Abstract
The Southern house mosquito, Culex quinquefasciatus, is an important human health pest as a vector of several pathogens, including agents of lymphatic filariasis and arboviruses like West Nile virus. We conducted preliminary experiments in Recife, Brazil, to explore applications of Culex oviposition attractants in combination with Bacillus thuringiensis variety israelensis (Bti) in an attract- and-kill approach. Simple, cost-effective oviposition traps, BR-OVT, loaded with Bti and baited with or without attractant, were deployed in 10 homes for 30 d in 2 consecutive yr. Significantly higher numbers of egg rafts were deposited in traps baited with skatole or infusion than the control water traps. In the first year, 2006, significantly higher numbers of eggs were deposited in infusion-baited traps, particularly in the first 15 d of the experiment, than in skatole traps, but in the following year no significant difference was observed between synthetic and natural attractants. The tests strongly demonstrate that skatole or infusion can be used to enhance the number of egg rafts deposited on Bti-treated oviposition traps.
Keywords: Bacillus thuringiensis variety israelensis, skatole, grass infusion, West Nile virus, ovitrap
Environmentally friendly strategies for controlling Culex mosquito populations are sorely needed, as they transmit pathogens that cause human diseases throughout the world, including filariasis and various types of encephalitis. In the United States, Culex mosquitoes spread West Nile virus while feeding on birds and humans. Culex quinquefasciatus resistance to pyrethroids in Africa (Chandre et al. 1998) is a significant hindrance to mitigate malaria with insecticide-treated net programs. Pyrethroid-treated nets provide limited protection against blood feeding by Cx. quinquefasciatus (Kulkarni et al. 2007, Irish et al. 2008), and this may lead to reduced public acceptance and effectiveness of the insecticide-treated nets for Anopheles (Kulkarni et al. 2007, Irish et al. 2008). Indeed, in many villages in Africa, local people unable to distinguish between Anopheles and Culex bites rush to the conclusion that the malaria control program is not working (A. J. Cornel, personal communication). Therefore, novel strategies for reducing biting by Culex mosquitoes would have broader impacts in medical entomology, including mitigation of malaria and some of the so-called neglected diseases. As with integrated pest management in agriculture, integrated vector management programs (Anonymous 2003), including insecticide sprays, semiochemical-based strategies, biological control, and other strategies, may lead to more effective management of Culex mosquito populations.
Larval development is a particularly vulnerable point in the life cycle of Culex mosquitoes, as hundreds of larvae confined to small areas become susceptible to a number of biotic and abiotic factors, including predation and microbial infections. In theory, one should be able to attract gravid mosquitoes to lay eggs in water pools treated with agents toxic to eggs or offspring, a strategy known as attract and kill. A number of Culex mosquito oviposition attractants have been identified, including the mosquito oviposition pheromone, (5R,6S)-6-acetoxy-5-hexadecanolide (Laurence and Pickett 1982), skatole (Millar et al. 1992, Beehler et al. 1994, Olagbemiro et al. 2004, Leal et al. 2008), nonanal (Du and Millar 1999, Leal et al. 2008), trimethylamine (Leal et al. 2008), etc. The efficacy of the environmentally friendly biological control agent Bacillus thuringiensis variety israelensis (Bti) as mosquito larvicide has already been demonstrated (Mulla et al. 1985). Earlier attempts to employ a combination of Culex oviposition attractants and Bti may have been discouraged by early reports demonstrating that in the laboratory Culex mosquitoes lay significantly less egg rafts in Bti-treated water than in untreated water (Zahiri and Mulla 2005). However, subsequent reports have proved quite promising, as various wild Aedes species were found to lay more eggs in Bti treated than water traps (Santos et al. 2003, Stoops 2005), and in fact, field tests strongly suggest that Bti itself may act as an attractant for Aedes mosquitoes (Carrieri et al. 2009). This dichotomy prompted us to conduct field experiments to determine whether Cx. quinquefasciatus egg laying in Bti-treated water traps could be enhanced with attractants. As a result of logistic limitations, we did not address the question as to whether Bti attracts or repells gravid Culex mosquitoes, but focused on a more important question, i.e., whether there is a potential use of oviposition attractants in attract-and-kill system. In this preliminary work, we compared the effects of infusion and a synthetic attractant, skatole, and observed significantly higher egg laying in traps baited with either infusion or skatole than in control traps, thus suggesting that these attractants and biopesticide agent may be used in attract-and-kill strategy for reducing Culex mosquito abundance.
Materials and Methods
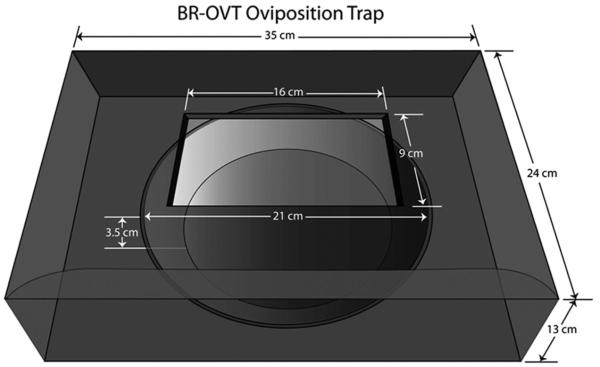
Our study was conducted during the rainy seasons of 2006 and 2009 using a newly developed, cost-effective ovitrap, BR-OVT (Fig. 1). This trap consisted of a simple, black plastic box (35 × 24 × 13 cm) with an entrance (16 × 9 cm) on the top and a black circular tray (internal diameter, 21 cm; 3.5 cm high) (Barbosa et al. 2007) in which both attractants and biological agent were loaded. Traps were deployed in 10 houses in Mustardinha, a neighborhood in Recife, Brazil, selected on the basis of year-round indoor infestation by Cx. quinquefasciatus. Three traps loaded with a commercial Bti formulation (Vectobac CG, Valent Biosciences, Libertyville, IL) at 0.45 mg/L were deployed in the backyard of each home with an intertrap distance of at least 1.5 m. This is 30× the LC50 (0.015 mg/L) for Bti against Recife populations of Cx. quinquefasciatus (Pei et al. 2002). Two of the traps were filled with 800 ml of tap water, one without attractant (control trap) and the other loaded with 100 μg of skatole in ethanol (Sigma-Aldrich, St. Louis, MO), whereas the third type of trap was loaded with 800 ml of a fermentation product (hereafter referred to as infusion or grass infusion) obtained by adding 30 g of fresh Eleusine indica (L.) Gaertn. (Poaceae) to 2 liters of tap water, which was kept at 27 ± 2°C for 7 d (Barbosa et al. 2007). In all experiments, traps were inspected every 3–4 d for a period of 30 d; egg rafts were removed, counted, and destroyed; and the traps were rotated. Previously, we confirmed that all the mosquitoes attracted to the traps in these locations were Cx. quinquefasciatus by morphological examination and polymerase chain reaction assay (Leal et al. 2008). Therefore, for this study egg rafts were not kept and hatched for species identification. In year 1 (August to September 2006), 15 d after the beginning of tests, traps were reloaded with fresh samples of infusion, Bti, and skatole. Experiments in year 2 (June 2007) were performed in the same homes, but the trap contents were not changed during the entire duration (30 d) of the tests. Data representing the number of egg rafts collected per inspection per trap were transformed to log (x + 1). All statistical analyses were based on transformed data, but untransformed means are presented. Data were analyzed by analysis of variance and compared by Tukey’s HSDs at 5% probability using KaleidaGraph (Synergy Software, Reading, PA).
Fig. 1.
Schematic view of the BR-OVT oviposition trap. Both oviposition attractant and biological agent (Bti) were loaded on the black tray, which was then placed inside the trap, just beneath the trap entrance.
Results and Discussion
A total of 1,475 egg rafts, comprising an estimated 312,000 eggs, was deposited in the 30 BR-OVT traps deployed in 10 residences during 30 d in 2006, suggesting removal of over 1,000 eggs per home per day. The following year, traps deployed in the same homes collected one-third less (588) egg rafts in 30 d. These results encourage us to conduct future evaluations to ascertain whether these attract-and-kill systems, particularly if loaded with odorless formulations (Leal et al. 2008), reduce mosquito populations and indoor biting frequencies.
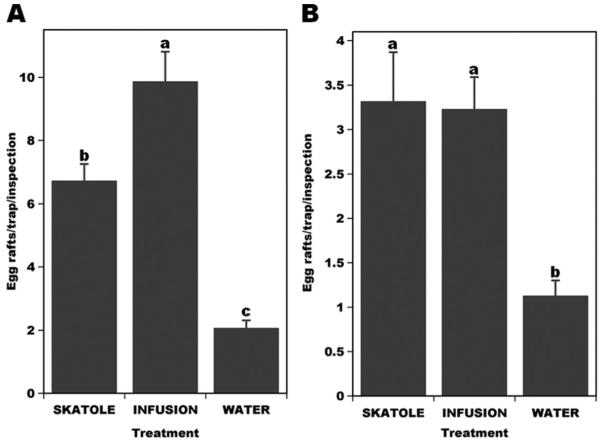
Throughout these experiments, traps baited with skatole and infusion captured significantly more egg rafts than traps devoid of attractants, i.e., water traps (Fig. 2, A and B). In 2006, the number of egg rafts deposited in infusion-baited traps was significantly higher than those in skatole-baited traps (Fig. 2A), but in 2007 there was no significant difference between the number of eggs laid in infusion- and skatole-baited traps (Fig. 2B). Intriguingly, infusion-baited traps out-performed skatole-baited traps by 3-fold in the first half of the experiments in 2006 (infusion, 13.97 ± 1.62a egg rafts per trap per inspection; skatole, 4.47 ± 0.5b; water, 1.67 ± 0.32c; treatments labeled with the same letters are not significantly different by Tukey’s HSD at 5% probability). However, skatole- and infusion-baited traps performed equally well in the second part of the experiments conducted with fresh trap contents (infusion, 10.12 ± 0.98a egg rafts per trap per inspection; skatole, 9 ± 0.78a; water, 2.47 ± 0.34b). This difference between the synthetic and natural attractants in the two phases of the experiment may be related to heterogeneity of infusion preparations. In the second year, without changing trap contents during the experiment, skatole- and infusion-baited traps performed equally well in the first and second part of the experiment (first 15 d; infusion, 3.5 ± 0.56a egg rafts per trap per inspection; skatole, 2.67 ± 0.45a; water, 0.8 ± 0.15b; last 15 d; infusion, 3.14 ± 0.47a egg rafts per trap per inspection; skatole, 4.47 ± 1.05a; water, 1.5 ± 0.29b).
Fig. 2.
Culex mosquito egg rafts deposited in traps containing Bti in combination with skatole, infusion, or water only (control traps). Traps were inspected every 3–4 d. (A) The number of egg rafts laid on skatole-baited traps in 2006 was significantly higher than those in control traps, but overall captures in infusion-baited traps were significantly higher than in skatole traps. (B) Lower numbers of egg rafts were deposited in 2007; there was no significant difference between natural and synthetic attractants, but both types performed significantly better than the control traps. Treatments labeled with the same letters are not significantly different (Tukey’s HSD, 5% probability).
These experiments clearly indicated that oviposition attractants, such as infusion and skatole, enhanced egg laying by Cx. quinquefasciatus in traps when loaded with the biological agent, Bti. We did not address, however, the question of whether Culex mosquito egg laying in traps baited with Bti was reduced or enhanced. Initially, we planned to extend the experiments to test the effect of Bti on egg laying, but this would require deployment of at least one more treatment, i.e., a negative control trap with water only, with a trade-off between addressing this question and compromising intertrap distance. Whether Bti itself deters or inhibits Culex oviposition remains to be investigated, but more importantly, the current experiments clearly indicate that the addition of an oviposition attractant to Bti traps do enhance egg laying and potentially serves as an effective attract-and-kill control strategy.
Further detailed experiments are required to determine unambiguously whether trapping Culex eggs in Bti-baited traps would affect mosquito abundance in home dwellings. The low cost of the BR-OVT traps and the availability of the oviposition attractants described in this study may facilitate attempts to reduce mosquito abundance by attract and kill in combination with other integrated vector management-based strategies to control Culex populations.
Acknowledgments
We thank Zainulabeuddin Syed, William Reisen, and Anthony Cornel (University of California, Davis) for their critique of an earlier version of the manuscript. This work was supported in part by the National Institutes of Health (National Institute of Allergy and Infectious Diseases).
References Cited
- Anonymous Guidelines for integrated vector management. 2003 http://www.afro.who.int/vbc/framework-guidelines/guide_integrated_vector_management.pdf.
- Barbosa RMR, Souto A, Eiras AE, Regis L. Laboratory and field evaluation of an oviposition trap for Culex quinquefasciatus (Diptera: Culicidae) Mem. Inst. Oswaldo Cruz. 2007;102:523–529. doi: 10.1590/s0074-02762007005000058. [DOI] [PubMed] [Google Scholar]
- Beehler JW, Millar JG, Mulla MS. Field evaluation of synthetic compounds mediating oviposition in Culex mosquitoes (Diptera: Culicidae) J. Chem. Ecol. 1994;20:281–291. doi: 10.1007/BF02064436. [DOI] [PubMed] [Google Scholar]
- Carrieri M, Masetti A, Albieri A, Maccagnani B, Bellini R. Larvacidal activity and influence of Bacillus thuringiensis var. insraelensis on Aedes albopictus oviposition in ovitraps during a two-week check interval protocol. J. Am. Mosq. Control Assoc. 2009;25:149–155. doi: 10.2987/08-5852.1. [DOI] [PubMed] [Google Scholar]
- Chandre F, Darriet F, Darder M, Cuany A, Doannio JM, Pasteur N, Guillet P. Pyrethroid resistance in Culex quinquefasciatus from west Africa. Med. Vet. Entomol. 1998;12:359–66. doi: 10.1046/j.1365-2915.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- Du YJ, Millar JG. Electroantennogram and oviposition bioassay responses of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) to chemicals in odors from Bermuda grass infusions. J. Med. Entomol. 1999;36:158–166. doi: 10.1093/jmedent/36.2.158. [DOI] [PubMed] [Google Scholar]
- Irish SR, Guessan RN, Boko PM, Metonnou C, Odjo A, Akogbeto M, Rowland M. Loss of protection with insecticide-treated nets against pyrethroid-resistant Culex quinquefasciatus mosquitoes once nets become holed: an experimental hut study. Parasites & Vectors. 2008;1:17. doi: 10.1186/1756-3305-1-17. doi: 10.1186/1756-3305-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni MA, Malima R, Mosha FW, Msangi S, Mrema E, Kabula B, Lawrence B, Kinung’hi S, Swilla J, Kisinza W, Rau ME, Miller JE, Schellenberg JA, Maxwell C, Rowland M, Magesa S, Drakeley C. Efficacy of pyrethroid-treated nets against malaria vectors and nuisance-biting mosquitoes in Tanzania in areas with long-term insecticide-treated net use. Trop. Med. Int. Health. 2007;12:1061–73. doi: 10.1111/j.1365-3156.2007.01883.x. [DOI] [PubMed] [Google Scholar]
- Laurence BR, Pickett JA. Erythro-6-acetoxy-5-hexadecanolide, the major compound of a mosquito attractant pheromone. J. Chem. Soc. Chem. Commun. 1982:59–60. 1982. [Google Scholar]
- Leal WS, Barbosa RM, Xu W, Ishida Y, Syed Z, Latte N, Chen AM, Morgan TI, Cornel AJ, Furtado A. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS One. 2008;3:e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JG, Chaney JD, Mulla MS. Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. J. Am. Mosq. Control Assoc. 1992;8:11–7. [PubMed] [Google Scholar]
- Mulla MS, Darwazeh HA, Ede L, Kennedy B, Dulmage HT. Efficacy and field evaluation of Bacillus thuringiensis (H-14) and B. sphaericus against floodwater mosquitoes in California. J. Am. Mosq. Control Assoc. 1985;1:310–315. [PubMed] [Google Scholar]
- Olagbemiro TO, Birkett MA, Mordue Luntz AJ, Pickett JA. Laboratory and field responses of the mosquito, Culex quinquefasciatus, to plant-derived Culex spp. oviposition pheromone and the oviposition cue skatole. J. Chem. Ecol. 2004;30:965–976. doi: 10.1023/b:joec.0000028461.86243.19. [DOI] [PubMed] [Google Scholar]
- Pei G, Oliveira CM, Yuan Z, Nielsen-LeRoux C, Silva-Filha MH, Yan J, Regis L. A strain of Bacillus sphaericus causes slower development of resistance in Culex quinquefasciatus. Appl. Environ. Microbiol. 2002;68:3003–3009. doi: 10.1128/AEM.68.6.3003-3009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SRA, Melo-Santos MAV, Regis L, Albuquerque CMR. Field evaluation of ovitraps consociated with grass infusion and Bacillus thuringiensis var. israelensis to determine oviposition rates. Dengue Bull. 2003;27:156–162. [Google Scholar]
- Stoops CA. Influence of Bacillus thuringiensis var. israelensis on oviposition of Aedes albopictus (Skuse) J. Vector Ecol. 2005;30:41–44. [PubMed] [Google Scholar]
- Zahiri NS, Mulla MS. Non-larvicidal effects of Bacillus thuringiensis israelensis and Bacillus sphaericus on oviposition and adult mortality of Culex quinquefasciatus Say (Diptera: Culicidae) J. Vector Ecol. 2005;30:155–162. [PubMed] [Google Scholar]