Abstract
Activation of serum complement triggers Th17 cell–dependent spontaneous autoimmune disease in an animal model. In genetically autoimmune-prone SKG mice, administration of mannan or β-glucan, both of which activate serum complement, evoked Th17 cell–mediated chronic autoimmune arthritis. C5a, a chief component of complement activation produced via all three complement pathways (i.e., lectin, classical, and alternative), stimulated tissue-resident macrophages, but not dendritic cells, to produce inflammatory cytokines including IL-6, in synergy with Toll-like receptor signaling or, notably, granulocyte/macrophage colony-stimulating factor (GM-CSF). GM-CSF secreted by activated T cells indeed enhanced in vitro IL-6 production by C5a-stimulated macrophages. In vivo, C5a receptor (C5aR) deficiency in SKG mice inhibited the differentiation/expansion of Th17 cells after mannan or β-glucan treatment, and consequently suppressed the development of arthritis. Transfer of SKG T cells induced Th17 cell differentiation/expansion and produced arthritis in C5aR-sufficient recombination activating gene (RAG)−/− mice but not in C5aR-deficient RAG−/− recipients. In vivo macrophage depletion also inhibited disease development in SKG mice. Collectively, the data suggest that complement activation by exogenous or endogenous stimulation can initiate Th17 cell differentiation and expansion in certain autoimmune diseases and presumably in microbial infections. Blockade of C5aR may thus be beneficial for controlling Th17-mediated inflammation and autoimmune disease.
There is recent evidence that IL-17–secreting CD4+ T cells (Th17 cells) play a key role in autoimmune diseases, such as rheumatoid arthritis (RA) and multiple sclerosis (Harrington et al., 2005; Veldhoen et al., 2006; Korn et al., 2009). It remains unclear, however, how pathogenic self-reactive Th17 cells are generated from naive T cells, and are activated by external or internal stimuli in autoimmune disease.
SKG mice, a mutant of the gene encoding ZAP-70 on the BALB/c background, spontaneously develop CD4+ T cell–mediated autoimmune arthritis clinically and immunologically resembling human RA (Sakaguchi et al., 2003). The mutation alters the sensitivity of developing T cells to positive and negative selection in the thymus, leading to thymic production of potentially arthritogenic autoimmune T cells (Sakaguchi et al., 2003; Hirota et al., 2007). The SKG arthritis is critically dependent on Th17 cells, as deficiency of either IL-17 or IL-6 completely inhibits the disease (Hirota et al., 2007). Importantly, they spontaneously develop severe arthritis in a microbially conventional environment but not under a specific pathogen–free (SPF) condition, suggesting that environmental stimuli such as microbial infection may expand or trigger the differentiation of arthritogenic Th17 cells (Yoshitomi et al., 2005). Indeed, injection of zymosan, a crude extract of yeast cell wall containing β-glucans or purified β-glucans, such as laminarin, activates innate immunity via Toll-like receptor (TLR) and Dectin-1, and drives preferential differentiation and expansion of Th17 cells, thereby triggering arthritis in SKG mice under a SPF condition (Yoshitomi et al., 2005; LeibundGut-Landmann et al., 2007). Because zymosan is also an activator of the alternative pathway of complement (Mullaly and Kubes, 2007) and β-glucan structure can be recognized by ficolin-L, an initiator of the lectin pathway (Garlatti et al., 2007), it is also likely that complement activation may contribute to triggering Th17-mediated autoimmune disease.
In this report, we show that complement activation via all three pathways (i.e., the lectin, classical, and alternative pathways) and the resulting generation of the common product C5a potently promote the differentiation/expansion of self-reactive T cells to Th17 cells that mediate autoimmune arthritis in SKG mice. The results indicate that exogenous or endogenous stimuli that activate complement can be a triggering cause of Th17-mediated autoimmune disease and that C5a is a key molecular target in controlling Th17-mediated autoimmunity as well as microbial immunity.
RESULTS AND DISCUSSION
Mannan triggers autoimmune arthritis by expanding Th17 cells
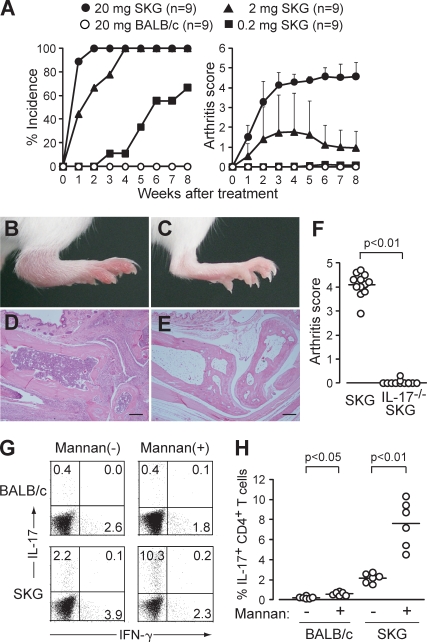
We first tested whether mannan, a prototypic activator of the lectin pathway of complement activation, was able to trigger arthritis in SKG mice (Fig. 1, A–E; Fujita, 2002). A single i.p. injection of 20 mg mannan triggered self-sustained chronic arthritis within 2 wk in all of the treated SKG mice but not in BALB/c mice. A small amount (200 µg) also elicited joint swelling, but only in a few small joints and in 50% of SKG mice. IL-17−/− SKG mice were completely resistant to arthritis induction by mannan (Fig. 1 F). The ratio of IL-17+ cells among CD4+ T cells was increased significantly (approximately fourfold) in regional (e.g., popliteal) lymph nodes of mannan-treated SKG mice with arthritis (e.g., in ankles) compared with control PBS-treated SKG mice without arthritis; the ratio also increased significantly, although to a much lesser degree, in mannan-treated BALB/c mice (Fig. 1, G and H). Thus, mannan can enhance the development of arthritogenic Th17 cells and evoke arthritis in SKG mice.
Figure 1.
Mannan triggers autoimmune arthritis by expanding Th17 cells. (A) Joint score of 8∼12-wk-old SKG or BALB/c mice that received a single i.p. injection of mannan at the indicated doses. A total of two independent experiments are shown. Error bars are means ± SD of scores. (B–E) A representative joint swelling and histology of an SKG (B and D) and a BALB/c mouse (C and E) 8 wk after mannan treatment (hematoxylin and eosin staining). Bars, 200 µm. (F) Joint scores of IL-17+/+ or IL-17−/− SKG mice 8 wk after mannan treatment (n = 12). Horizontal bars are the means of each group. (G) Intracellular staining of IL-17 and IFN-γ in CD4+ T cells in the popliteal lymph nodes from SKG or BALB/c mice 8 wk after mannan treatment (numbers indicate percentages). One representative staining out of six independent experiments is shown. (H) Percentages of IL-17+ cells in CD4+ T cells in each SKG or BALB/c group (n = 6), as shown in G. Horizontal bars are the means of each group.
C5aR is essential for driving Th17 cell differentiation and triggering arthritis
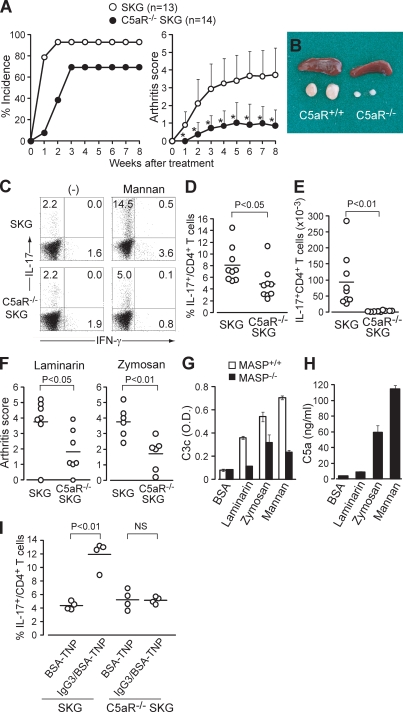
C5a, a key common product of all three complement activation pathways, is the most potent complement-derived mediator of inflammation; increases the production of IL-6, TNF, and IL-1 from TLR-stimulated macrophages; and suppresses IL-12 production (Guo and Ward, 2005; Hawlisch et al., 2005; Zhang et al., 2007). To examine possible effects of mannan treatment on C5a production via the lectin pathway, and consequently Th17 cell differentiation and expansion in SKG mice, we prepared SKG mice deficient in C5aR (CD88; Hawlisch et al., 2005). The incidence and severity of arthritis was significantly suppressed in mannan-treated C5aR−/− SKG mice (Fig. 2 A). The measurement of serum C3a and C5a revealed that the treatment strongly triggered complement activation for the first 3 d, with persisting low level activation over 28 d in mannan-elicited arthritic SKG mice (Fig. S1). Mannan-treated C5aR+/+ SKG mice, when examined 8 wk (Fig. 2, B–E) or 2 wk (Fig. S1) after treatment, showed a marked hypertrophy of the regional lymph nodes (Fig. 2 B), which contained a much higher ratio and absolute number of IL-17+ CD4+ T cells compared with similarly treated C5aR−/− SKG mice (Fig. 2, C–E) or BALB/c mice (not depicted).
Figure 2.
C5a drives Th17 cell differentiation and triggers arthritis. (A) Joint score of C5aR+/+ or C5aR−/− SKG mice treated by 20 mg mannan. A total of two independent experiments are shown. Error bars are means ± SD of scores. *, P < 0.05. (B) Spleen and popliteal lymph nodes of C5aR+/+ or C5aR−/− SKG mice 8 wk after mannan treatment. (C) Intracellular staining of IL-17 and IFN-γ in CD4+ T cells in the popliteal lymph nodes of C5aR+/+ or C5aR −/− SKG mice, as shown in B (numbers indicate percentages). One representative staining out of nine independent experiments is shown. (D and E) Percentages (D) and absolute numbers (E) of IL-17+ cells in CD4+ T cells in each group (n = 9), as shown in C. (F) Joint scores of C5aR+/+ or C5aR−/− SKG mice 8 wk after treatment by laminarin (n = 7) or zymosan (n = 6). (G) C3 deposition assay with laminarin-, zymosan-, or mannan-coated wells incubated with 2% sera from MASP-intact or -null C57BL/6 sera. (H) C5a ELISA for the supernatant of the C3 deposition assay with BALB/c sera, as shown in G. Error bars are means ± SD. (I) C5aR+/+ and C5aR−/− SKG mice were i.p. injected with TNP-BSA alone or IgG3 anti-TNP/TNP-BSA IC. The percentage of IL-17+ cells in the peritoneal CD4+ T cells was assessed on day 7 (n = 4). Horizontal bars are the means.
Similar to mannan treatment, arthritis elicitation by laminarin or zymosan was significantly suppressed in C5aR−/− SKG mice (Fig. 2 F). The C3 deposition assay, in which the amount of activated C3c was quantified by ELISA, showed that laminarin and zymosan activated complement (Fig. 2 G). The C3c deposition was reduced by the use of mannose-binding lectin-associated serine protease (MASP)–null serum, indicating the involvement of the lectin pathway (Fujita, 2002; Iwaki et al., 2006; Takahashi et al., 2008). C5a was indeed detected in the supernatant sera of the C3 deposition assay after complement activation (Fig. 2 H). These results collectively indicate that laminarin, zymosan, and mannan in particular can commonly activate complement to produce C5a, which critically contributes to evoking autoimmune arthritis in SKG mice.
Although the lectin and alternative pathways are stimulated by microbial products such as mannan or zymosan, the classical pathway can be activated by antigen–antibody immune complexes (ICs; Guo and Ward, 2005). ICs not only activate complement but also deliver signal through FcγR on APCs (Sylvestre et al., 1996). As such, we asked whether an IgG3-IC, which is known to activate the classical and alternative complement pathways without the involvement of FcγR, would expand Th17 cells in a C5a/C5aR-dependent manner (Fig. 2 I; Díaz de Ståhl et al., 2003). C5aR+/+ and C5aR−/− SKG mice were i.p. injected with trinitrophenyl (TNP) hapten–conjugated BSA alone or with ICs formed of TNP-BSA and a TNP-specific IgG3 mAb. Compared with the injection of BSA-TNP alone, IL-17+ cells were markedly increased in the peritoneal cavity of C5aR+/+ SKG but not C5aR−/− SKG mice after i.p. injection of the ICs, although this single-dose injection failed to elicit arthritis in the former. Collectively, activation of all three complement activation pathways facilitates Th17 cell differentiation and expansion via C5a/C5aR.
C5a acts on tissue-resident macrophages to drive Th17 cell differentiation
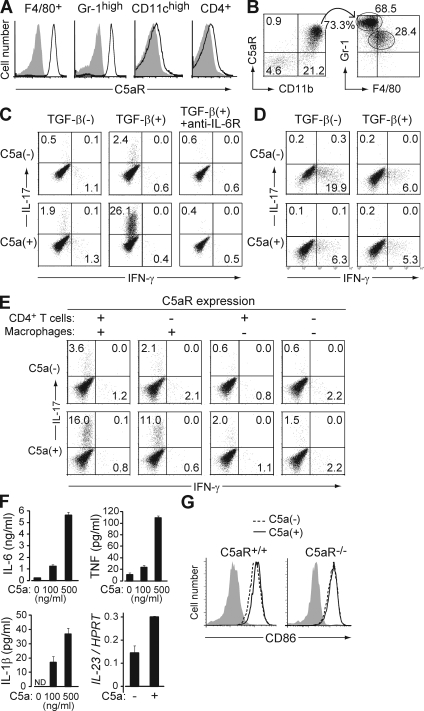
C5aR was highly expressed on neutrophils (as CD11bhighGr-1highF4/80− cells) and monocytes/macrophages (as CD11bhighF4/80+Gr-1low/− cells) in the peritoneal cavity or the spleen of nontreated SKG mice (Fig. 3 A), and in arthritic joints of mannan-treated SKG mice (Fig. 3 B). To determine the cell types that received C5aR signaling and drove Th17 cell differentiation, we cultured naive BALB/c CD4+ T cells with various types of APCs and stimulated them with anti-CD3 in the presence or absence of C5a and/or TGF-β. Notably, naive CD4+ T cells co-cultured with resident peritoneal macrophages (as CD11bhighF4/80+Gr-1− cells) in the presence of TGF-β spontaneously differentiated into IL-17+ cells, and addition of recombinant C5a dramatically increased IL-17+ cells but not IFN-γ+ cells; anti–IL-6R completely inhibited the increase (Fig. 3 C). This Th17-promoting effect of C5a was greater on naive (CD44lowCD45Rbhigh) CD4+ T cells than memory (CD44highCD45Rblow) CD4+ T cells (unpublished data). The in vitro C5a-mediated expansion of Th17 cells also occurred with thioglycollate-elicited peritoneal macrophages, but to a much lesser extent compared with resident peritoneal macrophages (Fig. S2). Importantly, these effects with macrophages were not observed with splenic DCs (Fig. 3 D) or with DCs from mannan-treated SKG mice (Fig. S2), even in the presence of TGF-β. Without TGF-β, CD4+ T cells cultured with DCs differentiated primarily into IFN-γ+ cells. Addition of C5a slightly decreased the percentage of IFN-γ+ cells but did not evoke Th17 cell differentiation (Fig. 3 D).
Figure 3.
C5a acts on tissue-resident macrophages to facilitate Th17 cell differentiation. (A) F4/80+ cells in the peritoneal cavity, and Gr-1high, CD11chigh, and CD4+ cells in the spleen of nontreated SKG mice were stained for C5aR (continuous line) or isotype control (shading). (B) Cells infiltrating in the arthritic joint of mannan-treated SKG mice were stained for C5aR, CD11b, Gr-1, and F4/80. C5aR+ CD11b+ cells are shown for the expression of Gr-1 and F4/80. (C) BALB/c CD4+ T cells were stimulated with anti-CD3 and cultured with resident peritoneal macrophages in the presence or absence of TGF-β, recombinant C5a, or anti–IL-6R, and stained for intracellular cytokines on day 3. (D) Intracellular cytokine staining of BALB/c CD4+ T cells cultured with splenic DCs in the presence or absence of TGF-β and C5a. (E) Intracellular cytokine staining of CD4+ T cells after criss-cross co-culture with C5aR+/+ or C5aR−/− CD4+ T cells and C5aR+/+ or C5aR−/− macrophages in the presence of TGF-β. (F) ELISA assessment (triplicates) for IL-6, TNF, and IL-1β produced by macrophages, or quantitative RT-PCR for their IL-23 mRNA expression, when macrophages were stimulated overnight by C5a at the indicated doses. Error bars are means ± SD. (G) CD86 expression on C5aR+/+ or C5aR−/− macrophages cultured with or without C5a for 4 h. Shading indicates isotype control. Results in A–G represent three independent experiments. Numbers in B–E indicate percentages.
To determine whether the induction of Th17 cells by C5a in CD4+ T cell/macrophage co-culture depended on a direct effect of C5a on CD4+ T cells or macrophages, we performed criss-cross co-cultures with C5aR+/+ or C5aR−/− CD4+ T cells and C5aR+/+ or C5aR−/− macrophages (Fig. 3 E). Th17 cell differentiation was inhibited when macrophages, but not CD4+ T cells, lacked C5aR, suggesting that cytokines produced by C5a-stimulated macrophages were responsible for inducing Th17 cell differentiation. Indeed, C5a elicited a dose-dependent production of large amounts of IL-6 and, to a lesser degree, TNF and IL-1β from resident peritoneal macrophages (Fig. 3 F). Production of IL-23, which is essential for the survival and expansion of Th17 cells, was up-regulated at the mRNA level in C5a-treated macrophages, although the cytokine was below the detection limit (30 pg/ml) of ELISA (Fig. 3 F). C5a also up-regulated co-stimulatory molecules such as CD86 on C5aR+/+ macrophages but not on C5aR−/− macrophages (Fig. 3 G; Strainic et al., 2008).
C5a drives Th17 cell differentiation in synergy with GM-CSF or TLR signaling
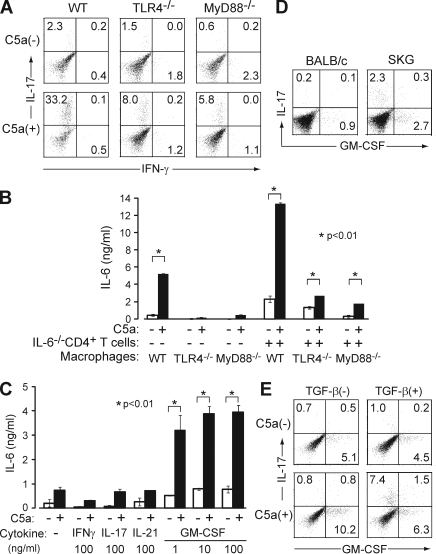
Next we asked whether the in vitro robust expansion of Th17 cells was mediated by C5a alone or by a synergy of C5a and LPS, because the recombinant C5a we used contained a trace amount of contaminated LPS, which could synergistically act to drive Th17 cell differentiation (see Materials and methods; Fang et al., 2009). To dissect TLR-dependent and -independent effects, we used macrophages from TLR4−/− or MyD88−/− mice. Although a deficiency of TLR4−/− or MyD88−/− in macrophages substantially reduced the C5a-mediated expansion of Th17 cells, a significant proportion of Th17 cells still developed (Fig. 4 A). Further, C5a derived from LPS-free human plasma expanded Th17 cells (unpublished data; Köhl, 1997). Notably, when TLR4−/− or MyD88−/− macrophages alone were stimulated by C5a, IL-6 production was nearly abolished (Fig. 4 B). However, in co-culture with anti-CD3–stimulated IL-6−/− CD4+ T cells, C5a significantly enhanced IL-6 production by TLR4−/− or MyD88−/− macrophages, although much less potently compared with wild types (Fig. 4 B).
Figure 4.
C5a drives Th17 cell differentiation in synergy with TLR or GM-CSF. (A) C57BL/6 CD4+ T cells were cultured with TLR4−/−, MyD88−/−, or wild-type C57BL/6 macrophages in the presence of TGF-β with or without C5a. (B) Macrophages alone from these mice or co-cultured with anti-CD3–stimulated CD4+ T cells from IL-6−/− mice were stimulated with C5a overnight. IL-6 in the supernatant was determined by ELISA. (C) MyD88−/− macrophages were stimulated by C5a overnight in the presence or absence of cytokines at the indicated doses. IL-6 in the supernatant was determined by ELISA (triplicates). Error bars are means ± SD. (D) Freshly isolated BABL/c or SKG splenic CD4+ T cells were stained for intracellular IL-17 and GM-CSF. (E) BALB/c CD4+ T cells were cultured with macrophages in the presence or absence of C5a and/or TGF-β, stimulated with anti-CD3, and stained for intracellular cytokines on day 3. Results in A–E represent three independent experiments. Numbers in A, D, and E indicate percentages.
To analyze how T cells contributed to the C5a-induced IL-6 production by macrophages, we assessed the effect of co-stimulatory molecules and T cell–derived cytokines that could alter macrophage function (Grabstein et al., 1986). IL-6 production was partially inhibited by blockade of CD40L (Fig. S3; Hirota et al., 2007). When TLR4−/− or MyD88−/− macrophages alone were stimulated with C5a in the presence or absence of various cytokines (e.g., IL-17, IL-21, IFN-γ, and GM-CSF), only GM-CSF significantly enhanced IL-6 production even at a low concentration (e.g., 1 ng/ml; Fig. 4 C and Fig. S3; Sonderegger et al., 2008). Although freshly isolated BALB/c naive CD4+ T cells contained only a small number of GM-CSF–secreting cells (Fig. 4 D), their co-culture with resident macrophages under anti-CD3 stimulation markedly increased the proportion of GM-CSF+ T cells, and C5a further increased the proportion (Fig. 4 E). GM-CSF+ T cells were distinct from Th17 cells, whose expansion in the presence of C5a and TGF-β accompanied a slight decrease in GM-CSF+ T cells (Fig. 4 E). In vivo, splenic CD4+ T cells in SKG mice contained sizable proportions of Th17 cells and GM-CSF+ CD4+ T cells; one half of the latter also secreted IFN-γ (Fig. 4 D and not depicted).
Collectively, the results in Fig. 3 and Fig. 4 indicate that C5a acts on macrophages in the joint and other tissues to provoke their production of IL-1, IL-6, TNF, and IL-23, and together with tissue TGF-β, promotes the differentiation and expansion of self-reactive T cells into Th17 cells. C5a stimulates macrophages to produce IL-6 in at least two ways: via synergy with TLR signaling and via T cell–macrophage interaction. In the latter, GM-CSF produced by activated T cells enhances IL-6 production by C5a-stimulated macrophages, indicating a novel pathway of promoting Th17 cell differentiation and expansion.
Macrophages are required for in vivo Th17 expansion and induction of arthritis
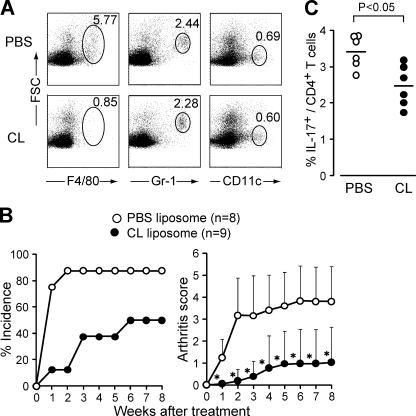
To determine the role of macrophages in vivo, we treated SKG mice with clodronate liposome (CL), which specifically depletes monocytes and macrophages (Solomon et al., 2005). The treatment indeed efficiently depleted C5aR+ monocytes/macrophages without affecting neutrophils or DCs in SKG mice (Fig. 5 A). CL administration before mannan injection markedly attenuated the development of arthritis (Fig. 5 B) and reduced the expansion of Th17 cells (Fig. 5 C).
Figure 5.
Macrophages are required for in vivo Th17 expansion and induction of arthritis. (A) Specific depletion of macrophages in SKG mice 3 d after i.v. injection of 200 µl CL. One representative out of three independent experiments is shown (numbers indicate percentages). (B) Joint scores of SKG mice i.v. injected with 200 µl PBS or CL 1 d before mannan treatment. *, P < 0.05. Error bars are means ± SD. (C) Percentage of IL-17+ CD4+ T cells in the popliteal lymph nodes of SKG mice 2 wk after mannan treatment. Mice were pretreated by an i.v. injection of PBS or CL (n = 6). Horizontal bars are the means.
C5aR signaling promotes spontaneous differentiation of CD4+ T cells to Th17 cells via homeostatic proliferation
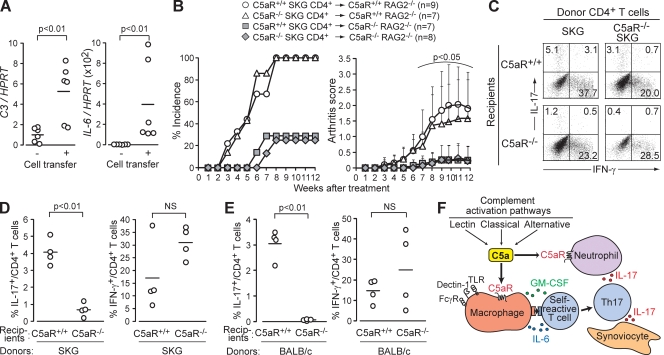
Similar to innate immune stimulation by microbial products, aseptic stimulation of SKG self-reactive T cells (e.g., via homeostatic proliferation in a lymphopenic environment) evokes spontaneous differentiation of Th17 cells and triggers autoimmune arthritis (Hirota et al., 2007). Because T cell–APC interaction induces local complement activation (Liu et al., 2008; Strainic et al., 2008), we asked whether such intrinsic complement activation would contribute to Th17 cell differentiation and arthritis induction. In RAG2−/− mice that developed arthritis after transfer of SKG CD4+ T cells, the joint tissue actively transcribed C3 and IL-6 mRNA, indicating complement activation and IL-6 production in the affected joint (Fig. 6 A). When CD4+ T cells from C5aR+/+ or C5aR−/− SKG mice were transferred to C5aR+/+ or C5aR−/− RAG2−/− mice, both CD4+ T cell populations induced arthritis at equivalent incidences and severities in C5aR+/+ RAG2−/− mice but were significantly lower in C5aR−/−RAG2−/− mice (Fig. 6 B). Thus, C5aR expression by the recipient cells, not donor CD4+ T cells, was required for disease induction. In accordance with the joint scores, the generation of Th17 cells from transferred CD4+ T cells was significantly suppressed in C5aR−/− recipients (Fig. 6, C and D). The generation of IFN-γ–secreting cells was not significantly affected, although there was a tendency toward a higher proportion of IFN-γ+ cells in C5aR−/− recipients. Inhibition of Th17 cell differentiation by C5aR deficiency was not restricted to SKG CD4+ T cells, as Th17 cell differentiation of BALB/c CD4+ T cells during homeostatic proliferation was also suppressed in C5aR-deficient recipients (Fig. 6 E). Collectively, these results indicate that interactions between self-reactive T cells and APCs can preferentially drive the differentiation of the former into Th17 effector cells via complement activation and the resulting C5a action on APCs.
Figure 6.
C5a promotes spontaneous differentiation of CD4+ T cells to Th17 cells via homeostatic proliferation. (A) Quantitative RT-PCR for C3 and IL-6 mRNA in the joints of RAG2−/− mice 12 wk after transfer of SKG CD4+ T cells (n = 6). (B) 106 C5aR+/+ or C5aR−/− SKG CD4+ T cells were transferred to C5aR+/+ or C5aR−/− RAG2−/− mice. Joint scores were assessed every week in two independent experiments. Error bars are means ± SD. (C) 2 × 106 C5aR+/+ or C5aR−/− SKG CD4+ T cells were transferred to C5aR+/+ or C5aR−/− RAG2−/− mice. Intracellular cytokines in recipient splenic CD4+ T cells were stained on day 7 (numbers indicate percentages). One representative out of four independent experiments is shown. (D and E) Percentages of IL-17+ or IFN-γ+ cells among CD4+ T cells in C5aR+/+ or C5aR−/− RAG2−/− mice (n = 4 each) after transfer of SKG (D) or BALB/c CD4+ T cells (E). (F) A model for the role of complement activation in Th17-mediated autoimmune arthritis. Horizontal bars in A, D, and E are the means.
Thus, extrinsic and intrinsic complement activation promotes Th17 cell differentiation and expansion, evoking autoimmune arthritis in SKG mice (Fig. 6 F). It was noted, however, that C5aR deficiency significantly suppressed but did not completely inhibit arthritis development triggered by laminarin, zymosan, or mannan (Fig. 2). This incomplete inhibition could be attributed to the fact that these microbial products not only activate complement but also directly stimulate macrophages and DCs via cell surface–expressed pattern recognition receptors such as TLR and C-type lectin receptors (e.g., Dectin-1, mannose receptor, and DC-SIGN; Yoshitomi et al., 2005; Robinson et al., 2006; Sheng et al., 2006). Indeed, in our co-culture experiments with macrophages, zymosan, laminarin, or mannan at a high dose (1 mg/ml) expanded Th17 cells, presumably via IL-6 production in an MyD88-dependent and -independent manner (Fig. S4). In addition, we observed a synergy between the signals from C5aR and MyD88 for cytokine production by macrophages (Fig. 4 A; Fang et al., 2009). Yet, it is of note that the prototypic TLR agonist LPS or CpG alone failed to elicit arthritis in SKG mice, in contrast to successful arthritis induction by mannan, a prototypic complement activator (Yoshitomi et al., 2005). This indicates that complement activation and C5a production can be a major pathway for driving Th17-dependent autoimmune arthritis in SKG mice.
Because C5a, IL-17, and GM-CSF are commonly capable of enhancing granulopoiesis and neutrophil recruitment (Höpken et al., 1996; Korn et al., 2009), together they would cause robust neutrophil accumulation (Fig. 3 B) and form a positive feedback loop of Th17-mediated inflammation (Fig. 6 H; Sonderegger et al., 2008). In addition, the cartilage surface lacks several complement inhibitors, which might render the joint highly susceptible to complement activation (Matsumoto et al., 2002). It is thus likely that transient synovial inflammation may frequently occur when an individual is exposed to complement-activating microbial products, ICs, autoantibodies, or physical trauma. Such synovial inflammation per se may not be sufficient to trigger chronic arthritis in normal individuals. Yet, if an individual harbors potentially arthritogenic CD4+ T cells (e.g., because of genetic predisposition), such complement-induced synovial inflammation may promote the differentiation/expansion of arthritogenic Th17 cells and instigate chronic arthritis. It is worth noting in this regard that genetic susceptibility to RA is in part determined by the polymorphism of the genes encoding C5 (TRAF1-C5), PTPN22 (which affects TCR proximal signaling, as observed with the SKG ZAP-70 mutation), or STAT4, which might alter Th17 cell function (Vang et al., 2005; Plenge et al., 2007; Remmers et al., 2007). These genetic polymorphisms could promote the production of arthritogenic T cells and their Th17 cell differentiation via complement activation. Although C5aR signaling plays a complex role depending on the cell types (e.g., macrophages or DCs) and additional receptors (e.g., TLRs; Guo and Ward, 2005; Weaver et al., 2010), this report provides evidence that complement activation and C5a production is critically involved in the initiation of certain autoimmune disease, and presumably microbial immunity, by driving Th17 development.
MATERIALS AND METHODS
Mice.
C5aR−/−, IL-17−/−, IL-6−/−, RAG2−/−, TLR4−/−, and MyD88−/− mice (TLR4- or MyD88-deficient mice were provided by S. Akira, Osaka University, Osaka, Japan) were described previously (Hawlisch et al., 2005; Yoshitomi et al., 2005; Hirota et al., 2007). MASP-null mice were generated by crossing MASP1/3−/− and MASP2/sMAP−/− mice (Iwaki et al., 2006; Takahashi et al., 2008). All mice were maintained in a SPF condition in our animal facility in accordance with the guidelines for animal care approved by the Institute for Frontier Medical Sciences, Kyoto University.
Reagents.
Recombinant mouse C5a was purchased from R&D Systems. Mannan from Saccharomyces cerevisiae was purchased from Sigma-Aldrich and was dissolved in 200 µl PBS before i.p. injection. The endotoxin level in the reagents used in the current study was 0.44 EU/ml for 1 µg/ml C5a and 6.14 EU/ml for 1 mg/ml mannan, 4.01 EU/ml for 1 mg/ml laminarin, and <0.001 EU/ml for plasma-derived C5a, as determined by the LAL test (Wako Chemicals USA, Inc.). Plasma-derived C5a was purified from human EGTA-plasma as previously described (Köhl, 1997). CL was a gift from Roche, and the liposomal preparation was prepared as previously described (Solomon et al., 2005). IgG3-IC was prepared as previously described (Díaz de Ståhl et al., 2003).
Antibodies.
For flow-cytometric analysis, anti-CD4 (RM4-5), anti–IL-17 (TC11-18H10.1), anti–IFN-γ (XMG1.2), anti-CD11b (M1/70), anti-CD11c (HL3), anti–Gr-1 (RB6-8C5), and isotype IgG were purchased from eBioscience; anti–GM-CSF (MP1-22E9) was purchased from BD; anti-C5aR (20/70) was purchased from Cedarlane; and anti-F4/80 (A3-1) was purchased from AbD Serotec.
Cell culture.
Resident peritoneal macrophages were sorted by MoFlo (Dako) for FSChigh, SSChigh, CD11bhigh cells from lavage of the BALB/c peritoneal cavity with 10 ml PBS containing 2% FCS and 2 mM EDTA. Thioglycollate-elicited peritoneal macrophages were prepared as previously described (Zhang et al., 2007). Splenic DCs were sorted for CD11chigh cells in BALB/c spleens treated with Librase Blenzyme II (Roche). 2.5 × 104 CD4+ T cells were cultured with 1.25 × 104 macrophages or DCs in RPMI 1640 medium (Sigma-Aldrich) containing 10% FCS, and were stimulated with 0.5 µg/ml anti-CD3 (2C11) with or without 500 ng/ml C5a (R&D Systems), 10 ng/ml TGF-β (PeproTech), or 10 µg/ml anti–IL-6R (MR16-1).
C3 deposition assay.
Maxi-Plates (Thermo Fisher Scientific) were coated with 100 µg/ml laminarin, zymosan, or mannan. After blocking, wells were incubated with 2% mouse sera diluted with TBS/Tween/Ca (0.1% BSA, 0.05% Tween 20, 5 mM CaCl2) at 37°C for 30 min. C3c deposited on the well surfaces was detected by anti–human C3c (Dako) followed by horseradish peroxidase–conjugated secondary antibody (Dako), and assessed by ELISA. Supernatants from the C3c deposition assay were assessed for the concentration of C5a by ELISA and expressed as the concentrations in the original sera.
Quantitative RT-PCR.
Total RNA of peritoneal macrophages was extracted 24 h after incubation with 500 ng/ml C5a using the RNeasy column (QIAGEN). Total RNA of joint tissues was extracted by Isogen (Wako Chemicals USA, Inc.) according to the manufacturer’s instructions. cDNA was transcribed by reverse transcription (SuperScript III; Invitrogen), and the genes were quantified by the SYBR Green I system using LightCycler (Roche). Gene expression was normalized to expression of the HPRT gene. IL-23, C3, and IL-6 primer sequences are as follows: IL-23 forward, 5′-TCCCTACTAGGACTCAGCCAAC-3′; IL-23 reverse, 5′-TGGGCATCTGTTGGGTCT-3′; C3 forward, 5′-TTCGTCCTCATCGCACTG-3′; C3 reverse, 5′-TGTAACTGGCTTCAATATACTCC-3′; IL-6 forward, 5′-CCACTTCACAAGTCGGAGGCTTA-3′; and IL-6 reverse, 5′-GCAAGTGCATCATCGTTGTTCATAC-3′.
ELISA.
ELISA for C3a, C5a, and cytokines was performed according to the manufacturer’s instructions (BD). For measuring in vivo complement activation, futhan (FUT-175; BD) was added to the plasma preparation to prevent ex vivo complement activation.
Clinical assessment of joint scores, intracellular cytokine staining, and preparation of synovial cells.
These were performed as described previously (Hirota et al., 2007).
Statistical analysis.
The in vivo joint scores were analyzed by the Mann-Whitney U test. Unless otherwise mentioned, the Student’s t test was used for statistical analysis. P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 shows C3a/C5a production and early Th17 cell expansion after mannan treatment. Fig. S2 depicts the dependency of C5a/TGF-β–induced Th17 cell development on the type of APCs. Fig. S3 shows the effect of co-stimulation and T cell–derived cytokines on IL-6 production by C5a-stimulated macrophages. Fig. S4 depicts TLR-dependent IL-6 production by laminarin, zymosan, or mannan. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092301/DC1.
Acknowledgments
The authors thank R. Ishii and M. Kakino for technical assistance, T. Matsushita for histology, Z. Fehervari and P. Gannon for critically reading the manuscript, K. Tsuchiya for advice on the LAL test, S. Akira for providing TLR4- or MyD88-deficient mice, and the members of our laboratory for valuable discussion.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CL
- clodronate liposome
- IC
- immune complex
- MASP
- mannose-binding lectin-associated serine protease
- RA
- rheumatoid arthritis
- SPF
- specific pathogen–free
- TLR
- Toll-like receptor
- TNP
- trinitrophenyl
References
- Díaz de Ståhl T., Dahlstrom J., Carroll M.C., Heyman B. 2003. A role for complement in feedback enhancement of antibody responses by IgG3. J. Exp. Med. 197:1183–1190 10.1084/jem.20022232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C., Zhang X., Miwa T., Song W.C. 2009. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 114:1005–1015 10.1182/blood-2009-01-198283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. 2002. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2:346–353 10.1038/nri800 [DOI] [PubMed] [Google Scholar]
- Garlatti V., Belloy N., Martin L., Lacroix M., Matsushita M., Endo Y., Fujita T., Fontecilla-Camps J.C., Arlaud G.J., Thielens N.M., Gaboriaud C. 2007. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 26:623–633 10.1038/sj.emboj.7601500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K.H., Urdal D.L., Tushinski R.J., Mochizuki D.Y., Price V.L., Cantrell M.A., Gillis S., Conlon P.J. 1986. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 232:506–508 10.1126/science.3083507 [DOI] [PubMed] [Google Scholar]
- Guo R.F., Ward P.A. 2005. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23:821–852 10.1146/annurev.immunol.23.021704.115835 [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- Hawlisch H., Belkaid Y., Baelder R., Hildeman D., Gerard C., Köhl J. 2005. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 22:415–426 10.1016/j.immuni.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., Sakaguchi S. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204:41–47 10.1084/jem.20062259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höpken U.E., Lu B., Gerard N.P., Gerard C. 1996. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 383:86–89 10.1038/383086a0 [DOI] [PubMed] [Google Scholar]
- Iwaki D., Kanno K., Takahashi M., Endo Y., Lynch N.J., Schwaeble W.J., Matsushita M., Okabe M., Fujita T. 2006. Small mannose-binding lectin-associated protein plays a regulatory role in the lectin complement pathway. J. Immunol. 177:8626–8632 [DOI] [PubMed] [Google Scholar]
- Köhl J. 1997. Anaphylatoxins. Complement: A Practical Approach. Dodds A.W., Sim R.B., Oxford University Press Inc, New York: 135–163 [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485–517 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- Liu J., Lin F., Strainic M.G., An F., Miller R.H., Altuntas C.Z., Heeger P.S., Tuohy V.K., Medof M.E. 2008. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J. Immunol. 180:5882–5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I., Maccioni M., Lee D.M., Maurice M., Simmons B., Brenner M., Mathis D., Benoist C. 2002. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat. Immunol. 3:360–365 10.1038/ni772 [DOI] [PubMed] [Google Scholar]
- Mullaly S.C., Kubes P. 2007. Mast cell-expressed complement receptor, not TLR2, is the main detector of zymosan in peritonitis. Eur. J. Immunol. 37:224–234 10.1002/eji.200636405 [DOI] [PubMed] [Google Scholar]
- Plenge R.M., Seielstad M., Padyukov L., Lee A.T., Remmers E.F., Ding B., Liew A., Khalili H., Chandrasekaran A., Davies L.R., et al. 2007. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N. Engl. J. Med. 357:1199–1209 10.1056/NEJMoa073491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers E.F., Plenge R.M., Lee A.T., Graham R.R., Hom G., Behrens T.W., de Bakker P.I., Le J.M., Lee H.S., Batliwalla F., et al. 2007. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357:977–986 10.1056/NEJMoa073003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.J., Sancho D., Slack E.C., LeibundGut-Landmann S., Reis e Sousa C. 2006. Myeloid C-type lectins in innate immunity. Nat. Immunol. 7:1258–1265 10.1038/ni1417 [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Takahashi T., Hata H., Nomura T., Tagami T., Yamazaki S., Sakihama T., Matsutani T., Negishi I., Nakatsuru S., Sakaguchi S. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 426:454–460 10.1038/nature02119 [DOI] [PubMed] [Google Scholar]
- Sheng K.C., Pouniotis D.S., Wright M.D., Tang C.K., Lazoura E., Pietersz G.A., Apostolopoulos V. 2006. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 118:372–383 10.1111/j.1365-2567.2006.02384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S., Rajasekaran N., Jeisy-Walder E., Snapper S.B., Illges H. 2005. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur. J. Immunol. 35:3064–3073 10.1002/eji.200526167 [DOI] [PubMed] [Google Scholar]
- Sonderegger I., Iezzi G., Maier R., Schmitz N., Kurrer M., Kopf M. 2008. GM-CSF mediates autoimmunity by enhancing IL-6–dependent Th17 cell development and survival. J. Exp. Med. 205:2281–2294 10.1084/jem.20071119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strainic M.G., Liu J., Huang D., An F., Lalli P.N., Muqim N., Shapiro V.S., Dubyak G.R., Heeger P.S., Medof M.E. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 28:425–435 10.1016/j.immuni.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre D., Clynes R., Ma M., Warren H., Carroll M.C., Ravetch J.V. 1996. Immunoglobulin G–mediated inflammatory responses develop normally in complement-deficient mice. J. Exp. Med. 184:2385–2392 10.1084/jem.184.6.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Iwaki D., Kanno K., Ishida Y., Xiong J., Matsushita M., Endo Y., Miura S., Ishii N., Sugamura K., Fujita T. 2008. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J. Immunol. 180:6132–6138 [DOI] [PubMed] [Google Scholar]
- Vang T., Congia M., Macis M.D., Musumeci L., Orrú V., Zavattari P., Nika K., Tautz L., Taskén K., Cucca F., et al. 2005. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 37:1317–1319 10.1038/ng1673 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Weaver D.J., Jr., Reis E.S., Pandey M.K., Köhl G., Harris N., Gerard C., Köhl J. 2010. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur. J. Immunol. 40:710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H., Sakaguchi N., Kobayashi K., Brown G.D., Tagami T., Sakihama T., Hirota K., Tanaka S., Nomura T., Miki I., et al. 2005. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201:949–960 10.1084/jem.20041758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Kimura Y., Fang C., Zhou L., Sfyroera G., Lambris J.D., Wetsel R.A., Miwa T., Song W.C. 2007. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 110:228–236 10.1182/blood-2006-12-063636 [DOI] [PMC free article] [PubMed] [Google Scholar]