Abstract
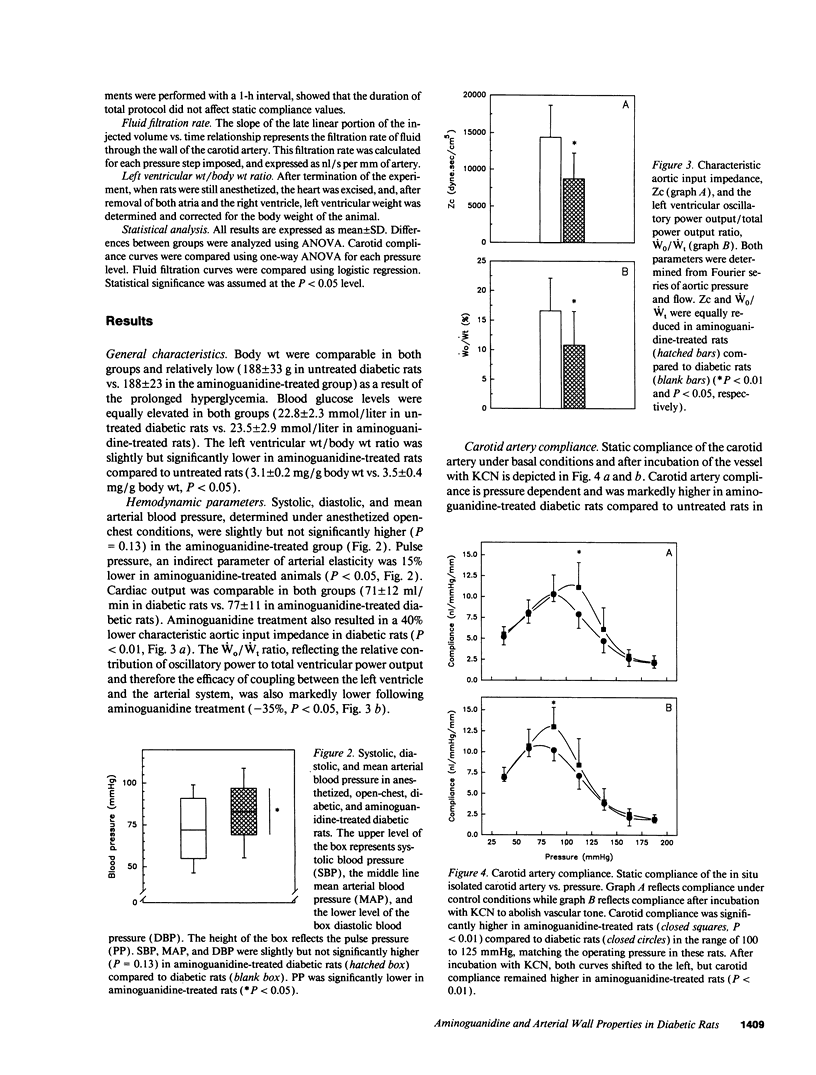
The accumulation of advanced glycosylation end-products (AGEs) on collagen and the subsequent stiffening of this matrix protein in diabetes has been described many years ago. Structural modification of collagen in the arterial wall might have important effects on arterial elasticity. Aminoguanidine is known to decrease the formation of AGEs. In this study we evaluated the effects of aminoguanidine treatment on different parameters reflecting arterial wall elasticity in diabetic rats. We demonstrated that treatment of diabetic rats with aminoguanidine resulted in a significant increase in carotid static compliance (+39%, P < 0.01 under control conditions, and +27%, P < 0.01 after abolition of vascular tone by KCN), and a decrease in characteristic aortic input impedance (-40%, P < 0.01). The arterial pulse pressure in aminoguanidine-treated rats was decreased (-15%, P < 0.05) and the pulsatile component of left ventricular power output was relatively diminished (-35%, P < 0.05). In addition, we observed a lower fluid filtration across the carotid wall. These results indicate an increased vascular elasticity, an improved left ventricular-arterial coupling, and a decreased vascular permeability in diabetic rats after aminoguanidine treatment, suggesting that AGE-accumulation on collagen negatively affects arterial wall properties in experimental diabetes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benessiano J., Levy B. I., Michel J. B. Instantaneous aortic blood flow measurement with range-gated Doppler flowmeter in anesthetized rat. J Pharmacol Methods. 1985 Sep;14(2):99–110. doi: 10.1016/0160-5402(85)90047-6. [DOI] [PubMed] [Google Scholar]
- Bollinger A., Frey J., Jäger K., Furrer J., Seglias J., Siegenthaler W. Patterns of diffusion through skin capillaries in patients with long-term diabetes. N Engl J Med. 1982 Nov 18;307(21):1305–1310. doi: 10.1056/NEJM198211183072103. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992 Dec;15(12):1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charonis A. S., Reger L. A., Dege J. E., Kouzi-Koliakos K., Furcht L. T., Wohlhueter R. M., Tsilibary E. C. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990 Jul;39(7):807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Edelstein D., Brownlee M. Aminoguanidine ameliorates albuminuria in diabetic hypertensive rats. Diabetologia. 1992 Jan;35(1):96–97. doi: 10.1007/BF00400859. [DOI] [PubMed] [Google Scholar]
- Edelstein D., Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992 Jan;41(1):26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen B. Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1986 May;29(5):282–286. doi: 10.1007/BF00452063. [DOI] [PubMed] [Google Scholar]
- Isnard R. N., Pannier B. M., Laurent S., London G. M., Diebold B., Safar M. E. Pulsatile diameter and elastic modulus of the aortic arch in essential hypertension: a noninvasive study. J Am Coll Cardiol. 1989 Feb;13(2):399–405. doi: 10.1016/0735-1097(89)90518-4. [DOI] [PubMed] [Google Scholar]
- Kumari K., Umar S., Bansal V., Sahib M. K. Inhibition of diabetes-associated complications by nucleophilic compounds. Diabetes. 1991 Aug;40(8):1079–1084. doi: 10.2337/diab.40.8.1079. [DOI] [PubMed] [Google Scholar]
- Lash J. M., Bohlen H. G. Structural and functional origins of suppressed acetylcholine vasodilation in diabetic rat intestinal arterioles. Circ Res. 1991 Nov;69(5):1259–1268. doi: 10.1161/01.res.69.5.1259. [DOI] [PubMed] [Google Scholar]
- Levy B. I., Benessiano J., Poitevin P., Safar M. E. Endothelium-dependent mechanical properties of the carotid artery in WKY and SHR. Role of angiotensin converting enzyme inhibition. Circ Res. 1990 Feb;66(2):321–328. doi: 10.1161/01.res.66.2.321. [DOI] [PubMed] [Google Scholar]
- Levy B. I., Poitevin P., Safar M. E. Effects of alpha 1-blockade on arterial compliance in normotensive and hypertensive rats. Hypertension. 1991 Apr;17(4):534–540. doi: 10.1161/01.hyp.17.4.534. [DOI] [PubMed] [Google Scholar]
- Litwin S. E., Raya T. E., Daugherty S., Goldman S. Peripheral circulatory control of cardiac output in diabetic rats. Am J Physiol. 1991 Sep;261(3 Pt 2):H836–H842. doi: 10.1152/ajpheart.1991.261.3.H836. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. D., Berwick L., Orci L., Winegrad A. I. Morphology and metabolism of an aortic intima-media preparation in which an intact endothelium is preserved. J Clin Invest. 1976 Mar;57(3):650–660. doi: 10.1172/JCI108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese G., Tilton R. G., Speedy A., Santarelli E., Eades D. M., Province M. A., Kilo C., Sherman W. R., Williamson J. R. Modulation of hemodynamic and vascular filtration changes in diabetic rats by dietary myo-inositol. Diabetes. 1990 Mar;39(3):312–322. doi: 10.2337/diab.39.3.312. [DOI] [PubMed] [Google Scholar]
- Safar M. E., London G. M. Arterial and venous compliance in sustained essential hypertension. Hypertension. 1987 Aug;10(2):133–139. doi: 10.1161/01.hyp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis-Liparota T., Cooper M., Papazoglou D., Clarke B., Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991 Oct;40(10):1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- Suárez G., Rajaram R., Bhuyan K. C., Oronsky A. L., Goidl J. A. Administration of an aldose reductase inhibitor induces a decrease of collagen fluorescence in diabetic rats. J Clin Invest. 1988 Aug;82(2):624–627. doi: 10.1172/JCI113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Chang K., Tilton R. G., Prater C., Jeffrey J. R., Weigel C., Sherman W. R., Eades D. M., Kilo C. Increased vascular permeability in spontaneously diabetic BB/W rats and in rats with mild versus severe streptozocin-induced diabetes. Prevention by aldose reductase inhibitors and castration. Diabetes. 1987 Jul;36(7):813–821. doi: 10.2337/diab.36.7.813. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Delbridge L., Handelsman D. J., Reeve T., Turtle J. R. The thermal stability of collagen in diabetic rats: correlation with severity of diabetes and non-enzymatic glycosylation. Diabetologia. 1983 Apr;24(4):282–285. doi: 10.1007/BF00282714. [DOI] [PubMed] [Google Scholar]



