Abstract
In mouse, a subset of dendritic cells (DCs) known as CD8α+ DCs has emerged as an important player in the regulation of T cell responses and a promising target in vaccination strategies. However, translation into clinical protocols has been hampered by the failure to identify CD8α+ DCs in humans. Here, we characterize a population of human DCs that expresses DNGR-1 (CLEC9A) and high levels of BDCA3 and resembles mouse CD8α+ DCs in phenotype and function. We describe the presence of such cells in the spleens of humans and humanized mice and report on a protocol to generate them in vitro. Like mouse CD8α+ DCs, human DNGR-1+ BDCA3hi DCs express Necl2, CD207, BATF3, IRF8, and TLR3, but not CD11b, IRF4, TLR7, or (unlike CD8α+ DCs) TLR9. DNGR-1+ BDCA3hi DCs respond to poly I:C and agonists of TLR8, but not of TLR7, and produce interleukin (IL)-12 when given innate and T cell–derived signals. Notably, DNGR-1+ BDCA3+ DCs from in vitro cultures efficiently internalize material from dead cells and can cross-present exogenous antigens to CD8+ T cells upon treatment with poly I:C. The characterization of human DNGR-1+ BDCA3hi DCs and the ability to grow them in vitro opens the door for exploiting this subset in immunotherapy.
DCs are key players in immune regulation and an important component of rational immunotherapeutic strategies in humans (Steinman, 2008). In mouse, rat, and human species, DCs can be broadly divided into two groups (Villadangos and Schnorrer, 2007; Naik, 2008; Heath and Carbone, 2009; Merad and Manz, 2009). Plasmacytoid DCs (pDCs) have variable antigen-presenting activity, but respond to viruses by producing IFN-α via a Toll-like receptor (TLR)–dependent pathway (Gilliet et al., 2008). In contrast, conventional DCs (cDCs) are known for their potent antigen-presenting activity and the ability to induce either T cell immunity or tolerance in response to self and foreign antigens. cDC have been extensively studied in mouse tissues, in particular the secondary lymphoid organs. In lymph nodes, cDCs encompass multiple subsets that are either derived from blood-borne precursors or from afferent lymph-borne DCs emigrating from tissues. Mouse spleen lacks an afferent lymph supply, and therefore contains only blood-derived cDCs. The latter express CD11c and are often divided into CD8α+ and CD8α− subsets, with CD8α− subsets further subdivided on the basis of CD4 expression into CD4+ and CD4− DCs (Villadangos and Schnorrer, 2007; Naik, 2008; Heath and Carbone, 2009; Merad and Manz, 2009). CD8α+ DCs have attracted much attention and have been studied extensively (Shortman and Heath, 2010). Compared with other DCs subsets, CD8α+ DCs express much higher levels of certain gene products such as CD8α, TLR3, CD36, CD103, and nectin-like 2 (Necl2) protein, but lower or undetectable levels of CD11b, TLR7, DCIR2 (also known as 33D1), RIG-I, MDA5, CD11b, and SIRPα (Edwards et al., 2003; Galibert et al., 2005; Dudziak et al., 2007; Luber et al., 2010; Shortman and Heath, 2010). Certain proteins are expressed by CD8α+ DCs and some DC subsets, but not by others. These include shared expression of DEC205 and Langerin (CD207) with Langerhans cells and a subset of dermal DCs, as well as shared expression of IRF8 with pDC (Inaba et al., 1995; Schiavoni et al., 2002; Takahara et al., 2002; Shortman and Heath, 2010). Notably, CD8α+ DCs, but not other DC subtypes have recently been shown to depend on the transcription factor Batf3 for their development, suggesting that they represent an ontogenetically distinct mouse leukocyte lineage (Hildner et al., 2008).
In addition to distinct gene expression profiles and ontogeny, CD8α+ DCs have several functional properties that distinguish them from other DC subtypes, albeit in a quantitative rather than qualitative manner. These include a superior capacity to cross-present exogenous antigens on MHC class I, to ingest material from dead or dying cells, and to produce IL-12 in response to innate and T cell–derived stimuli (for review see Shortman and Heath, 2010). Because of these properties, CD8α+ DCs have emerged as an attractive cellular target for vaccination strategies, in particular ones aimed at eliciting CTL responses against tumor or virus-infected cells. In addition, CD8α+ DCs can also induce conversion of antigen-specific T cells into regulatory T cells, suggesting that antigen delivery to CD8α+ DCs could be used to dampen immune reactivity (Yamazaki et al., 2008). Unfortunately, the translation of mouse experiments into human studies and clinical protocols has been hampered by the fact that CD8α+ DCs have not been identified in human (Naik, 2008). Nevertheless, distinct DC subsets can be phenotypically identified in human blood by HLA-DR expression, lack of antigens specific to other leukocyte lineages, and an additional set of markers known as blood DC antigens (BDCAs; Dzionek et al., 2000). For example, BDCA2 is a marker for circulating human pDCs, whereas BDCA3 (also known as CD141 or thrombomodulin) marks a distinct small subset of blood DCs (Dzionek et al., 2000; MacDonald et al., 2002). Interestingly, a recent microarray analysis has indicated that BDCA3+ DCs from human peripheral blood have a transcriptional signature that resembles that of mouse spleen CD8α+ DCs, despite the fact that they do not express CD8α (Robbins et al., 2008). In addition, blood BDCA3+ DCs have been reported to be expanded in volunteers treated with Flt3L (Galibert et al., 2005), a cytokine that also greatly promotes the expansion of CD8α+ DCs in mice (Maraskovsky et al., 1996). Finally, Necl2 is also selectively expressed by human blood BDCA3+ DCs (Galibert et al., 2005). Thus, human blood BDCA3+ DCs might constitute a DC population equivalent to the mouse CD8α+ DC subset (Shortman and Heath, 2010). However, these cells have not been fully characterized and, in particular, it is unclear whether they constitute a homogeneous population and whether the rare BDCA3+ DCs circulating in blood represent DCs in human secondary lymphoid organs.
We and others have recently identified the C-type lectin, DNGR-1, also known as CLEC9A, as a novel marker for mouse CD8α+ DCs and their CD8− CD24+ blood-borne precursors (Caminschi et al., 2008; Sancho et al., 2008). Interestingly, human DNGR-1 is highly restricted to BDCA3hi DCs among peripheral blood mononuclear cells (Caminschi et al., 2008; Huysamen et al., 2008; Sancho et al., 2008). We therefore hypothesized that the BDCA3 and DNGR-1 markers together would be useful for identifying putative equivalents of mouse CD8α+ DCs in human secondary lymphoid tissues and for purifying them for the purpose of characterization. Here, we report that DNGR-1+ BDCA3+ double-positive DCs can be identified in primary human spleen, as well as in the spleens of mice reconstituted with human hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs). Additionally, we describe a protocol for growing populations of these cells in vitro from human cord blood (CB) HSCs/HPCs. We characterize DNGR-1+ BDCA3+ DCs from all three sources and find that they have phenotypic and functional properties resembling those of mouse CD8α+ DCs. We endorse the view that DNGR-1+ BDCA3+ DCs constitute a population of human DCs similar to the mouse CD8α+ DC subset. The ability to generate these cells in humanized mice, and to grow them in vitro, offers tremendous opportunity for studying their properties and for using them in immunotherapeutic approaches.
RESULTS
A discrete population of DNGR-1+ BDCA3hi DCs is present in human spleen
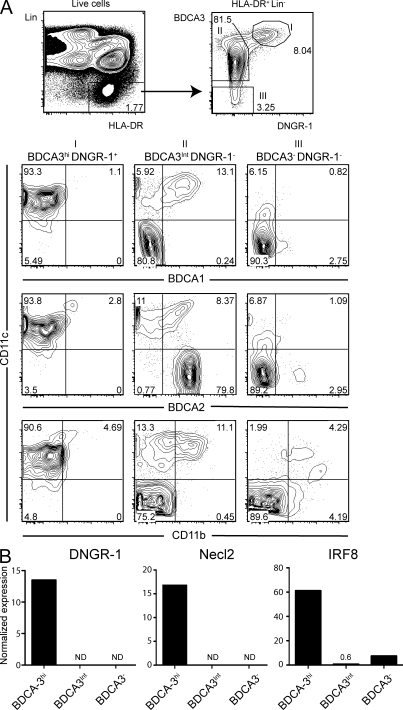
Mouse CD8α+ DCs have been primarily isolated from spleen, and we asked if the latter organ in humans contains a DNGR-1+ BDCA3+ DC subset. We screened samples from a bank of frozen cell suspensions that had been obtained less than 8 h post mortem by mechanical dissociation of healthy human spleens from cadaveric organ donors. Human DCs could be identified in all the samples by flow cytometry as HLA-DR+ cells lacking the lineage (Lin)-specific markers CD3, CD14, CD16, CD19, CD20, and CD56 (Fig. 1 A, top left, and Table S1). As in peripheral blood, DNGR-1 was found to be expressed exclusively by BDCA3hi DCs (population I; Fig. 1 A, top right). Like mouse CD8α+ DCs, human spleen DNGR-1+ BDCA3hi DCs were negative for CD11b and expressed slightly lower levels of CD11c than other DCs (Fig. 1 A and Fig. S1). Human spleen DNGR-1+ BDCA3hi DCs also did not express BDCA2, the pDC marker, or BDCA1 (CD1c), a more promiscuous marker also used to define human DC subtypes (Fig. 1 A and Fig. S1). The DNGR-1− population included cells lacking BDCA3 (BDCA3−, population III; Fig. 1 A, top right), as well as cells expressing moderate levels of the marker (BDCA3int; population II; Fig. 1 A, top right). BDCA3− DCs were relatively homogeneous and were mostly negative for CD11c, BDCA1, BDCA2, and CD11b (Fig. 1 A). In contrast, the BDCA3int population was heterogeneous and included CD11c− BDCA2+ pDC that lacked CD11b and DNGR-1. Interestingly, BDCA3int cells also included CD11c+ DCs that variably expressed BDCA1, BDCA2, and CD11b (Fig. 1 A and Fig. S1). We sorted BDCA1− BDCA2− cells into BDCA3hi, BDCA3int, and BDCA3− populations. BDCA3hi DCs expressed mRNA for DNGR-1, Necl2, and IRF8 (Fig. 1 B), similar to mouse CD8α+ DCs. BDCA3int and BDCA3− DCs expressed low or undetectable levels of DNGR-1, Necl2, and IRF8 mRNA (Fig. 1 B). We conclude that BDCA3 can be expressed by multiple human DC subsets, including pDC, but, at high levels of expression and especially in combination with DNGR-1, it marks a discrete population of human spleen DCs that resemble mouse CD8α+ DCs.
Figure 1.
DNGR-1+ BDCA3hi DCs are found in the spleens of humans. (A) Flow cytometry analysis of human spleen cell suspensions from cadaveric donors. Live HLA-DR+ Lin− cells were gated as shown (top left) and analyzed for expression of BDCA3 versus DNGR-1 (top right). Three populations were defined (gate I: BDCA3hi, DNGR-1+; gate II: BDCA3int, DNGR-1−; gate III: BDCA3−, DNGR-1−) and analyzed for the expression of CD11c versus BDCA1, BDCA2 or CD11b (bottom). Numbers indicate percentage of cells in each of the indicated gates or quadrants. Arrows show gating strategy. (B) Normalized expression of DNGR-1, Necl2, and IRF8 mRNA on sorted HLA-DR+ Lin− BDCA2− BDCA1− DCs, from human spleen, expressing high (BDCA3hi), intermediate (BDCA3int), or low levels of BDCA3 (BDCA3−). ND, not detectable. Data in A and B are representative of at least three cadaveric donors. Additional data analysis is shown in Fig. S1 and a summary of DC population frequency across three donors is presented in Table S1.
A discrete population of DNGR-1+ BDCA3hi DCs is found in spleens of humanized mice
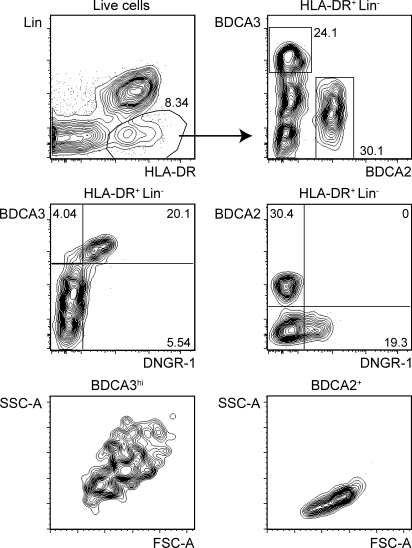
Because of restricted access, paucity of DCs, and difficulty in recovering live cells from frozen samples, we were limited in the amount of analysis that we could carry out on primary human spleen (Table S1). Therefore, we assessed whether DNGR-1+ DCs could be identified in the spleens of mice reconstituted with human stem cells. Two xenotransplantation mouse models were used for this purpose: NOD/SCID/β2m-null mice or NOD/SCID/γc-null mice reconstituted with purified human CB HSCs/HPCs. In three independent successful xenotransplantation cohorts, HLA-DR+ Lin− human DCs were found in the spleens of all mice at 8–12 wk after reconstitution, independent of the host mouse strain (Fig. 2 and not depicted). As in human spleen, distinct DC subsets could be defined on the basis of BDCA2, BDCA3, and DNGR-1 expression. They included DNGR-1− BDCA3− BDCA2− DCs, as well as DNGR-1− BDCA3int BDCA2+ cells that corresponded to pDCs. Notably, a separate population of cells that were negative for BDCA2, but bright for BDCA3 and positive for DNGR-1, could also be identified (Fig. 2). These DNGR-1+ BDCA3hi cells were larger than BDCA2+ pDCs (Fig. 2) and resembled the ones in human spleen (see above). Thus, DNGR-1+ BDCA3hi DCs can be found in the spleens of humanized mice, in addition to those of humans.
Figure 2.
DNGR-1+ BDCA3hi DCs are found in the spleens of humanized mice. HLA-DR+ Lin− live spleen cells from humanized mice (NOD/SCID/γc) were analyzed as in Fig. 1 for the expression of BDCA3 versus BDCA2 (top right), BDCA3 versus DNGR-1 (middle left), and BDCA2 versus DNGR-1 (middle right). The scatter profile of BDCA3+ and BDCA2+ cells is also shown (bottom). Numbers indicate percentage of cells in each of the indicated gates or quadrants. Arrows show gating strategy. Data are representative of multiple mice from three independent cohorts of mice engrafted with human HSCs/HPCs.
Ex vivo generation of DNGR-1+ BDCA3+ DCs from human HSCs/HPCs
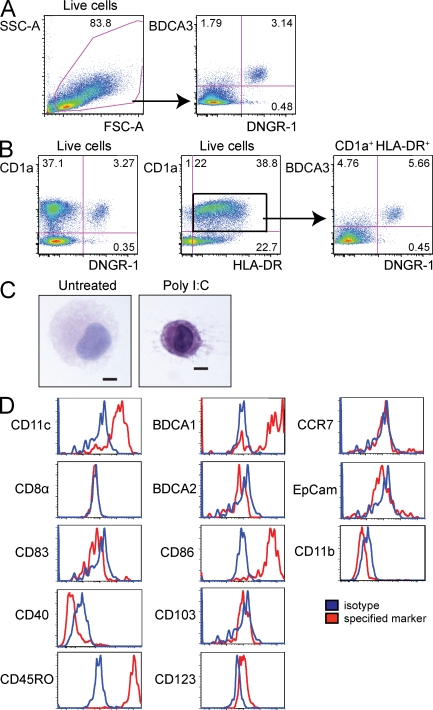
Although humanized mice proved a more useful source of DNGR-1+ BDCA3hi DCs than primary human spleen, variation in chimerism and the low number of cells obtained were limiting factors (unpublished data). Successful generation of DCs from hematopoietic stem cells has been achieved for Langerhans cells and dermal DC equivalents (Klechevsky et al., 2008). Therefore, we tried to generate DNGR-1+ DCs in vitro from human CB HSCs/HPCs (CB-derived DCs [CBDCs]). We set up cultures as a two step procedure: in the first step, HSCs/HPCs were amplified by culture in the presence of SCF, Flt3L, IL-3, and IL-6. The expanded cells were then aliquoted and tested in a variety of differentiation conditions for the ability to generate DNGR-1+ and/or BDCA3+ DCs. Flt3L was included in the differentiation “cocktail” because BDCA3+ DCs are expanded in Flt3L-treated human subjects (Galibert et al., 2005), and Flt3L is essential for mouse DCs development (Merad and Manz, 2009). In addition, we used GM-CSF and IL-4, as these cytokines have been used to generate DCs in mouse and human. Finally, SCF was added to maintain HSC/HPC viability. Using HLA-DR and CD1a expression as a phenotypic definition of in vitro HSC/HPC-derived DCs (Klechevsky et al., 2008), we found that this culture system generated ∼30–50% of Lin− HLA-DR+ CD1a+ cells, which included a small percentage of the desired DNGR-1+ BDCA3+ double-positive subset (Fig. 3 A). Omission of any one of the four cytokines, SCF, Flt3L, GM-CSF, or IL-4, from the differentiation cocktail prevented the emergence of DNGR-1+ BDCA3+ DCs by day 13 (Fig. S2 A), although small numbers of the cells could be recovered in the absence of SCF in longer cultures (e.g., 15 d; not depicted), consistent with the notion that SCF acts primarily to sustain progenitor cells. In addition, DNGR-1+ BDCA3+ DCs could still be obtained when substituting TGF-β for Flt3L in the differentiation cocktail (unpublished data). DNGR-1+ BDCA3+ cells were not seen at 6 d of the differentiation culture, but represented 3–6% of total live cells by 12 d, suggesting that they differentiate or expand as a late event. As for the cells in primary human spleen, CB-derived DNGR-1+ BDCA3+ DCs did not express CD14, CD11b, or BDCA2, but expressed CD11c (Fig. 3 D and Fig. S2 B). In contrast to primary human spleen DNGR-1+ BDCA3hi DCs, those from CBDCs expressed BDCA1 (Fig. 3 D), perhaps because of GM-CSF–driven induction of CD1a-c family members (Porcelli et al., 1992). CB-derived DNGR-1+ BDCA3+ DCs expressed CD86 and CD45RO, but only low levels of CD123 (Fig. 3 D), as reported for blood BDCA3+ DCs (MacDonald et al., 2002). In contrast to mouse CD8α+ DCs, DNGR-1+ BDCA3+ DCs did not express CD8α or CD103. They also did not express high levels of EpCam, indicating that they were not Langerhans cells (Bursch et al., 2007). Finally, CB-derived DNGR-1+ BDCA3+ DCs appeared to be immature, in that they did not express CD40, CD83, or CCR7 (Fig. 3 D). Consistent with immaturity, DNGR-1+ BDCA3+ DCs were not noticeably dendritic until the CBDC cultures were treated with poly I:C (to promote DC maturation (Fig. 3 C). Thus, immature DNGR-1+ BDCA3+ DCs phenotypically similar to primary human spleen DNGR-1+ BDCA3hi DCs and to blood BDCA3+ DCs can be generated in vitro from human HSCs/HPCs.
Figure 3.
Phenotype of in vitro–generated DNGR-1+ BDCA3+ CBDCs. CBDCs were generated as described in the Materials and methods. Live cells were analyzed for the expression of BDCA3 versus DNGR-1 (A), CD1a versus DNGR-1 (B, left), and CD1a versus HLA-DR (B, middle). Gated CD1a+ HLA-DR+ cells were analyzed for the expression of BDCA3 versus DNGR-1 (B, right). Numbers indicate percentage of cells in each of the indicated gates or quadrants. Arrows show gating strategy. (C) CBDCs were treated with or without poly I:C (10 µg/ml) overnight and subsequently sorted into live HLA-DR+ Lin− DNGR-1+ cells. Cytospins were prepared and morphology was assessed by hematoxylin and eosin staining. Bar, 5 µm. (D) Live DNGR-1+ DCs were analyzed for the expression (red) of CD11c, BDCA1, CD8α, BDCA2, CD83, CD86, CD40, CD103, CD45RO, CD123, CCR7, EpCam, and CD11b. Isotype-matched control mAb staining is shown in the blue histograms. Data in A–D are representative of multiple BDC cultures with two independent pools of CB-derived HSCs/HPCs.
Human DNGR-1+ DCs resemble mouse CD8α+ DCs in gene expression pattern
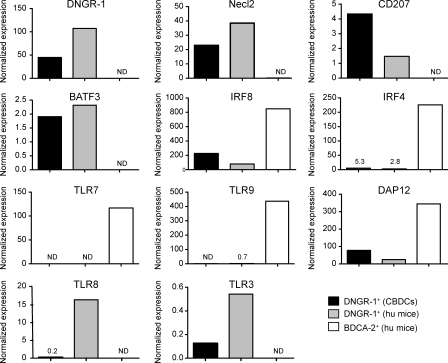
To establish a possible equivalence between DNGR-1+ BDCA3+ human DCs and mouse CD8α+ DCs, we analyzed the expression of selected gene products by quantitative PCR. As for the cells isolated from human spleen (Fig. 1 B), DNGR-1+ DCs purified from bulk CBDC cultures or from the spleens of humanized mice expressed mRNA for DNGR-1, Necl2, and IRF8 (Fig. 4). IRF8 (and DAP12) was expressed at higher levels by BDCA2+ pDCs isolated in parallel from humanized mice (Fig. 4), consistent with data in the mouse (Schiavoni et al., 2002). In contrast, the same pDCs did not express Necl2 or DNGR-1 (Fig. 4). Similar to mouse CD8α+ DCs, human DNGR-1+ DCs expressed CD207 and BATF3, but did not express mRNA for IRF4 or TLR7, which was restricted to pDCs (Fig. 4). Interestingly, TLR9 expression was also not expressed by DNGR-1+ DCs, but was expressed by pDCs (Fig. 4). This is different from the situation with mouse CD8α+ DCs, which express TLR9. Finally, DNGR-1+ DCs, but not pDCs from humanized mice, expressed TLR3 and TLR8 (Fig. 4). DNGR-1+ cells from CBDC cultures only expressed very low levels of the same TLRs (Fig. 4), possibly because of their immaturity. Consistent with the latter interpretation, DNGR-1+ CBDCs up-regulated TLR3 and TLR8, but not TLR7, upon treatment with type I IFN, a known inducer of nucleic acid–sensing TLRs (unpublished data).
Figure 4.
DNGR-1+ BDCA3+ DCs display a gene expression profile characteristic of mouse CD8α+ DCs. Normalized expression of DNGR-1, Necl2, CD207, BATF3, IRF8, IRF4, TLR7, TLR9, DAP12, TLR8, and TLR3 mRNA in BDCA3+ DNGR-1+ DCs (DNGR-1+) purified either from CBDCs or from pooled spleens of two to five humanized mice (hu mice). Expression was compared with that of BDCA2+ pDCs purified from the same humanized mouse spleens. Data are representative of two independent experiments. ND, not detectable.
Human DNGR-1+ DCs resemble mouse CD8α+ DCs in responsiveness to TLR agonists
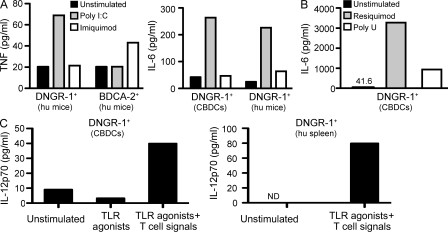
Mouse CD8α+ DCs express TLR3, but not TLR7, mRNA and respond to poly I:C, which is a TLR3 (and MDA5/RIG-I) agonist, but not to imiquimod (R837), an agonist for TLR7 (Edwards et al., 2003). We purified DNGR-1+ DCs from CBDC cultures or from humanized mouse spleen and stimulated them with poly I:C or imiquimod. Independent of the source, DNGR-1+ DCs consistently responded to poly I:C, but not imiquimod, with production of TNF, IL-6, and other cytokines (Fig. 5 A and not depicted). A similar response pattern was seen with total unfractionated CBDCs when measuring TNF production or surface HLA-DR up-regulation as a marker of DNGR-1+ DCs maturation (Fig. S3). In contrast, BDCA2+ pDCs from humanized mice responded to imiquimod, but not poly I:C, in the same experiments (Fig. 5 A). When trying various other TLR agonists, we noticed that DNGR-1+ DCs also responded to resiquimod (R848) and poly U (Fig. 5 B). In the mouse, where there is lack of functional TLR8 expression, these compounds have been defined as TLR7 agonists (Diebold et al., 2004). However, in the human, they are reported to additionally stimulate TLR8 (Heil et al., 2004). The potent response of DNGR-1+ BDCA3+ DCs to resiquimod and poly U, but not imiquimod, indicates that these cells can express functional TLR8.
Figure 5.
DNGR-1+ BDCA3+ DCs respond to poly I:C and to TLR8, but not TLR7 agonists. (A) Purified DNGR-1+ BDCA3+ DCs from CBDCs (DNGR-1+ CBDCs) or from humanized mice (DNGR-1+ hu mice), or BDCA2+ pDCs from the same humanized mice (BDCA2+ hu mice), were cultured with poly I:C (10 µg/ml), imiquimod (10 µg/ml), or medium alone. After overnight incubation, culture supernatant was tested for TNF and IL-6 content. (B) DNGR-1+ CBDCs as in (A) were cultured with resiquimod (10 µg/ml), poly U (10 µg/ml) or medium alone. Supernatant was tested for IL-6 after overnight culture. (C) DNGR-1+ BDCA3+ DCs purified from CBDCs (DNGR-1+ CBDCs) or human spleen (DNGR-1+ hu spleen) were cultured with a mix of TLR agonists and cytokines with or without antigen-specific T cells and antigen, as described in the Materials and methods. Supernatant was tested for IL-12 p70 after overnight culture. Data in A–C are representative of at least two independent experiments with independent sources of cells. ND, not detectable.
Human DNGR-1+ DCs produce IL-12 in response to innate and T cell–derived stimuli
A hallmark of mouse CD8α+ DCs is their ability to produce high levels of IL-12 in response to TLR agonists, especially in synergy with signals through the CD40, IL-4, GM-CSF, or IFN-γ receptors (Shortman and Heath, 2010). DNGR-1+ DCs purified from CBDC cultures produced no IL-12 p70 above background in response to stimulation with a cocktail of TLR agonists, even when these were given together with IL-4 and IFN-γ (Fig. 5 C). However, the same cells produced IL-12 p70 when antigen-specific T cells and antigen were added to the cultures (Fig. 5 C). Notably, this was also the case for primary DNGR-1+ DCs isolated directly from human spleen cell suspensions (Fig. 5 C). Thus, coordinate delivery of innate and T cell–derived stimuli reveals the ability of DNGR-1+ BDCA3+ DCs, including ones from human spleen, to produce bioactive IL-12.
Human DNGR-1+ DCs internalize dead cell material and cross-present exogenous proteins to CD8+ T cells
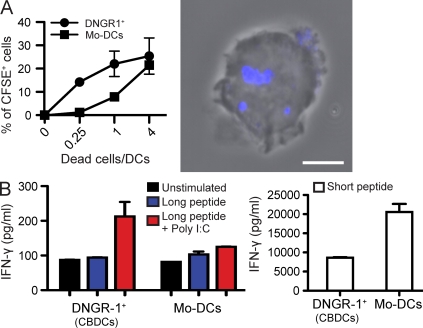
Another attribute of mouse CD8α+ DCs is their superior capacity to internalize debris from dead or dying cells (Iyoda et al., 2002; Schulz and Reis e Sousa, 2002). DNGR-1+ DCs were able to take dead cell material and were superior in this regard to monocyte-derived DCs (Mo-DCs) generated using GM-CSF + IL-4 (Sallusto et al., 1995; Fig. 6 A). By microscopy, internalized dead cell material took the appearance of multiple small inclusions within a single DNGR-1+ DC (Fig. 6 A). Finally, we assessed the ability of DNGR-1+ BDCA3+ DCs to cross-present exogenous antigen to CD8+ T cells, a functional characteristic of mouse CD8α+ DCs. In the first instance, we used unfractionated CBDC cultures and compared them to Mo-DCs. Unfortunately, the CBDC used for these experiments were only 50% HLA-A2+, as they were generated from HSCs/HPCs purified from pooled CB, whereas Mo-DCs were from a single donor and homogenous for HLA-A2 expression (unpublished data). This might be one reason why CBDCs were less efficient than Mo-DCs at presenting preprocessed antigenic peptides to HLA-A2–restricted T cell clones (Fig. S4). Nevertheless, CBDCs were at least as efficient as Mo-DCs at activating NY-ESO-1157-165–specific T cells in response to intact recombinant NY-ESO-1 protein, a clinically relevant antigen expressed by a broad range of tumor types (Chen et al., 2005; Fig. S4). The activation of the NY-ESO–specific T cell clone reflected true NY-ESO-1 protein processing and cross-presentation, as it was not seen with CBDCs that were fixed in glutaraldehyde, even though the latter were still able to present the preprocessed determinant (Fig. S4). Interestingly, this cross-presenting ability was only revealed upon treatment of DCs with poly I:C (Fig. S4), underscoring the importance of DC maturation in promoting antigen processing and presentation (Inaba et al., 2000; Delamarre et al., 2003). Finally, we tested purified DNGR-1+ BDCA3+ CBDCs. We used a different model of cross-presentation, comprising a long Melan-A–derived peptide that requires processing for its (cross-)presentation to a specific HLA-A2–restricted T cell line (Faure et al., 2009). As for the NY-ESO-1 protein, presentation of the long Melan-A peptide was only seen upon treatment of the DCs with poly I:C (Fig. 6 B). DNGR-1+ DCs were more efficient at cross-presenting the long peptide than Mo-DCs, even though the latter were more efficient at presenting the short peptide, representing the preprocessed determinant. We conclude that DNGR-1+ DCs have equal or superior cross-presentation ability to that of Mo-DCs.
Figure 6.
DNGR-1+ BDCA3+ DCs internalize dead cell debris and cross-present exogenous antigens. (A) CBDCs or Mo-DCs were incubated with labeled dead melanoma cells at the indicated ratios, as described in the Materials and methods. Uptake of dead cell material by DNGR-1+ DCs or Mo-DCs was quantified by flow cytometry (left) and confirmed by confocal microscopy (right; blue shows dead cell material). Data are mean ± SEM of three biological replicates from one experiment representative of two independent experiments. Bar, 5 µm. (B) 104 purified BDCA3+ DNGR-1+ CBDCs or Mo-DCs, as indicated, were pulsed for 2–3 h with or without 1 µM MelanA/MART-1 long peptide as antigen source for cross-presentation or the same concentration of short peptide as a processing-independent control, in the presence or absence of poly I:C. Cells were subsequently washed twice and co-cultured with a MelanA-specific CD8+ T cell clone at a 5:1 T:DCs ratio. IFN-γ accumulation in culture supernatants was assessed after 40 h. Data are the mean value of duplicate wells ± range and are representative of two independent experiments.
DISCUSSION
Analysis of leukocyte subsets in the mouse over the last 15 yr has highlighted the diversity of the DCs family and the notion that distinct DCs types perform different immune functions (Villadangos and Schnorrer, 2007; Naik, 2008; Heath and Carbone, 2009; Merad and Manz, 2009). Much attention has focused on the mouse CD8α+ DCs subset and its role in immunity and tolerance, which could potentially be harnessed for immunotherapy of multiple diseases (Shortman and Heath, 2010). However, equivalent cells in humans have not been described. Here, we characterize DNGR-1+ BDCA3hi DCs as possible human equivalents of mouse CD8α+ DCs. Like their putative murine counterparts, DNGR-1+ BDCA3hi DCs are large cells that express MHC class II, Necl2, Langerin, IRF8, BATF3, and TLR3, but not IRF4, CD11b, or TLR7. DNGR-1+ BDCA3hi further resemble mouse CD8α+ DCs in their capacity to take up dead cell material, to respond to poly I:C, but not TLR7 agonists, to produce IL-12 p70 in response to innate and T cell–derived stimuli and in their cross-presenting properties and dendritic morphology that are revealed upon poly I:C stimulation. Although it remains to be formally proven that DNGR-1+ BDCA3hi DCs are the human equivalent of mouse CD8α+ DCs, our data open the door for additional research to validate this concept and describe experimental systems in which this can be achieved.
We show that DNGR-1+ BDCA3hi DCs are present in normal human spleen, as well as in the spleens of humanized mice. Access to primary healthy human spleen samples was critical to establishing that the previously described BDCA3+ DCs in human blood are representative of DCs in lymphoid organs. Our ability to detect DNGR-1+ BDCA3hi DCs in human spleen cell suspensions is unlikely to be explained by blood contamination as DNGR-1+ BDCA3hi DCs represent 0.05% of PBMC (Caminschi et al., 2008; Huysamen et al., 2008; Sancho et al., 2008), yet make up 0.1% of total splenocytes (Table S1). Thus, DNGR-1+ BDCA3hi human spleen DCs likely correspond to the rare Necl2+ cells previously found by immunofluorescence in human spleen sections (Galibert et al., 2005). Their low frequency among spleen cells is consistent with them being a rare cell type, although it may also relate to the method used for preparation of the cell suspension as, in mouse, mechanical dissociation of spleen without enzymatic digestion selects against CD8α+ DC isolation (Vremec et al., 1992). It will be interesting to extend our analysis to additional donors to determine if DNGR-1+ BDCA3hi human spleen DCs can show signs of in vivo activation related to bacterial infections or extent of trauma preceding death (McIlroy et al., 2001).
BDCA3+ DCs have been previously reported in human spleen (Velásquez-Lopera et al., 2008) and in the spleen and bone marrow of humanized mice (Cravens et al., 2005), but it is not clear whether they precisely correspond to the DNGR-1+ BDCA3hi population characterized here. Indeed, we find that although BDCA3 is expressed at the highest levels by DNGR-1+ DCs, it is also expressed at lower levels by multiple DCs subtypes, including pDCs, in human and humanized mouse spleen. BDCA3 is also expressed by Lin+ non-DCs (MacDonald et al., 2002; unpublished data), and therefore caution must be exercised when using it as a marker. This is similar to the situation with DEC-205 in the mouse, which is expressed at highest level on CD8α+ DCs and Langerhans cells, but is also expressed by other cell types (Witmer-Pack et al., 1995). Worryingly, we have noticed that use of dim fluorophores or inefficient flow cytometer calibration can result in contamination of the BDCA3hi population with BDCA3int cells and, during cell sorting, lead to false PCR results (unpublished data). For this reason, we would advocate the dual use of DNGR-1 and BDCA3 as markers to define CD8α+ DC equivalents in human. Notably, we and others have shown that antigen targeting to DNGR-1 is a useful strategy for inducing CTL responses and antibody responses in mice (Caminschi et al., 2008; Sancho et al., 2008), and can additionally be used to generate Th1 and Th17 or for converting antigen-specific CD4+ T cells into regulatory T cells (Joffre et al., 2010). Given that DNGR-1 appears to specifically mark the CD8α+ DCs lineage across mouse and human species, DNGR-1 targeting could also prove a useful method for antigen delivery to DCs in humans.
In this paper, we additionally report on a set of culture conditions that allows the generation of DNGR-1+ BDCA3+ DCs from human HSCs/HPCs. The percentage of cells obtained was low, suggesting that the culture system will benefit from additional optimization. Interestingly, the same cultures generated DNGR-1− BDCA3− DCs subsets, in addition to DNGR-1+ BDCA3+ DCs. Although it is tempting to draw an analogy with Flt3L cultures of mouse bone marrow, which can generate both CD8α+-like and CD8α− DCs after 8–10 d of culture in Flt3L (Naik et al., 2005), we found that DNGR-1− BDCA3− DCs are not equivalent to CD8α− DCs. In fact, DNGR-1− BDCA3− DCs are a mixed population that contain, among others, precursors for DNGR-1+ BDCA3+ DCs (unpublished data). In this study, we ignored DNGR-1− DCs and focused exclusively on the DNGR-1+ BDCA3+ subset. Nevertheless, we believe that it may eventually be possible to use the same or a similar culture system to generate CD8α− DCs equivalents, thereby permitting a direct comparison between the various subsets in humans. In the absence of a CD8α− DCs comparator, we compared DNGR-1+ BDCA3+ to Mo-DCs. The latter are the most used cell type in studies of human DC biology, including studies of endocytic ability (Sallusto et al., 1995), and constitute the gold standard for cross-presentation studies in humans (Albert et al., 1998). We show that DNGR-1+ BDCA3+ DCs compare favorably to Mo-DCs in properties such as uptake of dead cells and cross-presentation of exogenous antigens to CD8+ T cells. This is reminiscent of the situation with mouse CD8α+ DCs, which are equal or superior to mouse bone marrow GM-CSF–derived DCs at cross-presenting exogenous antigens to CD8+ T cells. However, the APC functions of DNGR-1+ BDCA3+ DCs will need to be explored further, notably with regards to the ability to cross-present dead cell-associated antigens, as well as present antigens to MHC class II–restricted CD4+ T cells. We have not yet been able to obtain sufficient numbers of cells to perform those experiments in a satisfactory manner. Further work on validating the antigen-presenting properties of DNGR-1+ BDCA3+ DCs and the optimization of protocols for growing these cells in vitro may eventually allow for their use in antigen pulsing and adoptive transfer immunotherapy approaches, analogous to those currently using Mo-DCs.
Innate recognition pathways are key controllers of DCs function, and the repertoire of TLR expression by DC subsets can act as a determinant of their properties. Like mouse CD8α+ DCs, human DNGR-1+ BDCA3+ can express TLR3, but not TLR7, and can respond to poly I:C, but not imiquimod. pDCs show a reciprocal pattern of response (Fig. 5 A), a fact that could be exploited in vaccination strategies designed to mobilize one and/or the other cell type. Interestingly, human DNGR-1+ BDCA3+ are not identical to mouse CD8α+ DCs in regard to TLR9 expression, and it will be important to determine whether this might limit the effectiveness of CpG DNA oligonucleotides currently being tested as adjuvants in humans (Daubenberger, 2007). Nevertheless, the ability of DNGR-1+ BDCA3+ DCs to respond to TLR8 agonists suggests that RNA-based adjuvants would be useful alternatives for use in vaccination protocols aimed at targeting these cells in vivo. Interestingly, although TLR triggering was sufficient to promote secretion of IL-6 and TNF by DNGR-1+ BDCA3+ DCs, it was not sufficient to induce IL-12 p70 production, even when given together with cytokines known to up-regulate the limiting IL-12 p35 subunit (Hochrein et al., 2000). In our experiments, production of IL-12 p70 by DNGR-1+ BDCA3+ DCs required additional feedback signals from T cells, reminiscent of the situation with mouse CD8α+ DCs (Schulz et al., 2000). Notably, when normalized for DC number, the levels of secreted IL-12 p70 represented 5 fg/ml/cell, which is comparable to the levels obtained from mouse CD8α+ DCs or from human thymic DCs (Hochrein et al., 2000). Thus, DNGR-1+ BDCA3+ DCs could act as efficient producers of IL-12 in vivo upon appropriate stimulation.
Mouse CD8α+ DCs have been reported to be restricted to the thymus and secondary lymphoid tissues. In contrast, BDCA3+ cells have also been found in human bronchioalveolar lavage fluid, lung, tonsils, dermis, decidua, and kidney (Demedts et al., 2005; Lindstedt et al., 2005; Narbutt et al., 2006; Tsoumakidou et al., 2006; Ban et al., 2008; Fiore et al., 2008). However, Batf3-dependent CD11b− CD103+ Langerin+ DCs related to CD8α+ DCs have recently been identified in mouse peripheral tissues (Bedoui et al., 2009; Ginhoux et al., 2009; Edelson et al., 2010; Henri et al., 2010). Thus, there is a growing feeling that functional equivalents of CD8α+ DCs in mouse and in other species may not necessarily be restricted to lymphoid tissues. In this regard, analysis of the distribution of DNGR-1+ BDCA3hi DCs in normal and pathological human specimens may yet prove very informative.
MATERIALS AND METHODS
Human tissue.
Cell suspensions of human cadaveric spleen from healthy victims of traffic accidents (organ donors) were prepared <8 h post mortem by mechanical dissociation followed by Ficoll density centrifugation. Cells were frozen and stored in liquid nitrogen until used. The procedure was approved by the ULB-Erasme Ethics Committee of Hospital Erasme, Brussels, Belgium. CB was collected from mothers attending the Royal London Hospital, London, UK, after informed consent through a protocol approved by the East London and City Research Ethics Committee. Mononuclear cells were obtained by Ficoll density centrifugation and ammonium chloride red cell lysis. They were depleted for lineage marker positive cells using the StemSep system (STEMCELL Technologies Inc.) to generate Lin− HSCs/HPCs.
Humanized mice.
All animal protocols were approved by the London Research Institute Ethics Committee and were performed under the authority of a project license granted by the UK Home Office, in accordance with UK governmental regulations (Animal Scientific Procedures Act 1986). NOD/SCID/β2microglobulin-null mice and NOD/SCID/IL-2Rγ-null mice were bred at Charles Rivers Laboratories, housed in microisolators, and fed sterile food and acidified water. Mice aged 8–12 wk were sublethally irradiated (3.75 Gy) up to 24 h before i.v. injection of 50,000 Lin− human CB cells. Mice were analyzed 8–24 wk after reconstitution. Spleen cells were prepared by digestion with liberase and DNase, followed in some cases by an OptiPrep gradient to enrich for low-density cells (Sigma-Aldrich).
In vitro–differentiated DCs.
Human Lin− CB cells differentiated into DCs using a two-step protocol. In the first step (amplification), Lin− cells were cultured at 5 × 104 cells/ml in StemSpan serum-free medium (STEMCELL Technologies Inc.) with penicillin, streptomycin, 100 ng/ml SCF, 100 ng/ml Flt3L, 20 ng/ml IL-3, and 20 ng/ml IL-6 (R&D Systems). After 7–11 d of culture, the expanded cells were frozen until further use or were used immediately. In the second step (differentiation), 6.25 × 104 cells/ml were plated in RPMI 1640 supplemented with glutamine, penicillin, streptomycin, 2-ME (all from Invitrogen), 10% heat-inactivated fetal calf serum (Autogen Bioclear), 20 ng/ml SCF, 20 ng/ml GM-CSF, 20 ng/ml IL-4, and 100 ng/ml Flt3L (R&D Systems). Cultures were kept for 12–14 d and cytokines were replenished after 6–7 d. Monocyte-derived DCs were prepared as previously described (Sallusto et al., 1995; Salio et al., 2001).
Antibodies.
Anti-HLA-DR (L243), anti–Lineage-1 cocktail (CD3, CD14, CD16, CD19, CD20, and CD56), anti-BDCA-3/CD141 (1A4), anti-CD123 (7G3), anti-CD11c (B-ly6), anti-CD80 (L307.4), anti-CD8α (RPA-T8), anti-CD14 (M5E2), anti-CD3 (UCHT1), anti-CD16 (3G8), anti-CD19 (HIB19), and anti-CD56 (B159) were purchased from BD. Anti-BDCA-2/CD303 (AC144), anti-BDCA-1/CD1c (AD5-8E7), anti-CD83 (HB15), anti-EpCam/CD326 (HEA-125), and anti-CCR7/CD197 (FR11-11E8) were obtained from Miltenyi Biotec. Anti-CD1a (201B5.08) was purchased from Dendritics. Anti-CD45RO (UCHL1), anti-CD103 (B-Ly7), anti-CD40 (5C3), anti-CD11b (ICRF44), anti-CD20 (2H7), and isotype-matched control antibodies were purchased from eBioscience. Anti-hDNGR-1 was described previously (Sancho et al., 2008). All antibodies were tested for staining against appropriate positive controls.
Flow cytometry and cell sorting.
Cells were preincubated on ice with mouse serum (Jackson ImmunoResearch Laboratories) and purified IgG2a (BD) to block Fc receptors, and then stained with appropriate antibody combinations. To avoid cell clumping, primary human spleen cell suspensions were kept in DNase-containing buffer during staining. Multiparameter analysis was performed on an LSRII (BD) or FACSAria (BD) flow cytometer. Dead cells were excluded by a combination of scatter gating and DAPI exclusion. Analysis was performed using FlowJo software (Tree Star, Inc.). For cell sorting (FACSAria), primary human spleen cell suspensions, in vitro generated DCs, or low-density cells from humanized mice were stained and live Lin− HLA-DR+ cells were sorted into the indicated subsets.
Stimulation with TLR agonists.
Sorted DCs populations were cultured at 105 cells/ml with selected TLR agonists (Invivogen) used at predetermined optimal concentrations. Cytokine accumulation in supernatants was measured after 16 h using a Cytometric Bead Array (BD). Bulk CBDCs were stimulated for 16 h with varying concentrations of poly I:C or TLR7 agonists for assessment of cytokine secretion or HLA-DR up-regulation. For IL-12 p70, sorted HLA-A2–expressing DCs populations were cultured at 5 × 103 cells/well in 100 µl with a mixture of TLR 1–9 agonists (human TLR agonist 1–9; Invivogen; used at predetermined optimal concentrations), IL-4 (10 ng/ml), and IFN-γ (10 ng/ml; R&D Systems), in the presence or absence of 5 × 104 MelanA-specific HLA-A2–restricted CD8+ T cells and 1 µM of MelanA/MART-1 short peptide.
RNA isolation and quantitative RT-PCR.
RNA from FACS-sorted DCs subsets was extracted with an RNeasy Micro kit and treated with DNase I, according to the manufacturer’s protocol (QIAGEN). cDNA was synthesized from total RNA with random hexamer primers and Superscript II RT (Invitrogen). Quantitative PCR was performed with Taqman Universal PCR MasterMix (Applied Biosystems) and predesigned primers and probe mixes (Taqman Gene Expression Assays; Applied Biosystems). Measurements were performed using a sequence detection system (ABI PRISM 7700; Applied Biosystems). Levels of mRNA for the specific gene being measured were divided by those for GAPDH measured in parallel (normalized expression).
Uptake of dead cells.
Human melanoma cells were UV irradiated (2,400 J/cm2), incubated for 8 h at 37°C to allow apoptosis and secondary necrosis, and labeled with CFSE (for flow cytometry) or Alexa Fluor 633-SE (for confocal microscopy). Dead cells were added to 5 × 104 CBDCs or Mo-DCs at different ratios for 2 h at 4°C or 37°C. For confocal microscopy, cells were subsequently plated on fibronectin-coated coverslips for 15 min, fixed in 3.7% paraformaldehyde/PBS for 10 min, permeabilized in 0.1% Triton X-100/PBS for 3 min, blocked with 5% mouse serum, and stained for DNGR-1 using Alexa Fluor 546–coupled antibody. Coverslips were mounted in Fluoromount-G and imaged with a laser scanning confocal microscope (Axiovert 100M LSM 510; Carl Zeiss, Inc.) with a 63× Plan-Apochromat NA 1.4 oil objective. For flow cytometric analysis, cells were stained for DNGR-1 and BDCA3 and the percentage of CFSE+ DNGR-1+ BDCA3+ cells was calculated by subtracting the frequency of positive events at 4°C (binding) from the frequency at 37°C (binding + uptake).
Antigen presentation assays.
NY-ESO-1157–165 peptide (Chen et al., 2000) or NY-ESO-1 full-length protein (provided by the Ludwig Institute of Cancer Research, New York, NY) and the MelanA/MART-1 short (ELAGIGILTV) or long (KGHGHSYTTAEEAAGIGILTVILGVL) peptides were used as antigen sources. Antigen presentation assays were performed as previously described (Salio et al., 2001; Faure et al., 2009) using HLA-A2–restricted NY-ESO-1– or MelanA-specific CD8+ T cells.
Online supplemental material.
Table S1 show a summary of the analyses of human spleen DCs subsets in three different donors. Fig. S1 shows the phenotype of different DCs populations from human spleen defined on the basis of CD11c versus DNGR-1. Fig. S2 shows that DNGR-1+ BDCA3+ CB-derived DCs require Flt3L, GM-CSF, IL-4, and SCF to develop in vitro and do not express CD14. Fig. S3 demonstrates the differential response of bulk CBDC to poly I:C and imiquimod at the level of TNF production and HLA-DR up-regulation. Fig. S4 compares bulk CBDC and Mo-DCs for the ability to (cross-)present NY-ESO protein or peptide to antigen-specific T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092618/DC1.
Acknowledgments
We thank the FACS Laboratory and Equipment Park of the London Research Institute for technical support and Biological Resources for the care of the animals used in these studies. We are grateful to members of the Immunobiology Laboratory, Cancer Research UK for advice and discussions.
This work was funded by Cancer Research UK (core London Research Institute support to C. Reis e Sousa and D. Bonnet; grant C399/A2291 to V. Cerundolo) and by the Oxford Biomedical Research Centre Cancer Theme. L.F. Poulin is supported by a Marie-Curie Intra-European Fellowship (project number 235732). C. Reis e Sousa acknowledges additional financial support in the form of a prize from Fondation Bettencourt-Schueller.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BDCA
- blood DC antigen
- CB
- cord blood
- CBDC
- CB-derived DC
- cDC
- conventional DC
- HSC/HPC
- hematopoietic stem cells/hematopoietic progenitor cell
- Mo-DC
- monocyte-derived DC
- pDC
- plasmacytoid DC
- TLR
- Toll-like receptor
References
- Albert M.L., Sauter B., Bhardwaj N. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89 10.1038/32183 [DOI] [PubMed] [Google Scholar]
- Ban Y.-L., Kong B.-H., Qu X., Yang Q.-F., Ma Y.-Y. 2008. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin. Exp. Immunol. 151:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., et al. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488–495 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- Bursch L.S., Wang L., Igyarto B., Kissenpfennig A., Malissen B., Kaplan D.H., Hogquist K.A. 2007. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204:3147–3156 10.1084/jem.20071966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I., Proietto A.I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J.C., Rizzitelli A., Wu L., Vremec D., van Dommelen S.L., et al. 2008. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 112:3264–3273 10.1182/blood-2008-05-155176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Dunbar P.R., Gileadi U., Jäger E., Gnjatic S., Nagata Y., Stockert E., Panicali D.L., Chen Y.T., Knuth A., et al. 2000. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J. Immunol. 165:948–955 [DOI] [PubMed] [Google Scholar]
- Chen J.L., Stewart-Jones G., Bossi G., Lissin N.M., Wooldridge L., Choi E.M., Held G., Dunbar P.R., Esnouf R.M., Sami M., et al. 2005. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J. Exp. Med. 201:1243–1255 10.1084/jem.20042323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens P.D., Melkus M.W., Padgett-Thomas A., Islas-Ohlmayer M., Del P Martin M., Garcia J.V. 2005. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells. 23:264–278 10.1634/stemcells.2004-0116 [DOI] [PubMed] [Google Scholar]
- Daubenberger C.A. 2007. TLR9 agonists as adjuvants for prophylactic and therapeutic vaccines. Curr. Opin. Mol. Ther. 9:45–52 [PubMed] [Google Scholar]
- Delamarre L., Holcombe H., Mellman I. 2003. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 198:111–122 10.1084/jem.20021542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts I.K., Brusselle G.G., Vermaelen K.Y., Pauwels R.A. 2005. Identification and characterization of human pulmonary dendritic cells. Am. J. Respir. Cell Mol. Biol. 32:177–184 10.1165/rcmb.2004-0279OC [DOI] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- Dudziak D., Kamphorst A.O., Heidkamp G.F., Buchholz V.R., Trumpfheller C., Yamazaki S., Cheong C., Liu K., Lee H.W., Park C.G., et al. 2007. Differential antigen processing by dendritic cell subsets in vivo. Science. 315:107–111 10.1126/science.1136080 [DOI] [PubMed] [Google Scholar]
- Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D.W., Schmitz J. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037–6046 [DOI] [PubMed] [Google Scholar]
- Edelson B.T., Kc W., Juang R., Kohyama M., Benoit L.A., Klekotka P.A., Moon C., Albring J.C., Ise W., Michael D.G., et al. 2010. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207:823–836 10.1084/jem.20091627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.D., Diebold S.S., Slack E.M., Tomizawa H., Hemmi H., Kaisho T., Akira S., Reis e Sousa C. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33:827–833 10.1002/eji.200323797 [DOI] [PubMed] [Google Scholar]
- Faure F., Mantegazza A., Sadaka C., Sedlik C., Jotereau F., Amigorena S. 2009. Long-lasting cross-presentation of tumor antigen in human DC. Eur. J. Immunol. 39:380–390 10.1002/eji.200838669 [DOI] [PubMed] [Google Scholar]
- Fiore N., Castellano G., Blasi A., Capobianco C., Loverre A., Montinaro V., Netti S., Torres D., Manno C., Grandaliano G., et al. 2008. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 45:259–265 10.1016/j.molimm.2007.04.029 [DOI] [PubMed] [Google Scholar]
- Galibert L., Diemer G.S., Liu Z., Johnson R.S., Smith J.L., Walzer T., Comeau M.R., Rauch C.T., Wolfson M.F., Sorensen R.A., et al. 2005. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J. Biol. Chem. 280:21955–21964 10.1074/jbc.M502095200 [DOI] [PubMed] [Google Scholar]
- Gilliet M., Cao W., Liu Y.J. 2008. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8:594–606 10.1038/nri2358 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. 2009. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206:3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W.R., Carbone F.R. 2009. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10:1237–1244 10.1038/ni.1822 [DOI] [PubMed] [Google Scholar]
- Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529 10.1126/science.1093620 [DOI] [PubMed] [Google Scholar]
- Henri S., Poulin L.F., Tamoutounour S., Ardouin L., Guilliams M., de Bovis B., Devilard E., Viret C., Azukizawa H., Kissenpfennig A., Malissen B. 2010. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207:189–206: S1–S6 10.1084/jem.20091964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K., Edelson B.T., Purtha W.E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B.U., Unanue E.R., Diamond M.S., et al. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 322:1097–1100 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrein H., O’Keeffe M., Luft T., Vandenabeele S., Grumont R.J., Maraskovsky E., Shortman K. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192:823–833 10.1084/jem.192.6.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysamen C., Willment J.A., Dennehy K.M., Brown G.D. 2008. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J. Biol. Chem. 283:16693–16701 10.1074/jbc.M709923200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Swiggard W.J., Inaba M., Meltzer J., Mirza A., Sasagawa T., Nussenzweig M.C., Steinman R.M. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 163:148–156 10.1006/cimm.1995.1109 [DOI] [PubMed] [Google Scholar]
- Inaba K., Turley S., Iyoda T., Yamaide F., Shimoyama S., Reis e Sousa C., Germain R.N., Mellman I., Steinman R.M. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927–936 10.1084/jem.191.6.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda T., Shimoyama S., Liu K., Omatsu Y., Akiyama Y., Maeda Y., Takahara K., Steinman R.M., Inaba K. 2002. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195:1289–1302 10.1084/jem.20020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O.P., Sancho D., Zelenay S., Keller A.M., Reis e Sousa C. 2010. Efficient and versatile manipulation of the peripheral CD4(+) T cell compartment by antigen targeting to DNGR-1 / CLEC9A. Eur. J. Immunol. 40:1255–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K., et al. 2008. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 29:497–510 10.1016/j.immuni.2008.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt M., Lundberg K., Borrebaeck C.A. 2005. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 175:4839–4846 [DOI] [PubMed] [Google Scholar]
- Luber C.A., Cox J., Lauterbach H., Fancke B., Selbach M., Tschopp J., Akira S., Wiegand M., Hochrein H., O’Keeffe M., Mann M. 2010. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 32:279–289 10.1016/j.immuni.2010.01.013 [DOI] [PubMed] [Google Scholar]
- MacDonald K.P., Munster D.J., Clark G.J., Dzionek A., Schmitz J., Hart D.N. 2002. Characterization of human blood dendritic cell subsets. Blood. 100:4512–4520 10.1182/blood-2001-11-0097 [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Brasel K., Teepe M., Roux E.R., Lyman S.D., Shortman K., McKenna H.J. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953–1962 10.1084/jem.184.5.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy D., Troadec C., Grassi F., Samri A., Barrou B., Autran B., Debré P., Feuillard J., Hosmalin A. 2001. Investigation of human spleen dendritic cell phenotype and distribution reveals evidence of in vivo activation in a subset of organ donors. Blood. 97:3470–3477 10.1182/blood.V97.11.3470 [DOI] [PubMed] [Google Scholar]
- Merad M., Manz M.G. 2009. Dendritic cell homeostasis. Blood. 113:3418–3427 10.1182/blood-2008-12-180646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S.H. 2008. Demystifying the development of dendritic cell subtypes, a little. Immunol. Cell Biol. 86:439–452 10.1038/icb.2008.28 [DOI] [PubMed] [Google Scholar]
- Naik S.H., Proietto A.I., Wilson N.S., Dakic A., Schnorrer P., Fuchsberger M., Lahoud M.H., O’Keeffe M., Shao Q.X., Chen W.F., et al. 2005. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174:6592–6597 [DOI] [PubMed] [Google Scholar]
- Narbutt J., Lesiak A., Sysa-Jedrzejowska A., Smolewski P., Robak T., Zalewska A. 2006. The number and distribution of blood dendritic cells in the epidermis and dermis of healthy human subjects. Folia Histochem. Cytobiol. 44:61–63 [PubMed] [Google Scholar]
- Porcelli S., Morita C.T., Brenner M.B. 1992. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 360:593–597 10.1038/360593a0 [DOI] [PubMed] [Google Scholar]
- Robbins S.H., Walzer T., Dembélé D., Thibault C., Defays A., Bessou G., Xu H., Vivier E., Sellars M., Pierre P., et al. 2008. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 9:R17 10.1186/gb-2008-9-1-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M., Shepherd D., Dunbar P.R., Palmowski M., Murphy K., Wu L., Cerundolo V. 2001. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J. Immunol. 167:1188–1197 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389–400 10.1084/jem.182.2.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D., Mourão-Sá D., Joffre O.P., Schulz O., Rogers N.C., Pennington D.J., Carlyle J.R., Reis e Sousa C. 2008. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Invest. 118:2098–2110 10.1172/JCI34584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G., Mattei F., Sestili P., Borghi P., Venditti M., Morse H.C., III, Belardelli F., Gabriele L. 2002. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196:1415–1425 10.1084/jem.20021263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O., Reis e Sousa C. 2002. Cross-presentation of cell-associated antigens by CD8α+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 107:183–189 10.1046/j.1365-2567.2002.01513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O., Edwards A.D., Schito M., Aliberti J., Manickasingham S., Sher A., Reis e Sousa C. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462 10.1016/S1074-7613(00)00045-5 [DOI] [PubMed] [Google Scholar]
- Shortman K., Heath W.R. 2010. The CD8+ dendritic cell subset. Immunol. Rev. 234:18–31 10.1111/j.0105-2896.2009.00870.x [DOI] [PubMed] [Google Scholar]
- Steinman R.M. 2008. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 29:319–324 10.1016/j.immuni.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Takahara K., Omatsu Y., Yashima Y., Maeda Y., Tanaka S., Iyoda T., Clausen B.E., Matsubara K., Letterio J., Steinman R.M., et al. 2002. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int. Immunol. 14:433–444 10.1093/intimm/14.5.433 [DOI] [PubMed] [Google Scholar]
- Tsoumakidou M., Tzanakis N., Papadaki H.A., Koutala H., Siafakas N.M. 2006. Isolation of myeloid and plasmacytoid dendritic cells from human bronchoalveolar lavage fluid. Immunol. Cell Biol. 84:267–273 10.1111/j.1440-1711.2006.01428.x [DOI] [PubMed] [Google Scholar]
- Velásquez-Lopera M.M., Correa L.A., García L.F. 2008. Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes. Clin. Exp. Immunol. 154:107–114 10.1111/j.1365-2249.2008.03734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J.A., Schnorrer P. 2007. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7:543–555 10.1038/nri2103 [DOI] [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D.J., Ardavin C.F., Wu L., Shortman K. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176:47–58 10.1084/jem.176.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M.D., Swiggard W.J., Mirza A., Inaba K., Steinman R.M. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. II. Expression in situ in lymphoid and nonlymphoid tissues. Cell. Immunol. 163:157–162 10.1006/cimm.1995.1110 [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Dudziak D., Heidkamp G.F., Fiorese C., Bonito A.J., Inaba K., Nussenzweig M.C., Steinman R.M. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181:6923–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]