Abstract
IL-22 has both proinflammatory and tissue-protective properties depending on the context in which it is expressed. However, the factors that influence the functional outcomes of IL-22 expression remain poorly defined. We demonstrate that after administration of a high dose of bleomycin that induces acute tissue damage and airway inflammation and is lethal to wild-type (WT) mice, Th17 cell–derived IL-22 and IL-17A are expressed in the lung. Bleomycin-induced disease was ameliorated in Il22−/− mice or after anti–IL-22 monoclonal antibody (mAb) treatment of WT mice, indicating a proinflammatory/pathological role for IL-22 in airway inflammation. However, despite increased bleomycin-induced IL-22 production, Il17a−/− mice were protected from airway inflammation, suggesting that IL-17A may regulate the expression and/or proinflammatory properties of IL-22. Consistent with this, IL-17A inhibited IL-22 production by Th17 cells, and exogenous administration of IL-22 could only promote airway inflammation in vivo by acting in synergy with IL-17A. Anti–IL-22 mAb was delivered to Il17a−/− mice and was found to exacerbate bleomycin-induced airway inflammation, indicating that IL-22 is tissue protective in the absence of IL-17A. Finally, in an in vitro culture system, IL-22 administration protected airway epithelial cells from bleomycin-induced apoptosis, and this protection was reversed after coadministration of IL-17A. These data identify that IL-17A can regulate the expression, proinflammatory properties, and tissue-protective functions of IL-22, and indicate that the presence or absence of IL-17A governs the proinflammatory versus tissue-protective properties of IL-22 in a model of airway damage and inflammation.
IL-22 is a member of the IL-10 cytokine family and plays critical roles in inflammation, immune surveillance, and tissue homeostasis at mucosal sites (Ouyang et al., 2008; Colonna, 2009). IL-22 is produced by CD4+ Th17 cells, NK cells, CD11c+ myeloid cells, and lymphoid tissue inducer–like cells (Liang et al., 2006; Zheng et al., 2008; Cella et al., 2009; Takatori et al., 2009). The IL-22 receptor is composed of the IL-22R and IL-10R2 subunits, and receptor ligation results in phosphorylation of STAT1, STAT3, and STAT5 and activation of the p38 mitogen-activated protein kinase pathway (Kotenko et al., 2001; Lejeune et al., 2002). The IL-22 receptor is found on cells of nonhematopoietic origin in the skin, kidney, liver, lung, and gut, allowing for IL-22–mediated regulation of local epithelial, endothelial, and stromal cell responses after infection or exposure to inflammatory stimuli (Wolk et al., 2004; Ouyang et al., 2008). Despite significant insights into IL-22–IL-22R interactions, reports on the in vivo functions of this pathway have been conflicting (Zenewicz and Flavell, 2008). For example, after infection with Gram-negative bacteria, IL-22 can enhance maintenance of the epithelial barrier and act in synergy with the Th17 cell–coexpressed cytokine IL-17A to promote host protective immunity against infection (Liang et al., 2006; Aujla et al., 2008; Zheng et al., 2008). In addition to antimicrobial properties, several studies have reported tissue-protective properties of IL-22 in mouse models of inflammatory bowel disease and hepatitis (Pan et al., 2004; Radaeva et al., 2004; Zenewicz et al., 2007, 2008; Sugimoto et al., 2008; Pickert et al., 2009). In contrast, other studies have demonstrated that IL-22 has proinflammatory/pathological properties after Toxoplasma gondii infection and in mouse models of psoriasis and arthritis (Zheng et al., 2007; Ma et al., 2008; Geboes et al., 2009; Muñoz et al., 2009).
Although IL-22 is known to induce expression of antimicrobial peptides after Klebsiella pneumoniae infection in the lung (Aujla et al., 2008), the influence of the IL-22 pathway on the development, progression, and resolution of airway inflammation has not yet been examined. Using a model of high-dose bleomycin–induced acute tissue damage and airway inflammation (Snider et al., 1978; Nagai et al., 1992; Huaux et al., 2003; Matute-Bello et al., 2008), we demonstrate that a CD4+ Th17 cell response ensues after treatment of WT mice, characterized by the production of IL-22 and IL-17A in the lung. Administration of anti–IL-22 neutralizing mAb in WT mice or use of Il22−/− mice revealed a reduction in bleomycin-induced disease, indicative of a proinflammatory/pathological role for IL-22 in airway inflammation. As IL-17A and IL-22 are coexpressed and have been shown to act cooperatively (Liang et al., 2006; Aujla et al., 2008), we investigated the influence of IL-17A on IL-22 expression and function in the lung by using Il17a−/− mice. Il17a−/− mice exhibited enhanced levels of bleomycin-induced IL-22 expression because of a loss of IL-17A–mediated suppression of IL-22 production in Th17 cells. Despite increased IL-22 expression, Il17a−/− mice were protected from bleomycin-induced airway inflammation, indicating that IL-22 acts in synergy with IL-17A to promote airway inflammation. Consistent with this, exogenous IL-22 could only promote airway inflammation when coadministered with IL-17A. Treatment of Il17a−/− mice with anti–IL-22 mAb exacerbated bleomycin-induced inflammation, supporting a tissue-protective role for IL-22 in the absence of IL-17A. Furthermore, IL-22 protected airway epithelial cells against bleomycin-induced apoptosis, and this property was reversed with the coadministration of IL-17A. Collectively, these data are the first to demonstrate a pathological role for IL-22 in a model of airway inflammation and identify that IL-17A can govern the proinflammatory/pathological versus tissue-protective properties of IL-22 in the lung.
RESULTS
A Th17 cell response develops during bleomycin-induced airway inflammation
When administered at a high dose, bleomycin results in airway damage and acute inflammation, characterized by production of inflammatory mediators and infiltration of lymphocytes and granulocytes, resulting in the disruption of lung architecture, decreased pulmonary function, and death (Snider et al., 1978; Nagai et al., 1992; Huaux et al., 2003; Matute-Bello et al., 2008). These responses have been shown to be partially dependent on both T cells and the cytokines IL-6 and IL-12/23p40, as depletion or genetic deletion of any of these factors individually attenuates bleomycin-induced disease (Maeyama et al., 2001; Sakamoto et al., 2002; Saito et al., 2008). Expression of IL-6 and IL-12/23p40 also promotes Th17 cell differentiation and survival (Bettelli et al., 2006; Mangan et al., 2006; McGeachy et al., 2009). However, whether Th17 cells differentiate and extravasate to the lung after an instillation of high-dose bleomycin is unknown.
To test this, either PBS or a high dose of bleomycin that is lethal for WT mice was intratracheally instilled into C57BL/6 mice, and mRNA isolated from whole lung tissue was analyzed for cytokines associated with Th17 cell differentiation. Although levels of Il6, Tgfb1, and Il12b were not significantly increased, Il23a transcript was significantly elevated in samples from bleomycin-instilled mice compared with PBS controls (Fig. S1). Consistent with no significant changes in Tgfb1 mRNA, no up-regulation of active TGF-β protein could be observed in the lung tissue of mice receiving a high dose of bleomycin, as determined by ELISA (Fig. S2 A) or immunofluorescence staining (Fig. S2 B), in comparison to PBS-instilled controls. Furthermore, increased active TGF-β protein could only be observed in the lungs of mice receiving a low dose of bleomycin, which is known to induce lung fibrosis (Fig. S2, A and B).
An increase in mRNA encoding the Th17 effector cytokines Il17a and Il22 was observed in the lungs of bleomycin-instilled mice (Fig. 1 A). However, bleomycin-induced inflammation was not associated with expression of IL-17F, as IL-17F could not be detected in the lungs by real-time PCR (RT-PCR; not depicted) or intracellular staining (Fig. S3 A) compared with in vitro differentiated Th17 cells (Fig. S3 B).
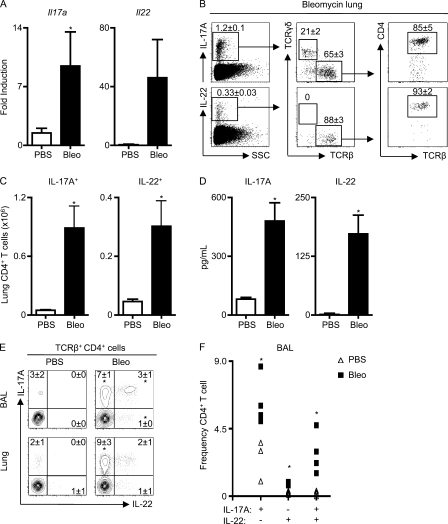
Figure 1.
A Th17 response develops after bleomycin-induced airway inflammation. C57BL/6 mice were intratracheally instilled with PBS or bleomycin (Bleo) and sacrificed on day 8. (A) cDNA prepared from lungs was analyzed by RT-PCR for Il17a and Il22 expression. (B) Live lung cells from bleomycin-instilled mice were analyzed for the frequency of IL-17A+ and IL-22+ T cells. Populations are sequentially gated on total live cells (left), total cytokine-positive cells (middle), and TCRβ+ cells (right). (C) Total numbers of IL-17A+ and IL-22+ CD4+ T cells obtained from the lungs of PBS or bleomycin-treated mice. (D) Lung cell suspensions were stimulated with anti-CD3 mAb for 48 h, and supernatants were analyzed for IL-17A and IL-22 secretion by ELISA. (E) CD4+ T cells from the BAL and lung cells were analyzed for the frequency of IL-17A+ and IL-22+ cells by flow cytometry. (F) Frequency from individual mice of IL-17A+, IL-22+, and IL-17A+/IL-22+ CD4+ T cells in the BAL. All data are representative of three or more independent experiments with a minimum of three to four mice per group. Data shown are means ± SEM. Significance was determined using the Mann-Whitney U test. *, P < 0.05.
A previous study reported that after high-dose bleomycin instillation, TCRγδ+ cells were a dominant source of IL-17A (Braun et al., 2008). To identify the cellular sources of IL-17A after bleomycin exposure, lung cell suspensions from bleomycin-instilled C57BL/6 mice were isolated, stimulated briefly ex vivo, and analyzed by flow cytometry for surface markers and intracellular cytokines. Although 21% of the total IL-17A+ cells were found to be TCRγδ+ cells, TCRβ+ cells constituted 65% of the total IL-17A+ cells (Fig. 1 B, top left and middle). The majority of TCRβ+ cells that expressed IL-17A were CD4+, indicating that bleomycin induced a dominant CD4+ TCRβ+ Th17 cell response (Fig. 1 B, top right). Although unclear at present, differences in local environment stimuli, origin of mice, or the source, dose, and kinetics of administered bleomycin may contribute to whether TCRγδ+ or TCRαβ+ T cells are the dominant sources of IL-17A in the airway after bleomycin exposure. Analysis of IL-22+ cell populations revealed that predominantly CD4+ TCRβ+ T cells produced IL-22 after bleomycin exposure (Fig. 1 B, bottom). Although these data do not definitively rule out other cellular sources of IL-17A and IL-22, they indicate that CD4+ TCRβ+ T cells are the dominant source of both cytokines after instillation of high-dose bleomycin. To determine if the observed IL-17A+ or IL-22+ CD4+ T cell populations were increased after bleomycin exposure, total numbers of cytokine-positive cells in the lungs were quantified. In comparison to mice receiving PBS, mice exposed to bleomycin exhibited a significant increase in the total number of CD4+ T cells that expressed IL-17A or IL-22 (Fig. 1 C), and there were significantly elevated levels of IL-17A and IL-22 protein in supernatants of lung cultures after polyclonal T cell stimulation (Fig. 1 D). To examine whether IL-17A and IL-22 were coexpressed in CD4+ Th17 cells after exposure to bleomycin, TCRβ+ CD4+ T cells from the bronchioalveolar lavage (BAL) and lung were analyzed by flow cytometry. In comparison to PBS-instilled controls, bleomycin-instilled mice exhibited a significant increase in the frequency of TCRβ+ CD4+ T cells that expressed IL-17A alone, IL-22 alone, or coexpressed IL-17A and IL-22 in the BAL (Fig. 1 E, top; and Fig. 1 F). Additionally in the lung, bleomycin-exposed WT mice exhibited a significant increase in the frequency of TCRβ+ CD4+ T cells that expressed IL-17A alone, and a trend toward an increase in the frequency of those that coexpressed IL-17A and IL-22 (Fig. 1 E, bottom). Collectively, these data demonstrate that after bleomycin instillation, a CD4+ Th17 cell response develops in WT mice, characterized by expression of IL-17A and IL-22 in the airway.
Neutralization of IL-22 protects mice from bleomycin-induced airway inflammation
IL-22 has proinflammatory and tissue-protective properties in several different disease settings (Zenewicz and Flavell, 2008). To examine the influence of IL-22 on acute airway damage and inflammation, bleomycin-exposed C57BL/6 mice were treated with either isotype control or anti–IL-22 neutralizing mAb. In comparison to PBS-instilled mice that had a BAL cellularity of 20 × 103 ± 9 × 103, bleomycin-instilled, isotype control mAb–treated mice exhibited a marked increase in cellular recruitment to the BAL (Fig. 2 A). Examination and differential quantification of hematoxylin and eosin (H&E)–stained cytocentrifuge BAL preparations isolated from isotype control mAb–treated bleomycin-instilled mice revealed that the cellular infiltrate was composed of neutrophils (Fig. 2 B, white arrows), lymphocytes, and macrophages (Fig. S4 A). In addition, a population of Gr-1+ SSChi neutrophils was found in dissociated lung tissue isolated from bleomycin-instilled mice (Fig. 2 C). The Gr-1+ cells in the lungs of bleomycin-treated WT mice were confirmed to be neutrophils, as the majority coexpressed CD11b (Fig. S5 A), Ly6C (Fig. S5 B), and Ly6G (Fig. S5 C) and lacked expression of F4/80 (Fig. S5 D) and MHCII (Fig. S5 E). Examination of H&E-stained lung sections from bleomycin-instilled mice revealed leukocyte infiltration (Fig. 2 D, black arrows) and disruption of lung architecture (Fig. 2 D, white arrows). Associated with airway inflammation, weight loss was observed in bleomycin-instilled mice (Fig. 2 E).
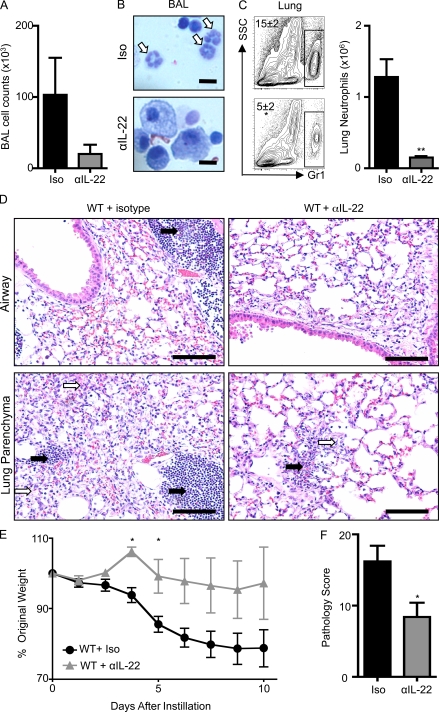
Figure 2.
Administration of anti–IL-22 mAb protects mice from bleomycin-induced airway inflammation. C57BL/6 mice were intratracheally instilled with bleomycin, treated with an isotype control (Iso) or anti–IL-22 (αIL-22) mAb, and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; neutrophils are highlighted by white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows) and disruption of lung architecture (white arrows). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. *, P < 0.05; **, P < 0.01.
In contrast, administration of anti–IL-22 neutralizing mAb to bleomycin-exposed mice resulted in a reduction in the total number of inflammatory cells infiltrating into the BAL (Fig. 2 A). A marked reduction in neutrophilia was observed by microscopic examination of BAL cell preparations (Fig. 2 B, white arrows; and Fig. S4 A), and a significant reduction in both the frequency and total number of neutrophils was observed by flow cytometric analysis of dissociated lung tissue in those mice receiving an anti–IL-22 neutralizing mAb (Fig. 2 C). Histological analysis revealed that blockade of IL-22 also resulted in reduced leukocyte recruitment (Fig. 2 D, black arrows) and less disruption of lung architecture (Fig. 2 D, white arrows). Associated with decreased inflammation, anti–IL-22–treated mice were protected from bleomycin-induced weight loss (Fig. 2 E). A pathological score combining weight loss and histological changes revealed that blockade of IL-22 significantly protected mice from bleomycin-induced pathology (Fig. 2 F).
Consistent with anti–IL-22 mAb treatment of WT mice, bleomycin-treated Il22−/− mice exhibited a decrease in cell recruitment to the BAL (Fig. S6 A), a reduction in airway neutrophilia (Fig. S6, B and C), less severe disruption in lung architecture (Fig. S6 D), significantly reduced weight loss (Fig. S6 E), and a significant reduction in pathology scoring (Fig. S6 F) compared with bleomycin-exposed Il22+/+ littermate controls. Collectively, these data indicate that IL-22 production is pathological in bleomycin-induced airway inflammation, promoting inflammatory cell recruitment, disruption of lung architecture, and weight loss.
Airway inflammation is reduced in Il17a−/− mice despite an increase in bleomycin-induced IL-22 expression
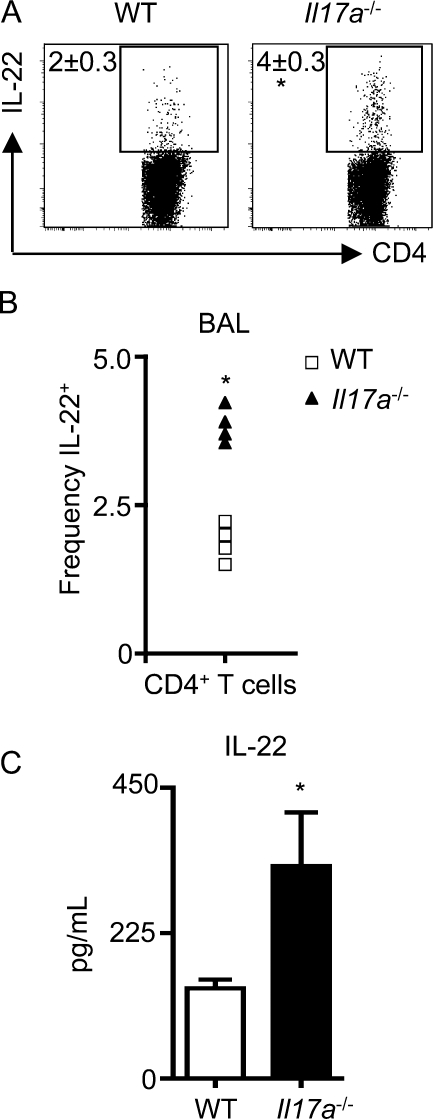
IL-22 and IL-17A are coexpressed by Th17 cells, and can act cooperatively in the induction of antimicrobial peptides and inflammatory mediators (Liang et al., 2006; Aujla et al., 2008). To test whether the expression or functions of IL-22 in the lung were dependent on the presence of IL-17A, bleomycin was instilled into WT and Il17a−/− mice. Flow cytometric analysis of the BAL revealed a significant increase in the frequency of IL-22+ CD4+ T cells from bleomycin-instilled Il17a−/− mice compared with WT mice (Fig. 3, A and B). In addition, there were significantly increased levels of IL-22 in supernatants of polyclonally stimulated lymphocytes isolated from the lungs of bleomycin-instilled Il17a−/− mice compared with WT mice (Fig. 3 C). To test if IL-17A regulates IL-22 expression, splenocytes were isolated from naive WT or Il17a−/− mice and polyclonally stimulated under conditions permissive for Th17 cell differentiation. There was a marked increase in both the frequency and mean fluorescence intensity of IL-22+ CD4+ T cells in splenocyte cultures from Il17a−/− mice compared with WT cultures (Fig. S7 A). Furthermore, addition of rIL-17A to splenocyte cultures from Il17a−/− mice suppressed the frequency and mean fluorescence intensity of IL-22+ CD4+ T cells in a dose-dependent manner (Fig. S7 B), resulting in a significant decrease in IL-22 protein levels in culture supernatants (Fig. S7 C). Conversely, IL-22 did not appear to regulate expression of IL-17A, as bleomycin-instilled Il22−/− mice produced equivalent levels of IL-17A to WT mice (Fig. S7 D), and addition of rIL-22 to splenocyte cultures isolated from Il22−/− mice did not suppress IL-17A production (Fig. S7 E). Collectively, these results indicate that IL-17A can inhibit expression of IL-22 in Th17 cells.
Figure 3.
IL-17A partially regulates the expression of IL-22. WT and Il17a−/− mice were intratracheally instilled with bleomycin and sacrificed on day 10. (A) CD4+ BAL T cells were analyzed for the frequency of IL-22+ cells by flow cytometry. (B) Frequency of individual mice for IL-22+ CD4+ T cell increases in the BAL. (C) Lung cell suspensions were stimulated with anti-CD3 mAb for 48 h, and supernatants were analyzed for IL-22 secretion by ELISA. All data are representative of two or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. Significance was determined using the Mann-Whitney U test. *, P < 0.05.
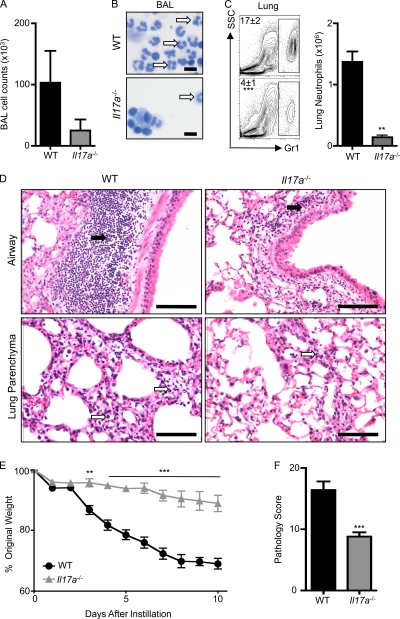
Given that the in vivo neutralization of IL-22 reduced bleomycin-induced inflammation in WT mice, we hypothesized that the elevated IL-22 levels in bleomycin-instilled Il17a−/− mice would correlate with exacerbated inflammation. However, there was a reduction in the cellularity of the BAL in Il17a−/− mice compared with WT mice after bleomycin instillation (Fig. 4 A). Further, there was a marked reduction in the bleomycin-induced neutrophil responses in the BAL of Il17a−/− mice compared with WT mice (Fig. 4 B, white arrows; and Fig. S4 B), correlating with a significant reduction in the frequency and total number of neutrophils in the lung parenchyma (Fig. 4 C). The decreased inflammatory cell recruitment in the BAL and lung of Il17a−/− compared with WT mice was associated with reduced leukocyte infiltrates (Fig. 4 D, black arrows), less disruption of lung architecture (Fig. 4 D, white arrows), and protection from bleomycin-induced weight loss (Fig. 4 E). Pathological scoring confirmed that the absence of IL-17A significantly protected mice from bleomycin-induced disease (Fig. 4 F), thereby demonstrating that abrogation of IL-17A also protects mice from bleomycin-induced airway inflammation.
Figure 4.
Il17a−/− mice are protected from bleomycin-induced pulmonary inflammation. WT or Il17a−/− mice were intratracheally instilled with bleomycin and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; neutrophils are highlighted with white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows) and disruption in lung architecture (white arrows). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. **, P < 0.01; ***, P < 0.001.
Previous studies demonstrated that IL-22 acts synergistically with IL-17A to promote inflammation and provide protection in the context of infection with a Gram-negative pathogen (Aujla et al., 2008), whereas administration of rIL-22 alone failed to promote neutrophil recruitment into the airway (Liang et al., 2007). Therefore, we hypothesized that blockade of either IL-17A or IL-22 protected against bleomycin-induced disease because the proinflammatory properties of IL-22 require synergy with IL-17A. To test this hypothesis, we intratracheally instilled rIL-17A alone, rIL-22 alone, or rIL-17A and rIL-22 in combination into Il17a−/− mice. Instillation of either IL-22 alone or IL-17A alone resulted in a significant increase in lung expression of Il6 but not of the neutrophil chemoattractant Cxcl1 (Fig. S8 A). Consistent with this, instillation of either IL-17A or IL-22 alone did not result in a significant increase in the frequency (Fig. S8, B and C) or total number (Fig. S8 D) of neutrophils in the BAL or lung. In contrast, coadministration of both IL-17A and IL-22 resulted in a significant increase in mRNA expression encoding Il6 and Cxcl1 in the lungs of mice (Fig. S8 A). Examination of the BAL and lungs revealed that mice receiving coadministration of both IL-17A and IL-22 exhibited a significant increase in the frequency (Fig. S8, B and C) and total number (Fig. S8 D) of neutrophils recruited to the airway. Collectively, these results indicate that in vivo IL-22 alone is not proinflammatory in the airway, but IL-22 can act synergistically with IL-17A to promote expression of inflammatory cytokines and chemokines, leading to the recruitment of neutrophils to the airway.
IL-22 is tissue protective in the absence of IL-17A
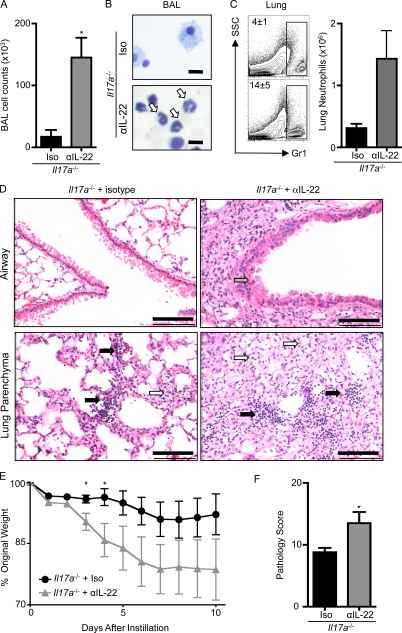
As Il17a−/− mice exhibited elevated levels of IL-22 yet failed to develop bleomycin-induced inflammation, we hypothesized that the function of IL-22 in airway inflammation may differ in the absence of IL-17A. To test this hypothesis, anti–IL-22 neutralizing mAb was administered to bleomycin-instilled Il17a−/− mice. Consistent with earlier findings (Fig. 4), Il17a−/− mice receiving an isotype control antibody demonstrated minimal signs of bleomycin-induced inflammatory cell recruitment (Fig. 5, A–C), less disruption in lung architecture (Fig. 5 D), and reduced weight loss (Fig. 5 E) compared with WT controls. In contrast, administration of anti–IL-22 neutralizing mAb to bleomycin-instilled Il17a−/− mice resulted in a significant increase in cell recruitment to the BAL (Fig. 5 A) and correlated with increased neutrophilia in the BAL (Fig. 5 B, white arrows; and Fig. S4 C) and lung (Fig. 5 C).
Figure 5.
Blockade of IL-22 reverses protection of Il17a−/− mice from bleomycin-induced pulmonary inflammation. Il17a−/− mice were intratracheally instilled with bleomycin, treated with an isotype control (Iso) or anti–IL-22 (αIL-22) mAb, and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; highlighted by white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows), disruption in lung architecture (white arrows), and epithelial hyperplasia (gray arrow). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. *, P < 0.05.
Microscopic examination of H&E-stained lung sections revealed increased leukocyte infiltration (Fig. 5 D, black arrows) and elevated disruption in lung architecture (Fig. 5 D, white arrows) in anti–IL-22–treated Il17a−/− mice. Epithelial cells in lung tissue examined from Il17a−/− mice in which IL-22 was neutralized demonstrated increased hyperplasia and loss of normal morphology in comparison to isotype control–treated Il17a−/− mice after bleomycin administration (Fig. 5 D, gray arrow), indicating that IL-22 may influence the integrity of the epithelial barrier in the absence of IL-17A. Neutralization of IL-22 led to exacerbated weight loss in bleomycin-instilled Il17a−/− mice compared with isotype control–treated Il17a−/− mice (Fig. 5 E), and pathological scoring confirmed a significant increase in bleomycin-induced inflammation and tissue destruction in Il17a−/− mice after blockade of IL-22 (Fig. 5 F). Blockade of IL-22 in Il17a−/− mice established a level of bleomycin-induced disease comparable to that observed in WT mice, as no statistically significant differences in the BAL cellularity, neutrophil recruitment, weight loss, or pathology scoring were observed between bleomycin-treated WT and anti–IL-22 mAb–treated Il17a−/− mice (unpublished data). The loss of IL-22–mediated protection in Il17a−/− mice revealed a pathway of bleomycin-induced inflammation that was independent of both IL-22 and IL-17A. Notwithstanding this, these data indicate that IL-17A may govern the proinflammatory and pathological properties of IL-22 in the lung. Specifically, in the presence of IL-17A, IL-22 appears to promote proinflammatory and pathological outcomes, whereas in the absence of IL-17A, IL-22 appears to confer a tissue-protective role in this model of airway damage and inflammation.
IL-17A regulates IL-22–mediated protection from bleomycin-induced airway epithelial cell apoptosis
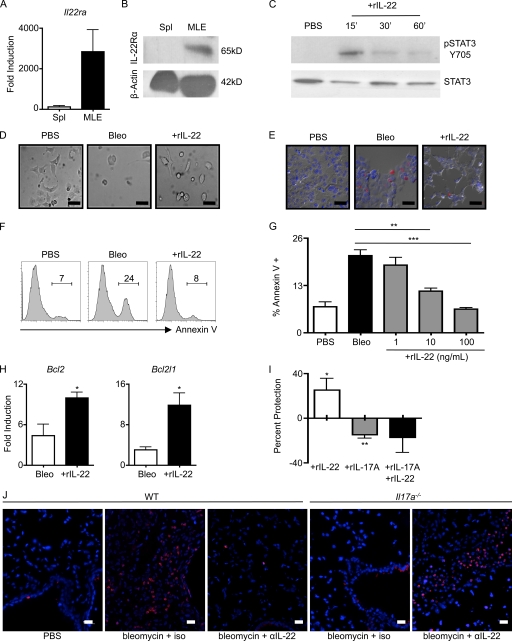
IL-22 acts on cells of nonhematopoietic origin and has been shown to promote epithelial cell repair mechanisms (Wolk et al., 2004; Aujla et al., 2008; Pickert et al., 2009). Given that administration of anti–IL-22 neutralizing mAb to Il17a−/− mice increased bleomycin-induced epithelial cell hyperplasia and disruption of lung alveolar architecture (Fig. 5 D, gray arrow), we hypothesized that IL-22 may protect epithelial cells from bleomycin-mediated damage. To test this, we used the mouse pulmonary epithelial cell line MLE-12 (Wikenheiser et al., 1993), which expressed Il22ra mRNA transcripts (Fig. 6 A) and IL-22Rα protein (Fig. 6 B). Treatment with rIL-22 led to STAT3 phosphorylation (Fig. 6 C), confirming that this cell line is responsive to IL-22. To test whether IL-22 influenced bleomycin-induced damage, bleomycin was added to epithelial cell cultures in the presence or absence of rIL-22. Administration of bleomycin resulted in disrupted epithelial cell morphology and a loss of confluency, as examined by microscopy (Fig. 6 D, middle). These visual alterations correlated with an induction of epithelial cell apoptosis, as determined by terminal deoxynucleotidyl trasferase dUTP nick-end labeling (TUNEL) staining (Fig. 6 E, middle). However, addition of rIL-22 resulted in a striking reduction in bleomycin-induced disruption of cell morphology (Fig. 6 D, right) and decreased epithelial cell apoptosis identified by TUNEL staining (Fig. 6 E, right). To quantify the IL-22–mediated protection, airway epithelial cell cultures were stained with an mAb recognizing annexin V and analyzed by flow cytometry. Addition of rIL-22 significantly protected epithelial cells from bleomycin-induced apoptosis (Fig. 6, F and G), and this protection was dose dependent (Fig. 6 G). IL-22–mediated protection was also associated with significantly increased levels of mRNA transcripts encoding the antiapoptotic genes Bcl2 and Bcl2l1 in comparison to cultures that did not receive IL-22 (Fig. 6 H).
Figure 6.
IL-17A regulates the tissue-protective properties of IL-22. (A) cDNA prepared from MLE cells and splenocytes (Spl) was examined for the presence of Il22ra transcripts by RT-PCR. (B) Immunoblot of MLE cell and total splenocyte lysates with antibody for IL-22Rα and β-actin. (C) MLE cells were stimulated with 10 ng/ml rIL-22 for the indicated times before lysis and immunostaining for phopho-STAT3 (pSTAT3) and total STAT3. (D) For imaging, MLE cell were treated overnight with bleomycin in the absence (Bleo) or presence (+rIL-22) of rIL-22. (E and F) Induction of apoptosis was identified by in situ TUNEL staining (E) or flow cytometry analysis for the frequency of annexin V+ cells (F). Bars, 10 µm. (G) Frequency of annexin V+ cells with the addition of increasing concentrations of rIL-22. (H) cDNA from bleomycin-treated MLE cells in the presence or absence of 100 ng/ml rIL-22 was prepared and analyzed for Bcl2 and Bcl2l1 transcripts by RT-PCR. (I) Percent protection with the addition of 10 ng/ml rIL-17A and 10 ng/ml rIL-22 was determined based on the frequency of annexin V+ cells above or below that obtained with bleomycin alone (set at 0). (J) In situ TUNEL staining of paraffin-embedded lung tissue. Bars, 10 µm. Data from in vitro studies are representative of two or more independent experiments with triplicate wells per condition. Data shown are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As IL-17A regulated the functional consequences of IL-22 expression in vivo, we hypothesized that IL-17A may influence IL-22–mediated protection of airway epithelial cells from bleomycin-induced apoptosis in vitro. Analysis of airway epithelial cells by RT-PCR and ELISA revealed the absence of IL-17A expression in PBS- and bleomycin-treated airway epithelial cells (unpublished data), indicating that there was no source of endogenous IL-17A in this system. As observed previously, administration of rIL-22 protected epithelial cells from bleomycin-induced apoptosis (25% increase in protection; Fig. 6 I) compared with PBS controls. However, the addition of exogenous IL-17A to cultures enhanced bleomycin-induced apoptosis of airway epithelial cells relative to PBS controls (15% decrease in protection; Fig. 6 I). Co-administration of rIL-17A and rIL-22 to cultures prevented IL-22–mediated protection and instead enhanced bleomycin-induced apoptosis relative to PBS controls (18% decrease in protection; Fig. 6 I). Collectively, these results indicate that IL-17A regulates the ability of IL-22 to protect airway epithelial cells from bleomycin-induced apoptosis.
To test whether the ability of IL-17A to regulate IL-22–mediated tissue protection in vivo was associated with alterations in epithelial cell apoptosis, TUNEL staining was performed on lung sections of bleomycin-instilled WT and Il17a−/− mice that were treated with an isotype control or with an anti–IL-22 neutralizing antibody. Bleomycin-instilled WT mice treated with an isotype control antibody exhibited a marked increase in apoptotic bodies in comparison to PBS-instilled controls (Fig. 6 J). Consistent with the pathological role of IL-22 in bleomycin-induced airway inflammation, blockade of IL-22 (WT plus anti–IL-22 mAb) resulted in a reduction of bleomycin-induced apoptotic bodies throughout the lung tissue (Fig. 6 J). In contrast, blockade of IL-22 in the absence of IL-17A (Il17a−/− anti–IL-22) demonstrated a marked increase in apoptotic bodies compared with isotype-treated controls (Fig. 6 J). Collectively, these results indicate that IL-17A governs the ability of IL-22 to protect airway epithelial cells from bleomycin-induced apoptosis in vivo.
DISCUSSION
Although significant advances have been made in characterizing the expression of IL-22 and IL-22R and the signal transduction pathways that are activated, there are conflicting reports on the biological consequences of IL-22 expression in mouse models of inflammation. For example, IL-22 production can be either pathological or tissue protective depending on the disease model examined (Zenewicz and Flavell, 2008). The results of the present study are the first to demonstrate that IL-22 expression can promote inflammation after bleomycin-induced airway inflammation. Critically, the functional outcomes of IL-22 expression in the lung were governed by coexpression of IL-17A. When coexpressed in vivo, IL-17A and IL-22 acted synergistically to promote chemokine expression, neutrophil recruitment, and airway inflammation. Conversely, in the absence of IL-17A, IL-22 expression was no longer proinflammatory and pathological but rather conferred tissue-protective functions by promoting the integrity of the epithelial barrier. Therefore, differential spatial and temporal expression of IL-17A and IL-22 may explain the divergent functions of IL-22 reported in different models of infection or inflammation.
After bleomycin exposure, a Th17 cell response developed, characterized by expression of IL-17A and IL-22 in the lung and BAL. Previous studies have identified that many of the factors that promote the differentiation of Th17 cells have been linked in the pathogenesis of bleomycin-induced airway inflammation, including IL-6 and IL-12/23p40 (Maeyama et al., 2001; Sakamoto et al., 2002; Saito et al., 2008). Consistent with a proinflammatory role for Th17 cells, neutralization of either Th17 cell–associated effector cytokine, IL-22 or IL-17A, was sufficient to provide protection against bleomycin-induced airway inflammation in WT mice, suggesting that a functional synergy between both cytokines can promote disease. Synergy between IL-22 and IL-17A has previously been observed in vitro (Liang et al., 2006) and in vivo after infection with the pulmonary pathogen K. pneumoniae (Aujla et al., 2008). This synergy promoted the production of inflammatory mediators and antimicrobial peptides (Liang et al., 2006), and was found to be beneficial for the host after pulmonary infection (Aujla et al., 2008). However, it was also found that administration of exogenous IL-22 itself was not enough to promote neutrophil recruitment to the airway (Liang et al., 2007), suggesting that IL-17A was required for the proinflammatory properties of IL-22. Consistent with this, we demonstrate that exogenous IL-22 is only able to promote inflammation in the airway in the presence of IL-17A. It is possible that in mouse models of psoriasis, arthritis, and protozoan infection, in which IL-22 was reported to be proinflammatory (Zheng et al., 2007; Ma et al., 2008; Geboes et al., 2009; Muñoz et al., 2009), the same synergy between IL-22 and IL-17A is operating to promote inflammation.
In the absence of IL-17A, it was found that there were increased levels of IL-22 after bleomycin instillation. This finding was consistent with previous studies that also observed increased IL-22 mRNA in the absence of IL-17A in a mouse model of colitis (O’Connor et al., 2009) or decreased IL-22 mRNA in splenocyte cultures with the addition of exogenous IL-17A (Smith et al., 2008; von Vietinghoff and Ley, 2009). In in vitro studies, we demonstrated that IL-17A could suppress the expression and secretion of IL-22 from Th17 cells in a dose-dependent manner. Interestingly, it has also been reported that IL-17A can inhibit IL-17F expression by Th17 cells (von Vietinghoff and Ley, 2009), suggesting a common pathway for IL-17A–mediated inhibition of Th17 cell effector cytokine expression. However, it has not yet been determined whether IL-17A is acting directly on the Th17 cells or through an accessory cell, and further investigation must be conducted to determine the mechanisms through which the suppression of IL-22 production occurs.
Despite elevated expression of IL-22 in the absence of IL-17A, Il17a−/− mice were not susceptible to bleomycin-induced disease, which is consistent with a loss of the proinflammatory properties of IL-22. However, blockade of IL-22 in the absence of IL-17A exacerbated bleomycin-induced disease, indicating a tissue-protective role for IL-22 in airway inflammation in the absence of IL-17A. Consistent with this hypothesis, we found that rIL-22 could protect airway epithelial cells from bleomycin-induced apoptosis in both in vitro and in vivo assays. Further, IL-22–mediated protection from epithelial cell apoptosis was reversed in the presence of IL-17A. Previous reports proposed a constitutive tissue-protective function for IL-22 in mouse models of inflammatory bowel disease and hepatitis (Pan et al., 2004; Radaeva et al., 2004; Zenewicz et al., 2007, 2008; Sugimoto et al., 2008; Pickert et al., 2009). However, after bleomycin-induced airway inflammation, IL-22 exhibited a constitutive proinflammatory effect in WT mice and was only tissue protective in the absence of IL-17A. One possible explanation for the constitutive proinflammatory effects of IL-22 in bleomycin-exposed WT mice in comparison to a constitutive tissue-protective role for IL-22 reported in mouse models of inflammation in the intestine or liver may be the differential coexpression of IL-17A and IL-22 in distinct tissues. For example, after bleomycin instillation the majority of IL-22–expressing cells in the lung coexpressed IL-17A and promoted inflammation. In contrast, subsets of gut-resident NK cells and skin-resident CD4+ T cells are reported to express IL-22 but do not coexpress IL-17A (Satoh-Takayama et al., 2008; Cella et al., 2009; Duhen et al., 2009; Trifari et al., 2009). Collectively, these reports support a model in which production of IL-22 by these cell populations in the absence of IL-17A may be important in promoting tissue-protective responses. Therefore, the cellular sources, anatomical location, and cytokine coexpression profile of resident and recruited cell populations may influence the functional properties of IL-22 and provide an explanation for the distinct functional roles of IL-22 in models of infection and inflammation in distinct peripheral tissues.
When anti–IL-22 mAb was administered in the absence of IL-17A, bleomycin-induced disease was comparable to that in WT mice and independent of IL-17A and IL-22. It is possible that other Th17 cell–derived cytokines such as IL-17F or TNF-α may play a significant role in this context, as well as other nonrelated inflammatory cytokines such as IFN-γ, all of which have been shown to contribute to airway inflammation in other model systems (Lukacs et al., 1995; Segel et al., 2003; Liang et al., 2007; Yang et al., 2008). Additionally, a recent report identified that in a model of bleomycin-induced fibrosis, IL-17A can act cooperatively with TGF-β to promote disease (Wilson et al., 2010). In the present study, the influence of IL-17A and IL-22 on bleomycin-induced tissue damage and acute airway inflammation occurred independently of any significant changes in the production of TGF-β protein (unpublished data). Notwithstanding that, future studies in a model of fibrosis will be required to examine the potential functional interactions between IL-17A, IL-22, and TGF-β in the development and/or progression of disease.
Based on the in vitro and in vivo findings reported here, we propose three mechanisms by which IL-17A regulates the functional consequences of IL-22 expression. First, IL-17A regulates the in vivo and in vitro expression levels of IL-22 by inhibiting IL-22 production from Th17 cells. Second, IL-17A promotes the proinflammatory properties of IL-22 by acting in synergy to induce expression of inflammatory cytokines, chemokines, and neutrophil recruitment. Third, IL-17A prevents the tissue-protective functions of IL-22 by suppressing the antiapoptotic effects of IL-22 on epithelial cells. Therefore, this complex regulation of IL-22 by IL-17A may underlie how IL-22 can promote both pathological or tissue-protective outcomes depending on the context in which it is expressed.
The ability of IL-22 to be either pathological or protective, depending on the context in which it is expressed, is a property shared by other cytokines that signal through STAT3, including IL-6 and IL-27, which can either promote or regulate inflammation dependent on the cytokine milieu and regulation of signal transduction (Yasukawa et al., 2003; Villarino et al., 2004). It is probable that the interplay between IL-17A and IL-22 signaling pathways will determine the balance between proinflammatory versus tissue-protective outcomes. IL-22 is known to signal through the STAT3 and p38 mitogen-activated protein kinase pathways, whereas IL-17A signals predominantly through the NF-κB pathway (Kotenko et al., 2001; Lejeune et al., 2002; Gaffen, 2009). The STAT3 and NF-κB pathways regulate a wide range of biological processes, including cell growth, differentiation, and apoptosis, and complex interactions have been reported between these two transcription factors (Alonzi et al., 2001; Uskokovic et al., 2007; Bollrath and Greten, 2009). Therefore, future investigation into the interplay between the signal transduction pathways and gene targets of both IL-17A and IL-22 will likely yield further insight into the ability of IL-17A to regulate the functional consequences of IL-22 expression. Notwithstanding this, the results of the present study provide the first demonstration that IL-22 can promote disease in a model of airway inflammation, and support a model in which IL-17A regulates the levels of expression, proinflammatory properties, and tissue-protective properties of IL-22, thereby determining the functional consequences of IL-22 expression in the lung. Differential temporal and spatial coexpression of IL-17A and IL-22 may underlie the conflicting reports of the biological effects of IL-22 in distinct disease models, and may offer selective therapeutic potential in the treatment of Th17 cell–associated inflammatory diseases.
MATERIALS AND METHODS
Mice, bleomycin instillation, and mAb treatments.
C57BL/6 mice were purchased from the Jackson Laboratory. C57BL/6 Il17a−/− mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). 129 Il22−/− mice were generated at Lexicon Genetics in collaboration with Pfizer and subsequently backcrossed to BALB/cBy at the Jackson Laboratory with colony mates used for all groups. All mice were maintained in specific pathogen–free facilities at the University of Pennsylvania. All protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC), and all experiments were performed according to the guidelines of the University of Pennsylvania IACUC. Bleomycin (NOVAPLUS) was administered intratracheally at either a lethal high dose of 0.009 mg/g or at a low dose of 0.0018 mg/g. Mice were sacrificed at 8–10 d or at reaching 70% of their original weight. Mice receiving antibody treatment were injected i.p. with 0.4 mg of either IL22-01 or an isotype control antibody (Pfizer) every 3 d starting on day 0.
Isolation and analysis of lung and BAL cells.
BAL cell and lung single-cell suspensions were obtained as previously described (Nair et al., 2009). Cells were stained with antibodies to the following markers: Gr-1, CD11b, Ly6G, Ly6C, F4/80, MHCII, CD4, CD8, TCRβ, and TCRγδ (eBioscience). For analysis of intracellular cytokine production, cells were stimulated directly ex vivo by incubation for 4 h with 50 ng/ml PMA, 750 ng/ml ionomycin, and 10 µg/ml brefeldin A (all obtained from Sigma-Aldrich), or stimulated for 48 h with soluble anti-CD3 (eBioscience) followed by analysis of cytokine secretion by ELISA. Intracellular staining was performed using FITC- and PE-conjugated IL-17A antibodies, Alexa Fluor 488–conjugated IL-17F (eBioscience), and Alexa Fluor 647–conjugated IL22-02 antibody (Pfizer), with the latter conjugated according to manufacturer’s instructions (Invitrogen). Dead cells were excluded from analysis using a violet viability stain (Invitrogen). Flow cytometry data collection was performed on a FACSCanto II (BD). Files were analyzed using FlowJo software (Tree Star, Inc.). Cytocentrifuge preparations of BAL cells were stained with H&E (Thermo Fisher Scientific).
RNA isolation, cDNA preparation, and RT-PCR.
RNA was isolated from whole lung tissue using mechanical homogenization and TRIzol isolation (Invitrogen) according to the manufacturer’s instructions. MLE cell RNA was isolated using RNeasy mini kits (QIAGEN). cDNA was generated using SuperScript reverse transcriptase (Invitrogen). RT-PCR was performed on cDNA using SYBR green chemistry (Applied Biosystems) and commercially available primer sets (QIAGEN). Reactions were run on an RT-PCR system (ABI7500; Applied Biosystems). Samples were normalized to β-actin and displayed as fold induction over naive or untreated controls unless otherwise stated.
Histological sections and pathology scoring.
Lungs were inflated with 4% paraformaldehyde and embedded in paraffin, and 5-µm sections were used for staining with H&E. The severity of bleomycin-induced pathology was scored according to weight loss (1, 1–10%; 2, 10–15%; 3, 15–20%; 4, 20–25%; and 5, 25–30%) and blind scoring of H&E-stained lung tissue sections according to consolidation (1–5), fibrosis (1–5), granulocyte recruitment (1–5), and lymphocyte recruitment (1–5), for an overall score out of 25.
ELISA and Immunofluorescence staining.
Standard IL-17A sandwich ELISAs were performed using commercially available antibodies (eBioscience). Sandwich ELISAs for IL-22 were performed using IL22-01 (Pfizer) as a capture antibody and biotin-conjugated IL22-03 (Pfizer) as a detection antibody. Active TGF-β was measured from lung homogenates as previously described (Huaux et al., 2003) using a commercially available ELISA kit (eBioscience). Immunofluorescence staining was performed as previously described (Nair et al., 2009) using a commercially available antibody for TGF-β (Santa Cruz Biotechnology, Inc.).
Instillation of recombinant cytokines.
rIL-17A (eBioscience) and rIL-22 (Pfizer) in sterile PBS were administered intratracheally to Il17a−/− mice daily over a 3-d period at a dose of 1 µg/mouse. Mice were sacrificed on day 4 for analysis of the BAL and lung.
In vitro splenocyte activation.
Single-cell suspensions were obtained from spleens of mice and red blood cells were lysed. Splenocytes were cultured with 1 µg/ml of soluble anti-CD3 and anti-CD28 (eBioscience) in the presence of 10 ng/ml rIL-6, 1 ng/ml rTGF-β, 10 ng/ml rIL-23, 10 µg/ml anti–IL-4, and 10 µg/ml anti–IFN-γ. rIL-17A and rIL-22 were added to cultures at the designated concentrations. After 3–5 d of stimulation, cells were restimulated with PMA and ionomycin in the presence of BFA for 4 h, followed by staining for intracellular cytokines.
Electrophoresis and immunostaining.
Analysis was performed using standard SDS-PAGE and immunoblotting techniques. Biotin-conjugated anti–IL-22Rα1 (R&D Systems), anti-actin, anti–phospho-STAT3 (Tyr705), and total anti-STAT3 (Cell Signaling Technology) were used as primary antibodies, followed by either streptavidin-conjugated horseradish peroxidase (R&D Systems) or donkey anti–rabbit–conjugated streptavidin (GE Healthcare). Blots were developed using ECL detection reagents (GE Healthcare).
MLE cell line and in vitro apoptosis assay.
The MLE cell line (American Type Culture Collection) was a gift from M.F. Beers (University of Pennsylvania, Philadelphia, PA) and maintained in RPMI 1640 media supplemented with 2% FBS, 5 µg/ml insulin, 10 µg/ml transferrin, 20 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, 10 mM Hepes, and 2 mM l-glutamine. After seeding and adherence, apoptosis was induced by administration of 2.5 U/ml bleomycin to cultures or PBS as a control. rIL-22 or rIL-17A was added to cultures at designated concentrations and incubated for ∼8 h before harvesting using 0.05% trypsin-EDTA. Cell suspensions were subsequently stained for annexin V according to the manufacturer’s protocols (BD). Cell cultures and paraffin-embedded lung sections were stained using the TMR Red In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s protocol.
Statistical analysis.
Results represent means ± SEM. Statistical significance was determined by the Student’s t test unless otherwise noted in the figure legends (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Online supplemental material.
Fig. S1 demonstrates a significant increase in Il23a transcript after bleomycin instillation. Fig. S2 shows that TGF-β protein is not up-regulated after administration of high-dose bleomycin. Fig. S3 shows that IL-17F protein is not up-regulated after administration of high-dose bleomycin. Fig. S4 displays the differential BAL counts in different experimental treatment groups after administration of bleomycin. Fig. S5 shows that the phenotype of infiltrating Gr-1+ SSChi cells after bleomycin instillation is consistent with that of neutrophils. Fig. S6 demonstrates that Il22−/− mice are protected from bleomycin-induced airway inflammation. Fig. S7 demonstrates that IL-17A regulates IL-22 expression in Th17 cells but IL-22 does not regulate IL-17A expression. Fig. S8 demonstrates that IL-17A and IL-22 act synergistically to promote airway inflammation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092054/DC1.
Acknowledgments
We would like to acknowledge members of the Artis laboratory for helpful discussions and critical reading of the manuscript. We thank the Morphology Core and Pilot Feasibility Program of the National Institute of Diabetes and Digestive and Kidney Disease Center (DK50306) and Vet School Pathology Service for technical expertise, K. Lam and A. Root for purification of neutralizing and control antibodies, and R. Askew for discussion regarding the design of the IL-22–deficient mice.
Work in the Artis laboratory is supported by the National Institutes of Health (grants AI61570, AI74878, and AI083480 to D. Artis, and grant T32AI007532-08 to G.F. Sonnenberg), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award to D. Artis), the Crohn’s and Colitis Foundation of America (M.G. Nair), and the University of Pennsylvania (pilot grants from the Center for Infectious Diseases and University Research Fund to D. Artis). C. Zaph was funded by the Irvington Institute Fellowship Program of the Cancer Research Institute.
L.A. Fouser is employed by Pfizer. The authors have no further conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchioalveolar lavage
- RT-PCR
- real-time PCR
- TUNEL
- terminal deoxynucleotidyl trasferase dUTP nick-end labeling
References
- Alonzi T., Maritano D., Gorgoni B., Rizzuto G., Libert C., Poli V. 2001. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell. Biol. 21:1621–1632 10.1128/MCB.21.5.1621-1632.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275–281 10.1038/nm1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bollrath J., Greten F.R. 2009. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 10:1314–1319 10.1038/embor.2009.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R.K., Ferrick C., Neubauer P., Sjoding M., Sterner-Kock A., Kock M., Putney L., Ferrick D.A., Hyde D.M., Love R.B. 2008. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 31:167–179 10.1007/s10753-008-9062-6 [DOI] [PubMed] [Google Scholar]
- Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., Colonna M. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 457:722–725 10.1038/nature07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. 2009. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 31:15–23 10.1016/j.immuni.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857–863 10.1038/ni.1767 [DOI] [PubMed] [Google Scholar]
- Gaffen S.L. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556–567 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes L., Dumoutier L., Kelchtermans H., Schurgers E., Mitera T., Renauld J.C., Matthys P. 2009. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 60:390–395 10.1002/art.24220 [DOI] [PubMed] [Google Scholar]
- Huaux F., Liu T., McGarry B., Ullenbruch M., Phan S.H. 2003. Dual roles of IL-4 in lung injury and fibrosis. J. Immunol. 170:2083–2092 [DOI] [PubMed] [Google Scholar]
- Kotenko S.V., Izotova L.S., Mirochnitchenko O.V., Esterova E., Dickensheets H., Donnelly R.P., Pestka S. 2001. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 276:2725–2732 10.1074/jbc.M007837200 [DOI] [PubMed] [Google Scholar]
- Lejeune D., Dumoutier L., Constantinescu S., Kruijer W., Schuringa J.J., Renauld J.C. 2002. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 277:33676–33682 10.1074/jbc.M204204200 [DOI] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Long A.J., Bennett F., Whitters M.J., Karim R., Collins M., Goldman S.J., Dunussi-Joannopoulos K., Williams C.M., Wright J.F., Fouser L.A. 2007. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179:7791–7799 [DOI] [PubMed] [Google Scholar]
- Lukacs N.W., Strieter R.M., Chensue S.W., Widmer M., Kunkel S.L. 1995. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J. Immunol. 154:5411–5417 [PubMed] [Google Scholar]
- Ma H.L., Liang S., Li J., Napierata L., Brown T., Benoit S., Senices M., Gill D., Dunussi-Joannopoulos K., Collins M., et al. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeyama T., Kuwano K., Kawasaki M., Kunitake R., Hagimoto N., Hara N. 2001. Attenuation of bleomycin-induced pneumopathy in mice by monoclonal antibody to interleukin-12. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1128–L1137 [DOI] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- Matute-Bello G., Frevert C.W., Martin T.R. 2008. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295:L379–L399 10.1152/ajplung.00010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J., Chen Y., Tato C.M., Laurence A., Joyce-Shaikh B., Blumenschein W.M., McClanahan T.K., O’Shea J.J., Cua D.J. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10:314–324 10.1038/ni.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Heimesaat M.M., Danker K., Struck D., Lohmann U., Plickert R., Bereswill S., Fischer A., Dunay I.R., Wolk K., et al. 2009. Interleukin (IL)-23 mediates Toxoplasma gondii–induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 206:3047–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A., Aoshiba K., Ishihara Y., Inano H., Sakamoto K., Yamaguchi E., Kagawa J., Takizawa T. 1992. Administration of alpha 1-proteinase inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am. Rev. Respir. Dis. 145:651–656 [DOI] [PubMed] [Google Scholar]
- Nair M.G., Du Y., Perrigoue J.G., Zaph C., Taylor J.J., Goldschmidt M., Swain G.P., Yancopoulos G.D., Valenzuela D.M., Murphy A., et al. 2009. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206:937–952 10.1084/jem.20082048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor W., Jr., Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y., Kolls, and J.K., Flavell R.A. 2009. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10:603–609 10.1038/ni.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 28:454–467 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Hong F., Radaeva S., Gao B. 2004. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell. Mol. Immunol. 1:43–49 [PubMed] [Google Scholar]
- Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., et al. 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206:1465–1472 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S., Sun R., Pan H.N., Hong F., Gao B. 2004. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 39:1332–1342 10.1002/hep.20184 [DOI] [PubMed] [Google Scholar]
- Saito F., Tasaka S., Inoue K., Miyamoto K., Nakano Y., Ogawa Y., Yamada W., Shiraishi Y., Hasegawa N., Fujishima S., et al. 2008. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 38:566–571 10.1165/rcmb.2007-0299OC [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Zhao L.H., Jain F., Kradin R. 2002. IL-12p40(−/−) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp. Mol. Pathol. 72:1–9 10.1006/exmp.2001.2409 [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., et al. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 29:958–970 10.1016/j.immuni.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Segel M.J., Izbicki G., Cohen P.Y., Or R., Christensen T.G., Wallach-Dayan S.B., Breuer R. 2003. Role of interferon-gamma in the evolution of murine bleomycin lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L1255–L1262 [DOI] [PubMed] [Google Scholar]
- Smith E., Stark M.A., Zarbock A., Burcin T.L., Bruce A.C., Vaswani D., Foley P., Ley K. 2008. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J. Immunol. 181:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider G.L., Hayes J.A., Korthy A.L. 1978. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: pathology and stereology. Am. Rev. Respir. Dis. 117:1099–1108 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K., Blumberg R.S., Xavier R.J., Mizoguchi A. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118:534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori H., Kanno Y., Watford W.T., Tato C.M., Weiss G., Ivanov I.I., Littman D.R., O’Shea J.J. 2009. Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41 10.1084/jem.20072713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S., Kaplan C.D., Tran E.H., Crellin N.K., Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10:864–871 10.1038/ni.1770 [DOI] [PubMed] [Google Scholar]
- Uskokovic A., Dinic S., Mihailovic M., Grigorov I., Ivanovic-Matic S., Bogojevic D., Grdovic N., Arambasic J., Vidakovic M., Martinovic V., et al. 2007. STAT3/NFkappaB interplay in the regulation of alpha2-macroglobulin gene expression during rat liver development and the acute phase response. IUBMB Life. 59:170–178 10.1080/15216540701272612 [DOI] [PubMed] [Google Scholar]
- Villarino A.V., Huang E., Hunter C.A. 2004. Understanding the pro- and anti-inflammatory properties of IL-27. J. Immunol. 173:715–720 [DOI] [PubMed] [Google Scholar]
- von Vietinghoff S., Ley K. 2009. IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. J. Immunol. 183:865–873 10.4049/jimmunol.0804080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser K.A., Vorbroker D.K., Rice W.R., Clark J.C., Bachurski C.J., Oie H.K., Whitsett J.A. 1993. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc. Natl. Acad. Sci. USA. 90:11029–11033 10.1073/pnas.90.23.11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Madala S.K., Ramalingam T.R., Gochuico B.R., Rosas I.O., Cheever A.W., Wynn T.A. 2010. Bleomycin and IL-1β–mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 207:535–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity. 21:241–254 10.1016/j.immuni.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Chang S.H., Park H., Nurieva R., Shah B., Acero L., Wang Y.H., Schluns K.S., Broaddus R.R., Zhu Z., Dong C. 2008. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205:1063–1075 10.1084/jem.20071978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., et al. 2003. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 4:551–556 10.1038/ni938 [DOI] [PubMed] [Google Scholar]
- Zenewicz L.A., Flavell R.A. 2008. IL-22 and inflammation: leukin’ through a glass onion. Eur. J. Immunol. 38:3265–3268 10.1002/eji.200838655 [DOI] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Karow M., Flavell R.A. 2007. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 27:647–659 10.1016/j.immuni.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. 2008. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 29:947–957 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 445:648–651 10.1038/nature05505 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]