Abstract
Little is known about how the microbiota regulates T cell proliferation and whether spontaneous T cell proliferation is involved in the pathogenesis of inflammatory bowel disease. In this study, we show that stimulation of innate pathways by microbiota-derived ligands and antigen-specific T cell stimulation are both required for intestinal inflammation. Microbiota-derived ligands promoted spontaneous T cell proliferation by activating dendritic cells (DCs) to produce IL-6 via Myd88, as shown by the spontaneous proliferation of T cells adoptively transferred into specific pathogen–free (SPF) RAG−/− mice, but not in germfree RAG−/− mice. Reconstitution of germfree RAG−/− mice with cecal bacterial lysate–pulsed DCs, but not with IL-6−/− or Myd88−/− DCs, restored spontaneous T cell proliferation. CBir1 TCR transgenic (CBir1 Tg) T cells, which are specific for an immunodominant microbiota antigen, induced colitis in SPF RAG−/− mice. Blocking the spontaneous proliferation of CBir1 Tg T cells by co-transferring bulk OT II CD4+ T cells abrogated colitis development. Although transferred OT II T cells underwent spontaneous proliferation in RAG−/− mice, the recipients failed to develop colitis because of the lack of cognate antigen in the intestinal lumen. Collectively, our data demonstrate that induction of colitis requires both spontaneous proliferation of T cells driven by microbiota-derived innate signals and antigen-specific T cell proliferation.
The pathogenesis of inflammatory bowel disease (IBD) involves the presence of one or more genetically determined defects that result in an abnormal mucosal immune system that overreacts to normal constituents of the microbiota (Elson et al., 2005; Sartor, 2006; Strober et al., 2007). The complex intestinal microbiota provides an abundant source of immunostimulatory organisms containing innate stimulatory materials and antigens that can activate innate and adaptive immune responses, respectively. In multiple experimental models of colitis, the microbiota plays a crucial role, in that the mice develop colitis with a conventional microbiota but fail to develop colitis when housed under germfree conditions (Taurog et al., 1994; Rath et al., 1996; Sellon et al., 1998; Frank et al., 2007). It is now generally accepted that commensal enteric bacteria provide constant antigenic stimulation that can activate pathogenic T cells and lead to chronic intestinal inflammation. Data from our own studies and others suggest that an excessive adaptive immune response to gut microflora antigens is indispensable for intestinal inflammation (Lodes et al., 2004; Xavier and Podolsky, 2007). Transfer of CD4+ T cells reactive to a single commensal flagellin antigen or to a single bacteria-associated antigen is able to induce colitis development in immunodeficient mice (Iqbal et al., 2002; Lodes et al., 2004; Watanabe et al., 2006). All the subsets of CD4+ effector T cells reactive to commensal bacterial antigens have been implicated in the pathogenesis of IBD. IFN-γ–Th1 and IL-23/IL-17–Th17 pathways are associated with Crohn’s disease, whereas the IL-13–Th2 pathway is associated with ulcerative colitis. It has also been shown that microbial components are necessary for the onset of abnormal inflammatory responses because of their capacity to stimulate innate cells via membrane-bound and endosomal Toll-like receptors (TLRs). TLR4 deficiency abrogates colitis development in myeloid-specific signal transducer and activator of transcription (STAT)-3−/− mice (Kobayashi et al., 2003) and Myd88 deficiency blocks colitis development in IL-10–deficient mice (Rakoff-Nahoum et al., 2006). However, the mechanism by which the Myd88 pathway supports intestinal inflammation is still largely unknown. It is also unknown whether microbiota stimulation of innate responses via TLR ligands and adaptive immune responses to microbiota antigens contributes to intestinal inflammation independently or whether they interact with another.
Homeostatic mechanisms control the overall size and composition of the mature T cell pool to keep the overall number of T cells relatively constant during life (Jameson, 2002; Min et al., 2005; Surh and Sprent, 2008). Accumulating data indicate a role for T cell homeostatic proliferation in the pathogenesis of autoimmune diseases (Theofilopoulos et al., 2001; King et al., 2004; Krupica et al., 2006; Milner et al., 2007; Chang et al., 2008; McPherson et al., 2009), as well as in controlling HIV reservoir size and persistence in latently infected CD4+ T cells (Chomont et al., 2009). In response to lymphopenia, naive T cells proliferate and transition into a memory-like state through a process termed “homeostatic proliferation” (Jameson, 2002). T cell homeostasis is regulated by two distinct modes of cell proliferation: slow IL-7/IL-15-dependent homeostatic proliferation and rapid MHC/antigen interaction-dependent spontaneous proliferation (Schluns et al., 2000; Ku et al., 2000; Goldrath et al., 2002, 2004; Min et al., 2004). A recent work demonstrates that IL-6 is responsible for spontaneous proliferation in an adoptive transfer model of colitis, in that blockade of IL-6 inhibited CD8+ T cell spontaneous proliferation as well as colitis development in RAG−/− recipient mice (Tajima et al., 2008). However, the factors that stimulate IL-6 production, the cellular source of IL-6, and the exact role of IL-6 in T cell spontaneous proliferation are still unknown. The intestinal microbiota has been shown to be required for T cell spontaneous proliferation. Germfree mice have underdeveloped mucosal and systemic immune systems with decreased cellularity of gut associated lymphoid tissues, mesenteric lymph nodes, and spleen (Cebra, 1999; Mazmanian et al., 2008). Adoptively transferred polyclonal CD4+ T cells do not undergo spontaneous proliferation in germfree SCID mice but do in conventionally reared SCID mice (Kieper et al., 2005). However, it is unclear whether the microbiota-driven T cell spontaneous proliferation is caused by microbiota antigenic stimulation or their interaction with host innate cells via TLR–TLR ligand interactions, and whether microbiota-driven T cell spontaneous proliferation is required for the induction of colitis.
In this study, we investigated the effect of the microbiota on T cell spontaneous proliferation and the role of T cell spontaneous proliferation in the pathogenesis of intestinal inflammation. A mouse TCR transgenic line (CBir1 Tg) which is specific for the immunodominant microbiota flagellin antigen, CBir1, present in the intestinal lumen (Cong et al., 2009), as well as a TCR transgenic line (OT II) specific for ovalbumin which is not present in the intestinal lumen was used. We demonstrate that the microbiota provided innate stimulatory activity to promote T cell spontaneous proliferation by activating DCs to produce IL-6 via TLR–TLR ligand interaction through a Myd88-dependent pathway. Both microbiota innate stimulation-driven T cell spontaneous proliferation and microbiota-induced antigen-specific T cell activation were required for the induction of colitis.
RESULTS
Transfer of CBir1 Tg CD4+ T cells, but not OT II CD4+ T cells, induces colitis in B6.RAG−/− mice under SPF conditions
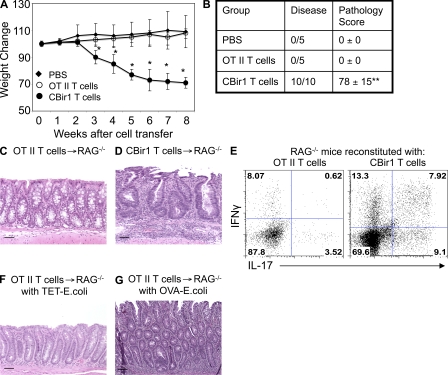
To determine the role of commensal bacterial antigens in induction of colitis in lymphopenic mice, 106 CD4+ T cells from B6.CBir1 Tg mice specific for a microbiota flagellin present in intestinal lumen and CD4+ T cells from B6.OT II mice specific for OVA were separately transferred into specific pathogen–free (SPF) B6.RAG−/− mice. RAG−/− recipients of CBir1 Tg CD4+ T cells, but not OT II CD4+ T cells, lost weight starting from week 3 (Fig. 1 A). All recipients that received CBir1 Tg CD4+ T cells developed severe colonic inflammation when sacrificed 8 wk after cell transfer. In contrast, none of the recipients that received OT II CD4+ T cells developed colitis (Fig. 1, B–D), indicating that activation by cognate antigen in the intestine plays a crucial role in the induction of colitis. Both CBir1 Tg CD4+ T cells and OT II CD4+ T cells proliferated, and substantial numbers of CD4+ T cells were recovered from the intestinal lamina propria (LP) of recipients. Notably, more LP CD4+ T cells were recovered from the RAG−/− recipients of CBir1 Tg CD4+ T cells (3.2 × 105/mouse) than from the recipients of OT II CD4+ T cells (1 × 105/mouse). Th1 and Th17 cells have been implicated in the induction of colitis in various animal models of colitis and in patients with IBD (Cong et al., 2002; Iqbal et al., 2002; Hue et al., 2006; Kullberg et al., 2006; Elson et al., 2007; Kobayashi et al., 2008; Brand, 2009; Zenewicz et al., 2009). To determine if there is different cytokine production in the recipients reconstituted with CBir1 Tg CD4+ T cells vs. with OT II CD4+ T cells, LP CD4+ T cell production of IFN-γ and IL-17 was determined by flow cytometry 8 wk after cell transfer. LP CD4+ T cells from the recipients of OT II T cells produced IFN-γ (8.69%) and IL-17 (4.14%) at a low frequency, whereas LP CD4+ T cell from the recipients of CBir1 Tg T cells produced IFN-γ (21.2%) and IL-17 (17.0%) at a much higher frequency. Interestingly, a substantial proportion of IL-17+IFN-γ+ double positive LP CD4+ T cells were present in colitic RAG−/− recipient mice reconstituted with CBir1 Tg T cells but not in OT II T cell reconstituted recipients (Fig. 1 E). Whether such IL-17+IFN-γ+ CD4+ T cells are the pathogenic T cells that drive colitis development is unclear at present.
Figure 1.
Adoptive transfer of CBir1 Tg CD4+ T cells but not OT II CD4+ T cells induces colitis in RAG−/− mice. Groups of 5 B6.RAG−/− mice were injected with 106 CBir1 Tg CD4+ T cells, 106 OT II CD4+ T cells, or PBS as control. Recipient mice were weighed weekly. 8 wk after transfer, the mice were sacrificed and assessed for histopathology. LP CD4+ T cell cytokine production was determined by flow cytometry. (A) Weight changes of the recipient mice. *, P < 0.05 compared with the PBS group. (B) Pathological scores of the recipient mice. **, P < 0.01 compared with the PBS group. (C and D) Colonic histopathology of the recipient mice. (E) Intestinal LP CD4+ T cell IL-17 and IFN-γ production. (F and G) Groups of 5 B6.RAG−/− mice were injected with 5 × 106 OT II CD4+ T cells and then intrarectally administered with TET-E. coli (F) or with OVA-E. coli (G) every week. Data are from one of two independent experiments with similar results. Bars: (C, D, and F) 50 µm; (E) 100 µm.
To investigate whether lack of colitis induction in the RAG−/− recipient mice that received OT II T cells was caused by significantly less proliferation of OT II T cells than CBir1 Tg T cells upon transfer into RAG−/− mice and independent of antigen recognition, 5 × 106 naive or OT II CD4+ T cells that had been activated with OVA for a week were transferred into SPF B6.RAG−/− mice, and the recipient mice were then colonized with OVA-expressing Escherichia coli (OVA-E. coli) or irrelevant Tetanus toxin fragment C (TET)–expressing E. coli (TET-E. coli). When sacrificed 8 wk later, neither naive nor preactivated OT II T cells induced colitis in the recipients colonized with TET-expressing E. coli (Fig. 1 F), but all recipients colonized with OVA-expressing E. coli did develop colitis with a pathology score of 64 ± 21 (Fig. 1 G), indicating that lack of colitis in the OT II T cell recipients was not caused by the number of OT II T cells, but by lack of cognate antigen in the colon. Collectively, these data demonstrated a crucial role of bacterial-associated antigens in the induction of colitis.
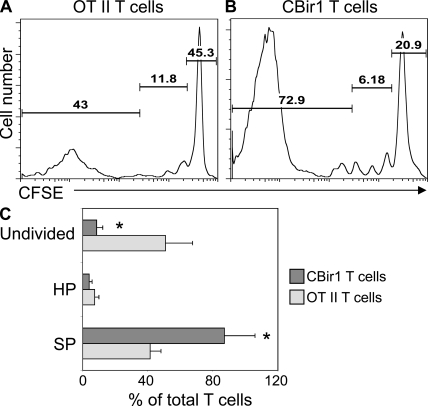
To determine whether the inability of OT II T cells to induce colitis in the RAG−/− recipients is caused in part by the lack of homeostatic proliferation and/or spontaneous proliferation of OT II T cells in RAG−/− mice, CBir1 Tg CD4+ T cells and OT II CD4+ T cells were labeled with CFSE and transferred separately into RAG−/− mice. As shown in Fig. 2, both CBir1 Tg CD4+ T cells and OT II CD4+ T cells underwent homeostatic proliferation and spontaneous proliferation. Approximately 43% of OT II T cells underwent spontaneous proliferation, 11.8% underwent homeostatic proliferation, and 45.3% remained undivided (Fig. 2, A and C). A higher proportion of CBir1 Tg CD4+ T cells underwent spontaneous proliferation (Fig. 2, B and C). Approximately 72.9% of CBir1 Tg T cells underwent spontaneous proliferation, 6.18% underwent homeostatic proliferation, and 20.9% stayed undivided (Fig. 2 B). This could be caused by differences of TCR affinity between OT II T cells and CBir1 Tg T cells, or by the presence of CBir1 flagellin, but not OVA, in the intestine, which could further stimulate CBir1 Tg T cell spontaneous proliferation.
Figure 2.
More CBir1 Tg CD4+ T cells undergo spontaneous proliferation than do OT II CD4+ T cells in RAG−/− mice. 106 CFSE-CBir1 Tg CD4+ T cells or 106 CFSE-OT II CD4+ T cells were transferred into RAG−/− mice, and the recipient mice were sacrificed 10 d later. CD4+ T cell proliferation in the spleen was analyzed by CFSE dilution by flow cytometry. (A) OT II CD4+ T cell proliferation in RAG−/− mice. (B) CBir1 Tg CD4+ T cell proliferation in RAG−/− mice. Numbers in each plot represent the percentage of donor cells undergoing spontaneous proliferation, homeostatic proliferation, and staying undivided, respectively. Data are representative of at least four individual mice of each group from three independent experiments. (C) Percentage of transferred T cells in spontaneous proliferation (SP), homeostatic proliferation (HP), and undivided from three independent experiments. *, P < 0.05 for CBir1 Tg T cells against OT II T cells.
CD4+ T cells do not undergo spontaneous proliferation in germfree RAG−/− mice
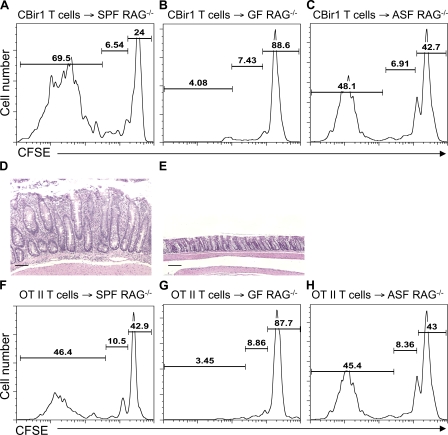
In multiple experimental colitis models, mice do not develop colitis under germfree conditions. We next asked whether microbiota could affect colitis induction via regulation of T cell spontaneous proliferation. As reported previously (Cebra, 1999; Mazmanian et al., 2008), the total number of splenic CD4+ and CD8+ T cells in germfree mice are only ∼80% of that of conventional SPF mice, indicating a mild lymphopenic condition. To further investigate the role of intestinal microbiota in T cell spontaneous proliferation, CFSE-labeled CBir1 Tg CD4+ T cells or OT II CD4+ T cells were transferred into RAG−/− mice housed in conventional SPF conditions or in germfree conditions. 10 d later, T cell proliferation was determined by flow cytometry based on CFSE dilution. As previously shown, CBir1 Tg CD4+ T cells and OT II CD4+ T cells underwent both homeostatic proliferation and spontaneous proliferation in SPF RAG−/− mice (Fig. 3, A and F), whereas both TCR Tg T cells underwent only homeostatic proliferation, but not spontaneous proliferation, in RAG−/− mice housed in germfree conditions (Fig. 3, B and G). Interestingly, both TCR Tg T cells underwent homeostatic proliferation and spontaneous proliferation in RAG−/− mice reconstituted with Altered Schaedler’s Flora (ASF; Fig. 3, C and H). As previously shown, CBir1 Tg T cells induced colitis in SPF RAG−/− mice, but not in germfree RAG−/− mice, 8 wk after cell transfer (Fig. 3, D and E). These data are consistent with antigenic stimulation by the microbiota driving T cell spontaneous proliferation, as previously suggested (Kieper et al., 2005; Surh and Sprent, 2008); however, they do not exclude a role for microbiota innate stimulation in T cell spontaneous proliferation.
Figure 3.
CD4+ T cells do not undergo spontaneous proliferation in germfree RAG−/− mice. 106 CFSE-CBir1 Tg CD4+ T cells (A–C) or 106 CFSE-OT II CD4+ T cells (F–H) were transferred into RAG−/− mice housed under SPF conditions (A and F), germfree (GF) conditions (B and G), or ASF conditions (C and H). 10 d after the transfer, division of the CFSE-labeled CD4+ T cells was determined by CFSE dilution. Numbers in each plot represent the percentage of donor cells undergoing spontaneous proliferation, homeostatic proliferation, and staying undivided, respectively. Data are representative of at least three individual mice of each group from three independent experiments. Histopathology of the colon of CBir1 Tg T cell recipients at 5 mice/group under SPF conditions (D) and germfree conditions (E) was assessed 8 wk after cell transfer. Bars, 100 µm. Data are representative of three independent experiments with similar results.
Microbiota stimulation of DC IL-6 production via Myd88 promotes T cell spontaneous proliferation
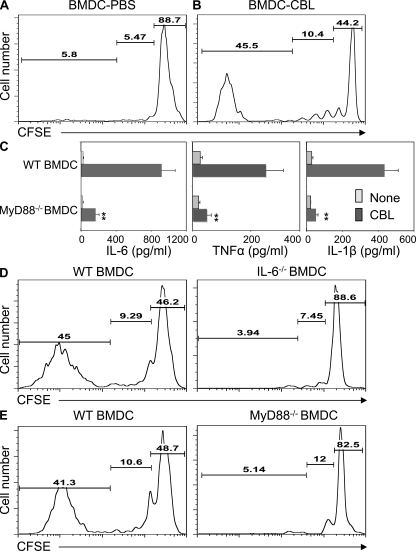
To determine whether microbiota-DC interactions affect T cell homeostatic proliferation and/or spontaneous proliferation, RAG−/−.OT II CD4+ T cells were transferred into germfree RAG−/− mice together with BM-derived DCs (BMDCs) that were or were not pulsed with cecal bacterial lysates (CBLs) from conventional SPF mice. RAG−/−.OT II CD4+ T cells underwent only homeostatic proliferation in the RAG−/− recipients reconstituted with control PBS-pulsed BMDCs (Fig. 4 A). However, they underwent both homeostatic proliferation and spontaneous proliferation in the RAG−/− recipients reconstituted with CBL-pulsed BMDCs (Fig. 4 B), indicating that microbiota-stimulated DCs can result in nonspecific stimulation of CD4+ T cell spontaneous proliferation.
Figure 4.
Microbiota stimulation of DC IL-6 production via Myd88 pathway promotes T cell spontaneous proliferation. BMDCs generated from B6 mice were pulsed with PBS or 100 µg/ml of CBLs for 24 h. 106 CFSE-RAG−/−.OT II CD4+ T cells were transferred together with 106 PBS-pulsed BMDCs (A) or with CBL-pulsed BMDCs (B) into germfree RAG−/− mice. 10 d after the transfer, division of the CFSE-labeled CD4+ T cells was determined by flow cytometry. Numbers in each plot represent the percentage of donor cells undergoing spontaneous proliferation, homeostatic proliferation, and staying undivided, respectively. (C) BMDCs generated from B6 mice or B6.Myd88−/− mice were stimulated with 100 µg/ml of CBL for 24 h. To determine IL-1β production, 2 mM ATP was added in the last 10 min of the culture. IL-6, TNF, and IL-1β production in the supernatant was determined by ELISA. **, P < 0.01 compared with WT DCs. (D) 106 CFSE-RAG−/−.OT II CD4+ T cells were transferred together with 106 CBL-pulsed B6.BMDCs or CBL-pulsed B6.IL-6−/− BMDCs into germfree RAG−/− mice. (E) 106 CFSE-RAG−/−.OT II CD4+ T cells were transferred together with 106 CBL-pulsed B6.BMDCs or CBL-pulsed B6.Myd88−/− BMDCs into germfree RAG−/− mice. 10 d after the transfer, division of the CFSE-labeled CD4+ T cells was determined by flow cytometry. Numbers in each plot represent the percentage of donor cells undergoing spontaneous proliferation, homeostatic proliferation, and undivided, respectively. Data are representative of at least four mice of each group from two independent experiments.
IL-6 has been shown to be a vital cytokine in CD8+ T cell spontaneous proliferation in a recent study (Tajima et al., 2008). We next determined the role of IL-6 in the promotion of CD4+ T cell spontaneous proliferation by CBL-pulsed DCs. CBL-pulsed BMDCs produced high amounts of IL-6, as well as TNF and IL-1β in vitro, and such IL-6 and other cytokine production was impaired in CBL-pulsed Myd88−/− BMDCs, indicating that the Myd88 is involved in CBL-stimulated DC IL-6 production (Fig. 4 C). RAG−/−.OT II CD4+ T cells were then transferred into germfree RAG−/− mice together with WT or IL-6−/− CBL-pulsed BMDCs. RAG−/−.OT II CD4+ T cells underwent both homeostatic proliferation and spontaneous proliferation in the recipients reconstituted with CBL-pulsed WT BMDCs, whereas they only underwent homeostatic proliferation but not spontaneous proliferation in the recipients reconstituted with CBL-pulsed IL-6−/− BMDCs (Fig. 4 D), demonstrating that DC IL-6 production plays a crucial role in the CD4+ T cell spontaneous proliferation driven by the microbiota.
Multiple microbial products stimulate DC cytokine production via TLR–TLR ligand interaction through Myd88 (Medzhitov et al., 1998; Beutler, 2004; Akira et al., 2006; Medzhitov, 2007). To determine the role of Myd88 pathway in microflora-driven CD4+ T cell spontaneous proliferation in vivo, RAG−/−.OT-II CD4+ T cells were transferred into germfree RAG−/− mice together with CBL-pulsed Myd88−/− BMDCs or control CBL-pulsed WT BMDCs. As shown previously, CD4+ T cells underwent homeostatic and spontaneous proliferation in the germfree RAG−/− recipient mice reconstituted with CBL-pulsed WT BMDCs. However, in the RAG−/− recipient mice reconstituted with CBL-pulsed Myd88−/− BMDCs, RAG−/−.OT II T cells only underwent homeostatic proliferation, but not spontaneous proliferation (Fig. 4 E), confirming that the Myd88-dependent TLR pathway plays a crucial role in microbiota-driven CD4+ T cell spontaneous proliferation through the induction of DC IL-6 production.
IL-6 mediates colitis development induced by CBir1 Tg CD4+ T cells in RAG−/− mice
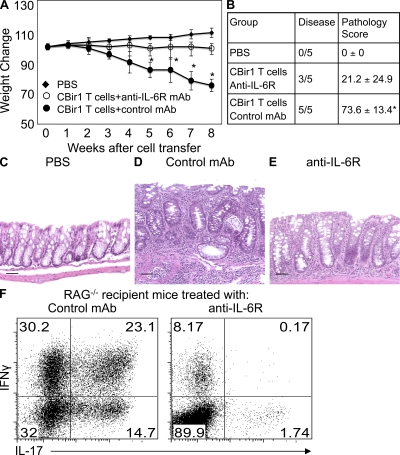
To determine whether IL-6 is also important for the induction of colitis, CBir1 Tg CD4+ T cells were transferred into conventional SPF RAG−/− mice. A group of 5 mice were injected with 100 µg anti–IL-6R mAb i.p. at the time of cell transfer and weekly thereafter, or with control mAb as control. Treatment with anti–IL-6R mAb but not control mAb inhibited weight loss (Fig. 5 A). When sacrificed 8 wk after cell transfer, all RAG−/− recipient mice treated with control mAb developed severe colitis, whereas only 3 out of 5 recipient mice that were treated with anti–IL-6R mAb developed mild colitis, and the colon of the other 2 recipient mice was normal (Fig. 5, B–E). The colonic LP CD4+ T cells in colitic recipient mice treated with control mAb produced high amounts of IFN-γ and IL-17, and a substantial proportion of CD4+ T cells produced both IFN-γ and IL-17 (Fig. 5 F). Blockade of IL-6 signaling dramatically inhibited colonic IL-17 and IFN-γ single-positive CD4+ T cells, as well as double-positive CD4+ T cells. These data indicate that IL-6 not only mediates microbiota innate stimulation-driven CD4+ T cell spontaneous proliferation, but also induces colitis development driven by CD4+ T cells reactive to microbiota bacterial antigens.
Figure 5.
Administration of anti–IL-6R mAb inhibits colitis development induced by CBir1 Tg CD4+ T cells in RAG−/− mice. RAG−/− mice were reconstituted with 106 CBir1 Tg CD4+ T cells. Groups of 5 recipient mice were injected with 100 µg anti–IL-6R mAb or control mAb i.p. weekly. A group of five recipient mice were injected i.p. with PBS as control. Recipient mice were weighed weekly. 8 wk after cell transfer, the mice were sacrificed and assessed for histopathology. LP CD4+ T cell cytokine production was determined by flow cytometry. (A) Weight changes of the recipient mice. *, P < 0.05 compared with anti–IL-6R mAb–treated mice. (B) Colonic pathology scores of the recipient mice. *, P < 0.05 compared with anti–IL-6R mAb–treated mice (nonparametric Mann-Whitney test). (C–E) Histopathology of the recipient mice. Bars, 50 µm. (F) Intestinal LP CD4+ T cell IL-17 and IFN-γ production. The representative experiment shown was performed twice with similar data.
Co-transfer of bulk CD4+ T cells restrains CBir1 Tg CD4+ T cell spontaneous proliferation and abrogates colitis development
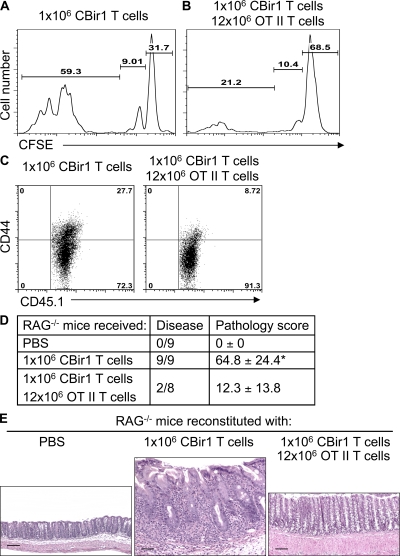
It has been shown that co-transfer of large numbers of CD4+ T cells into lymphopenic hosts restrains excessive activation and proliferation of transferred CD45RBhi T cells and protects the recipient mice from intestinal inflammation (Barthlott et al., 2003). To investigate the role of T cell spontaneous proliferation in the induction of colitis, we first determined whether co-transfer of large numbers of irrelevant RAG−/−.OT II CD4+ T cells could control the spontaneous proliferation of CBir1 Tg CD4+ T cells. Both regulatory T (T reg) cells and competition with neighboring T cells for limited space and resources are involved in the inhibition of spontaneous proliferation (Barthlott et al., 2003; Moses et al., 2003; Stockinger et al., 2004; Bourgeois and Stockinger, 2006; Winstead et al., 2008). T reg cells are also able to inhibit colitis development induced by T cells (Cong et al., 2002; Mottet et al., 2003; Izcue et al., 2009). We used RAG−/−.OT II CD4+ T cells, which are all naive and contain no T reg cells, to exclude the role of T reg cells in this system. 106 CFSE-labeled CD4+ T cells from CD45.1 CBir1 Tg mice were co-transferred with 12 × 106 CD4+ T cells from CD45.2 RAG−/−.OT II mice into RAG−/− mice. 10 d later, T cell spontaneous proliferation in these mice was compared with that in the recipients receiving 106 CFSE-labeled CD45.1 CBir1 Tg CD4+ T cells alone. When transferred alone, CBir1 Tg CD4+ T cells proliferated massively in RAG−/− mice, and most cells underwent spontaneous proliferation (59.3%; Fig. 6 A). In contrast, CBir1 Tg CD4+ T cells proliferated much less when transferred together with 12 × 106 RAG−/−.OT II CD4+ T cells. A smaller number of CBir1 Tg T cells underwent spontaneous proliferation (21.2%), and most CBir1 Tg T cells remained undivided (68.5%; Fig. 6 B), indicating that large numbers of naive OT II T cells restrained CBir1 Tg T cell spontaneous proliferation. Co-transfer of bulk OT II T cells inhibited CBir1 Tg T cell activation, in that the level of CD44 expression by CBir1 Tg T cells was much lower when co-transferred with 12 × 106 RAG−/−.OT II T cells compared with transfer of 106 CBir1 Tg T cells alone (Fig. 6 C).
Figure 6.
Co-transfer of large numbers of OT II CD4+ T cells restrains CBir1 Tg T cell spontaneous proliferation and inhibits colitis induction. 106 CFSE-CD45.1 CBir1 Tg CD4+ T cells were transferred into RAG−/− mice alone (A) or together with 12 × 106 CD45.2 RAG−/−.OT II CD4 T cells (B). 10 d after the transfer, division of the CFSE-labeled CBir1 Tg T cells was determined by CFSE dilution by gating on CD45.1+ cells. Numbers in each plot represent the percentage of donor cells undergoing spontaneous proliferation, homeostatic proliferation, and staying undivided, respectively. (C) CD44 expression of CBir1 Tg T cells was determined by flow cytometry by gating on CD45.1+ cells. Data are from one of two independent experiments with similar results. (D) The recipient mice were sacrificed 8 wk after cell transfer and the total pathology score of cecum and large intestine was assessed. *, P < 0.05 compared with the group that received CBir1 Tg T cells and bulk OT II T cells (nonparametric Mann-Whitney test). Data are sum of two independent experiments. (E) Histopathology of colon in the recipient mice. Bars: (E, left) 100 µm; (E, middle and right) 50 µm. The representative experiment shown was performed twice with similar data.
To determine whether restraining CBir1 Tg T cell spontaneous proliferation affects their ability to induce colitis, recipient RAG−/− mice were sacrificed 8 wk after cell transfer, and the histopathology was determined. All RAG−/− recipients that received CBir1 Tg CD4+ T cells alone developed severe colitis (9 out of 9), whereas the recipients that were co-transferred with CBir1 Tg CD4+ T cells and RAG−/−.OT II T cells developed either mild or no colonic inflammation (Fig. 6, D and E). These data indicate that T cell spontaneous proliferation is a prerequisite for colitis development in lymphopenic hosts.
DISCUSSION
Microbiota activation of T cells plays an important role in the pathogenesis of IBD, particularly the microbiota stimulation of bacterial antigen-specific T cells that induce colitis (Lodes et al., 2004; Kieper et al., 2005; Sartor, 2006; Strober et al., 2007; Xavier and Podolsky, 2007; Surh and Sprent, 2008). We report here that beyond the well-appreciated role in driving pathogenic bacterial antigen-specific T cells via the T cell receptor, the microbiota also provide innate stimulation to promote T cell clonal expansion via spontaneous proliferation, which is dependent on innate cell IL-6 production. This microbiota-driven T cell spontaneous proliferation is necessary for colitis development, but not sufficient in itself as microbiota antigen stimulation to reach a threshold number of T cells producing IFN-γ, and IL-17 is also required.
Two distinct types of cell proliferation occur after adoptive transfer of naive T cells into lymphopenic mice. Slow homeostatic proliferation is IL-7 and IL-15 dependent, in that blockade of either IL-7 or IL-15 abrogates it (Min et al., 2005). Rapid spontaneous proliferation is dependent on antigen recognition via TCR and MHC, either by self-antigens or by commensal bacterial antigens (Min et al., 2005). Although our data demonstrated that naive CD4+ T cells underwent rapid spontaneous proliferation in SPF RAG−/− mice but not in germfree RAG−/− mice, which is consistent with previous observations (Kieper et al., 2005), we show that reconstitution with BMDCs pulsed with CBLs from conventional SPF mice restored spontaneous proliferation of adoptively transferred OT II CD4+ T cells in germfree RAG−/− mice. These data indicate that the microbiota promotes T cell spontaneous proliferation in lymphopenic mice, in part, via innate stimulation rather than antigen stimulation. Interestingly, reconstitution of germfree RAG−/− mice with CBL-pulsed IL-6−/− BMDCs failed to restore spontaneous proliferation of CD4+ T cells, confirming a crucial role of IL-6 in spontaneous proliferation. Our results are in line with a recent study which demonstrated an important role of IL-6 in CD8+ T cell spontaneous proliferation in lymphopenic mice (Tajima et al., 2008). Microbiota CBLs stimulated BMDCs to produce IL-6 predominantly via Myd88, in that Myd88 deficiency impaired CBL-stimulated DC IL-6 production in vitro and in vivo. Although our data definitely indicate an involvement of Myd88 to induce IL-6 production, the Myd88 pathway is complex and many different cell processes are affected by this mutation, thus other factors could also contribute to this process. Collectively, these data indicate that T cell spontaneous proliferation in lymphopenic mice is predominantly driven by microbiota TLR ligand–stimulated innate cell IL-6 production. Notably, CBir1 Tg T cells specific for a bacterial flagellin present in the intestinal lumen underwent more extensive spontaneous proliferation in SPF RAG−/− mice than did OT II T cells specific for OVA, which is not present in the intestinal lumen. Also, although transfer of CBL-pulsed BMDCs rescued OT II T cell spontaneous proliferation in germfree RAG−/− mice to a comparable level to OT II T cell spontaneous proliferation in SPF RAG−/− mice, it was still reduced compared with flagellin-specific CBir1 Tg T cells in SPF RAG−/− mice. This is consistent with antigenic stimulation by microbiota further promoting T cell clonal expansion, which is initiated by the innate stimulation of microbiota.
How important is microbiota-driven T cell spontaneous proliferation in the pathogenesis of colitis? It has long been speculated that lymphopenia-induced homeostatic proliferation plays an important role in the pathogenesis of autoimmune diseases (Theofilopoulos et al., 2001; King et al., 2004; Krupica et al., 2006; Milner et al., 2007; Chang et al., 2008; McPherson et al., 2009). In an experimental model of autoimmune retinitis, autoimmune disease in the retina occurs only with lymphopenia-induced proliferation of naive CD4 T cells (McPherson et al., 2009). Furthermore, germline mutation of Foxp3 in scurfy mice causes lymphopenia in the spleen and massive T cell spontaneous proliferation in neonates, which together with the T reg cell defect leads to fatal autoimmune disease (Chang et al., 2008). As shown in Fig. 6, CBir1 Tg T cells underwent massive spontaneous proliferation and induced colitis in RAG−/− mice. Consistent with a previous study by Barthlott et al. (2003), co-transfer of large numbers of OT II T cells inhibited CBir1 Tg T cell spontaneous proliferation through homeostatic regulation. Interestingly, inhibition of CBir1 Tg T cell spontaneous proliferation also abrogated colitis induction by CBir1 Tg T cells in RAG−/− mice, which provides direct evidence for the requirement for T cell spontaneous proliferation in the induction of colitis in this transfer model. In line with this argument, it has been shown that colitis development in mice with myeloid-specific STAT3 deficiency is abrogated in TLR4-deficient mice (Kobayashi et al., 2003), and intestinal inflammation in IL-10−/− mice is abolished by concomitant Myd88 deficiency (Rakoff-Nahoum et al., 2006), both of which might be caused by the lack of spontaneous proliferation driven by microbiota stimulation on innate cells in the gut. How bulk OT II T cells inhibit CBir1 Tg T cell spontaneous proliferation is currently unknown, but competition for DC occupancy, availability of cytokines, and limited space are potentially involved. It is also unknown whether bulk OT II T cell inhibition of CBir1 Tg T cell–induced colitis is caused by inhibition of CBir1 Tg T cell activation or proinflammatory cytokine production in addition to inhibition of CBir1 Tg T cell spontaneous proliferation. The mechanism of such inhibition is under active investigation.
An enhanced T cell response to microbiota antigens has been demonstrated in patients with IBD, as well as in multiple experimental colitis models (Duchmann et al., 1995; Cong et al., 1998; Blumberg et al., 1999). Adoptive transfer of CD4+ T cells reactive to cecal bacterial antigens, or even to a single commensal antigen, CBir1 flagellin, induced colitis in immunodeficient mice (Lodes et al., 2004). Our current data further emphasize the importance of microbiota antigen stimulation of specific CD4+ T cells in driving colitis development. Both CBir1 Tg T cells and OT II T cells underwent rapid spontaneous proliferation in RAG−/− recipient mice; however, only CBir1 Tg T cells, which are reactive to a microbiota flagellin present in the intestinal lumen, induced colitis development. OT II T cells underwent spontaneous proliferation but did not induce colitis in RAG−/− mice. However, if the RAG−/− recipient mice were colonized with OVA-expressing E. coli in the intestine, but not with E. coli that express an irrelevant Tetanus toxin fragment C antigen, OT II CD4+ T cells were able to induce colitis in RAG−/− recipient. This is consistent with the previous report that adoptive transfer of BALB/c.DO11.10 Th1 and Th2 cells, which are also specific for OVA, into BALB/c.SCID mice, did not induce colitis in the recipient mice. However, they were able to induce colitis in SCID recipients if the SCID recipient mice were colonized with OVA-expressing E. coli in the intestine, but not with E. coli that express an irrelevant TET antigen (Iqbal et al., 2002). An interesting feature of CBir1 Tg T cell adoptive transfer model, as well as most other experimental colitis models, is that CD4+ T cells only induce inflammation in the cecum and colon, but not in the small intestine, despite the presence of CBir1 Tg T cells in both small and large intestinal LP. Interestingly, the bacteria that produce CBir1-like flagellins (Duck et al., 2007) are only detected in the cecum and colon but not in the small intestine (unpublished data). Collectively, these data indicate that both microbiota innate stimulation-driven T cell spontaneous proliferation and microbiota antigen-induced TCR activation are required for the induction of colitis.
Microbiota stimulation of innate IL-6 production could have more than one function in driving colitis development. IL-6 promotes T cell spontaneous proliferation and is required for induction of Th17 cells. Th17 cell response has been implicated in patients with Crohn’s disease and in experimental colitis. Blockade of Th17 pathway by administration of anti–IL-23 or anti–IL-23R antibodies inhibited colitis development in immunodeficient mice adoptively transferred with cecal bacterial antigen-specific T cells or CD4+CD45RBhi T cells (Elson et al., 2007; Lee et al., 2009). IL-6 acts as a crucial cytokine in driving Th17 cell differentiation together with TGFβ. Thus, innate IL-6 production stimulated by the microbiota may stimulate Th17 cells in the intestine. In addition to substantial numbers of IFN-γ+ CD4+ T cells and IL-17+ CD4+ T cells, large numbers of IFN-γ+IL-17+ CD4+ T cells were also present in the colonic LP of colitic RAG−/− recipient mice of CBir1 Tg T cells, but not in the RAG−/− recipient mice of OT II T cells, indicating that both IL-6 and TCR activation are required to generate these IFN-γ+IL-17+ CD4+ T cells. It has been shown recently that Th17 cells can convert into IFN-γ–producing Th1 cells both in vitro and in vivo (Lee et al., 2009; Yang et al., 2008), but there is no evidence showing Th1 cell conversion to Th17 cells. A recent study using genome-wide mapping of H3K4me3, a permissive histone modification found at promoters and enhancers of active genes, and H3K27me3, which is present in broad domains that encompass inactive genes, indicates that both Rorc and IL-17 gene loci have repressive hallmarks in Th1 cells. This suggests that it may be more difficult to induce IL-17 production in a polarized Th1 cell (Wei et al., 2009). The blockade of IL-6 inhibited both Th17 cell development and the generation of IFN-γ+IL-17+ CD4+ T cells. Thus, IFN-γ+IL-17+CD4+ T cells could represent the transition stage of Th17 conversion to Th1 cells.
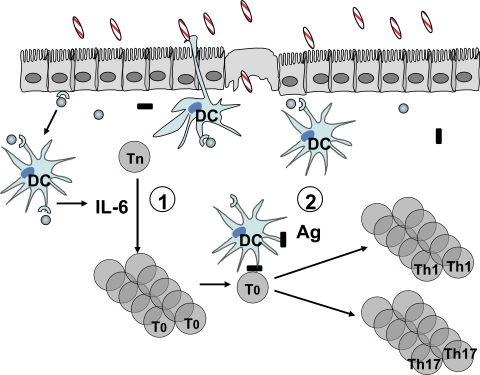
In summary, our data demonstrate that microbiota stimulation of innate cell IL-6 production via Myd88 drives T cell spontaneous proliferation. Such T cell spontaneous proliferation is necessary but not sufficient for the induction of colitis in immunodeficient mice, which also requires further stimulation of T cell receptors by antigens of the microbiota. These data are consistent with a two-hit model of the role of microbiota in the pathogenesis of colitis. First, microbiota promotes T cell clonal expansion by stimulating spontaneous proliferation through innate IL-6 production. Second, T cells must encounter their cognate microbiota antigen, which further stimulates antigen-specific T cell activation and effector T cell differentiation to drive the development of colitis (Fig. 7).
Figure 7.
Two-hit model of the role of microbiota in the pathogenesis of colitis. (1) Microbiota stimulates intestinal innate cell IL-6 production through TLR–TLR ligand interactions. IL-6, and probably other factors, promotes spontaneous proliferation of T cells, which are in T0 status and do not produce effector cytokines. (2) Once T0 cells encounter their cognate microbiota antigen, they will proliferate in a TCR-dependent matter and differentiate into effector T cells, which produce effector cytokines IFN-γ and IL-17 locally in the colon and induce inflammation.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) mice, B6.OT II mice, B6.IL-6−/− mice, B6.Myd88−/− mice, and B6.RAG-1−/− mice were obtained from The Jackson Laboratory and maintained in the Animal Facility at the University of Alabama at Birmingham (UAB). B6.RAG-1−/−.OT II mice were obtained from Taconic. B6.CBir1-specific TCR transgenic (CBir1 Tg) mice were generated and maintained in the Animal Facility at UAB (Cong et al., 2009). Germfree B6.RAG-1−/− mice were derived by hysterectomy and maintained in Trexler type isolators according to standard gnotobiotic techniques using germfree SW mice (Taconic) as foster mothers (Trexler, 1983). Isolators were monitored for contamination monthly by examination of Gram-stained films of fresh fecal samples and by aerobic and anaerobic bacterial culture and fungal culture of fresh fecal samples and swabs of water bottle sipper tubes and isolator interiors. ASF was established to generate ASF B6.RAG-1−/− mice according to Taconic’s instructions and confirmed by examination of Gram-stained films of fresh fecal samples. 8–10-wk-old female mice were used in these experiments. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of UAB.
Reagents and materials.
Reagents and materials were purchased from the following sources: anti–mouse CD4, IL-17, IFN-γ, CD45.1, and CD 44 antibodies were purchased from BD; DNase and collagenase type IV were purchased from Sigma-Aldrich; CFSE was purchased from Invitrogen; and GM-CSF was purchased from R&D Systems.
CD4+ T cell purification and labeling with CFSE.
CD4+ T cells were isolated as previously described (Elson et al., 2007) by using anti–mouse CD4-magnetic beads (BD). For some experiments, CD4+ T cells were labeled with 2.5 µM CFSE following manufacture’s protocol at 37°C in the presence of 0.1% BSA.
Generation of BMDCs.
BM cells were isolated as described previously (Elson et al., 2007) by culturing BM cells in the presence of 20 ng/ml GM-CSF in 6-well plates (Corning) at 37°C in 5% CO2 in humid air for 8 d.
Isolation of LP cells.
Small and large intestines were removed, sliced, and washed with ice-cold PBS to remove fecal content. After removing epithelium by gentle shaking in Ca2+/Mg2+-free HBSS supplemented with 1 mM EDTA and 2% FBS for 40 min at 37°C, the tissue was resuspended in digestion medium containing RPMI 1640, 5% FBS, 0.5 mg/ml collagenase type IV, and 10 µg/ml DNase, and then incubated for 40 min at 37°C by gentle shaking two times. Cells were passed through a mesh, centrifuged, and the pellet was resuspended in 40% Percoll and carefully overlaid onto 70% Percoll. The interface containing the LP leukocytes was collected and washed with PBS supplemented with 1% FBS.
Intracellular staining for cytokine production.
As described previously (Elson et al., 2007), 5 × 105 cells were stimulated for 5 h with PMA and ionomycin. Monensin was added for the last 3 h of culture. After surface staining for CD4, the cells were fixed and permeabilized using Cytofix/Cytoperm solution (BD). IL-17 and IFN-γ staining was done by using PE- or APC-conjugated antibodies (BD).
Adoptive transfer of OT II and CBir1 Tg T cells and induction of colitis.
As previously described, CFSE-labeled or unlabeled CD4+ T cells were injected i.v. into groups of five RAG−/− mice housed in SPF or germfree condition. In some experiments, certain groups of the OT II T cell recipients were given OVA-expressing E. coli or TET-expressing E. coli into the colon weekly. OVA-expressing E. coli and TET-expressing E. coli were generated as described previously (Iqbal et al., 2002). 2 mo later, the mice were sacrificed and histopathology was examined.
Histopathologic assessment.
At necropsy, the small intestine, cecum, and colon were separated and Swiss rolls of each were prepared. Tissues were fixed in 10% buffered formalin and paraffin embedded. The sections (5 µm) were stained with hematoxylin and eosin. All slides were scored by an experienced veterinary pathologist (T. Schoeb, University of Alabama, Birmingham, AL) without knowledge of their origin. Histological scoring was performed using a modification of the previously reported scoring system (Iqbal et al., 2002). In brief, longitudinal sections were examined for crypt epithelial hyperplasia, degeneration, loss; goblet cell loss; crypt exudate; LP and submucosal inflammatory cell accumulation; submucosal edema; mucosal ulceration; and transmural inflammation, with the identity of specimens concealed from the pathologist. Each lesion component was scored 1, 2, or 3 for mild, moderate, or severe, respectively (intensity), and 0 for absent, or 1, 2, 3, or 4 for ∼25, 50, 75, or 100% of the tissue affected, respectively (extent). The total lesion severity score was calculated by summation of the products of extent and intensity scores for each individual lesion component.
Statistical analysis.
The nonparametric Mann-Whitney U test was used. The differences were considered statistically significant at P < 0.05.
Acknowledgments
This work was supported by research grants from National Institutes of Health grants DK079918, DK071176, Digestive Diseases Research Development Center grant DK064400, and National Institutes of Health RR-20136.
No authors have conflicting financial interests.
Footnotes
Abbreviations used:
- ASF
- Altered Schaedler’s Flora
- BMDC
- BM-derived dendritic cell
- CBL
- cecal bacterial lysate
- IBD
- inflammatory bowel disease
- LP
- lamina propria
- SPF
- specific pathogen-free
- STAT
- signal transducer and activator of transcription
- TET
- Tetanus toxin fragment C
- Tg
- transgenic
- TLR
- Toll-like receptor
References
- Akira S., Uematsu S., Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Barthlott T., Kassiotis G., Stockinger B. 2003. T cell regulation as a side effect of homeostasis and competition. J. Exp. Med. 197:451–460 10.1084/jem.20021387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 430:257–263 10.1038/nature02761 [DOI] [PubMed] [Google Scholar]
- Blumberg R.S., Saubermann L.J., Strober W. 1999. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 11:648–656 10.1016/S0952-7915(99)00032-1 [DOI] [PubMed] [Google Scholar]
- Bourgeois C., Stockinger B. 2006. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J. Immunol. 177:4558–4566 [DOI] [PubMed] [Google Scholar]
- Brand S. 2009. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 58:1152–1167 10.1136/gut.2008.163667 [DOI] [PubMed] [Google Scholar]
- Cebra J.J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S–1051S [DOI] [PubMed] [Google Scholar]
- Chang X., Zheng P., Liu Y. 2008. Homeostatic proliferation in the mice with germline FoxP3 mutation and its contribution to fatal autoimmunity. J. Immunol. 181:2399–2406 [DOI] [PubMed] [Google Scholar]
- Chomont N., El-Far M., Ancuta P., Trautmann L., Procopio F.A., Yassine-Diab B., Boucher G., Boulassel M.R., Ghattas G., Brenchley J.M., et al. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15:893–900 10.1038/nm.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Brandwein S.L., McCabe R.P., Lazenby A., Birkenmeier E.H., Sundberg J.P., Elson C.O. 1998. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J. Exp. Med. 187:855–864 10.1084/jem.187.6.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Weaver C.T., Lazenby A., Elson C.O. 2002. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J. Immunol. 169:6112–6119 [DOI] [PubMed] [Google Scholar]
- Cong Y., Feng T., Fujihashi K., Schoeb T.R., Elson C.O. 2009. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 106:19256–19261 10.1073/pnas.0812681106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchmann R., Kaiser I., Hermann E., Mayet W., Ewe K., Meyer zum Büschenfelde K.H. 1995. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 102:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck L.W., Walter M.R., Novak J., Kelly D., Tomasi M., Cong Y., Elson C.O. 2007. Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm. Bowel Dis. 13:1191–1201 10.1002/ibd.20237 [DOI] [PubMed] [Google Scholar]
- Elson C.O., Cong Y., McCracken V.J., Dimmitt R.A., Lorenz R.G., Weaver C.T. 2005. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 206:260–276 10.1111/j.0105-2896.2005.00291.x [DOI] [PubMed] [Google Scholar]
- Elson C.O., Cong Y., Weaver C.T., Schoeb T.R., McClanahan T.K., Fick R.B., Kastelein R.A. 2007. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 132:2359–2370 10.1053/j.gastro.2007.03.104 [DOI] [PubMed] [Google Scholar]
- Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 104:13780–13785 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Sivakumar P.V., Glaccum M., Kennedy M.K., Bevan M.J., Benoist C., Mathis D., Butz E.A. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Luckey C.J., Park R., Benoist C., Mathis D. 2004. The molecular program induced in T cells undergoing homeostatic proliferation. Proc. Natl. Acad. Sci. USA. 101:16885–16890 10.1073/pnas.0407417101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S., Ahern P., Buonocore S., Kullberg M.C., Cua D.J., McKenzie B.S., Powrie F., Maloy K.J. 2006. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203:2473–2483 10.1084/jem.20061099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N., Oliver J.R., Wagner F.H., Lazenby A.S., Elson C.O., Weaver C.T. 2002. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 195:71–84 10.1084/jem.2001889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27:313–338 10.1146/annurev.immunol.021908.132657 [DOI] [PubMed] [Google Scholar]
- Jameson S.C. 2002. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2:547–556 [DOI] [PubMed] [Google Scholar]
- Kieper W.C., Troy A., Burghardt J.T., Ramsey C., Lee J.Y., Jiang H.Q., Dummer W., Shen H., Cebra J.J., Surh C.D. 2005. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J. Immunol. 174:3158–3163 [DOI] [PubMed] [Google Scholar]
- King C., Ilic A., Koelsch K., Sarvetnick N. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 117:265–277 10.1016/S0092-8674(04)00335-6 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Kweon M.N., Kuwata H., Schreiber R.D., Kiyono H., Takeda K., Akira S. 2003. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111:1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Okamoto S., Hisamatsu T., Kamada N., Chinen H., Saito R., Kitazume M.T., Nakazawa A., Sugita A., Koganei K., et al. 2008. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 57:1682–1689 10.1136/gut.2007.135053 [DOI] [PubMed] [Google Scholar]
- Krupica T., Jr., Fry T.J., Mackall C.L. 2006. Autoimmunity during lymphopenia: a two-hit model. Clin. Immunol. 120:121–128 10.1016/j.clim.2006.04.569 [DOI] [PubMed] [Google Scholar]
- Ku C.C., Murakami M., Sakamoto A., Kappler J., Marrack P. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678 10.1126/science.288.5466.675 [DOI] [PubMed] [Google Scholar]
- Kullberg M.C., Jankovic D., Feng C.G., Hue S., Gorelick P.L., McKenzie B.S., Cua D.J., Powrie F., Cheever A.W., Maloy K.J., Sher A. 2006. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203:2485–2494 10.1084/jem.20061082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity. 30:92–107 10.1016/j.immuni.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes M.J., Cong Y., Elson C.O., Mohamath R., Landers C.J., Targan S.R., Fort M., Hershberg R.M. 2004. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113:1296–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Round J.L., Kasper D.L. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453:620–625 10.1038/nature07008 [DOI] [PubMed] [Google Scholar]
- McPherson S.W., Heuss N.D., Gregerson D.S. 2009. Lymphopenia-induced proliferation is a potent activator for CD4+ T cell-mediated autoimmune disease in the retina. J. Immunol. 182:969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature. 449:819–826 10.1038/nature06246 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C.A., Jr 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 2:253–258 10.1016/S1097-2765(00)80136-7 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Ward J.M., Keane-Myers A., Paul W.E. 2007. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. USA. 104:576–581 10.1073/pnas.0610289104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Foucras G., Meier-Schellersheim M., Paul W.E. 2004. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc. Natl. Acad. Sci. USA. 101:3874–3879 10.1073/pnas.0400606101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Yamane H., Hu-Li J., Paul W.E. 2005. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 174:6039–6044 [DOI] [PubMed] [Google Scholar]
- Moses C.T., Thorstenson K.M., Jameson S.C., Khoruts A. 2003. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc. Natl. Acad. Sci. USA. 100:1185–1190 10.1073/pnas.0334572100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet C., Uhlig H.H., Powrie F. 2003. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170:3939–3943 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Hao L., Medzhitov R. 2006. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 25:319–329 10.1016/j.immuni.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Rath H.C., Herfarth H.H., Ikeda J.S., Grenther W.B., Hamm T.E., Jr., Balish E., Taurog J.D., Hammer R.E., Wilson K.H., Sartor R.B. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Invest. 98:945–953 10.1172/JCI118878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R.B. 2006. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3:390–407 10.1038/ncpgasthep0528 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Kieper W.C., Jameson S.C., Lefrançois L. 2000. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432 10.1038/80868 [DOI] [PubMed] [Google Scholar]
- Sellon R.K., Tonkonogy S., Schultz M., Dieleman L.A., Grenther W., Balish E., Rennick D.M., Sartor R.B. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Barthlott T., Kassiotis G. 2004. The concept of space and competition in immune regulation. Immunology. 111:241–247 10.1111/j.1365-2567.2004.01831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W., Fuss I., Mannon P. 2007. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 117:514–521 10.1172/JCI30587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. 2008. Homeostasis of naive and memory T cells. Immunity. 29:848–862 10.1016/j.immuni.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Tajima M., Wakita D., Noguchi D., Chamoto K., Yue Z., Fugo K., Ishigame H., Iwakura Y., Kitamura H., Nishimura T. 2008. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J. Exp. Med. 205:1019–1027 10.1084/jem.20071133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J.D., Richardson J.A., Croft J.T., Simmons W.A., Zhou M., Fernández-Sueiro J.L., Balish E., Hammer R.E. 1994. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180:2359–2364 10.1084/jem.180.6.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A.N., Dummer W., Kono D.H. 2001. T cell homeostasis and systemic autoimmunity. J. Clin. Invest. 108:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler P.C. 1983. Gnotobiotics. In The Mouse in Biomedical Research. Vol. III. Normative Biology, Immunology, and Husbandry. Foster HL, Small JD., Fox JG., :1–16 [Google Scholar]
- Watanabe T., Kitani A., Murray P.J., Wakatsuki Y., Fuss I.J., Strober W. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 25:473–485 10.1016/j.immuni.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T.Y., Watford W.T., et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 30:155–167 10.1016/j.immuni.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead C.J., Fraser J.M., Khoruts A. 2008. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J. Immunol. 180:7305–7317 [DOI] [PubMed] [Google Scholar]
- Xavier R.J., Podolsky D.K. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 448:427–434 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Nurieva R., Martinez G.J., Kang H.S., Chung Y., Pappu B.P., Shah B., Chang S.H., Schluns K.S., Watowich S.S., et al. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 29:44–56 10.1016/j.immuni.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Watanabe T., Usui T., Matsunaga Y., Shirai Y., Yamori M., Itoh T., Habu S., Chiba T., Kita T., Wakatsuki Y. 2001. CD4 T cells monospecific to ovalbumin produced by Escherichia coli can induce colitis upon transfer to BALB/c and SCID mice. Int. Immunol. 13:1561–1570 10.1093/intimm/13.12.1561 [DOI] [PubMed] [Google Scholar]
- Zenewicz L.A., Antov A., Flavell R.A. 2009. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol. Med. 15:199–207 10.1016/j.molmed.2009.03.002 [DOI] [PubMed] [Google Scholar]