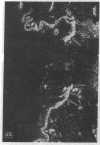
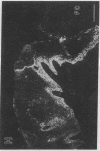
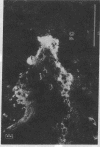
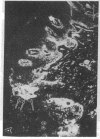
Abstract
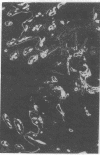
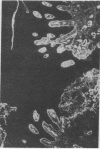
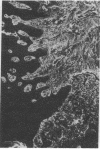
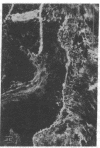
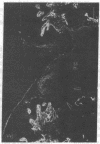
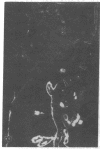
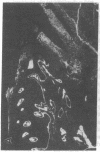
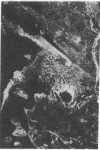
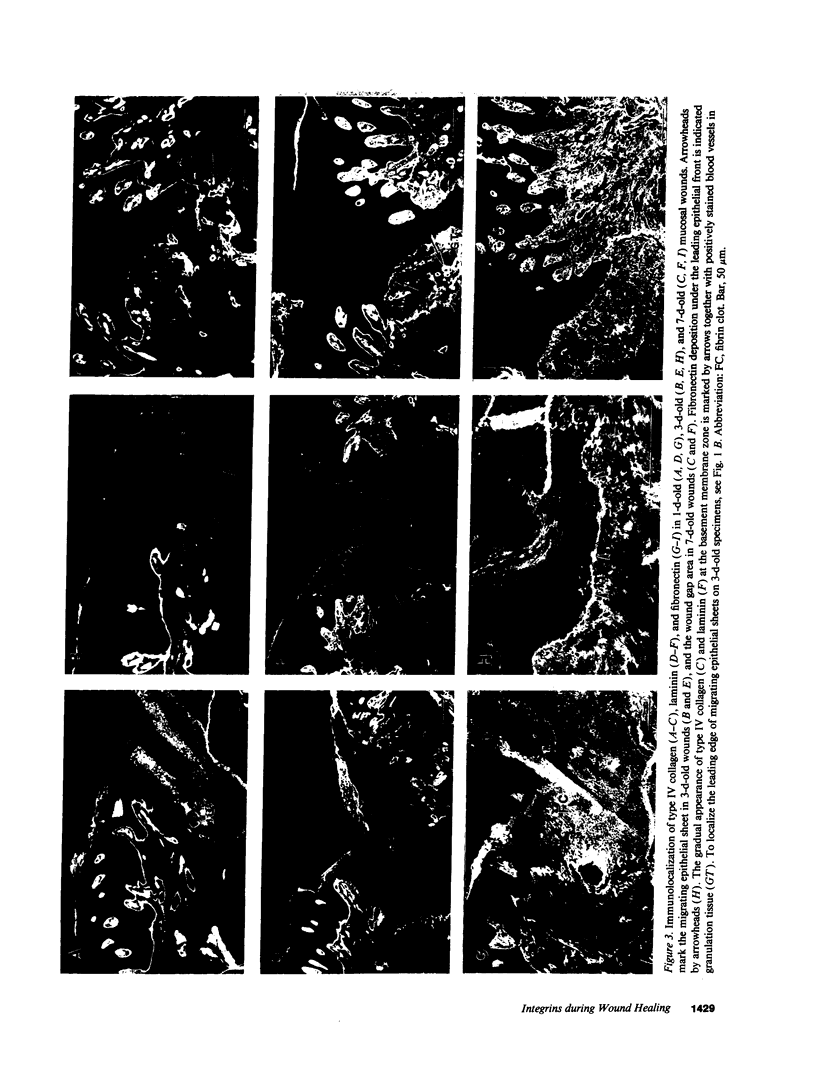
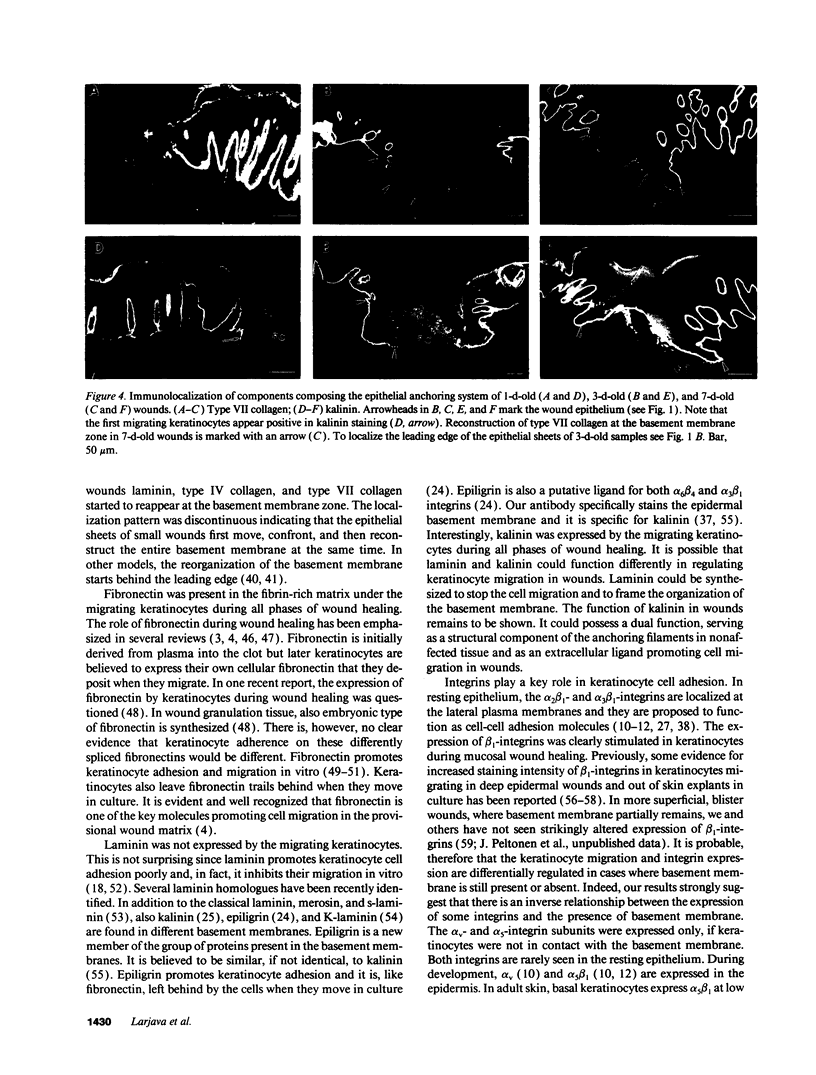
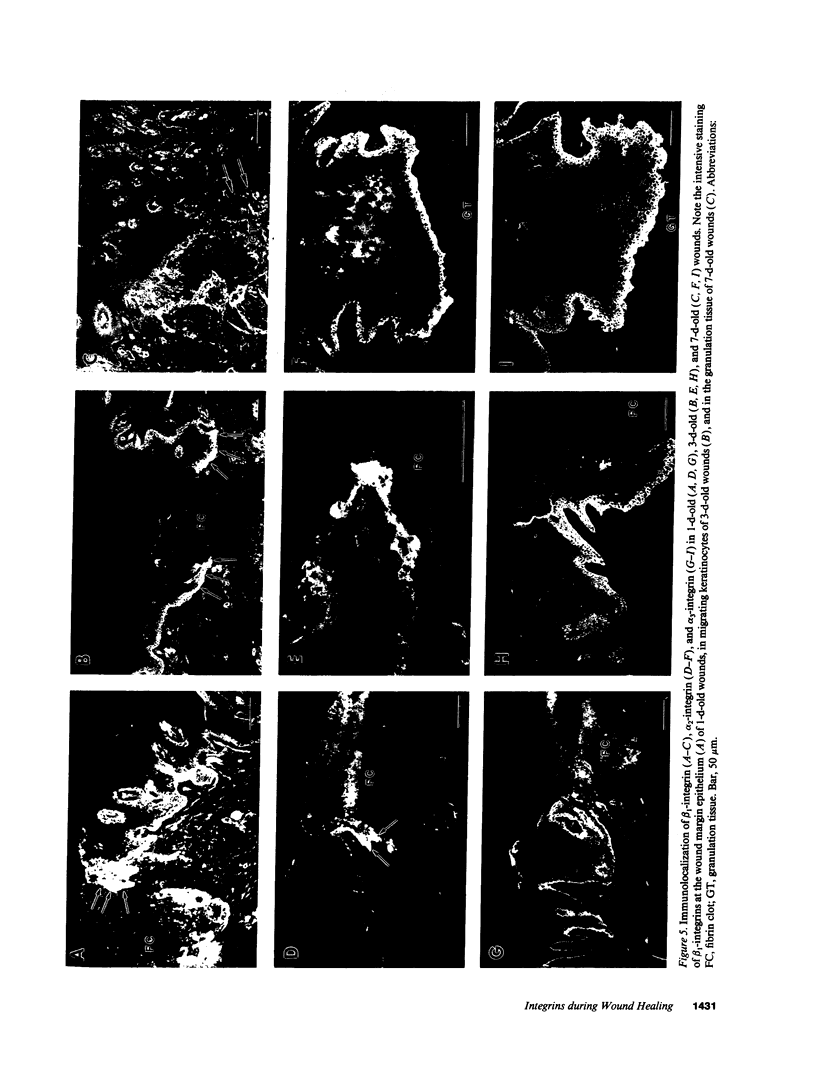
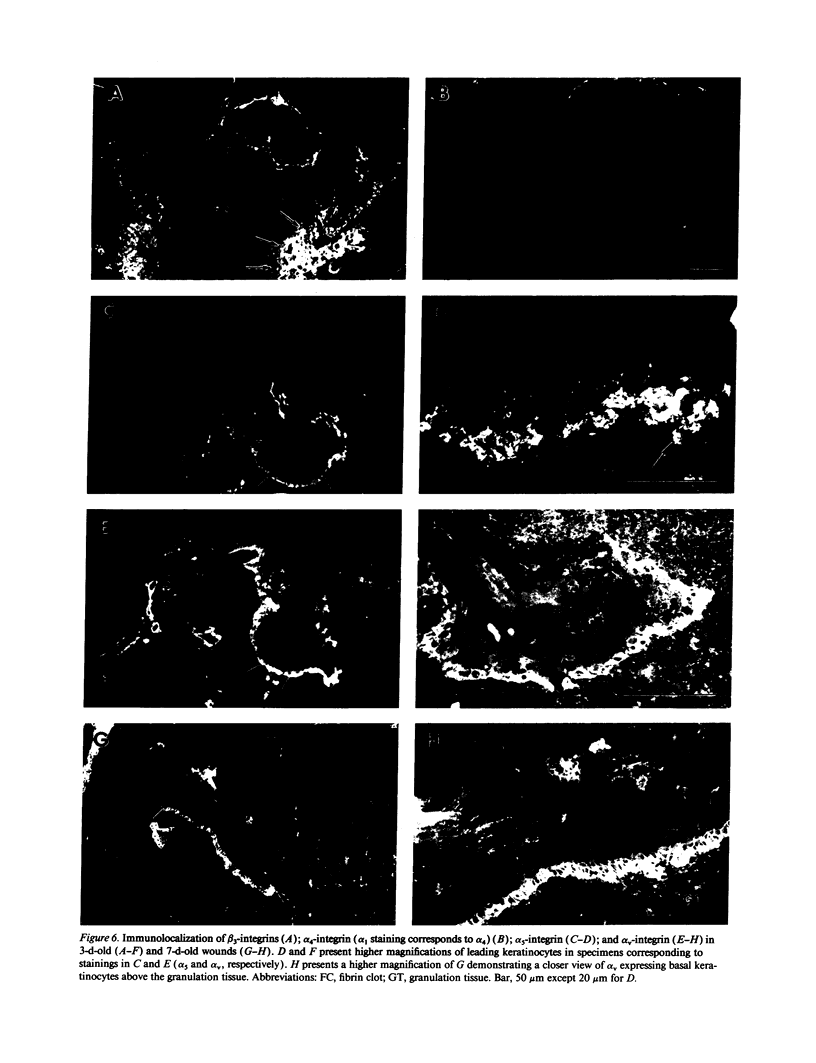
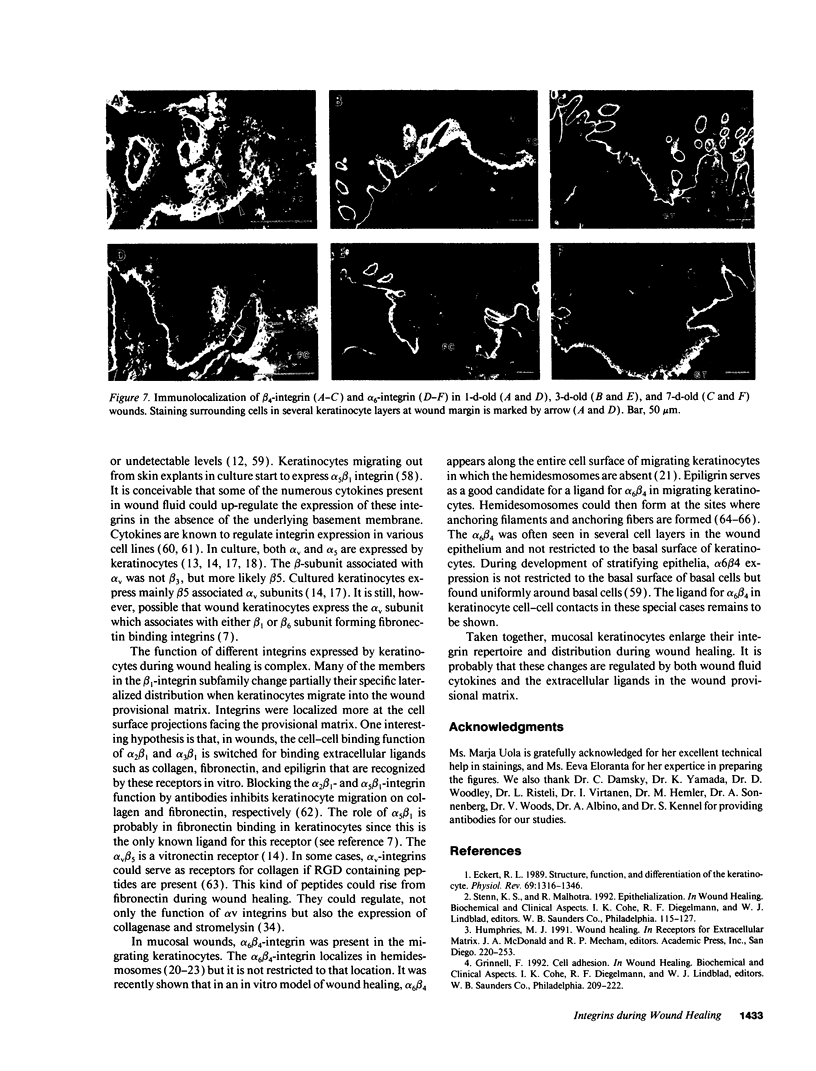
Extracellular matrix proteins and their cellular receptors, integrins, play a fundamental role in keratinocyte adhesion and migration. During wound healing, keratinocytes detach, migrate until the two epithelial sheets confront, and then regenerate the basement membrane. We examined the expression of different integrins and their putative ligands in keratinocytes during human mucosal wound healing. Migrating keratinocytes continuously expressed kalinin but not the other typical components of the basement membrane zone: type IV collagen, laminin, and type VII collagen. When the epithelial sheets confronted each other, these missing basement membrane components started to appear gradually through the entire wound area. The expression of integrin beta 1 subunit was increased in keratinocytes during migration. The beta 1-associated alpha 2 and alpha 3 subunits were expressed constantly by wound keratinocytes whereas the alpha 5 subunit was present only in keratinocytes during reepithelialization. Furthermore, migrating cells started to express alpha v-integrins which were not present in the nonaffected epithelium. All keratinocytes also expressed the alpha 6 beta 4 integrin during migration. In the migrating cells, the distribution of integrins was altered. In normal mucosa, beta 1-integrins were located mainly on the lateral plasma membrane and alpha 6 beta 4 at the basal surface of basal keratinocytes in the nonaffected tissue. In wounds, integrins were found in filopodia of migrating keratinocytes, and also surrounding cells in several cell layers of the migrating sheet. The results indicate that migrating keratinocytes, in deep human wounds enlarge their integrin repertoire. The changes in integrin expression take place concomitantly with changes in the basement membrane composition, suggesting a close interplay of these two groups of molecules during wound healing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Adams J. C., Watt F. M. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991 Nov;115(3):829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrez M. V., Bates R. C., Boyd A. W., Burns G. F. Arg-Gly-Asp-containing peptides expose novel collagen receptors on fibroblasts: implications for wound healing. Cell Regul. 1991 Dec;2(12):1035–1044. doi: 10.1091/mbc.2.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto Y., Obinata A., Endo H., Hirano H. Immunohistochemical study of basement membrane reconstruction by an epidermis-dermis recombination experiment using cultured chick embryonic skin: induction of tenascin. J Histochem Cytochem. 1992 Aug;40(8):1129–1137. doi: 10.1177/40.8.1377733. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Burgeson R. E., Lunstrum G. P., Rokosova B., Rimberg C. S., Rosenbaum L. M., Keene D. R. The structure and function of type VII collagen. Ann N Y Acad Sci. 1990;580:32–43. doi: 10.1111/j.1749-6632.1990.tb17915.x. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Wertz R. L. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J Invest Dermatol. 1985 May;84(5):378–383. doi: 10.1111/1523-1747.ep12265466. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- De Luca M., Tamura R. N., Kajiji S., Bondanza S., Rossino P., Cancedda R., Marchisio P. C., Quaranta V. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6888–6892. doi: 10.1073/pnas.87.17.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L. Structure, function, and differentiation of the keratinocyte. Physiol Rev. 1989 Oct;69(4):1316–1346. doi: 10.1152/physrev.1989.69.4.1316. [DOI] [PubMed] [Google Scholar]
- Engvall E., Earwicker D., Haaparanta T., Ruoslahti E., Sanes J. R. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990 Sep;1(10):731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I. K., Grill S. M., Spurr S. J., Brennan S. J. Hemidesmosome formation in vitro. J Cell Biol. 1983 Sep;97(3):849–857. doi: 10.1083/jcb.97.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S. J., Tisdale A. S. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol. 1988 Apr;126(2):253–262. doi: 10.1016/0012-1606(88)90136-4. [DOI] [PubMed] [Google Scholar]
- Guo M., Kim L. T., Akiyama S. K., Gralnick H. R., Yamada K. M., Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp Cell Res. 1991 Aug;195(2):315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- Guo M., Toda K., Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990 Jun;96(Pt 2):197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- Heino J., Massagué J. Transforming growth factor-beta switches the pattern of integrins expressed in MG-63 human osteosarcoma cells and causes a selective loss of cell adhesion to laminin. J Biol Chem. 1989 Dec 25;264(36):21806–21811. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Hertle M. D., Adams J. C., Watt F. M. Integrin expression during human epidermal development in vivo and in vitro. Development. 1991 May;112(1):193–206. doi: 10.1242/dev.112.1.193. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992 Jun;89(6):1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin N. A., Watt F. M. Transcriptional and post-translational regulation of beta 1 integrin expression during keratinocyte terminal differentiation. J Biol Chem. 1992 Jul 25;267(21):14852–14858. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Kurpakus M. A., Cooper H. M., Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991 Jun;2(6):427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R. R., Mattes M. J., Lloyd K. O., Old L. J., Albino A. P. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem. 1987 Nov 5;262(31):15158–15165. [PubMed] [Google Scholar]
- Kennel S. J., Foote L. J., Falcioni R., Sonnenberg A., Stringer C. D., Crouse C., Hemler M. E. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem. 1989 Sep 15;264(26):15515–15521. [PubMed] [Google Scholar]
- Kim J. P., Zhang K., Kramer R. H., Schall T. J., Woodley D. T. Integrin receptors and RGD sequences in human keratinocyte migration: unique anti-migratory function of alpha 3 beta 1 epiligrin receptor. J Invest Dermatol. 1992 May;98(5):764–770. doi: 10.1111/1523-1747.ep12499947. [DOI] [PubMed] [Google Scholar]
- Konter U., Kellner I., Klein E., Kaufmann R., Mielke V., Sterry W. Adhesion molecule mapping in normal human skin. Arch Dermatol Res. 1989;281(7):454–462. doi: 10.1007/BF00510080. [DOI] [PubMed] [Google Scholar]
- Kurpakus M. A., Quaranta V., Jones J. C. Surface relocation of alpha 6 beta 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991 Dec;115(6):1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Zhou C., Larjava I., Rahemtulla F. Immunolocalization of beta 1 integrins in human gingival epithelium and cultured keratinocytes. Scand J Dent Res. 1992 Oct;100(5):266–273. doi: 10.1111/j.1600-0722.1992.tb01069.x. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Bondanza S., Cremona O., Cancedda R., De Luca M. Polarized expression of integrin receptors (alpha 6 beta 4, alpha 2 beta 1, alpha 3 beta 1, and alpha v beta 5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J Cell Biol. 1991 Feb;112(4):761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich M. P., Lunstrum G. P., Keene D. R., Burgeson R. E. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992 Nov;119(3):695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Riser B. L., Dixit V. M., Varani J. Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol. 1988 Sep;132(3):543–551. [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Payne R. E., Jr, Russell N., Woodley D. T. Spreading and enhanced motility of human keratinocytes on fibronectin. J Invest Dermatol. 1985 Aug;85(2):125–130. doi: 10.1111/1523-1747.ep12276531. [DOI] [PubMed] [Google Scholar]
- Olerud J. E., Gown A. M., Bickenbach J., Dale B., Odland G. F. An assessment of human epidermal repair in elderly normal subjects using immunohistochemical methods. J Invest Dermatol. 1988 Jun;90(6):845–850. doi: 10.1111/1523-1747.ep12462083. [DOI] [PubMed] [Google Scholar]
- PUCHTLER H., SWEAT F., DOSS N. O. A ONE-HOUR PHOSPHOTUNGSTIC ACID-HEMATOXYLIN STAIN. Am J Clin Pathol. 1963 Sep;40:334–337. doi: 10.1093/ajcp/40.3_ts.334. [DOI] [PubMed] [Google Scholar]
- Parente M. G., Chung L. C., Ryynänen J., Woodley D. T., Wynn K. C., Bauer E. A., Mattei M. G., Chu M. L., Uitto J. Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6931–6935. doi: 10.1073/pnas.88.16.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Risteli L., Timpl R. Isolation and characterization of pepsin fragments of laminin from human placental and renal basement membranes. Biochem J. 1981 Mar 1;193(3):749–755. doi: 10.1042/bj1930749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Rousselle P., Lunstrum G. P., Keene D. R., Burgeson R. E. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991 Aug;114(3):567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Morris N. P., Burgeson R. E. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986 Oct;103(4):1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santala P., Heino J. Regulation of integrin-type cell adhesion receptors by cytokines. J Biol Chem. 1991 Dec 5;266(34):23505–23509. [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Hogervorst F., Osterop A., Veltman F. E. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988 Oct 5;263(28):14030–14038. [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrando P., Hsi B. L., Yeh C. J., Pisani A., Serieys N., Ortonne J. P. Monoclonal antibody GB3, a new probe for the study of human basement membranes and hemidesmosomes. Exp Cell Res. 1987 May;170(1):116–128. doi: 10.1016/0014-4827(87)90121-2. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., Bachmann P. M., O'Keefe E. J. Laminin inhibits human keratinocyte migration. J Cell Physiol. 1988 Jul;136(1):140–146. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Bachmann P. M., O'Keefe E. J. The role of matrix components in human keratinocyte re-epithelialization. Prog Clin Biol Res. 1991;365:129–140. [PubMed] [Google Scholar]
- Ylänne J., Hormia M., Järvinen M., Vartio T., Virtanen I. Platelet glycoprotein IIb/IIIa complex in cultured cells. Localization in focal adhesion sites in spreading HEL cells. Blood. 1988 Nov;72(5):1478–1486. [PubMed] [Google Scholar]