Abstract
Acute viral infections induce robust adaptive immune responses resulting in virus clearance. Recent evidence suggests that there may be depots of viral antigen that persist in draining lymph nodes (DLNs) after virus clearance and could, therefore, affect the adaptive immune response and memory T cell formation. The nature of these residual antigen depots, the mechanism of antigen persistence, and the impact of the persistent antigen on memory T cells remain ill defined. Using a mouse model of influenza virus infection of the respiratory tract, we identified respiratory dendritic cells (RDCs) as essential for both sampling and presenting residual viral antigen. RDCs in the previously infected lung capture residual viral antigen deposited in an irradiation-resistant cell type. RDCs then transport the viral antigen to the LNs draining the site of infection, where they present the antigen to T cells. Lastly, we document preferential localization of memory T cells to the DLNs after virus clearance as a consequence of presentation of residual viral antigen by the migrant RDC.
The induction of the adaptive immune response to an infectious agent consists of a defined series of events prompted by invasion of the body by the organism, followed by replication of the organism and the subsequent initiation of an innate and adaptive immune response. For organisms that enter at a body surface and confine replication primarily to that site, for example, infection of the respiratory tract by seasonal type A influenza, innate responses are triggered by infection of cells at the body surfaces and activation of sentinel innate immune cells in the surrounding tissues. Induction of the adaptive immune response typically requires the uptake/infection by the organism or uptake of its constituents by tissue-resident professional APCs, most notably immature DCs (Banchereau and Steinman, 1998; Lambrecht et al., 2001; de Heer et al., 2005). These professional APCs transport the organism or its products to secondary lymphoid organs, usually LNs draining the site of infection (draining LN [DLN]) where adaptive immune (T and B cells) responses are initiated. In the case of experimental mouse influenza infection, at least two distinct subsets of respiratory DCs (RDCs) have been implicated as critical APCs in the induction of primary T cell responses to this virus (GeurtsvanKessel et al., 2008; Kim and Braciale, 2009).
The outcome of the interaction between an invading microorganism and the immune system is either clearance of the agent or the development of chronic persistent infection. Viruses such as HIV, HCV, and, in the mouse model, LCMV, can produce chronic persistent infection with high titers of the virus because of the ability of these viruses to subvert or suppress the host immune response (Ahmed et al., 1984; Bevan and Braciale, 1995; Letvin and Walker, 2003; Wherry et al., 2003; Rehermann and Nascimbeni, 2005). Persistence of viral antigen at high levels in these infections may contribute to the dysregulation of the adaptive immune response observed in these infections (Shin and Wherry, 2007). In contrast, acute infection with many viruses results in rapid virus clearance (even with an initial high level of virus replication) and, presumably, elimination of virus-infected cells by the action of the adaptive and innate immune response.
Recently, however, evidence has emerged to suggest that after acute viral infection viral antigen can be detectable for an extended period, i.e., weeks to months, after clearance of infectious virus. This phenomenon is not only observed with viruses capable of establishing persistent infection in vitro, for example, respiratory syncytial virus (RSV; P’ringle et al., 1978; Schwarze et al., 2004) and Sendai virus (Mori et al., 1995), but has also been observed for classically lytic viruses, such as type A influenza (Jelley-Gibbs et al., 2005; Zammit et al., 2006) and VSV (Turner et al., 2007), which are not believed to produce chronic persistent infection of cells. In these instances, viral antigen was detected using the proliferative response of adoptively transferred TCR transgenic (Tg) T cells into previously infected animals as a sensitive measure of antigen persistence. Although the nature and the mechanism of antigen persistence in these instances was not defined, several studies provided evidence that the presentation of this residual viral antigen to T cells may influence the quality of the memory T cell response to infection (Jelley-Gibbs et al., 2005, 2007; Zammit et al., 2006; Turner et al., 2007; Woodland and Kohlmeier, 2009).
In several studies reporting persistence of viral antigen after acute infection and subsequent virus clearance, the reservoir of antigen (as detected by adoptive transfer of virus-specific T cells) was localized within secondary lymphoid organs, most notably the LN draining the site of infection (Jelley-Gibbs et al., 2005, 2007; Zammit et al., 2006). In this paper, we describe a series of experiments to investigate the underlying mechanism of the persistence of antigen presentation after respiratory tract infection with type A influenza virus. We found that a reservoir of viral antigen (i.e., RNA and protein) is present for an extended period after an acute influenza virus infection at the site of infection (i.e., the lungs) and localized to both nonhematopoietic (CD45−) and hematopoietic (CD45+) cell types within residual mild lung inflammatory foci. We further demonstrate that the residual viral antigen deposited in the previously infected lung is captured and transported by RDC to the regional LN, where the migrant RDC function in presenting the residual antigen to T cells. In addition, we provide evidence that lung follicular DCs (FDCs) may play an important role in regulating the response of RDC. Finally, our results suggest that as a consequence of presentation of residual viral antigen by the migrant RDC to T cells in the LNs draining the site of infection, there is preferential localization of memory T cells to the DLN after virus clearance. The implications of these observations are discussed.
RESULTS
Viral antigen persistence in the influenza-infected lungs
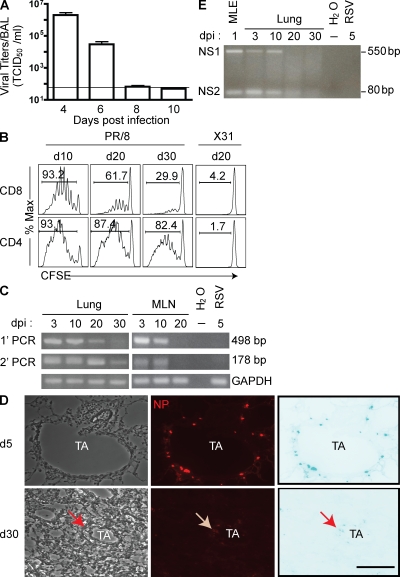
In the mouse model of influenza infection, type A influenza viruses are rapidly cleared from the lungs after intranasal (i.n.) infection, with infectious virus typically becoming undetectable in the lungs by days 8–10 post infection (p.i.; Fig. 1 A). Several groups have, however, reported that influenza viral antigen can persist in vivo for weeks after virus clearance in the form of processed antigen recognized by adoptively transferred CD8+ (or CD4+) virus-specific TCR Tg T cells (Jelley-Gibbs et al., 2005, 2007; Zammit et al., 2006). Indeed, after sublethal i.n. infection with the type A influenza A/PR/8 virus, we could also reproducibly detect the presence of viral antigen in the draining mediastinal LN (MLN) for at least 30 d, as measured by the proliferation of A/PR/8-specific naive CD8+ or CD4+ TCR Tg T cells in the draining MLN after adoptive transfer of the naive T cells at this time (Fig. 1 B). Adoptive transfer of these Tg T cells into mice infected with the antigenically distinct type A X-31 influenza strain did not result in proliferation (Fig. 1 B), verifying the antigen dependence and specificity of proliferation.
Figure 1.
Viral antigen persistence in the influenza-infected lungs. (A) Kinetic analyses of infectious virus recovery from the influenza (A/PR/8)-infected lungs. Bronchoalveolar lavages were collected at the indicated days p.i. and assayed for infectious virus titers using MDCK cells. The horizontal line indicates the limit of detection. Viral titers are represented as mean TCID50 (median tissue culture infective dose) ± SEM. (B) Duration of antigen presentation in the regional LN draining the lung. Previously, influenza-infected mice received i.v. CFSE-labeled HA-specific TCR Tg CD8+ (Cl-4) or CD4+ (TS1) T cells at the indicated days p.i. Proliferation in vivo was monitored by CFSE dilution 4 d later in the draining MLN. Antigen specificity was measured by transferring the reporter Tg T cells into mice that were previously infected with an antigenically irrelevant type A influenza virus (X31). Data are representative of at least five independent experiments. (C–E) Viral antigen persistence in the infected lung. Total RNA was extracted from MLN or lungs at the indicated days p.i. and subjected to RT-PCR analyses to detect NP RNA (with two rounds of PCR; C) or unspliced and spliced variants of NS gene (one round of PCR; E). RNA from RSV-infected lungs or an influenza-infected mouse lung epithelial cell line (MLE) was included as a control for primer specificity. Lung sections were stained for viral NP at day 5 (d5; top) or day 30 (bottom) p.i. and visualized by immunofluorescence microscopy (20×, D). Left, light fields; middle, anti-NP immunostaining; right, inverted view of the anti-NP immunostaining. TA, terminal airway. Data are representative of at least four independent experiments. Arrows indicate NP-positive cells. Bar, 200 µm.
In view of the rapid clearance of infectious virus from the lungs in the face of antigen persistence, we performed a companion analysis to determine the presence of viral RNA over time in the lungs and MLN of infected mice by RT-PCR. As Fig. 1 C demonstrates, the influenza gene encoding the abundant nucleocapsid protein (NP) is readily detectable in both the lungs and the MLN for up to 10 d p.i. Noninfectious virus introduced by the i.n. route was rapidly eliminated (i.e., input virion NP genes were eliminated from lungs by day 5 after inoculation.). The NP gene was also detectable in the lungs for up to 30 d when a sensitive nested RT-PCR strategy was used for detection. Consistent with these findings, we could readily detect (as expected) NP-expressing (NP+) cells among the lung airway epithelial cells by immunofluorescence at day 5 p.i. (Fig. 1 D, top). It is of interest that we could also detect NP+ cells at day 30 p.i., but the cells were clustered in rare foci associated with sites of residual mild inflammation and fibrosis (Fig. 1 D, bottom; and Fig. S1). NP+ cells were demonstrable both among CD45− lung parenchyma cells and hematopoietic (CD45+) cells within these inflammatory foci, with predominant NP expression among the CD45− nonhematopoietic cell types (Fig. S1). It should be noted that compared with the day 5 p.i. time point, NP expression in these cells was very low, thereby requiring high-gain microscopic settings to visualize these rare cells and precluding additional characterization of the NP+ cells by flow cytometry because of their rarity and low signal intensity. The influenza NP-specific antibody did not stain the lungs of mice infected with the antigenically distinct RSV (Fig. S1). The presence of viral antigen as late as 30 d p.i. raised the possibility that there was viral mRNA present in the previously infected lungs at this late time. The presence of influenza viral mRNA was verified by the detection of the mRNA encoding the NEP (NS2) protein, which is produced by alternative splicing of the NS1 gene segment (Fig. 1 E). We extended these results to include a quantitative analysis of the kinetics of expression of a third influenza gene, i.e., the spliced M2 mRNA, in the lungs using real-time RT-PCR (Fig. S2).
It is particularly noteworthy that we were unable to detect the presence of influenza viral RNA in the MLN after day 10 p.i., even using the sensitive nested RT-PCR (Fig. 1 C), in spite of the presence of viral antigen in the MLN for at least 30 d as detected by proliferation of naive TCR Tg T cells (Fig. 1 B). Therefore, the viral antigen appears to be deposited/retained within the draining MLN long after viral transcripts have disappeared from this site. It is noteworthy, however, that although viral RNA and protein were detectable in the infected lungs out to day 30 p.i., we and others (Román et al., 2002; Lawrence and Braciale, 2004) had previously reported that influenza-specific naive T cells are unable to activate and proliferate in the infected lungs even at the height of infection, i.e., days 3–5 p.i., when viral antigen and virus-infected cells are highly abundant.
Role of RDCs in antigen presentation in the draining MLN.
The findings in the previous section suggested that the previously infected lungs represented a depot of influenza virus antigen. However, presentation of this persistent viral antigen was restricted to the draining MLN, raising the possibility that this depot of residual antigen within lungs needed to be transported to the MLN. We recently reported that two distinct subsets of RDC, i.e., CD103+ RDC and CD11bhi RDC, play a central role as APC for induction of naive influenza virus–specific CD8+ T cell responses in the draining MLN after their migration from influenza-infected lungs to this site (Sung et al., 2006; Hao et al., 2008; Kim and Braciale, 2009). We therefore wanted to explore the possibility that one or both of these RDC subsets may continue to capture the now residual viral antigen in lungs and transport it to the draining MLN after virus clearance and recovery from infection.
These two CD11c+MHC IIhi RDC subsets are present, along with CD11c+MHC IIlo monocyte-like RDC (MoRDC), in the uninfected lungs (Fig. 2 A; Sung et al., 2006). Although, as previously reported (GeurtsvanKessel et al., 2008; Kim and Braciale, 2009), these RDC subsets transiently migrate out of the lungs into the draining MLN (see following paragraph) in response to influenza infection, these two MHC IIhi RDC subsets begin to return to a normal percentage distribution among the CD11c+ cells in lungs by day 40 p.i. (Fig. 2 A), with a slightly higher absolute numbers of these two RDC subsets at this time than in the naive lungs (5.4 ± 0.5 vs. 11 ± 0.6 × 104 for CD103+ RDC, P < 0.001, n > 6; and 4.2 ± 0.9 vs. 10.7 ± 0.6 × 104 for CD11bhi RDC, P = 0.001, n > 6 per lung set at day 0 and day 40 p.i., respectively). At day 20 p.i., the CD103+ RDC and CD11bhi RDC in the lungs express low levels of the costimulatory ligands CD40/80/86 (Fig. 2 B), which are comparable to the corresponding tissue-resident immature RDC isolated from the uninfected lungs (Fig. S3; Kim and Braciale, 2009).
Figure 2.
Characterization of RDC subsets in the lung after i.n. influenza virus infection. (A) CD103+ RDC (I), CD11bhi RDC (II), and MoRDC (III) in the uninfected and infected lungs are identified among CD45+CD11c + lung cells by flow cytometry at the indicated days p.i. Data are representative of analyses from >20 individual mice performed at each time point. (B) Surface marker expression on subsets of RDC. Lung-residing DC subsets (CD103+ RDC, I; CD11bhi RDC, II; and MoRDC, III) were examined at day 20 p.i. for surface expression of costimulatory molecules as indicated (open histograms). Staining with isotype-matched control antibodies was included (filled histograms). Data are representative of results from >10 mice analyzed in three independent experiments.
DCs with cell surface markers characteristic of these two MHC IIhi RDC subsets are present at low frequency in the draining MLN before infection (Fig. 3 A; Kim and Braciale, 2009). The presence of these RDCs in the LN presumably reflects the well recognized low-level homeostatic migration of tissue DC into the draining nodes in the absence of an inflammatory stimulus (Jakubzick et al., 2008). As previously reported (Kim and Braciale, 2009), the frequency of these two RDC subsets found in the draining MLN increases significantly by day 3 after influenza infection (Fig. 3 A). By day 20 p.i., DCs with phenotypic markers characteristic of these two RDC subsets are still detectable in the draining MLN (Fig. 3 A). These DCs in the enlarged day-20-p.i. LNs are present in elevated numbers compared with the normal (preinfection) LNs (0.5 ± 0.1 vs. 5.8 ± 1.2 × 103 for CD103+ RDC, P = 0.0005, n = 4–6; and 0.6 ± 0.09 vs. 10.8 ± 1.4 × 103 for CD11bhi RDC, P = 0.0001, n = 4–6 per MLN at day 0 and day 20 p.i., respectively). Among the other CD11c+ cells in the enlarged MLN are B220+ plasmacytoid DC (pDC) and the CD8αα+ LN-resident DC. We were able to further confirm the respiratory tract origin of the CD103+ and CD11bhi DC in the draining MLN by administration of fluorescent-labeled antigen (OVA-DQ) into the respiratory tract of mice at day 20 p.i. When the DC populations in the draining MLN were analyzed for the uptake of antigen 24 h later, antigen uptake was largely restricted to the CD103+ and CD11bhi DC subsets in the nodes (Fig. 3 B), which was as expected if these cells were migrant RDCs, which had sampled antigen deposited in the lungs before their migration to the MLN.
Figure 3.
Migrant RDC in the regional DLN of the previously infected mice support antigen-specific T cell proliferation. (A) Flow cytometry–based categorization of RDC subpopulations in the MLN of previously infected mice. The CD11c+ cells found in the MLN are divided into three major subsets: pDC (B220+ MHC IIlo, I), CD8α+ LNDC (CD8α+ MHC IIint, II), and migrant RDC (CD8α−B220−MHC IIhi, III). The migrant RDCs are further divided into two populations based on CD103 marker expression: CD103+ (CD103+ RDC) and CD103− (CD11bhi RDC) cells. Data are representative of more than five mice analyzed in five independent experiments. (B) In vivo uptake by RDC of soluble protein delivered to the respiratory tract before RDC migration to the DLN. Mice (day 20 p.i.) were given i.n. fluorescent dye–labeled OVA (OVA-DQ) or unlabeled OVA proteins. Migrant DCs that had taken up the soluble OVA protein in the respiratory tract were identified in the MLN 1 d later. Data are representative of four mice in two independent experiments. (C) Phenotypic features of DC subsets in the MLN of influenza-infected mice (day 20 p.i.). Levels of costimulatory molecule expression on CD8α+ LNDC, CD103+ RDC, and CD11bhi RDC were determined at either day 3 (gray) or day 20 (open) after infection. Black histograms represent isotype-matched control Ab stainings. Data are representative of two mice in two independent experiments. (D) Migrant RDC isolated from MLN can stimulate proliferation of naive Tg CD8+ T cells directly ex vivo. The indicated individual DC subsets were separated by cell sorting of MLN cell suspensions from pooled LN of previously infected mice (day 20 p.i., n = 15–20 mice/experiment) and then co-cultured with CFSE-labeled HA-specific naive TCR Tg CD8+ T cells for 4 d in cultures. Sorted DC preparations were incubated with the preprocessed synthetic cognate peptide (HA533-541) before co-culture with the responding T cells as a positive control. Data are representative of three independent experiments.
In contrast to their RDC counterparts found in lungs at day 20 p.i. (Fig. 2 B), the CD103+ and CD11bhi RDC in the draining MLN expressed elevated levels of costimulatory ligands (Fig. 3 C). When the major LN DC subsets with potential APC capacity, i.e., CD8αα+ DC, pDC, and the migrant CD103+ and CD11bhi RDC, were isolated from the draining MLN at day 20 p.i. and analyzed directly ex vivo for APC activity, only the two RDC subsets were capable of stimulating proliferation of naive influenza-specific Tg CD8+ T cells after in vitro co-culture (Fig. 3 D). CD8αα+ DC and pDC pulsed with a high concentration of processed peptide could, like CD103+ and CD11bhi RDC, support naive T cell activation in vitro. These data suggested that these two migrant RDC subsets, which have captured residual viral antigen in the lungs before their migration to the DLNs, account for the residual antigen-presenting activity observed in the MLN. The corresponding immature CD103+ and CD11bhi RDC isolated from the lungs at day 20 p.i. did contain processed viral antigen, as they could stimulate naive CD8+ T cells in vitro (Fig. S4). As expected, however, these lung-resident immature CD103+ and CD11bhi RDC only weakly stimulated proliferation of naive T cells, presumably reflecting the weak costimulatory capacity of these immature lung DC.
Residual antigen presentation to CD8+ T cells in vivo in the MLN depends on migrant RDC.
The findings in the previous section raised the possibility that residual viral antigen in the lung continues to be captured and transported by RDC to the draining MLN after virus clearance and recovery from infection. To further establish the role of RDC in residual antigen uptake and presentation in vivo, we used Tg mice expressing diphtheria toxin (DTx) receptor (DTR) under CD11c promoter (CD11c-DTR) to deplete CD11c+ DC (Jung et al., 2002) from the influenza-infected mice after virus clearance and resolution of infection. We have previously demonstrated that i.n. administration of a low dose of DTx into the CD11c-DTR mice preferentially depletes the CD103+ and CD11bhi RDC from the naive lung and that DTx treatment consequently eliminates the migration to and accumulation of these RDC in the inflamed MLN after influenza virus infection (Kim and Braciale, 2009). When low-dose DTx was delivered i.n. to the CD11c-DTR mice at day 20 p.i., we observed that CD103+ and CD11bhi RDC were preferentially eliminated from the lung at 24 h after DTx treatment (Fig. 4 A), as has been observed in the uninfected CD11c-DTR lungs (Kim and Braciale, 2009). Consistent with the lung origin of CD103+ and CD11bhi found in the lung-draining MLN, the accumulation of those DC subsets in the MLN was markedly diminished when DC populations in the MLN were examined 3 d after DTx treatment (Fig. 4 B). These data further support the notion that continual emigration of tissue-derived DC to DLN is the major contributor to DC subsets, with these phenotypic characteristics found in tissue DLNs before and/or after inflammatory stimuli like virus infection.
Figure 4.
Residual antigen presentation to CD8+ T cells in vivo in the MLN depends on migrant RDC. (A and B) Selective ablation of CD103+ and CD11bhi RDC from the previously infected lungs resulted in near absence of the corresponding populations in the lung-draining MLN. CD11c-DTR mice recovering from influenza virus (day 20 p.i.) were given DTx i.n., and depletion of RDC in the lung was analyzed 24 h later (percentage of DC among CD11c+SiglecF− cells: 15.2 ± 3.7 vs. 1.5 ± 0.7 for CD103+, P < 0.012, and 47.5 ± 6.4 vs. 17.6 ± 5.3 for CD11bhi RDC, P < 0.006, for PBS-treated vs. DTx-treated mouse panels, respectively; mean ± SEM; A) or in the DLN at 72 h (percentage of DC among total LN cells: 0.09 ± 0.01 vs. 0.03 ± 0.006 for CD103+, P < 0.003, and 0.12 ± 0.02 vs. 0.04 ± 0.001 for CD11bhi RDC, P < 0.008, for PBS-treated vs. DTx-treated mouse panels, respectively; mean ± SEM; B) after DTx treatments. Data in A and B is representative of at least three independent experiments with two animals per experiments. (C and D) The ability of T cells to proliferate in vivo in the lung-draining MLN was abrogated with the depletion of migrant RDC. PR/8-infected CD11c-DTR mice (day 20 p.i.) were treated with DTx (or PBS). CFSE-labeled HA-specific CL-4 CD8+ Tg T cells were then transferred i.v. into the previously infected treated mice 3 d later. The ability of the reporter T cells to proliferate in the MLN was examined 4 d after the adoptive transfer and depicted as the percentage of divided cells (mean ± SEM; P = 0.0003; C). Transferred T cells in the DLN of migrant RDC-depleted mice proliferate in response to i.v. influenza virus administration (D). Data in C and D are representative of two to four individual recipient mice analyzed in two separate experiments.
To monitor the impact of RDC depletion on residual viral antigen presentation activity in the MLN, we adoptively transferred CFSE dye-labeled naive CL-4 CD8+ T cells into the DTx-treated CD11c-DTR mice at day 3 after DTx treatment and analyzed T cell proliferation in the MLN 4 d later (Fig. 4 C). In contrast to the control group, where a substantial fraction of transferred CD8+ T cells underwent several rounds of cell division, the Tg T cells in the MLN of DTx-treated mice failed to proliferate. The inability of the transferred CD8+ T cell to proliferate was not a result of the simultaneous depletion of antigen-presenting DC both in the LN and in the lungs after DTx administration. As reported previously by us (Kim and Braciale, 2009), Tg CD8+ T cells can proliferate in the MLN when infectious influenza virus is delivered to the DTx-treated CD11c-DTR mice via the i.v. route, where the virus in the bloodstream can gain direct access to the LN-resident DC (Fig. 4 D).
The depot of viral antigen in the lung transported by RDC is radio resistant
The findings in the previous section implicated migrant RDC as the cell type presenting residual viral antigen to T cells in the MLN. The results of immunofluorescence analysis of viral antigen deposition in the lungs (Fig. 1 and Fig. S1) indicated that the residual viral antigen is deposited both in CD45+ cells of hematopoietic origin, presumably including CD103+ and CD11bhi RDC, and in CD45− parenchymal cells present in the infected lung. To explore the contribution of radiation-resistant, likely nonhematopoietic, cells, we irradiated previously influenza virus–infected mice at day 20 p.i. and reconstituted them with BM cells isolated from naive CD11c-DTR mice (Fig. 5 A). To examine the ability of the newly repopulated RDC in the lungs of these previously infected mice to present antigen and activate T cells in the MLN, at 4–5 wk after reconstitution (i.e., ∼7 wk after primary infection with either A/PR/8 virus or as a control the X-31 virus strain) these mice, along with nonchimeric wild-type (unirradiated/nonreconstituted) infected mice, received, by adoptive transfer, CFSE dye-labeled Tg CD4+ or CD8+ Tg T cells as used in Fig. 1. We analyzed T cell proliferation in the MLN 4 d after transfer. Both hemagglutinin (HA)-specific TCR Tg CD4+ and CD8+ T cells had proliferated in the chimeric mice that had been previously infected with A/PR/8 influenza virus (Fig. 5 B, middle), which is in contrast to the minimal proliferation observed in control mice (i.e., X-31 infected; Fig. 5 B, right). Moreover, more pronounced proliferative expansion was observed in CD4+ than CD8+ T cells (Fig. 5 B). The greater expansion of CD4+ than CD8+ T cells was also observed in the MLN of the nonchimeric mice previously infected with A/PR/8 virus ∼50 d before (Fig. 5 B, left). These data indicate that the depot of viral antigen in the lung is surprisingly long lasting and contained within or dependent on a cell type that is resistant to irradiation and, most likely, of nonhematopoietic origin (CD45−).
Figure 5.
The depot of viral antigen in the lung transported by RDC is radio resistant. (A) Schematic depiction of irradiation and BM transplantation. Wild-type mice recovering from acute influenza virus infection (∼3 wk after infection) underwent total body irradiation with a lethal dose and then were reconstituted with BM cells prepared from naive (uninfected) CD11c-DTR Tg mice. Mice were analyzed >4 wk after BM transplantation. (B) Antigen presentation persists in the DLN of the irradiated and BM reconstituted mice. Irradiated/reconstituted and control unirradiated mice received either CFSE-labeled Cl-4 (CD8+) or TS1 (CD4+) T cells by i.v. at day 50 p.i. T cell proliferation (CFSE dilution) in the DLN was measured 4 d later. X31-infected mice were included as a control for antigen specificity of the T cell response. (C) Engraftment of CD11c-DTR–derived RDC in the previously infected non-Tg mice. Reconstituted mice were treated i.n. with DTx (or PBS) route at >4 wk after BM reconstitution. Depletion of two major RDC subsets (CD103+ and CD11bhi) from the lungs was determined 24 h after DTx treatment. Percentages (mean ± SEM) of DC among CD11c+MHC IIhi cells were 13.2 ± 2.5 versus 0.6 ± 0.08 for CD103+ (P < 0.003) and 42 ± 5.5 versus 8.2 ± 1.1 for CD11bhi RDC (P < 0.001) for PBS-treated versus DTx-treated mouse panels, respectively. Data in B and C is representative of two independent experiments using two mice per experiment. (D) BM donor RDC were required for the presentation of residual vial antigen in the DLN of the previously infected mice. RDC-depleted mice, as described in C, were infused i.v. with CFSE-labeled reporter CD8+ or CD4+ Tg T cells 3 d after DTx (right) or PBS (left) treatments, and T cell proliferation in vivo was examined 4 d after transfer. Percentages (mean ± SEM) of divided cells (CFSElow) were 25.2 ± 4.2 versus 5.1 ± 0.9 for CD8 T cells (P < 0.004) and 65.1 ± 3.5 versus 15.3 ± 2.6 for CD4 T cells (P = 0.0001) for PBS-treated versus DTx-treated mice, respectively. (E) CD8+ T cells were capable of proliferating in the MLN when antigen was delivered via the i.v. route. Mice treated as in D received i.v. infectious A/PR/8 virus at the time of T cell transfer. Data in D and E are representative of three independent experiments using one to two mice per experiment.
To further verify that antigen transportation and presentation of residual antigen in the chimeric mice is also dependent on RDC, we depleted the CD103+ and CD11bhi RDC from the lungs of the reconstituted mice by i.n. DTx treatment 4 wk after BM reconstitution (Fig. 5 A). As expected, the CD11chiMHC IIhi RDC (i.e., CD103+ and CD11bhi RDC) in the reconstituted hosts were preferentially depleted from the lung after i.n. DTx administration, with analysis at 24 h after treatment (Fig. 5 C), and also from the MLN 3 d after treatment, which is indicative of the BM origin of and DTR expression by the CD11c+ RDC in the chimeric mice. To measure the antigen-presenting activity in the MLN of the RDC-depleted mice, labeled Tg T cells were adoptively transferred into the DTx-treated mice 3 d after treatment. The T cell proliferation in the MLN of the RDC-depleted chimeric mice was greatly diminished when examined 4 d later (Fig. 5 D). This diminished T cell proliferation seen in the DTx-treated chimeric mice was not a result of depletion of DC with antigen-presenting capacity in the MLN. The Tg T cells in the MLN were fully capable of activation and proliferation when influenza virus was delivered directly to the LNs by the i.v. route (Fig. 5 E). These findings suggest that there may be a reservoir of viral antigen present in a population of radiation-resistant, possibly CD45−, cells in the previously infected lungs and that RDC, particularly CD103+ and CD11bhi, are the crucial cell types for sampling residual viral antigen deposited in the radio-resistant lung cells.
Infection with influenza virus represents a strong inflammatory stimulus to the mouse lungs and results in the development of inducible bronchial-associated lymphoid tissue (iBALT; Moyron-Quiroz et al., 2004). A feature of iBALT is the formation and/or accumulation of CD45− FDC (as well as CD45+ B lymphocytes) within these inflammatory cell foci (Fig. S5 A; GeurtsvanKessel et al., 2009; Halle et al., 2009). Given the well recognized role of the FDC as an antigen depot (Mandels et al., 1980), we examined the impact of elimination of FDC from the lungs of previously infected mice on the ability of RDC to capture antigen in the lungs and stimulate the proliferation of T cells in the DLN. To eliminate FDC, we blocked lymphotoxin-β signaling to FDC (Mackay et al., 1997; Huber et al., 2005; GeurtsvanKessel et al., 2009) by administration of soluble lymphotoxin-β receptor (LTβR)–Ig to the previously infected mice before adoptive transfer of TCR Tg T cells as described (Fig. S5 B). Consistent with a diminution of FDC found in the lung (>50%) at the late days of infection (unpublished data), LTβR-Ig–mediated depletion of FDC (Fig. S5 A) markedly inhibited the ability of RDC to support T cell proliferation in the draining MLN (Fig. S5, C and D). This was not simply the result of an impairment of T cell homing into the immune-reactive LN, possibly caused by the destruction of lymphoid architecture and/or reduction of chemokine (i.e., CCL21), because this regimen at an earlier day after infection (i.e., day 15)—when antigen depots in the lungs are not restricted to a small subsets of lung cells—has no or minimal impact on recruitment (and subsequent proliferation) of the reporter T cells in the DLN (Fig. S6). These findings raise the possibility that a major reservoir of residual viral antigen may lie within iBALT structures.
Persistent antigen presentation by migrant RDC contributes to the elevated memory T cell pool in the draining MLN
The demonstration of a residual depot of viral antigen after virus infection has led to speculation that such antigen depots may play a role in the maintenance and trafficking of the memory T cell pool generated during infection or vaccination (Fazilleau et al., 2007b; Woodland and Kohlmeier, 2009). To date, much of the information concerning the role of residual antigen in the regulation of T cell memory has come from adoptive transfer studies using TCR Tg T cells (typically naive T cells) to analyze memory T cell formation/maintenance in conjunction with the duration of antigen persistence (Jelley-Gibbs et al., 2005, 2007; Zammit et al., 2006; Fazilleau et al., 2007a).
To evaluate the impact of residual viral antigen on the maintenance of the endogenous memory T cell pool after infection, we first examined the frequency of polyclonal influenza–specific memory CD8+ and CD4+ T cells in the draining MLN at days 30 and 70 p.i. by enumerating IFN-γ–secreting cells in response to stimulation by influenza virus–infected BMDC. In parallel, we determined the frequency of virus-specific memory T cells in the nondraining peripheral LNs (pLNs), as these secondary lymphoid tissues would be minimally, if at all, colonized by migrant RDC from the infected lungs. As observed in Fig. 6 A, elevated numbers of CD4+ and CD8+ IFN-γ–secreting T cells were found in the draining MLN at both 30 and 70 d p.i. Importantly, the frequency (and total number) of these responding cells was significantly greater in the draining MLN nodes relative to that of these memory cells in the nondraining pLN. These findings support the emerging view that after antigen exposure, the maintenance of antigen-specific memory T cells, as well as the distribution of the memory T cells among various secondary lymphoid tissues, may be regulated by the local availability of residual antigen within the specific site and adjoining tissues (Jelley-Gibbs et al., 2005; Zammit et al., 2006; Fazilleau et al., 2007a,b; Woodland and Kohlmeier, 2009). We further analyzed the proliferative potential and activation state of the influenza NP-specific memory T cells enriched in the DLNs. When evaluated at 40 d p.i., the cells demonstrated no significant BrdU uptake (12-h in vivo labeling), displayed background levels of expression of the proliferation marker Ki-67, and expressed elevated levels of the early activation marker CD69 (Fig. S7).
Figure 6.
Enrichment of memory T cell numbers in the DLN requires the continuous recruitment of antigen-bearing migrant RDC. (A) Enrichment of antigen-specific memory T cells in the LN draining the initial site of virus replication. Single cell suspensions prepared from lung–draining (MLN) or axillary and inguinal LN (pLN) of mice previously infected with A/PR/8 at the indicated time points were co-cultured with A/PR/8-infected BMDC, as APC and IFN-γ-secreting T cells were detected by intracellular cytokine staining. Data for each day analyzed are representative of four independent experiments using one mouse per experiment. (B–D) Lung-resident CD11chi DCs are required for maintaining the elevated number of antigen-specific polyclonal T cells in the lung DLN. (B) Schematic depiction of the time course of ablation of RDC from previously infected DTx-treated CD11c-DTR mice and reconstitution of the RDC-depleted lungs with DC. CD11c-DTR mice (day 30 p.i.) were given DTx i.n. The DTx-treated DTR mice then received either DC or alveolar macrophages (AM) i.n. 6 h after DTx treatment. Antigen-specific memory T cells were enumerated 4 d later. (C and D) Absolute numbers of IFN-γ–secreting polyclonal CD8+ (C) or CD4+ (D) T cells in the DLN upon restimulation with infected BMDC in vitro for 5 h as described in A. Data in C and D represent mean ± SEM values from four to seven individual experiments each using one animal per experiment.
Because lymphoid tissues like LNs can undergo substantial morphological changes (e.g., increase in size and number/distribution of HEV, etc.) in response to inflammatory stimuli like virus infection (Soderberg et al., 2005), the difference in the distribution of influenza-specific memory T cells between the DLN and non-DLN could simply be a reflection of the anatomical changes associated with the induction and development of immune responses in the DLN. If, however, in the influenza model, migrant RDCs displaying residual antigen obtained from the respiratory tract depot are playing a more direct role in controlling the distribution and frequency of memory cells, then depletion of the CD103+ and CD11bhi RDC from the respiratory tract might be expected to diminish the number of detectable memory cells selectively within the draining MLN. To explore this possibility, we transiently depleted these two RDC subsets from the lungs of DTR mice 30 d after influenza infection by low-dose DTx administration and determined the number of endogenous antigen-specific CD8+ and CD4+ memory T cells in the draining MLN 4 d later (Fig. 6 B). Transient depletion of RDC resulted in a significant (∼50%) decrease in the number of IFN-γ+CD8+ (Fig. 6 C) and CD4+ (Fig. 6 D) T cells in the draining MLN, with a minimal impact on those in the pLN. The effect of DTx treatment could be directly attributed to the loss of RDC from lungs. In a companion analysis, i.n. adoptive transfer of uninfected wild-type DC into infected DTx-treated DTR mice 4 h after toxin treatment restored antigen-specific IFN-γ–secreting T cell numbers in the draining MLN, whereas i.n. transfer of alveolar macrophages failed to restore antigen-specific T cell numbers (Figs. 6, C and D).
DISCUSSION
In this paper, we have analyzed antigen presentation in influenza-infected mice using the induction of T cell responses in vivo to monitor viral antigen persistence after the resolution of an acute respiratory virus infection. We have found that processed viral antigen may persist for an extended time period, i.e., up to day 70 p.i., in the lung-draining MLN, as measured by the ability of the transferred antigen-specific TCR Tg T cells to proliferate in the MLN. This finding is in agreement with previously reported observations (Jelley-Gibbs et al., 2005, 2007; Zammit et al., 2006; Fazilleau et al., 2007a), which implicated the secondary lymphoid organs (e.g., LNs draining the site of the initial infection) as sites of persistent antigen deposition after infection or vaccination. However, we have gone on to demonstrate that, in the case of respiratory virus infection with influenza, this small persistent reservoir of viral antigen (in the form of influenza viral RNA and protein) is localized to the site of infection, i.e., the lungs. Viral antigen (influenza NP) colocalized with both CD45− and, to a lesser extent, CD45+ lung cells within residual small inflammatory loci. We were able to reconcile this apparent discrepancy, i.e., MLN for the site of T cell activation and lungs for antigen persistence, by demonstrating that RDCs capture the residual viral antigen deposited in the previously infected lung and then migrate to the MLN where they present the in vivo–processed antigen to T cells. Our results further suggest that the presentation of residual viral antigen by the migrant RDC in the MLN appears to have a previously unrecognized role in regulating the memory T cell pool in the draining MLN after infectious virus clearance and recovery from the infection.
There is accumulating evidence in several models of experimental respiratory tract viral infection, i.e., VSV, RSV, or influenza virus, that viral antigen in a form of processed peptide persists in the LN, draining the site of initial pathogen entry (Schwarze et al., 2004; Jelley-Gibbs et al., 2005; Turner et al., 2007). It has been speculated that the viral antigen in the DLN may be retained in cells capable of presenting the antigen to antigen-specific memory T cells. In spite of efforts to identify the cell type that supports the proliferation of T cells in LNs draining sites of infection, the nature of antigen-presenting cells in the DLN has not been elucidated. Our analysis suggests that, in the case of acute viral infection of the respiratory tract, it is these two subsets of CD11chiMHC Class IIhi RDCs, i.e., CD103+ and CD11bhi RDCs (Sung et al., 2006; Kim and Braciale, 2009), which carry out this APC activity in the DLN after their migration from the previously infected lungs. Two lines of evidence support this view: CD103+ and CD11bhi RDC are the only DC subsets capable of supporting significant T cell proliferation when isolated from the lung-draining MLN and analyzed directly ex vivo, and elimination of these two RDC subsets from the previously infected respiratory tract abolishes the capacity of the draining MLN to support T cell activation/proliferation. In this regard, it is noteworthy that resident DCs within secondary lymphoid organs, for example, conventional DCs and pDCs, are thought to have relatively short life spans (from 3 to 7 d; Kamath et al., 2002), and these blood-derived DCs are thus unlikely to retain a pool of antigen within the DLN because of their rapid turnover. RDCs display a basal level of migration out of the respiratory tract to the draining MLN (without an acute inflammatory stimulus; Jakubzick et al., 2008; Kim and Braciale, 2009). As we demonstrate in this paper, these RDCs continued to migrate from the lungs to the draining MLN well after nominal clearance of infectious virus, probably in response to the low level of residual inflammation in the infected lungs at late times after infection. This property makes RDCs ideal candidates to capture antigen from a reservoir of viral protein within the previously infected lungs and transport it to the DLN.
We could detect mRNA encoding the influenza NP gene product, as well as mRNA for the alternatively spliced NS gene product NEP and spliced M gene product M2, in the lungs for up to 30 d p.i, which is well after infectious virus clearance from lungs (Fig. 1). Moreover, immunofluorescent staining of lung tissue revealed that the viral NP could likewise be detected in the previously infected lungs for up to 30 d p.i., largely within CD45− cells located in sites of focal inflammation reminiscent of iBALT. The presence of viral transcripts and viral protein in the lungs at these later times after infection in the absence of detectable viral gene expression in the draining MLN makes this depot of viral antigen the likely ultimate source of the viral antigen presented by migrant RDC to T cells in the draining nodes. It is also noteworthy that viral gene expression persists in the face of a vigorous adaptive immune response, which results in the elimination of both infectious virus and the vast majority of virally infected cells in the respiratory tract. We cannot formally determine whether infectious virions are assembled and released from this viral antigen reservoir, as high levels of circulating neutralizing antibodies in these animals preclude detection of infectious virions in the lung tissue at these later times p.i. The cell types expressing the influenza viral genes and the mechanism by which these cells escape elimination by the immune system remain to be determined. However, beyond 30 d p.i., although viral RNA and/or protein is not demonstrable, viral antigen is present and retained in some recognizable form by influenza-specific CD8+ and, more notably, by CD4+ T cells because T cell proliferation is demonstrable in the draining MLN for >70 d p.i. (Jelley-Gibbs et al., 2005; unpublished data). The nature of this reservoir of the viral antigen is open to speculation. One potential cellular reservoir of the antigen, which is long lived, irradiation resistant, and CD45−, is FDC (Mandels et al., 1980). Although these cells are normally not found in the lungs of naive mice after influenza infection and the formation of iBALT, FDCs can localize in follicles formed within the iBALT (Moyron-Quiroz et al., 2004). Our findings using in vivo depletion of FDC by LTβR blockade (Fig. S5) support the idea that FDC present in the previously infected lungs may, like CD11chiMHCIIhi RDC, play an important role in controlling the retention of memory T cells within the DLNs. Whether these cells serve as the ultimate, or even most effective, reservoir for residual viral antigen remains to be determined. Nevertheless, although not formally explored in this paper, we would speculate that iBALT-like structures within the previously infected lungs may be important sites of influenza viral antigen deposition where CD45− cells, such as FDC, may interact with CD45+ RDC to regulate memory T cell populations within the DLNs.
The preferential localization of memory CD8+ and CD4+ T cells to the LNs draining the site of infection, observed by us in this paper and by others (Hogan et al., 2001; Marshall et al., 2001; Jelley-Gibbs et al., 2005; Zammit et al., 2006), is a potentially useful adaptation for the adaptive immune system to deal with repeat infections by a given pathogen. This strategy allows for rapid mobilization of the memory response through a concentration of central memory cells in the LNs draining the likely site of subsequent infection. Using the DTR mouse model, we demonstrate that elimination of the critical CD103+ and CD11bhi RDC from the influenza-infected respiratory tract before the RDC migration to the DLN substantially decreases the numbers of virus-specific endogenous (wild type) memory CD8+ and CD4+ T cells present in the DLN within days of RDC elimination. Furthermore, adoptive transfer of DC (but not alveolar macrophages) into the respiratory tract of DTx-treated DTR mice restores memory T cell numbers within the DLN. Although strong inflammatory stimuli can alter the architecture of LNs draining the site of the inflammatory stimulus, resulting in preferential accumulation of T cells within the DLN (Soderberg et al., 2005), our findings, in particular the rapid loss of memory T cells from the DLN after RDC depletion from the lungs, are more consistent with an active role for these migrant viral antigen-bearing RDCs in retaining memory cell numbers within the DLN. The nature of the interaction between the memory T cells and the RDC leading to T cell retention in the DLN remains to be elucidated. Our analysis (Fig. S7) does, however, indicate that the accumulation of the memory T cells in the draining MLN appears not to be a result of ongoing proliferation of the memory T cells. Rather, the elevated expression of CD69 would favor a model where the interaction of memory T cells with the migrant RDC results in the CD69-dependent (Shiow et al., 2006) selective retention of the memory T cells within the draining nodes.
In summary, our study provides insight into the process by which viral antigen persists after acute respiratory virus infection and the mechanism through which the prolonged in vivo antigen presentation occurs. Our results suggest that RDCs found at the site of primary infection (i.e., the lungs) play a pivotal role in sampling the residual viral antigen and, upon migration to the DLN, directly present the in vivo–processed peptides to the antigen-specific T cells. As a consequence, this ongoing residual antigen recognition by memory T cells may be an important event resulting in the selective enrichment of virus-specific memory T cells in the DLN after the clearance of infectious agent. This enriched population of antigen-specific T cells maintained in the antigen DLN would likely provide a more rapid and robust recall T cell response to a reinfection. These findings suggest that immunization strategies using long-lasting vaccines deposited at body surfaces where the target pathogen enters the body (e.g., the respiratory tract or GI tract) may be the most effective way to sustain and recall a memory adaptive immune response.
MATERIALS AND METHODS
Mice and infection.
Female BALB/c mice (H-2d) were purchased from Taconic. Tg mice expressing nonhuman primate DTR under the control of the mouse CD11c-promoter (C.FVB-Tg Itgax-DTR/EGFP 57Lan/J, H-2d) and Thy-1.1 Clone 4 (Cl-4) TCR Tg mice (H-2d) were obtained from The Jackson Laboratory. Thy-1.2 Cl-4 TCR Tg mice (H-2d) were a gift from R.W. Dutton (Trudeau Institute, Saranac Lake, NY). TS1 TCR Tg mice (I-A/Ed) were provided by A. Caton (Wistar Institute, Philadelphia, PA), which are bred and housed in a pathogen-free environment and used at 9–13 wk of age for all experiments. To delete CD11c+ cells, DTR Tg mice were injected i.n. with 30 ng DTx/60 µl PBS (Sigma-Aldrich). All animal experiments were performed in accordance with protocols approved by the University of Virginia Animal Care and Use Committee.
Type A influenza viruses A/PR/8/34 (H1N1) or X-31 (H3N2) were grown in day 10 chicken embryo allantoic cavities as described previously (Lawrence and Braciale, 2004). Mice were infected either i.n. in 50 µl with 350 egg infectious doses (EID) or i.v. in 500 µl via tail vein with 1.7 × 107 EID.
Preparation of tissue leukocytes.
Preparation of lung single cell suspensions after enzymatic digestion was described in detail elsewhere (Stevens et al., 2007). For DC isolation from LN, tissues were minced and enzymatically digested with Type II collagenase (37°C for 20 min), followed by passing through Cell Strainer (BD). RBCs in the cell suspensions were lysed using ammonium chloride. Cells were washed and resuspended in FACS buffer containing PBS, 2% FBS, 10 mM EDTA, and 0.01% NaN3 for Ab staining or MACS buffer containing PBS, 2% FBS, and 10 mM EDTA for sorting. Viable nucleated cells in the suspensions were counted using a hemacytometer after exclusion of dead cells using Trypan blue dye.
Antibodies.
The mAbs were purchased from BD or eBioscience (unless stated), and clones were previously described in detail (Kim and Braciale, 2009). Anti–mouse CD16/32 used for Fc receptor blocking was isolated and purified in our laboratory. For biotinylated mAbs, samples were incubated with streptavidin (SA)-RPE, SA-APC or SA-APC-Cy7.
Flow cytometry analysis and intracellular staining.
Cell suspensions were blocked with 2.4G2 and then incubated with specific mAbs or isotype controls for 25 min on ice. Surface marker staining and intracellular cytokine staining were described previously (Stevens et al., 2007; Kim and Braciale, 2009). For identification of antigen-specific polyclonal CD8 or CD4 T cells in the tissues, single cell suspensions were co-cultured with infectious virus-pulsed BMDC for 5 h to stimulate antigen-specific T cells as described previously (Sun et al., 2009). Flow cytometry was performed on either FACSCalibur or FACSCanto flow cytometers (BD), and data were analyzed using FlowJo (Tree Star, Inc.).
T cell and DC isolation and in vitro co-culture.
The isolation of CD8+ T cell, CD4+ T cell, or DC subsets and their co-culture were described elsewhere in detail (Kim and Braciale, 2009).
Adoptive transfer.
Purified splenic naive CL-4 Tg CD8+ or TS1 Tg CD4+ T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Invitrogen) as previously described (Lawrence and Braciale, 2004). A total of 2 × 106 CFSE-labeled T cells were i.v. injected into the tail vein of Thy-mismatched congenic mice at the indicated time points. For adoptively transferring DC into DTx-treated CD11c-DTR mice, BMDC (106/50 µl) grown in tissue culture medium supplemented with GM-CSF for 6 d (Sun et al., 2009) were introduced i.n. As a control, freshly isolated alveolar macrophages (106/50 µl) were purified from lungs of naive animals as described previously (Stevens et al., 2007).
Immunofluorescence staining.
Lungs were first inflated by intratracheally injecting a final volume of 800 µl of OCT medium (50% in PBS) and then they were snap frozen in liquid nitrogen. 5-µm sections were then fixed in cold methanol for 15 min at −20°C and permeabilized in PBS with 0.5% Triton X-100 for 20 min at room temperature. Nonspecific binding was blocked by incubating the cells with 10% goat serum for 10 min, before incubation with an Alexa Fluor 555–conjugated mouse antibody that detected virus nucleoprotein (NP, clone H16, 1:200; gift from J. Yewdell, National Institutes of Health, Bethesda, MD) for 1 h at room temperature. The slide was then coated with antifade mounting solution (BioMeda) and sealed with a coverslip. Fluorescent images from serial planes, 1 µm apart, for multiple representative fields on each slide were obtained after merging using the deconvolution function of Openlab software (version 3.1.5; PerkinElmer; The laboratory of D. Kedes, University of Virginia, Charlottesville, VA).
RT-PCR analyses.
Total RNA was extracted from tissues using Tri Reagent (Molecular Research Center, Inc.), according to the manufacturer’s instructions. cDNA was synthesized from 2 µg of total RNA using random hexamers. For synthesis of initial PCR products, a set of primers specific for the NS (forward, 5′-CACTGTGTCAAGCTTTCAGG-3′; reverse, 5′-TCCATTCAAGTCCTCCGATG-3′) was used. This primer set amplifies two gene segments of NS gene: unspliced (NS1, 550 bp) and spliced variant (NS2, 80 bp), respectively. NP genes were amplified for two consecutive rounds of PCR using two different sets of primers: outer primers (forward, 5′-GATGGCATGCCATTCTGCCG-3′; reverse, 5′-GTACTCCTCTGCATTGTCTC-3′; 498 bp) and inner primers for the nested (second round) PCR (forward, 5′-GAACAACCGTTATGGCAGCA-3′; reverse, 5′-CATGTCAAAGGAAGGCACGA-3′; 178 bp), using 1 µl of the first round of PCR products as a template. Each round of PCR reaction was 35 cycles. The final PCR products were visualized on 2% agarose gel with EtBr staining.
i.n. instillation of OVA-DQ.
Mice were given i.n. either 0.2 mg OVA-DQ in100 µl (Invitrogen) or 0.2 mg of unlabeled OVA in 100 µl (Sigma-Aldrich) as a control. The amounts of OVA taken up by DC found in the MLN of infected mice at day 20 p.i. were assessed at 24 h after instillation by flow cytometry and compared with unlabeled OVA. It is of note that OVA-DQ acquires fluorescence after dequenching through proteolytic enzyme cleavage, permitting the analyses of both antigen uptake and processing by flow cytometry.
Irradiation and BM cell transplantation.
Mice were total body irradiated with 9.4 Gy and then i.v. injected with RBC-lysed BM cells (1–2 × 106) prepared from uninfected CD11c-DTR mice. Engraftment of DC was confirmed by depletion of the CD11c+ DC in lungs of the BM-reconstituted mice upon i.n. DTx administration 24 h later.
Statistics.
A two-tailed unpaired Student t test was used to analyze differences in mean values between groups. These statistical analyses were performed using Prism3 software (for Macintosh; GraphPad Software, Inc.). Results are expressed as means ± SD or SEM as indicated. Values of P < 0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows distribution of viral NP antigen among CD45+ or CD45− cells in the lung. Fig. S2 shows quantification of M gene spliced variant M2 mRNA in the lungs after influenza virus infection. Fig. S3 shows costimulatory molecule expression of DC subsets in the lung before viral infection. Fig. S4 shows the analysis of RDC present in the previously infected lungs for retention of residual viral antigens and stimulation of T cells ex vivo. Fig. S5 demonstrates that the blockade of signaling mediated by LTβRs in vivo diminished antigen presentation in the DLN of the previously influenza-infected lungs. Fig. S6 demonstrates that the blockade of LTβR-mediated signaling at earlier time points had no or minimal impact on the recruitment and subsequent proliferation of T cells in the DLN of the previously influenza-infected lungs. Fig. S7 demonstrates that enrichment of memory T cell numbers in the DLN is mediated by CD69-dependent retention rather than local de novo proliferation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092017/DC1.
Acknowledgments
We thank B. Small and S. Gill for excellent technical assistance and the members of Braciale laboratory for insightful discussions.
These studies were supported by grants from the National Institutes of Health (R01 AI–15608, R01 AI–37293, R01 HL–33391, and U-19 AI-83024) to T.J. Braciale and an American Lung Association fellowship (RT 82757 N) to T.S. Kim.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- DLN
- draining LN
- DTR
- DTx receptor
- DTx
- diphtheria toxin
- FDC
- follicular DC
- HA
- hemagglutinin
- iBALT
- inducible bronchial-associated lymphoid tissue
- i.n.
- intranasal
- LTβR
- lymphotoxin-β receptor
- MLN
- mediastinal LN
- MoRDC
- monocyte-like RDC
- NP
- nucleocapsid protein
- pDC
- plasmacytoid DC
- p.i.
- post infection
- pLN
- peripheral LN
- RDC
- respiratory DC
- RSV
- respiratory syncytial virus
- Tg
- transgenic
References
- Ahmed R., Salmi A., Butler L.D., Chiller J.M., Oldstone M.B. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 10.1084/jem.160.2.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Bevan M.J., Braciale T.J. 1995. Why can’t cytotoxic T cells handle HIV? Proc. Natl. Acad. Sci. USA. 92:5765–5767 10.1073/pnas.92.13.5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer H.J., Hammad H., Kool M., Lambrecht B.N. 2005. Dendritic cell subsets and immune regulation in the lung. Semin. Immunol. 17:295–303 10.1016/j.smim.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Fazilleau N., Eisenbraun M.D., Malherbe L., Ebright J.N., Pogue-Caley R.R., McHeyzer-Williams L.J., McHeyzer-Williams M.G. 2007a. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 8:753–761 10.1038/ni1472 [DOI] [PubMed] [Google Scholar]
- Fazilleau N., McHeyzer-Williams L.J., McHeyzer-Williams M.G. 2007b. Local development of effector and memory T helper cells. Curr. Opin. Immunol. 19:259–267 10.1016/j.coi.2007.04.003 [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., van Rijt L.S., Muskens F., Kool M., Baas C., Thielemans K., Bennett C., Clausen B.E., Hoogsteden H.C., et al. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621–1634 10.1084/jem.20071365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., Bergen I.M., van Rijt L.S., Muskens F., Elewaut D., Osterhaus A.D., Hendriks R., Rimmelzwaan G.F., Lambrecht B.N. 2009. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 206:2339–2349 10.1084/jem.20090410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S., Dujardin H.C., Bakocevic N., Fleige H., Danzer H., Willenzon S., Suezer Y., Hämmerling G., Garbi N., Sutter G., et al. 2009. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 206:2593–2601 10.1084/jem.20091472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X., Kim T.S., Braciale T.J. 2008. Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 82:4908–4919 10.1128/JVI.02367-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan R.J., Usherwood E.J., Zhong W., Roberts A.A., Dutton R.W., Harmsen A.G., Woodland D.L. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813–1822 [DOI] [PubMed] [Google Scholar]
- Huber C., Thielen C., Seeger H., Schwarz P., Montrasio F., Wilson M.R., Heinen E., Fu Y.X., Miele G., Aguzzi A. 2005. Lymphotoxin-beta receptor-dependent genes in lymph node and follicular dendritic cell transcriptomes. J. Immunol. 174:5526–5536 [DOI] [PubMed] [Google Scholar]
- Jakubzick C., Bogunovic M., Bonito A.J., Kuan E.L., Merad M., Randolph G.J. 2008. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205:2839–2850 10.1084/jem.20081430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs D.M., Brown D.M., Dibble J.P., Haynes L., Eaton S.M., Swain S.L. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202:697–706 10.1084/jem.20050227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs D.M., Dibble J.P., Brown D.M., Strutt T.M., McKinstry K.K., Swain S.L. 2007. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J. Immunol. 178:7563–7570 [DOI] [PubMed] [Google Scholar]
- Jung S., Unutmaz D., Wong P., Sano G., De los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., et al. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 17:211–220 10.1016/S1074-7613(02)00365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath A.T., Henri S., Battye F., Tough D.F., Shortman K. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 100:1734–1741 [PubMed] [Google Scholar]
- Kim T.S., Braciale T.J. 2009. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 4:e4204 10.1371/journal.pone.0004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B.N., Prins J.B., Hoogsteden H.C. 2001. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 18:692–704 [PubMed] [Google Scholar]
- Lawrence C.W., Braciale T.J. 2004. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 173:1209–1218 [DOI] [PubMed] [Google Scholar]
- Letvin N.L., Walker B.D. 2003. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9:861–866 10.1038/nm0703-861 [DOI] [PubMed] [Google Scholar]
- Mackay F., Majeau G.R., Lawton P., Hochman P.S., Browning J.L. 1997. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol. 27:2033–2042 10.1002/eji.1830270830 [DOI] [PubMed] [Google Scholar]
- Mandels T.E., Phipps R.P., Abbot A., Tew J.G. 1980. The follicular dendritic cell: long term antigen retention during immunity. Immunol. Rev. 53:29–59 10.1111/j.1600-065X.1980.tb01039.x [DOI] [PubMed] [Google Scholar]
- Marshall D.R., Turner S.J., Belz G.T., Wingo S., Andreansky S., Sangster M.Y., Riberdy J.M., Liu T., Tan M., Doherty P.C. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA. 98:6313–6318 10.1073/pnas.101132698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Komatsu T., Takeuchi K., Nakakuki K., Sudo M., Kimura Y. 1995. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J. Gen. Virol. 76:1251–1254 10.1099/0022-1317-76-5-1251 [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz J.E., Rangel-Moreno J., Kusser K., Hartson L., Sprague F., Goodrich S., Woodland D.L., Lund F.E., Randall T.D. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10:927–934 10.1038/nm1091 [DOI] [PubMed] [Google Scholar]
- P’ringle C.R., Shirodaria P.V., Cash P., Chiswell D.J., Malloy P. 1978. Initiation and maintenance of persistent infection by respiratory syncytial virus. J. Virol. 28:199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B., Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 10.1038/nri1573 [DOI] [PubMed] [Google Scholar]
- Román E., Miller E., Harmsen A., Wiley J., Von Andrian U.H., Huston G., Swain S.L. 2002. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196:957–968 10.1084/jem.20021052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze J., O’Donnell D.R., Rohwedder A., Openshaw P.J. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169:801–805 10.1164/rccm.200308-1203OC [DOI] [PubMed] [Google Scholar]
- Shin H., Wherry E.J. 2007. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19:408–415 10.1016/j.coi.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440:540–544 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Soderberg K.A., Payne G.W., Sato A., Medzhitov R., Segal S.S., Iwasaki A. 2005. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA. 102:16315–16320 10.1073/pnas.0506190102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens W.W., Kim T.S., Pujanauski L.M., Hao X., Braciale T.J. 2007. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 327:63–74 10.1016/j.jim.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C.L., Braciale T.J. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15:277–284 10.1038/nm.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S.S., Fu S.M., Rose C.E., Jr., Gaskin F., Ju S.T., Beaty S.R. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176:2161–2172 [DOI] [PubMed] [Google Scholar]
- Turner D.L., Cauley L.S., Khanna K.M., Lefrançois L. 2007. Persistent antigen presentation after acute vesicular stomatitis virus infection. J. Virol. 81:2039–2046 10.1128/JVI.02167-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D.L., Kohlmeier J.E. 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9:153–161 10.1038/nri2496 [DOI] [PubMed] [Google Scholar]
- Zammit D.J., Turner D.L., Klonowski K.D., Lefrançois L., Cauley L.S. 2006. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 24:439–449 10.1016/j.immuni.2006.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]