Abstract
Objectives
Besifloxacin is a novel fluoroquinolone that was recently approved for topical treatment of bacterial conjunctivitis. The compound was shown to be active in vitro against a broad spectrum of bacteria, including isolates resistant to other antibacterials. Here, the bactericidal activity of besifloxacin was evaluated against the most common bacterial conjunctivitis pathogens.
Methods
MIC, MBC and time–kill experiments with besifloxacin and comparators were performed according to CLSI guidelines. Quinolone resistance-determining regions (QRDRs) were sequenced using standard PCR-based techniques.
Results
MIC and MBC data indicated that besifloxacin was the most potent fluoroquinolone tested against Staphylococcus aureus (n = 30), Staphylococcus epidermidis (n = 15) and Streptococcus pneumoniae (n = 35), while all fluoroquinolones were highly active against Haemophilus influenzae (n = 40). Besifloxacin MBC:MIC ratios were ≤4 for 97.5% of all isolates tested (n = 120). All fluoroquinolones tested, as well as tobramycin, were bactericidal, while azithromycin was bactericidal against S. pneumoniae and H. influenzae, but bacteriostatic against the staphylococci. Time–kill assays with all four species showed that besifloxacin caused ≥1000-fold killing within 2 h for 11 of 12 isolates. Only one isolate treated with moxifloxacin and three ciprofloxacin-treated isolates achieved the same level of bactericidal activity under the same conditions. Unlike the comparator fluoroquinolones, besifloxacin maintained a high potency and bactericidal activity even against strains that contained multiple mutations in the genes encoding DNA gyrase and topoisomerase IV.
Conclusions
Overall, besifloxacin demonstrated rapid bactericidal activity against the four major human pathogens tested here, including isolates that showed in vitro resistance to other fluoroquinolones, β-lactams, macrolides or aminoglycosides.
Keywords: fluoroquinolones, time–kill, bactericidal activity
Introduction
Fluoroquinolones are widely used bactericidal drugs, and several are approved for the treatment of bacterial conjunctivitis.1 Besifloxacin is a novel member of the fluoroquinolone class of antibacterial agents that was recently approved for topical treatment of bacterial conjunctivitis. The compound demonstrates potent antibacterial activity in vitro against a wide range of pathogens including Gram-positive and -negative aerobic and anaerobic bacteria.2 To date, besifloxacin studies have reported primarily MIC results, which define only the inhibitory, or bacteriostatic, nature of an agent’s activity. While bacteriostatic agents can be sufficient to eradicate some bacterial infections, bactericidal agents may have an advantage in immunoprivileged sites such as the eye.
This study was conducted to assess how time and concentration of drug exposure, as well as mutations that confer resistance to other fluoroquinolones, influence the bactericidal activity of besifloxacin. Accordingly, MBC, time–kill and molecular sequencing experiments were performed with recent ocular isolates of Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae and Haemophilus influenzae, which are the four most prevalent bacterial conjunctivitis pathogens.
Similar to tobramycin and other fluoroquinolones used for ophthalmic indications, besifloxacin showed low MBC:MIC ratios typical of bactericidal agents. However, compared with moxifloxacin and ciprofloxacin, besifloxacin demonstrated overall improved bactericidal potency and speed of action against both fluoroquinolone-susceptible (FQS) and fluoroquinolone-resistant (FQR) isolates of the four species tested.
Materials and methods
Bacterial isolates
Isolates were collected by Eurofins Medinet (Chantilly, VA, USA). The test organisms for the MBC:MIC assays were recent (2006–07), predominantly ocular, isolates of United States origin, with the exception of one FQR strain isolated in 2005. Isolates consisted of non-consecutive, non-duplicate clinical isolates collected from >50 sites distributed across the USA. Isolates were selected to provide a geographically diverse challenge set that reflected current rates of important resistance phenotypes [e.g. methicillin-resistant S. aureus (MRSA), fluoroquinolone resistance etc.] among ocular pathogens. The only non-ocular isolates were the ATCC quality control strains (listed below) and the FQR S. pneumoniae and H. influenzae isolates due to the rarity of these phenotypes. Overall, a total of 30 S. aureus and 15 S. epidermidis (including methicillin- and ciprofloxacin-resistant isolates), 35 S. pneumoniae (including penicillin-intermediate and -resistant isolates and a levofloxacin-resistant isolate) and 40 H. influenzae (including β-lactamase-positive isolates) were tested. Quality control strains were S. aureus ATCC 29213, S. aureus ATCC 25923, S. pneumoniae ATCC 49619 and H. influenzae ATCC 49247.
For time–kill assays, individual isolates with specific resistance phenotypes were selected from the ocular pathogens described above. In addition, a non-ocular isolate of H. influenzae was included to represent the rare β-lactamase-negative, moxifloxacin non-susceptible phenotype. The test organisms included four isolates each of S. aureus and S. epidermidis that were methicillin susceptible (MSSA and MSSE, respectively) or methicillin resistant (MRSA and MRSE, respectively) and that were FQS or FQR. In addition, two isolates of S. pneumoniae (both penicillin susceptible, but FQS or FQR) and two isolates of H. influenzae [both β-lactamase negative, but FQS or fluoroquinolone non-susceptible (FQNS)] were evaluated.
Antibacterial drugs
The sources of the test agents were as follows: besifloxacin (Bausch + Lomb, Inc., Rochester, NY, USA); levofloxacin (Johnson & Johnson, Inc., Langhorne, PA, USA); penicillin, oxacillin and tobramycin (Sigma-Aldrich, St Louis, MO, USA); and ceftazidime, azithromycin, ciprofloxacin, moxifloxacin and gatifloxacin (TREK Diagnostic Systems, Inc., Cleveland, OH, USA).
MIC and MBC determination
MICs were determined by broth microdilution using frozen panels per CLSI reference methods and antibacterial susceptibilities were reported using CLSI interpretive criteria.3–5 The highest drug concentration tested in this assay was 32 mg/L for tobramycin and 8 mg/L for the fluoroquinolones and azithromycin. Prior to incubation, growth control wells were sampled and plated to confirm the initial inocula. After primary incubation, MICs were recorded, and MBCs were determined by spotting 10 µL from wells at and above MICs on drug-free agar medium. The cfu results from sampled test wells were used to determine MBCs that caused ≥3 log bacterial killing.3
Time–kill assay methodology
Time–kill assays were performed at 1×, 2×, 4× and 8× MIC values per CLSI methods.3 Briefly, growth-phase bacterial cultures at 5 × 105 to 5 × 106 cfu/mL were treated with antibacterial agents, and cfu were determined at 0, 2, 4, 6 and 24 h. For time–kill experiments, bactericidal activity was defined as a ≥3 log decrease in cfu/mL 24 h after treatment.
Molecular characterization of FQR strains used in time–kill assays
The molecular basis of fluoroquinolone resistance was investigated in the six FQR isolates used for time–kill experiments. The quinolone resistance-determining regions (QRDRs) of DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE, also known as grlA and grlB), which are commonly associated with fluoroquinolone resistance, were amplified and sequenced as described in the Supplementary methods [available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)].
Results
Table 1 summarizes the MIC and MBC values of besifloxacin and comparators for 120 isolates of the four most prevalent ocular pathogens. Where appropriate, β-lactam-resistant, macrolide-resistant or FQR isolates were included in the sample population to reflect current antibacterial resistance trends. Eleven of 30 S. aureus and 6 of 15 S. epidermidis isolates were resistant to ciprofloxacin. Since the MIC or MBC values in some cases exceeded the highest drug concentration tested, MBC:MIC ratios could not be calculated for all isolates and thus some MIC90 or MBC90 values were reported as >8. For the Gram-positive pathogens, besifloxacin and, to a lesser extent, moxifloxacin had the lowest MIC50 and MBC50 values of all agents tested. Against H. influenzae, ciprofloxacin and gatifloxacin were the most active drugs, followed closely by besifloxacin and moxifloxacin. MBC:MIC ratios were ≤4 for most antibacterial agents and test strains, indicating a bactericidal mode of action. The only exception was azithromycin, which was bacteriostatic against S. aureus and S. epidermidis.
Table 1.
In vitro activity and MBC:MIC ratios of besifloxacin and comparator agents against ocular isolates of S. aureus, S. epidermidis, S. pneumoniae and H. influenzae
| MIC and MBC values for 50% and 90% of all isolates (N) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Organism (number of isolates) | Test agent | MIC50 (mg/L) | MBC50 (mg/L) | MIC90 (mg/L) | MBC90 (mg/L) | Number (n) of MIC:MBC ratios obtaineda | Percentage of n with MBC:MIC ratios ≤ 2 | Percentage of n with MBC:MIC ratios ≤ 4 |
| S. aureus (N = 30) | BES | 0.03 | 0.06 | 4 | 4 | 30 | 83.3 | 90.0 |
| MXF | 0.06 | 0.06 | >8 | >8 | 25 | 84.0 | 84.0 | |
| GAT | 0.06 | 0.25 | >8 | >8 | 26 | 80.8 | 92.3 | |
| CIP | 0.5 | 1 | >8 | >8 | 21 | 76.2 | 90.5 | |
| AZM | 1 | >8 | >8 | >8 | 10 | 10.0 | 20.0 | |
| TOB | 0.5 | 1 | >32 | >32 | 24 | 75.0 | 91.7 | |
| S. epidermidis (N = 15) | BES | 0.03 | 0.06 | 4 | 4 | 15 | 93.3 | 100.0 |
| MXF | 0.06 | 0.12 | >8 | >8 | 12 | 91.7 | 100.0 | |
| GAT | 0.06 | 0.12 | >8 | >8 | 12 | 83.3 | 100.0 | |
| CIP | 0.12 | 0.25 | >8 | >8 | 10 | 90.0 | 100.0 | |
| AZM | >8 | >8 | >8 | >8 | 3 | 33.3 | 33.3 | |
| TOB | 0.06 | 0.12 | 8 | 16 | 14 | 85.7 | 100.0 | |
| S. pneumoniae (N = 35) | BES | 0.06 | 0.06 | 0.06 | 0.12 | 35 | 97.1 | 100.0 |
| MXF | 0.06 | 0.12 | 0.12 | 0.25 | 35 | 91.4 | 100.0 | |
| GAT | 0.12 | 0.25 | 0.25 | 0.5 | 35 | 100.0 | 100.0 | |
| CIP | 0.5 | 1 | 1 | 2 | 34 | 100.0 | 100.0 | |
| AZM | 0.06 | 0.25 | >8 | >8 | 25 | 68.0 | 92.0 | |
| TOB | 16 | 32 | 32 | 64 | 35 | 88.6 | 100.0 | |
| H. influenzae (N = 40) | BES | 0.015 | 0.015 | 0.015 | 0.03 | 40 | 92.5 | 100.0 |
| MXF | 0.015 | 0.03 | 0.03 | 0.03 | 40 | 97.5 | 100.0 | |
| GAT | 0.008 | 0.008 | 0.008 | 0.015 | 40 | 97.5 | 100.0 | |
| CIP | 0.008 | 0.015 | 0.008 | 0.015 | 40 | 100.0 | 100.0 | |
| AZM | 0.5 | 1 | 1 | 4 | 38 | 73.7 | 89.5 | |
| TOB | 2 | 2 | 4 | 4 | 40 | 100.0 | 100.0 | |
BES, besifloxacin; MXF, moxifloxacin; GAT, gatifloxacin; CIP, ciprofloxacin; AZM, azithromycin; TOB, tobramycin.
aMIC50, MBC50, MIC90 and MBC90 values were determined based on all isolates (N) tested for one species. Because some MIC or MBC values were higher than the tested range, the number of isolates (n) that yielded an MBC:MIC ratio was in some cases lower than the total number of isolates (N). The percentage of isolates with MBC:MIC ratios ≤4 was based only on the number of isolates that yielded MIC and MBC values within the test range (n).
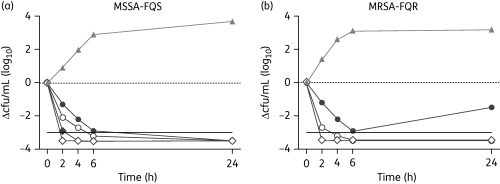
Time–kill assays were conducted for 12 isolates of FQS and FQR bacteria at concentrations that were 1×, 2×, 4× and 8× the respective MIC. Figure 1 shows representative examples of the concentration-dependent killing of FQS and FQR S. aureus strains 2, 4, 6 and 24 h after the addition of besifloxacin. Similar results were obtained for the other 10 test isolates after accounting for differences in their MICs [see Figure S1, available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)].
Figure 1.
Change in cfu/mL over time after the addition of besifloxacin. The methicillin- and ciprofloxacin-susceptible S. aureus (MSSA-FQS) isolate 1711525 (a) and the methicillin- and ciprofloxacin-resistant S. aureus (MRSA-FQR) isolate 1711529 (b) were treated with 1× (filled circles), 2× (open circles), 4× (filled diamonds) or 8× (open diamonds) the MIC of besifloxacin. MIC values were 0.03 mg/L for strain 1711525 and 1 mg/L for strain 1711529. A no-treatment growth control is identified by filled triangles. A 3 log reduction in viable cells is indicated by a continuous horizontal line.
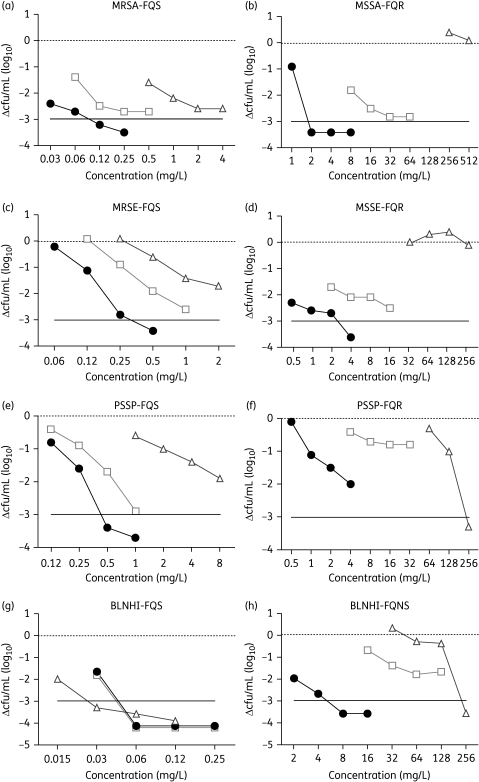
The relationship between drug concentration and the decrease in cfu 2 h after treatment with 1×, 2×, 4× and 8× the MIC of besifloxacin, moxifloxacin and ciprofloxacin is shown in Figure 2.
Figure 2.
Change in cfu/mL 2 h after the addition of 1×, 2×, 4× or 8× the MIC of besifloxacin (filled circles), moxifloxacin (open squares) or ciprofloxacin (open triangles). A 3 log reduction in viable cells is indicated by a continuous horizontal line. (a) S. aureus (MRSA-FQS). (b) S. aureus (MSSA-FQR). (c) S. epidermidis (MRSE-FQS). (d) S. epidermidis (MSSE-FQR). (e) S. pneumoniae (PSSP-FQS). (f) S. pneumoniae (PSSP-FQR). (g) H. influenzae (BLNHI-FQS). (h) H. influenzae (BLNHI-FQNS). PSSP, penicillin-susceptible S. pneumoniae; BLNHI, β-lactamase-negative H. influenzae.
Besifloxacin had the lowest MIC values for S. aureus and S. epidermidis and was the only agent that was able to reduce the cfu by >1000-fold after 2 h (Figure 2a–d). High levels of resistance prevented the testing of ciprofloxacin at 4× and 8× the MIC for FQR isolates of S. aureus (Figure 2b). Ciprofloxacin was not able to reduce the number of viable cells of FQR S. aureus and S. epidermidis (Figure 2b and d). The potency and bactericidal activity of moxifloxacin falls between that of besifloxacin and ciprofloxacin. Resistance to methicillin (oxacillin) in S. aureus and S. epidermidis did not affect the bactericidal activity of the fluoroquinolones, since methicillin-susceptible and -resistant strains had similar time–kill profiles [see Figure S2, available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)].
Against the FQS isolate of S. pneumoniae (Figure 2e), besifloxacin exhibited killing that was more rapid than that of either moxifloxacin or ciprofloxacin. Bactericidal activity against the FQR isolate was greater for ciprofloxacin than the other test agents; however, the ciprofloxacin MIC values were 16-fold higher than those of moxifloxacin and 128-fold higher than those of besifloxacin (Figure 2f). A 1000-fold reduction in cfu was observed after 6 h for besifloxacin and 24 h for moxifloxacin (data not shown).
Rapid bactericidal activity was observed against the FQS isolate of H. influenzae (Figure 2g), where ciprofloxacin had a 2-fold lower MIC value than besifloxacin and moxifloxacin. However, against the FQNS isolate, besifloxacin killed more rapidly than the other agents and did so at a concentration that was 8- to 16-fold lower than the comparators.
The nucleotide sequences of the QRDRs of the gyrA, gyrB, parC and parE genes for the six FQR isolates examined in the time–kill assays were determined and compared with those previously published for FQS strains; only nucleotide substitutions that were not present in any of the FQS reference strains are shown in Table 2. Codon 84 of GyrA in the staphylococci and H. influenzae, and the corresponding codon 81 in S. pneumoniae, encodes a Ser residue in FQS isolates. This was changed to a Leu, Phe or Tyr in the six FQR strains. Similarly, Ser-80 of the staphylococcal ParC (Ser-79 in S. pneumoniae and Ser-84 in H. influenzae) was replaced by a Tyr, Phe or Arg residue in FQR strains. While no other mutations were found in S. epidermidis, additional amino acid changes in gyrA, parC or parE were found in FQR isolates of S. aureus, S. pneumoniae and H. influenzae. Across all four species listed in Table 2, besifloxacin was 4- to 8-fold more potent than moxifloxacin and 16- to 256-fold more potent than ciprofloxacin against these FQR isolates.
Table 2.
Mutations associated with fluoroquinolone resistance in FQR isolates tested in time–kill assays
| MIC (mg/L) |
QRDR mutation(s)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Organism | Strain (phenotype) | BES | MXF | CIP | GyrA | GyrB | ParC | ParE |
| S. aureus | 1970149 (MSSA) | 1 | 8 | 256 | S84L | none | S80Y, E84G | none |
| 1711529 (MRSA) | 1 | 8 | 256 | S84L | none | S80Y, E84G | none | |
| S. epidermidis | 1711557 (MSSE) | 0.5 | 2 | 32 | S84F | none | S80Y | none |
| 1711555 (MRSE) | 0.5 | 2 | 32 | S84Y | none | S80F | none | |
| S. pneumoniae | 1115445 | 0.5 | 4 | 64 | S81Y | none | S79Y, K137N | none |
| H. influenzae | 1408337 | 2 | 16 | 32 | S84F, D88Y | none | S84R | D420N, S458A, S474N |
BES, besifloxacin; MXF, moxifloxacin; CIP, ciprofloxacin.
Discussion
Besifloxacin is a novel fluoroquinolone that was recently (May 2009) approved as a topical agent for the treatment of bacterial conjunctivitis. It was therefore of interest to assess the bactericidal activity of besifloxacin against the most common causes of this disease. MIC and MBC results in this study confirmed that besifloxacin had potent in vitro activity against clinical isolates of relevant species, and besifloxacin was also the most potent fluoroquinolone tested against S. aureus, S. epidermidis and S. pneumoniae. Besifloxacin, moxifloxacin and ciprofloxacin all demonstrated similar, potent activity against H. influenzae. MBC values of besifloxacin were within 4-fold of the MIC value for the majority of tested isolates, which is consistent with other fluoroquinolones shown to be bactericidal.6 There was no apparent correlation between the besifloxacin MBC:MIC ratios and resistance to either other fluoroquinolones or β-lactams such as methicillin (data not shown). The MBC:MIC ratios observed for besifloxacin were similar to those observed for other fluoroquinolones, although the MIC, MBC and time–kill profile of besifloxacin generally reflected better potency relative to the other fluoroquinolone comparators among FQR subpopulations. Similar activity against FQR isolates was also evident in a recent study that evaluated the antibacterial spectrum of besifloxacin against 40 Gram-positive and -negative species.2 Compared with FQS isolates, MIC values for FQR isolates generally increased to a lesser degree for besifloxacin than for the other fluoroquinolones tested. This trend of improved activity against FQR isolates was observed for various species of streptococci, staphylococci, Enterobacteriaceae, pseudomonads and Haemophilus sp.2
Similar to the fluoroquinolones, the MBC:MIC values showed that tobramycin was bactericidal against all four species tested. However, azithromycin was only bactericidal against S. pneumoniae and H. influenzae, but bacteriostatic against the staphylococci. This finding is consistent with previous reports, which showed that azithromycin was bactericidal against some species and bacteriostatic against others.7,8 In addition, azithromycin resistance was common among clinical isolates, necessitating drug concentrations that made it impractical to measure reliable MIC or MBC values for those isolates.
The time–kill assay results for selected isolates of S. aureus, S. epidermidis, S. pneumoniae and H. influenzae demonstrated the rapid bactericidal activity of besifloxacin, regardless of fluoroquinolone resistance phenotype. Besifloxacin achieved a 3 log kill for 11 of 12 isolates within 2 h and within 6 h for all 12 isolates. For many isolates, the profile of bacterial killing was more rapid than that of the other agents tested, even though the concentration of besifloxacin was in some cases as much as 256-fold lower than the other fluoroquinolone comparators.
Besifloxacin and moxifloxacin maintained bactericidal activity against S. aureus and S. epidermidis, regardless of the methicillin or fluoroquinolone resistance phenotype of the isolate. This is in contrast to ciprofloxacin, where only a bacteriostatic effect was observed against FQR isolates. Similar results were reported by Ince et al.,9 who noted that ciprofloxacin was similar to moxifloxacin in killing a ‘moderately resistant’ strain of S. aureus containing one Ser-80→Phe mutation in ParC. However, against a ‘highly resistant’ strain containing GyrA (Ser-84→Leu) and ParC (Ser-80→Phe) mutations, moxifloxacin maintained bactericidal activity, while ciprofloxacin was bacteriostatic.9 All four FQR isolates of S. aureus and S. epidermidis in the present time–kill study contained a Ser-84 mutation in GyrA and a Ser-80 mutation in ParC. In addition, the two S. aureus isolates contained an additional Glu-84 mutation in ParC, which might account for the higher MIC values compared with the two S. epidermidis isolates or the strain used by Ince et al.9
Besifloxacin and, to a lesser extent, moxifloxacin, were the most potent agents against FQS and FQR S. pneumoniae isolates, while ciprofloxacin had 8- to 128-fold higher MIC values. Besifloxacin was the fastest killing drug against the FQS S. pneumoniae isolate, while ciprofloxacin was the most rapidly bactericidal agent against FQR S. pneumoniae. However, the required drug concentration was 16- to 128-fold higher than that of moxifloxacin and besifloxacin, respectively. All three agents were rapidly bactericidal against FQS H. influenzae. With the exception of ciprofloxacin at 256 mg/L, besifloxacin was the only agent that was able to reduce the number of viable cells by >99.9% after 2 h. The time–kill kinetics of FQNS H. influenzae have not been studied in great detail and this is the first report that compares the bactericidal activity of besifloxacin with that of moxifloxacin and ciprofloxacin. Our results show that besifloxacin and moxifloxacin are bactericidal against FQS and FQR isolates of S. aureus, S. epidermidis, S. pneumoniae and H. influenzae; however, besifloxacin is active at equal or, in most cases, lower absolute drug concentrations.
Nucleotide sequencing of the QRDRs of gyrA, gyrB, parC and parE genes of the FQR isolates used for time–kill assays showed that each of the six strains, regardless of the species, contained at least two mutations. The Gram-positive species S. aureus, S. epidermidis and S. pneumoniae all contained one mutation in GyrA and one or two mutations in ParC, but no mutations in GyrB or ParE were observed. The H. influenzae isolate contained two mutations in GyrA and one mutation in ParC. While no GyrB mutations were found in this isolate, ParE contained three amino acid substitutions. Work by various investigators has found the same mutations described here and have shown that, while individual mutations can increase resistance to fluoroquinolones, high-level resistance is the result of multiple mutations that involve GyrA and ParC.10–18 Despite those multiple mutations, besifloxacin, and to a lesser extent moxifloxacin, maintained low MICs and potent bactericidal activity in the 24 h test period. In contrast, ciprofloxacin had notably higher MIC values for all species tested and lost bactericidal activity against FQR isolates of S. aureus and S. epidermidis.
Depending upon the pathogen, earlier generation fluoroquinolones, such as ciprofloxacin, preferably target either DNA gyrase or topoisomerase IV.19 Subsequent fluoroquinolones, such as moxifloxacin, and now besifloxacin, have shown progressively more balanced dual targeting activity, often inhibiting both enzymes with similar affinity.19 As a result, bacteria usually must accumulate two or more mutations to exhibit substantial in vitro resistance to the newer fluoroquinolones. Taken together with previous genetic and biochemical studies,19 the sequencing and MIC data presented here support the hypothesis that besifloxacin has well-balanced dual targeting activity, since the besifloxacin MICs were relatively low (0.5–2 mg/L) for strains with multiple QRDR mutations that had ciprofloxacin MICs of 32–256 mg/L.
Besifloxacin has previously been shown to have a broad spectrum of antibacterial activity in vitro,2 a favourable pharmacokinetic profile in animal conjunctiva and corneal tissue, as well as human tears,20,21 and efficacy in the treatment of bacterial conjunctivitis in clinical trials of 0.6% besifloxacin ophthalmic suspension.22–24 Following a single topical administration to healthy human subjects, the maximum besifloxacin concentration in tear fluid was 610 µg/g (i.e. ∼610 mg/L), with concentrations decreasing to ∼1.6 µg/g at 24 h.20 Besifloxacin concentrations in human tear fluid were sustained for at least 4 h at concentrations (approximately ≥85 mg/L) that were >20-fold above the highest MIC90 or MBC90 value measured in the present study for the four most prevalent ocular pathogens.20 Following a single instillation, the total area under the 24 h concentration–time profile (AUC24) for besifloxacin in human tear fluid was 1263 μg·h/g.20 Based on this single-dose AUC24 estimate, an AUC24/MIC90 ratio of ∼316 was achieved for S. aureus and S. epidermidis, and an AUC24/MIC90 ratio of >21 000 was obtained for S. pneumoniae and H. influenzae. After a single dose, besifloxacin exposure on the ocular surface achieved Cmax/MIC and AUC24/MIC90 ratios that were well above the generally accepted pharmacodynamic ratios required for fluoroquinolone efficacy (i.e. Cmax/MIC90 ≥ 10 and AUC24/MIC90 ≥ 30–50 for Gram-positive bacteria or ≥100–125 for Gram-negative bacteria).25–27 Taken together, these data indicate that the rapid, concentration-dependent in vitro bactericidal activity described here for besifloxacin at concentrations ranging from 0.015 to 8 mg/L are well within the clinically observed tear concentrations of this agent, and further support the empirical use of besifloxacin for treating ocular surface infections.20
Funding
This work was supported by a research grant from Bausch + Lomb, Rochester, NY, USA.
Transparency declarations
W. H., C. K. H., C. M. S. and T. W. M. are employees of Bausch + Lomb, Inc., Rochester, NY, USA. C. M. P. is an employee of Eurofins Medinet, Inc.
Supplementary data
Supplementary Material
Acknowledgements
Parts of this study were presented in poster form at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, 2009 (Poster D907).
We would like to thank Gary E. Zurenko and Jacqueline C. Lee of Micromyx (Kalamazoo, MI, USA) for editorial comments and Joel Proksch (Bausch + Lomb) for providing input on the pharmacokinetics and pharmacodynamics of besifloxacin.
References
- 1.Wolfson JS, Hooper DC, Swartz MN. Mechanisms of action and resistance to quinolone antimicrobial agents. In: Wolfson JS, Hooper DC, editors. Quinolone Antimicrobial Agents. Washington, DC: ASM Press; 1989. pp. 5–34. [Google Scholar]
- 2.Haas W, Pillar CP, Zurenko GE, et al. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53:3552–60. doi: 10.1128/AAC.00418-09. doi:10.1128/AAC.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline M26-A. Wayne, PA, USA: CLSI; 1999. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard M7-A7. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S18. Wayne, PA, USA: CLSI; 2008. [Google Scholar]
- 6.Schwalbe R, Steele-Moore L, Goodwin AC. Antimicrobial Susceptibility Testing Protocols. New York: CRC Press; 2007. [Google Scholar]
- 7.Ferrara A, Dos SC, Cimbro M, et al. Comparative antimicrobial activity and post-antibiotic effect of azithromycin, clarithromycin and roxithromycin against some respiratory pathogens. Int J Antimicrob Agents. 1996;7:181–6. doi: 10.1016/s0924-8579(96)00320-2. doi:10.1016/S0924-8579(96)00320-2. [DOI] [PubMed] [Google Scholar]
- 8.Retsema J, Girard A, Schelkly W, et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987;31:1939–47. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ince D, Zhang X, Hooper DC. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1410–5. doi: 10.1128/AAC.47.4.1410-1415.2003. doi:10.1128/AAC.47.4.1410-1415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ba BB, Arpin C, Vidaillac C, et al. Activity of gatifloxacin in an in vitro pharmacokinetic-pharmacodynamic model against Staphylococcus aureus strains either susceptible to ciprofloxacin or exhibiting various levels and mechanisms of ciprofloxacin resistance. Antimicrob Agents Chemother. 2006;50:1931–6. doi: 10.1128/AAC.01586-05. doi:10.1128/AAC.01586-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies TA, Kelly LM, Hoellman DB, et al. Activities and postantibiotic effects of gemifloxacin compared to those of 11 other agents against Haemophilus influenzae and Moraxella catarrhalis. Antimicrob Agents Chemother. 2000;44:633–9. doi: 10.1128/aac.44.3.633-639.2000. doi:10.1128/AAC.44.3.633-639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horii T, Suzuki Y, Monji A, et al. Detection of mutations in quinolone resistance-determining regions in levofloxacin- and methicillin-resistant Staphylococcus aureus: effects of the mutations on fluoroquinolone MICs. Diagn Microbiol Infect Dis. 2003;46:139–45. doi: 10.1016/s0732-8893(03)00037-3. doi:10.1016/S0732-8893(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 13.Jones ME, Critchley IA, Karlowsky JA, et al. In vitro activities of novel nonfluorinated quinolones PGE 9262932 and PGE 9509924 against clinical isolates of Staphylococcus aureus and Streptococcus pneumoniae with defined mutations in DNA gyrase and topoisomerase IV. Antimicrob Agents Chemother. 2002;46:1651–7. doi: 10.1128/AAC.46.6.1651-1657.2002. doi:10.1128/AAC.46.6.1651-1657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaPlante KL, Rybak MJ, Tsuji B, et al. Fluoroquinolone resistance in Streptococcus pneumoniae: area under the concentration–time curve/MIC ratio and resistance development with gatifloxacin, gemifloxacin, levofloxacin, and moxifloxacin. Antimicrob Agents Chemother. 2007;51:1315–20. doi: 10.1128/AAC.00646-06. doi:10.1128/AAC.00646-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Deguchi T, Yasuda M, et al. Alteration in the GyrA subunit of DNA gyrase and the ParC subunit of DNA topoisomerase IV in quinolone-resistant clinical isolates of Staphylococcus epidermidis. Antimicrob Agents Chemother. 1998;42:3293–5. doi: 10.1128/aac.42.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Mariano N, Rahal JJ, et al. Quinolone-resistant Haemophilus influenzae in a long-term-care facility: nucleotide sequence characterization of alterations in the genes encoding DNA gyrase and DNA topoisomerase IV. Antimicrob Agents Chemother. 2004;48:3570–2. doi: 10.1128/AAC.48.9.3570-3572.2004. doi:10.1128/AAC.48.9.3570-3572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Vazquez M, Roman F, Aracil B, et al. Laboratory detection of Haemophilus influenzae with decreased susceptibility to nalidixic acid, ciprofloxacin, levofloxacin, and moxifloxacin due to GyrA and ParC mutations. J Clin Microbiol. 2004;42:1185–91. doi: 10.1128/JCM.42.3.1185-1191.2004. doi:10.1128/JCM.42.3.1185-1191.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Yoshida J, Hatou S, et al. Mutations in the quinolone resistance determining region in Staphylococcus epidermidis recovered from conjunctiva and their association with susceptibility to various fluoroquinolones. Br J Ophthalmol. 2008;92:848–51. doi: 10.1136/bjo.2007.129858. doi:10.1136/bjo.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cambau E, Matrat S, Pan XS, et al. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009;63:443–50. doi: 10.1093/jac/dkn528. doi:10.1093/jac/dkn528. [DOI] [PubMed] [Google Scholar]
- 20.Proksch JW, Granvil CP, Mermet-Siou R, et al. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J Ocul Pharmacol Ther. 2009;25:335–44. doi: 10.1089/jop.2008.0116. doi:10.1089/jop.2008.0116. [DOI] [PubMed] [Google Scholar]
- 21.Ward KW, Lepage JF, Driot JY. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J Ocul Pharmacol Ther. 2007;23:243–56. doi: 10.1089/jop.2006.0137. doi:10.1089/jop.2006.0137. [DOI] [PubMed] [Google Scholar]
- 22.Karpecki P, DePaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31:514–26. doi: 10.1016/j.clinthera.2009.03.010. doi:10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116:1615–23. doi: 10.1016/j.ophtha.2009.05.014. doi:10.1016/j.ophtha.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Tepedino ME, Heller WH, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25:1159–69. doi: 10.1185/03007990902837919. doi:10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 25.Wright DH, Brown GH, Peterson ML, et al. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46:669–83. doi: 10.1093/jac/46.5.669. doi:10.1093/jac/46.5.669. [DOI] [PubMed] [Google Scholar]
- 26.Allen GP, Kaatz GW, Rybak MJ. In vitro activities of mutant prevention concentration-targeted concentrations of fluoroquinolones against Staphylococcus aureus in a pharmacodynamic model. Int J Antimicrob Agents. 2004;24:150–60. doi: 10.1016/j.ijantimicag.2004.03.011. doi:10.1016/j.ijantimicag.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Metzler K, Hansen GM, Hedlin P, et al. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int J Antimicrob Agents. 2004;24:161–7. doi: 10.1016/j.ijantimicag.2004.02.021. doi:10.1016/j.ijantimicag.2004.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.