1093/cercor/7.2.110. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Turesson HK. Speech production: how does a word feel? Curr Biol. 2008;18:R1142–R1144. doi: 10.1016/j.cub.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci. 1999;11:473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- Goldring JE, Dorris MC, Corneil BD, Ballantyne PA, Munoz DP. Combined eye-head gaze shifts to visual and auditory targets in humans. Exp Brain Res. 1996;111:68–78. doi: 10.1007/BF00229557. [DOI] [PubMed] [Google Scholar]
- Grant S, Hilgetag CC. Graded classes of cortical connections: quantitative analyses of laminar projections to motion areas of cat extrastriate cortex. Eur J Neurosci. 2005;22:681–696. doi: 10.1111/j.1460-9568.2005.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007;502:924–952. doi: 10.1002/cne.21326. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Karmos G, Schroeder CE, Ulbert I, Sterbing-D'Angelo SJ, D'Angelo WR, Kajikawa Y, Blumell S, de la ML. Neurosurgical access to cortical areas in the lateral fissure of primates. J Neurosci Methods. 2005;141:103–113. doi: 10.1016/j.jneumeth.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Lomber SG. Auditory cortex projections target the peripheral field representation of primary visual cortex. Exp Brain Res. 2008;190:413–430. doi: 10.1007/s00221-008-1485-7. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Nelson MD, Aronchick DM. Spatial characteristics of visual-auditory summation in human saccades. Vision Res. 1998;38:3955–3963. doi: 10.1016/s0042-6989(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Augath M, Logothetis NK. Functional imaging reveals visual modulation of specific fields in auditory cortex. J Neurosci. 2007;27:1824–1835. doi: 10.1523/JNEUROSCI.4737-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK. Visual modulation of neurons in auditory cortex. Cereb Cortex. 2008;18:1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J Neurosci. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Pincze Z, Fu KM, Javitt DC, Karmos G, Schroeder CE. Timing of pure tone and noise-evoked responses in macaque auditory cortex. Neuroreport. 2005;16:933–937. doi: 10.1097/00001756-200506210-00011. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Herdener M, Esposito F, Hubl D, di Salle F, Scheffler K, Bach DR, Federspiel A, Kretz R, Dierks T, et al. Differential patterns of multisensory interactions in core and belt areas of human auditory cortex. Neuroimage. 2006;31:294–300. doi: 10.1016/j.neuroimage.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Murray MM, Michel CM, Thiran JP, Maeder PP, Clarke S, Meuli RA. Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex. 2007;17:1672–1679. doi: 10.1093/cercor/bhl077. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Salejarvi WA, Hillyard SA. Involuntary orienting to sound improves visual perception. Nature. 2000;407:906–908. doi: 10.1038/35038085. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Rockland KS, Van Hoesen GW. Localization of area prostriata and its projection to the cingulate motor cortex in the rhesus monkey. Cereb Cortex. 2000;10:192–203. doi: 10.1093/cercor/10.2.192. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Schroeder CE. Neuronal mechanisms, response dynamics and perceptual functions of multisensory interactions in auditory cortex. Hear Res. 2009 doi: 10.1016/j.heares.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Gattass R, Desimone R, Ungerleider LG. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaques. J Neurosci. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Gatenby JC, Gore JC. A comparison of bound and unbound audio-visual information processing in the human cerebral cortex. Brain Res Cogn Brain Res. 2002;14:129–138. doi: 10.1016/s0926-6410(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Rosa MGP. A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision. Eur J Neurosci. 2006;24:2389–2405. doi: 10.1111/j.1460-9568.2006.05113.x. [DOI] [PubMed] [Google Scholar]
- Pekkola J, Ojanen V, Autti T, Jaaskelainen IP, Mottonen R, Sams M. Attention to visual speech gestures enhances hemodynamic activity in the left planum temporale. Hum Brain Mapp. 2006;27:471–477. doi: 10.1002/hbm.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;91:2578–2589. doi: 10.1152/jn.00834.2003. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MG, Casagrande VA, Preuss T, Kaas JH. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti) J Neurophysiol. 1997;77:3193–3217. doi: 10.1152/jn.1997.77.6.3193. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Foxe JJ. The timing and laminar profile of converging inputs to multisensory areas of the macaque neocortex. Brain Res Cogn Brain Res. 2002;14:187–198. doi: 10.1016/s0926-6410(02)00073-3. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Falchier A. Multisensory connections of monkey auditory cerebral cortex. Hear Res. 2009 doi: 10.1016/j.heares.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol. 2007;502:894–923. doi: 10.1002/cne.21325. [DOI] [PubMed] [Google Scholar]
- Sousa AP, Pinon MC, Gattass R, Rosa MG. Topographic organization of cortical input to striate cortex in the Cebus monkey: a fluorescent tracer study. J Comp Neurol. 1991;308:665–682. doi: 10.1002/cne.903080411. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- van Atteveldt NM, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43:271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH, Bixby JL. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Vaudano E, Legg CR, Glickstein M. Afferent and efferent connections of temporal association cortex in the rat: a horseradish peroxidase study. Eur J Neurosci. 1991;3:317–330. doi: 10.1111/j.1460-9568.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Vezoli J, Falchier A, Jouve B, Knoblauch K, Young M, Kennedy H. Quantitative analysis of connectivity in the visual cortex: extracting function from structure. Neuroscientist. 2004;10:476–482. doi: 10.1177/1073858404268478. [DOI] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- Wang Y, Celebrini S, Trotter Y, Barone P. Visuo-auditory interactions in the primary visual cortex of the behaving monkey: electrophysiological evidence. BMC Neurosci. 2008;9:79. doi: 10.1186/1471-2202-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RE, Kaas JH. Cortical projections of the dorsolateral visual area in owl monkeys: the prestriate relay to inferior temporal cortex. J Comp Neurol. 1985;234:35–59. doi: 10.1002/cne.902340104. [DOI] [PubMed] [Google Scholar]
- Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G. Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex. 2003;13:1034–1043. doi: 10.1093/cercor/13.10.1034. [DOI] [PubMed] [Google Scholar]