Abstract
We evaluated the relationship between prepregnancy and early pregnancy uterine blood flow (UBF) and resistance index (RI). Nineteen nulliparous subjects were studied during cycle day 8 ± 4, and early pregnancy (13.4 ± 1.6 wks). Color Doppler ultrasound of both uterine arteries and maternal heart was performed to calculate uterine RI, volumetric UBF and cardiac output (CO), respectively. We observed a strong negative association of uterine RI with prepregnancy UBF (r = −0.82, p < 0.001) that weakened, but remained significant in early pregnancy (r = − 0.48, p = 0.04). Prepregnancy uterine index (UBF/CO) was significantly associated with early pregnancy uterine index; r = 0.48, p = 0.04). There was also a trend associating prepregnancy and early pregnancy volumetric UBF (r = 0.44, p = 0.068). Prepregnancy UBF may be a determinant of early pregnancy UBF and UBF may have independent value as a predictor of adverse pregnancy outcome.
Keywords: prepregnancy, pregnancy, uterine blood flow, resistance index, uterine hemodynamics
Introduction
Maternal physiology during pregnancy undergoes significant changes to accommodate the needs of the fetus. Studies in sheep show a dramatic increase in uterine blood flow (UBF) and cardiac output (CO) during pregnancy as well as a striking redistribution of CO to the uterus 1, 2. These findings are echoed in more recent studies performed in pregnant women where maternal systemic CO also increases and UBF increases up to ten times from 50 mL/min in early pregnancy to 500 mL/min in late pregnancy 3, 4. Additionally, during pregnancy, maternal uteroplacental arteries transition to lower resistance vessels facilitating increased uterine and placental perfusion 5-9. Evidence suggests that uterine artery resistance index (RI) may begin to decrease as early as the luteal phase of the menstrual cycle and continues to decrease in the first trimester of pregnancy through the second trimester, slowing during the third trimester 10-13. Evidence of increased vascular impedance and reduced uterine blood flow in the uterine circulation has been associated with an increased risk for both fetal growth restriction and preeclampsia, particularly early onset disease 12, 14-17.
Increasing evidence points to important relationships of either prior preeclampsia or delivery of a growth restricted infant with later maternal cardiovascular disease outside of pregnancy 18. These studies support the hypothesis that the finding of either intrauterine growth restriction (IUGR) or preeclampsia during pregnancy reveals latent hemodynamic abnormalities in mothers which pre-date pregnancy. Consistent with this theory we have hypothesized that prepregnancy cardiovascular phenotype may specifically influence the risk of developing preeclampsia and in particular, preterm disease.
Though many studies have used Doppler ultrasound to evaluate uterine artery velocity waveforms in early and mid-pregnancy with the goal of predicting adverse pregnancy outcome, few have evaluated how prepregnancy UBF and RI inform early pregnancy UBF and RI 9. Further, distinct information may be gathered when performing wave form analysis versus assessing volumetric blood flow in early pregnancy. We suggest that prepregnancy status may have implications for the maternal accommodation to pregnancy. The central aim of this study was to investigate the value of prepregnancy uterine blood flow and resistance index in predicting early pregnancy uterine blood flow and resistance index.
Methods
Thirty four nulligravid women interested in conception were enrolled in this research study through an open advertisement. Women were provided with ovulation detection kits (Quidel Corporation, San Diego, CA) to assist with achieving a successful conception. Thirty women subsequently conceived. Eight subjects conceived before baseline pre-pregnancy studies were performed; one subject had a first trimester miscarriage; one subject was lost to follow-up; and one subject missed her early pregnancy research study visit but returned for study later in pregnancy. The remaining 19 subjects, all of whom conceived singleton pregnancies, who had complete prepregnancy and early pregnancy evaluations, comprise the current report. Women were enrolled consecutively over a 33 month period, from May 2004 through February 2007. Study subjects were between 18 and 40 years of age, nonsmokers, and free from major medical illness, including cardiovascular disease or diabetes mellitus. Prior to each study visit subjects were provided a 3500-mg sodium-balanced diet for 72 hours. Each subject was asked to abstain from alcohol and caffeine for at least 24 hours before the study and to avoid the use of decongestants and nonsteroidal medications for at least 48 hours before the study. All prepregnancy assessments were performed during the follicular phase. Assessments during pregnancy were performed between 11 and 15 menstrual weeks. Ovulation detection and early pregnancy ultrasound assessments were used to calculate gestational age. The research protocols were approved by the University of Vermont Human Investigational Committees. All women provided written informed consent.
Each periodic assessment was conducted between 8 AM and 10 AM. Subjects were admitted to the University of Vermont General Clinical Research Center on the day of the study after an overnight fast. For subjects’ prepregnancy visit, first-void urine was obtained to confirm nonpregnant state. Following height and weight determination, subjects rested in the supine position for the remainder of the study with a minimum of 30 minutes prior to hemodynamic assessment.
Uterine blood flow was assessed using color Doppler ultrasound with an 8.0 MHz transvaginal transducer employing a Vivid 7 General Electric ultrasound unit, (Milwaukee WI). Uterine artery measurements were obtained lateral to the cervix at the level of the internal os. Vessel diameter was measured between the inner surface of the vessel walls during real time power color Doppler imaging. Five estimates of vessel diameter were made for each uterine artery. For determination of average mean velocity angle correction was employed and all angles of sono-incidence were less than or equal to 60 degrees. Five velocity measurements were taken of each vessel and average mean velocity was calculated over an observation window of 1-3 minutes. Mean vessel diameter was used to calculate volumetric blood flow after integration with average mean velocity to express flow in mL/min: [(time averaged mean velocity) X cross sectional area (calculated as π r2)] X 60. Right and left uterine artery blood flow calculations were added to obtain total uterine blood flow.
Cardiac output was determined by Doppler echocardiographic examination. Doppler-derived forward stroke volume (FSV) across the aortic valve was calculated as the product of the left ventricular outflow tract area and the outflow tract velocity time integral as assessed by pulsed Doppler using previously described methods 19. Five complete spectral envelopes with the largest Doppler shift were recorded and averaged for each patient. The Doppler stroke volume was calculated as the product of the outflow tract area and the velocity time integral. CO is expressed as mLs/minute after integration of stroke volume with pulse rate.
Uterine index (UI) was calculated as uterine blood flow divided by CO. This measurement was important to determine if there were global generalized changes in blood flow or a redistribution of systemic blood flow favoring the uterine vascular bed during pregnancy.
Pregnancy outcome was assessed by chart review and is reported as a function of prepregnancy and early pregnancy observations.
Data Analysis
Comparisons between prepregnancy and early pregnancy hemodynamic measures were performed using paired t-tests. Pearson’s correlation coefficients were used to examine the relationship between UBF, RI, UI and CO both prior to pregnancy and during early pregnancy. Partial correlation coefficients were used to examine the relationship between prepregnancy measures and those assessed on the same women during early pregnancy. Hemodynamic measures were compared between women who developed preeclampsia and those that did not based on two sample t-test for the prepregnancy assessment. Because hemodynamic measures can vary depending on gestational age, pregnancy day was adjusted for and analyses of covariance were used to assess hemodynamic measurement comparisons for early pregnancy. All statistical analyses were performed using SAS Version 9.1 statistical software (SAS Institute, Cary NC).
Results
Subject characteristics
All pregnancies were singletons, and the majority of the subjects were Caucasian, 89% (17/19). Clinical and demographic characteristics are presented in Table 1.
Table 1. Characteristics of the nineteen women enrolled in this study.
| Characteristic | Mean ± SE |
|---|---|
| Maternal age (years) | 29.8 ± 3.0 |
| Body mass index (kg/m2) | 23.2 ± 3.3 |
| Prepregnancy cycle day | 8 ± 4 |
| Early pregnancy study day | 94 ± 11 |
UBF, RI, UI and CO
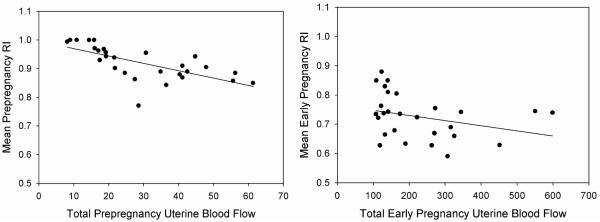
Early pregnancy UBF, CO and UI were all significantly increased relative to prepregnancy while RI was significantly decreased relative to early pregnancy (Table 2). Prepregnancy UBF and RI were highly correlated and negatively associated (r = −.82, p < 0.001) (Figure 1A). Early pregnancy UBF and RI were also significantly correlated, but exhibited a weaker negative association (r = −.48, p = 0.03) (Figure 1B). After adjusting for influence of pregnancy day, there was a significant positive correlation between prepregnancy UI and early pregnancy UI (partial r = .48, p = 0.04) (Figure 2). A similar relationship was observed between prepregnancy and early pregnancy UBF, though it did not achieve statistical significance. (partial r = .44, p = 0.067). Prepregnancy and early pregnancy RI were not significantly correlated (r = 0.09, p = 0.72). Prepregnancy and early pregnancy CO were strongly correlated (r = 0.72, p <0.001), however, CO was not significantly associated with UBF or RI either prepregnancy or in early pregnancy.
Table 2. Prepregnancy and early pregnancy values (mean ± SE) for uterine blood flow (UBF), resistance index (RI), uterine index (UI) and cardiac output (CO).
| Hemodynamic Measure | Prepregnancy | Early Pregnancy | p-value§ |
|---|---|---|---|
| CO (mL/min) | 4619 ± 904 | 5230 ± 1055 | .003 |
| UBF (mL/min) | 21.14 ± 9.1 | 218.0 ± 95.3 | < .001 |
| RI | 0.93 ± 0.06 | 0.70 ± 0.06 | < .001 |
| UI (%) | 0.48 ± 0.25 | 4.22 ± 1.67 | < .001 |
Statistical significance based on paired t-test
Figure 1.
A: Mean prepregnancy resistance index is negatively and significantly correlated to total prepregnancy uterine blood flow (r = −.82, p < 0.001) and B: mean early pregnancy RI is negatively and significantly correlated with total early pregnancy uterine blood flow though this association in early pregnancy is less than in prepregnancy (r = − .48, p = 0.03).
Figure 2.
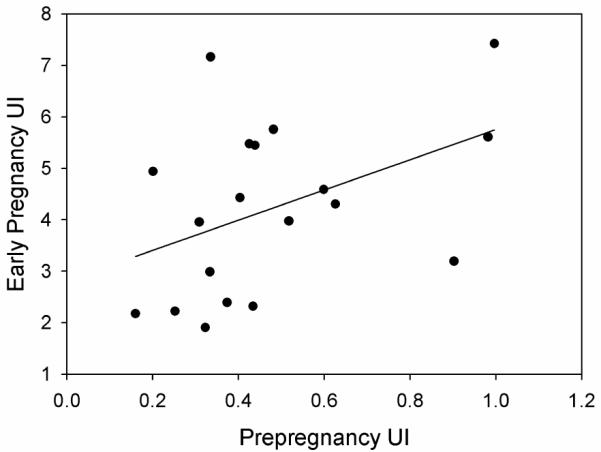
Early pregnancy uterine index is positively and significantly correlated with prepregnancy uterine index (partial r = .48, p = 0.04).
Pregnancy Outcome
Mean birth weight for the 19 pregnancies was 3402 ± 593 g. Mean birth weight percentile was 48th ± 28 ranging from the 1st to 95th. Mean gestational age was 39.4 ± 1.4 weeks. There were two women who developed preeclampsia. One subject developed preeclampsia at 34 weeks and delivered a growth restricted newborn at 37 weeks. The second woman developed preeclampsia at 40 weeks and delivered a newborn with a birth weight at the 30th percentile. We compared hemodynamic measures of the two women who developed preeclampsia to the remaining 17 women who did not. There were no significant differences between groups prior to pregnancy in maternal age, body mass index, or mean arterial pressure. Prior to pregnancy, CO and UBF tended to be lower in those who developed preeclampsia, but these differences were not significantly different (CO: non-preeclamptic: 4740 ± 860 mL/min, preeclamptic: 3560 ± 660 mL/min; p = .08; UBF: non-preeclamptic: 22.2 ± 8.2 mL/min, preeclamptic: 12 ± 5.5 mL/min; p = .14). In addition, there were no significant differences between the two groups in RI or UI prepregnancy or during early pregnancy. In early pregnancy, UBF was not significantly different (non-preeclamptics: 224.3 ± 98.8 mL/min, preeclamptics: 165.2 ± 34.5 mL/min; p = .14), however, maternal CO was significantly lower in the two women who went on to develop preeclampsia (CO: 5400 ± 960 mL/min, preeclamptic 3690 ± 210 mL/min; p = .02).
Discussion
Historically, most studies have investigated the relationship between early or mid pregnancy uterine artery hemodynamics and pregnancy outcome 13-15, 20-22. While elevated uterine artery RI and uterine waveform notching during pregnancy have been associated with low birth weight and preeclampsia, neither prepregnancy RI or UBF have been routinely assessed as predictors of either early pregnancy observations or pregnancy outcome. Observations now demonstrate that women who have had IUGR and preeclampsia are at increased risk for cardiovascular disease later in life 18, 23-25. These observations raise the question that there is a preexisting deficiency in vascular function that may contribute to pregnancy specific events and later, cardiovascular disease. In this regard, we hypothesized that uterine physiology and hemodynamics prior to pregnancy would be an important determinant of early pregnancy uterine physiology.
Because of the importance of UBF in pregnancy disease, we were interested in how UBF and UI, the relationship of UBF to CO, change between observations made prior to pregnancy and in early pregnancy. Furthermore, we were interested in how prepregnancy uterine artery RI and volumetric flow inform similar measures in early pregnancy.
We found that UBF increased ten-fold from prepregnancy to early pregnancy supporting prior observations of large physiological accommodations made by the body quite early in pregnancy. In support of this, UI also increased approximately ten-fold between prepregnancy and early pregnancy indicating that the percentage of blood directed to the uterus increases greatly in early pregnancy. Though few studies have evaluated UI during pregnancy, existing research suggests that the proportion of CO perfusing the uterine arteries is 3.5% in early pregnancy, a value that is consistent with our assessment of UI during pregnancy 9. Of particular interest are our observations that indicate that prepregnancy UI is significantly correlated with early pregnancy UI suggesting that prepregnancy UI predicts early pregnancy UI and accounts for approximately 25% of the variance observed.
We aimed to discern the relationship between prepregnancy and early pregnancy RI. We found that RI decreases significantly from prepregnancy to early pregnancy. This finding is consistent with the literature as RI is known to decrease linearly beginning in the luteal phase of the menstrual cycle and continue to decrease throughout pregnancy. We did not observe a correlation between prepregnancy and early pregnancy RI. Not surprisingly, prepregnancy UBF was significantly and negatively associated with prepregnancy RI, however, the strength of this association lessened during early pregnancy. The weakened association of UBF and RI in early pregnancy suggests that during early pregnancy, there may be other factors that contribute to increased UBF outside of decreased vascular impedance. Further, the apparent change in the strength of the association between UBF and RI raises the provocative question of whether UBF might have exclusive and separate value in predicting important pregnancy hemodynamic status and, by proxy, adverse pregnancy outcome. This theory is supported by our UI data showing that UI is significantly correlated between prepregnancy and early pregnancy. Most studies use uterine artery waveform indices as a measure of UBF rather than evaluating volumetric blood flow rates. Our observations suggest that volumetric analysis of UBF may have an independent contribution as compared to analysis of uterine artery waveforms.
Inadequate uterine perfusion has been suggested as a contributing factor to IUGR 26, 27. In women, although studies investigating volumetric UBF and pregnancy outcome are lacking, studies show that an elevated RI can suggest an increased risk for preeclampsia and IUGR14, 15, 21, 28, 29. More recent research suggests that Doppler indices obtained within the first trimester may have value in predicting pregnancy outcome 15, 30. Though our study was originally not intended to evaluate hemodynamic status in preeclamptics, it is interesting to note that of those who progressed to clinical disease, there were no differences in RI between women who developed term preeclampsia and those with normal outcome. Further, our preliminary findings indicate a tendency for lower prepregnancy CO in the preeclamptic subgroup and a significantly lower CO within this group during early pregnancy as compared to the women who did not develop preeclampsia. This observation is consistent with studies suggesting that women who develop preeclampsia have lower CO than those with normal pregnancies 31, 32. However, we recognize that due to very small sample size, our observations within the preeclamptic subgroup are not conclusive, rather, hypothesis-generating, and may suggest an existing maternal phenotype that may predispose women to preeclampsia.
We have not been able to identify prior publications investigating the relationship between prepregnancy volumetric UBF and RI and early pregnancy UBF and RI within individual women. This study has demonstrated a negative correlation between UBF and RI as assessed by Doppler ultrasound both prior to pregnancy and in early pregnancy. Further, we suggest that volumetric UBF analysis may have predictive value in assessing maternal hemodynamics that is independent of RI, particularly in early pregnancy.
As increasing evidence points to maternal preexisting vascular risk that materializes under conditions of vascular stress, such as pregnancy, we suggest that studying maternal physiology prior to, and during early pregnancy may provide insights into the pathophysiologic sequences associated with the development of both preeclampsia and fetal growth restriction that are not recognized when evaluating hemodynamics exclusively during pregnancy.
Acknowledgments
Grant support: NIH HL 71944
Footnotes
Research performed at the University of Vermont, Burlington, VT
References
- 1.Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977 Mar;232(3):H231–235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld CR, Morriss FH, Jr., Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5(5-6):252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 3.Maini CL, Rosati P, Galli G, Bellati U, Bonetti MG, Moneta E. Non-invasive radioisotopic evaluation of placental blood flow. Gynecol Obstet Invest. 1985;19(4):196–206. doi: 10.1159/000299034. [DOI] [PubMed] [Google Scholar]
- 4.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol Heart Circ Physiol. 1989 April 1;256(4):H1060–1065. doi: 10.1152/ajpheart.1989.256.4.H1060. 1989. [DOI] [PubMed] [Google Scholar]
- 5.Dickey RP, Hower JF. Ultrasonographic features of uterine blood flow during the first 16 weeks of pregnancy. Hum Reprod. 1995 Sep;10(9):2448–2452. doi: 10.1093/oxfordjournals.humrep.a136317. [DOI] [PubMed] [Google Scholar]
- 6.Kaminopetros P, Higueras MT, Nicolaides KH. Doppler study of uterine artery blood flow: comparison of findings in the first and second trimesters of pregnancy. Fetal Diagn Ther. 1991;6(1-2):58–64. doi: 10.1159/000263625. [DOI] [PubMed] [Google Scholar]
- 7.Murakoshi T, Sekizuka N, Takakuwa K, Yoshizawa H, Tanaka K. Uterine and spiral artery flow velocity waveforms in pregnancy-induced hypertension and/or intrauterine growth retardation. Ultrasound Obstet Gynecol. 1996 Feb;7(2):122–128. doi: 10.1046/j.1469-0705.1996.07020122.x. [DOI] [PubMed] [Google Scholar]
- 8.Schulman H, Fleischer A, Farmakides G, Bracero L, Rochelson B, Grunfeld L. Development of uterine artery compliance in pregnancy as detected by Doppler ultrasound. Am J Obstet Gynecol. 1986 Nov;155(5):1031–1036. doi: 10.1016/0002-9378(86)90340-6. [DOI] [PubMed] [Google Scholar]
- 9.Thaler I, Manor D, Itskovitz J, et al. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990 Jan;162(1):121–125. doi: 10.1016/0002-9378(90)90834-t. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein IM, Ziegler WF, Leavitt T, Badger GJ. Uterine artery hemodynamic adaptations through the menstrual cycle into early pregnancy. Obstet Gynecol. 2002 Apr;99(4):620–624. doi: 10.1016/s0029-7844(01)01787-2. [DOI] [PubMed] [Google Scholar]
- 11.Coppens M, Loquet P, Kollen M, De Neubourg F, Buytaert P. Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol. 1996 Feb;7(2):114–121. doi: 10.1046/j.1469-0705.1996.07020114.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh G, Breborowicz A, Brazert M, et al. Evaluation of third trimester uterine artery flow velocity indices in relationship to perinatal complications. J Matern Fetal Neonatal Med. 2006 Sep;19(9):551–555. doi: 10.1080/14767050600852510. [DOI] [PubMed] [Google Scholar]
- 13.Hollis B, Prefumo F, Bhide A, Rao S, Thilaganathan B. First-trimester uterine artery blood flow and birth weight. Ultrasound Obstet Gynecol. 2003;22(4):373–376. doi: 10.1002/uog.231. [DOI] [PubMed] [Google Scholar]
- 14.Coleman MA, McCowan LM, North RA. Mid-trimester uterine artery Doppler screening as a predictor of adverse pregnancy outcome in high-risk women. Ultrasound Obstet Gynecol. 2000 Jan;15(1):7–12. doi: 10.1046/j.1469-0705.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 15.Dugoff L, Lynch AM, Cioffi-Ragan D, et al. First trimester uterine artery Doppler abnormalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol. 2005;193(3, Supplement 1):1208–1212. doi: 10.1016/j.ajog.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Fratelli N, Rampello S, Guala M, Platto C, Frusca T. Transabdominal uterine artery Doppler between 11 and 14 weeks of gestation for the prediction of outcome in high-risk pregnancies. J Matern Fetal Neonatal Med. 2008;21(6):403–406. doi: 10.1080/14767050802053073. [DOI] [PubMed] [Google Scholar]
- 17.Özkaya U, Özkan S, Özeren S, Çorakç A. Doppler examination of uteroplacental circulation in early pregnancy: Can it predict adverse outcome? J Clin Ultrasound. 2007;35(7):382–386. doi: 10.1002/jcu.20370. [DOI] [PubMed] [Google Scholar]
- 18.Smith G, Walker M, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200(1) doi: 10.1016/j.ajog.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984 Sep;70(3):425–431. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- 20.Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1991 Dec;42(Suppl):S14–20. [PubMed] [Google Scholar]
- 21.Melchiorre K, Wormald B, Leslie K, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32(2):133–137. doi: 10.1002/uog.5400. [DOI] [PubMed] [Google Scholar]
- 22.Rang S, van Montfrans GA, Wolf H. Serial hemodynamic measurement in normal pregnancy, preeclampsia, and intrauterine growth restriction. Am J Obstet Gynecol. 2008 May;198(5):519, e511–519. doi: 10.1016/j.ajog.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Bellamy L, Casas J, Hingorani A, Williams D. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007 November 10;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. The Lancet. 2005;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 25.Wolf M, Hubel CA, Lam C, et al. Preeclampsia and Future Cardiovascular Disease: Potential Role of Altered Angiogenesis and Insulin Resistance. J Clin Endocrinol Metab. 2004 December 1;;89(12):6239–6243. doi: 10.1210/jc.2004-0548. 2004. [DOI] [PubMed] [Google Scholar]
- 26.Nylund L, Lunell NO, Lewander R, Sarby B. Uteroplacental blood flow index in intrauterine growth retardation of fetal or maternal origin. Br J Obstet Gynaecol. 1983 Jan;90(1):16–20. doi: 10.1111/j.1471-0528.1983.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 27.Lang U, Baker RS, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow--a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110(Supplement 1):S55–S61. doi: 10.1016/s0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 28.Harrington K, Fayyad A, Thakur V, Aquilina J. The value of uterine artery Doppler in the prediction of uteroplacental complications in multiparous women. Ultrasound Obstet Gynecol. 2004;23(1):50–55. doi: 10.1002/uog.932. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Gudnason H, Olofsson P, Dubiel M, Gudmundsson S. Increased uterine artery vascular impedance is related to adverse outcome of pregnancy but is present in only one-third of late third-trimester pre-eclamptic women. Ultrasound Obstet Gynecol. 2005 May;25(5):459–463. doi: 10.1002/uog.1895. [DOI] [PubMed] [Google Scholar]
- 30.Papageorghiou AT, Campbell S. First trimester screening for preeclampsia. Curr Opin Obstet Gynecol. 2006 Dec;18(6):594–600. doi: 10.1097/GCO.0b013e328010beda. [DOI] [PubMed] [Google Scholar]
- 31.Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008 Aug 13;32(5):682–686. doi: 10.1002/uog.5311. [DOI] [PubMed] [Google Scholar]
- 32.Bamfo JEAK, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in fetal growth-restricted and non-growth-restricted small-forgestational age pregnancies. Ultrasound Obstet Gynecol. 2007;29(1):51–57. doi: 10.1002/uog.3901. [DOI] [PubMed] [Google Scholar]