Abstract
Purpose of review
A better understanding of the molecular events underlying stroke recovery might be useful to optimize restorative therapies. Measurement of these events, however, is generally inaccessible in humans, at least at the molecular level. Substitute measures, or biomarkers, that are accessible and that provide useful insights might provide deeper insights into spontaneous recovery in humans. This review considers advances in insights into using biomarkers to understand recovery from stroke, and to serve as a surrogate measure of stroke recovery, including in a clinical trial context.
Recent findings
Among the key recent findings is that measures of brain function and injury are the strongest predictors of treatment effect, moreso than behavioral measures are despite the reliance on behavioral measures as study entry criteria. Functional neuroimaging studies have provided insights into therapeutic mechanism of action. In addition, measures of CNS function have been used to estimate individual therapy needs, findings that suggest the potential to tailor restorative therapies to the specific needs of individual patients.
Summary
Many therapies are emerging as potentially useful to promote improved recovery after stroke. Continued advances in biomarkers are providing new insights into the neurobiology of both spontaneous and therapy-induced brain repair after stroke.
Keywords: stroke, plasticity, recovery, therapy
Introduction
Stroke is a major source of disability. Most patients do not reach the hospital in time to receive acute therapies. The majority of those who do receive such therapies nevertheless still have significant disability, as spontaneous stroke recovery is generally incomplete[1]. One area of therapeutics that has the potential to further reduce disability revolves around restorative therapeutics. A restorative therapy can be defined as one that aims to improve outcome by promoting repair and restoration rather than by salvaging acutely threatened tissue. Restorative interventions under study have included growth factors, cells, small molecules, intensive and activity-based therapy, robotics, neuroprosthetics, electromagnetic brain stimulation, and cognitive strategies such as motor imagery[2]. Restorative therapies for stroke span the spectrum in terms of readiness for patient application, including publication of positive phase 3 trial results[3,4]. Whether the focus is on spontaneous recovery or on therapy-induced repair, a better understanding of the underlying cellular and molecular events might be useful to improve patient outcomes.
Measurement of the events underlying recovery, however, is generally inaccessible in humans, at least at the molecular level. Substitute measures, or biomarkers, that are accessible and that provide useful insights might provide deeper insights into spontaneous recovery in humans, and might also serve as surrogate markers in clinical trials of restorative therapies.
Biomarkers: general considerations
In the simplest case, a biomarker of stroke recovery would be a measure that correlates with clinical status or with clinical evolution. Such a marker might be useful in constructing or testing a biological model of recovery. A well-designed functional neuroimaging study can also provide biomarker data around a specific subset of neural events, for example, providing insights into specific neurochemical components of plasticity[5,6].
Biomarkers in a clinical trial might serve as a surrogate marker, which has been defined as “a laboratory measurement…used as a substitute for a clinically meaningful endpoint.…”[7] One of the earliest examples in stroke recovery was from Carey et al[8], who hypothesized that affected limb training would be associated with increased reliance on the ipsilesional motor system, that is, that measures of ipsilesional motor system function were a biomarker for treatment effects. These authors found that, in subjects with chronic stroke prior to training, affected hand finger tracking movements activated contralesional brain areas. However, after training at this task, the normal pattern of laterality of brain activation was restored, with activation shifting to ipsilesional brain regions.
In general, surrogate markers are particularly useful in phase 2 trials, for example, to probe biological activity of a proposed therapy, to inform the decision whether or not to proceed to phase 3[9,10], or by helping define a target population[11].” Examples include blood pressure as a surrogate marker for vascular death, HIV RNA levels as a marker of progression to AIDS, or tumor size as a marker of survival. Biomarker data might also be used to guide features of therapy in ways that behavioral or demographic data can not. This perspective has received increased attention in the acute stroke setting, where some studies have suggested that diffusion-perfusion mismatch might be a useful surrogate marker of salvable penumbra[12-14].
A number of caveats exist when considering the utility of a biomarker in this context. Surrogate endpoints are generally easier to measure than behavioral endpoints are, easier to standardize, and save time and money[9,15]--but these features are not always applicable for many of the imaging or physiological measures proposed as recovery-related surrogate endpoints. A measure has reduced potential to serve as a valid surrogate endpoint when it is not in the causal pathway of the disease process, when the therapeutic intervention selectively affects physiology of the surrogate, or when the surrogate measure does not fully capture the net effect of treatment on the clinical outcome[9,16]. The usual clinometrics considered for any outcome measure, such as validity, reliability, sensitivity, and specificity (see Figure 1), are pertinent to any discussion of biomarkers but in general have been incompletely assessed in the setting of stroke recovery[17-20]. Numerous influences and other covariates relevant to other aspects of stroke[21] are likely important to biomarkers of recovery and vastly complicate assessment of these markers.
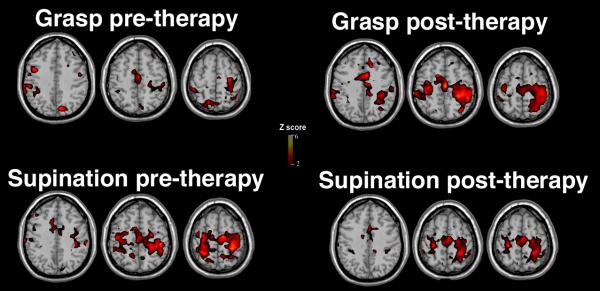
Figure 1.
Specificity of fMRI measures in relation to post-stroke therapy. Ten patients with chronic left brain hemiparetic stroke underwent fMRI scanning during two tasks, received three weeks of robotic therapy, then underwent repeat fMRI scanning. The content of the robotic therapy emphasized grasp/release movements. Group fMRI maps are displayed for 9 (bottom row) to 10 (top row) patients in whom such data were available. The top row shows evolution of fMRI activation for the task that was extensively practiced as part of therapy, with a significant 20-fold increase in left primary sensorimotor cortex activation. The bottom row shows that this increase was absent for a control task, pronation-supination, that relied on similar muscles but that was not rehearsed as part of therapy. The results suggest specificity of therapy effects on an fMRI biomarker[17].
Recovery after stroke
Animal studies have provided insights into the cellular and molecular events underlying spontaneous and treatment-induced stroke recovery for which human biomarkers might be useful. As reviewed elsewhere[22-24], these include structural changes in axons, dendrites, and synapses; increased activation and migration of endogenous neural stems; plus changes in extracellular matrix, growth factor levels, glia, angiogenesis, and excitability. In some cases, these changes arise bilaterally after a unilateral stroke. Cortical representational maps can shift and expand in relation to injury, behavioral recovery, and training.
Human studies of stroke recovery also provide information useful for selecting biomarkers of recovery. Overall, the best spontaneous return of behavior is associated with the greatest return of activity in primary cortex. Useful compensatory brain responses include increased activation in secondary areas that are normally connected to the injured zones through a distributed network, a shift in interhemispheric lateralization towards the contralesional hemisphere, and shifts in representational maps surrounding the infarcted zone. In general, the best behavioral outcomes after stroke are associated with the greatest return of brain function towards the normal state of organization[25]. Some authors have suggested that those events that support spontaneous recovery are likely to be the same events that are measured as biomarkers[26].
A biomarker might be selected on any of several bases. For example, a biomarker might be selected as it correlates with a cross-sectional estimate of behavioral status, with change in behavioral status over time, with response to a therapeutic intervention, or in relation to a key covariate of interest. The choice of test used might vary across these situations, but as described below, likely substantial overlap exists. Choice of biomarker might also emphasize pragmatic issues: most patients with stroke are often infirm with reduced capacity for extended testing; many have a range of stroke-related deficits, such as inattention, aphasia, or depression, that reduce capacity to undergo testing; and have relevant concomitant medical and demographic issues, such as median age in the eight decade.
Biomarkers of potential value to recovery after stroke
A number of measures of potential interest are available to serve as a biomarker of recovery. The most elementary examples are simple behavioral measures, such as short-distance measures of gait as a marker of overall vascular or social status[27], index finger extension as a predictor of arm recovery[28], or a blood test as a marker of subsequent clinical outcome[29]. Simple brain imaging measures also have potential value, for example, with total infarct volume correlating with, and predicting, overall neurological status[30,31].
More complex anatomical imaging measures are also of potential value as biomarkers of recovery. Imaging of region pertinent to specific neurotransmitters might have value for specific hypotheses such as those related to memory[32]. High resolution MRI can detect changes in cortical thickness[33], sometimes increased with treatment[34]. Diffusion tensor imaging (DTI) measures the tendency of water movement to be directional, and so reflects injury when stroke reduces the linearity of water movement, or fractional anisotropy, within a directional tract such as the corticospinal. DTI data provide a measure of white matter tract status that not only describes injury[35], but also can provide insights into repair[36]. DTI, like fMRI[37], is of particular value to translational science in brain repair because the same measurements obtained in animal models can be used in human subjects. Multimodality approaches provide rich insights in this context[38,39].
Measures of CNS function might also be useful biomarkers. Physiological measures such as motor system transcranial magnetic stimulation (TMS) can yield information on the size of a cortical representational map, or on the speed/magnitude of the motor evoked potential (MEP), reflections of CNS injury[40]. Brain function can be measured with functional MRI (fMRI) or positron emission tomography (PET), as well as with electroencephalography or magnetoencephalography. Use of such methods can measure the volume of regional activation, such as the volume of Broca’s area activated during a speech output task; the magnitude of activation, such as the height of parietal activation during a spatial attention task; or the balance of activation across hemispheres, sometimes reported as the laterality index (LI). PET can provide a direct measure of many processes including regional blood flow, metabolism, and receptor ligand occupation. Other tissue function measures include measures of cortical excitability or inhibition, or assessments of the connectivity between various cortical regions[41].
Measurement of tissue function introduces complexities that do not arise with measurement of tissue anatomy. To activate the brain with fMRI or PET, the subject must engage in a specific behavioral paradigm, correctly, according to instructions, on cue--at times a challenge after stroke. The behavioral paradigm must be carefully selected to probe the brain functional circuit of interest. These issues influence the utility of such a biomarker, and do so in a manner that might vary across sites.
There are many types of relationship that a biomarker might have with the process or behavior of interest. A biomarker might simply be a direct correlation with the level of activity in a specific brain area. For example, better outcome has been found to correlate with larger activation maps within ipsilesional motor cortex using a range of techniques[42,43]. In such cases, a TMS, fMRI, or PET measure might be used to determine which patients have more of a useful recovery-related process ongoing. A useful biomarker might also have an inverse correlation, for example, with reduced blood flow[44], activation[25], or evoked potentials[45] in specific brain areas at times corresponding to better behavioral outcome. A biomarker might involve excitatory or inhibitory[46] measures.
Although a preponderance of evidence suggests that the most critical processes for behavioral recovery take place in the ipsilesional hemisphere, a number of observations suggest that events useful to recovery can also be measured in the contralesional hemisphere[47-51]. In studies of brain activation, the LI[52] has been a useful metric for defining the activation balance between hemispheres, with recent studies providing refined methods for its determination[53].
Potential applications of stroke recovery biomarkers
A biomarker for recovery that is found to be valid and reliable might be employed in several different ways to improve patient outcomes. Functional neuroimaging measures might be used to guide dose of therapy, much as serum TSH is used to guide treatment of hypothyroidism or exercise treadmill testing is used to guide treatment of chest pain. Repeated measurement of fMRI laterality measurements[54] or motor evoked potential by TMS[55] have been found useful in this regard, with change over time in these measures suggesting potential modifications of a restorative therapy dose. Given that individual patient responses to a restorative therapy are highly variable, great promise exists in the use of biomarkers to guide qualitative or quantitative treatment choices in order to derive maximum patient gains.
A biomarker that can substantially improve prediction of spontaneous outcome[56], or of response to treatment, might be of enormous value to clinicians and clinical trialists. One candidate measure is fMRI-based. In patients with chronic stroke, smaller ipsilesional primary motor cortex activation during fMRI predicted gains from motor related therapy, and did so more strongly than many other clinical or imaging metrics did[57] (Figure 2). Stinear et al[35] achieved good success at predicting arm motor gains from therapy using DTI-based measures of white matter integrity, TMS-based measures of motor system function, demographics, and behavioral status.
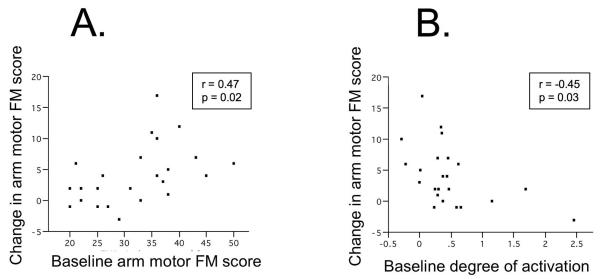
Figure 2.
Potential of fMRI to provide an independent measure for predicting response to therapy in chronic stroke. Twenty four patients underwent baseline behavioral and fMRI testing received six weeks of therapy, and had repeat behavioral testing. Measures of (A) baseline motor status, the arm motor Fugl-Meyer (FM) score and of (B) baseline brain function, the degree of activation in ipsilesional primary motor cortex as measured from fMRI brain mapping each predicted behavioral gains from subsequent therapy, and survived as the only such predictors in a multivariate model. Note that variables not surviving in the model included the classic stroke outcome predictors of age, time post-stroke, and infarct volume. In this population, better baseline motor status predicted greater gains from therapy. Also, lesser baseline motor cortex function during distal upper extremity movements predicted greater gains, suggesting underuse of available motor cortex resource at baseline. The two variable model, while exciting in its emphasis that behavioral and fMRI measures can have independent and complementary value, nevertheless only explained 40% of the variance in response to therapy[57].
Much work remains to bring biomarkers of stroke recovery to the point of utility. The degree of variance in behavioral outcome after therapy explained by biomarkers remains limited. For example, a measure of CNS injury based on DTI accounted for only 38% of the variance in clinical gains in one study[35]; an fMRI-based measure of CNS function, only 20%[57]. Also, a recent meta-analysis[58] examined functional neuroimaging as a biological marker of treatment effects. Concerns highlighted included that published data are skewed given that most studies using biomarkers in this context have preferentially enrolled patients with good baseline behavioral status, and so the value of recovery biomarkers in most patients remained less explored. Also, many studies using recovery biomarkers have focused on patients in the chronic phase of stroke, but data on patients in the initial weeks post-stroke are needed given that such biomarkers might also prove important, and vary from chronic phase findings[59,60], during this acute time period. The optimal technique to use for deriving biomarker data remains unclear, with few publications directly comparing measurements across modalities.
Nevertheless, recent studies investigating biomarkers of recovery following stroke have provided useful insights into treatment mechanism, patient selection, and prognosis. These findings are facilitating emergence of restorative therapies in patients with stroke, and thus deliver on the promise to reduce disability after stroke.
Conclusion
Biomarkers that measure CNS injury and function have the potential to provide insights into recovery, serve as surrogate markers in clinical trials, and possibly guide therapeutic decision making in a manner than can improve outcomes for individual patients. Recent findings are exciting and heuristic, but further studies are needed for such biomarkers to reach the point of widespread utility.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Gresham G, Duncan P, Stason W, Adams H, Adelman A, Alexander D, Bishop D, Diller L, Donaldson N, Granger C, et al. U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; Rockville, MD: Post-Stroke Rehabilitation. 1995
- 2.Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 3.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D, Clark PC. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. **This report provides phase 3 data showing that an intervention in patients with chronic stroke can produce significant, meaningful, and enduring behavioral gains.
- 5.Butefisch C, Davis B, Wise S, Sawaki L, Kopylev L, Classen J, Cohen L. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardy J, Pariente J, Leger A, Dechaumont-Palacin S, Gerdelat A, Guiraud V, Conchou F, Albucher JF, Marque P, Franceries X, et al. Methylphenidate modulates cerebral post-stroke reorganization. Neuroimage. 2006;33:913–922. doi: 10.1016/j.neuroimage.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Temple R. A regulatory authority’s opinion about surrogate endpoints. In: Nimmo W, Tucker G, editors. Clinical Measurement in Drug Evaluation. J. Wiley; 1995. [Google Scholar]
- 8.Carey J, Kimberley T, Lewis S, Auerbach E, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 9.Fleming T, DeMets D. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Stephen RM, Gillies RJ. Promise and progress for functional and molecular imaging of response to targeted therapies. Pharm Res. 2007;24:1172–1185. doi: 10.1007/s11095-007-9250-3. [DOI] [PubMed] [Google Scholar]
- 11.Collins JM. Functional imaging in phase I studies: decorations or decision making? J Clin Oncol. 2003;21:2807–2809. doi: 10.1200/JCO.2003.05.100. [DOI] [PubMed] [Google Scholar]
- 12.Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, Albers GW. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke. 2007;38:1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 15.Boissel J, Collet J, Moleur P, Haugh M. Surrogate endpoints: a basis for a rational approach. Eur J Clin Pharmacol. 1992;43:235–244. doi: 10.1007/BF02333016. [DOI] [PubMed] [Google Scholar]
- 16.Bucher H, Guyatt G, Cook D, Holbrook A, McAlister F. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence-Based Medicine Working Group. Jama. 1999;282:771–778. doi: 10.1001/jama.282.8.771. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2007 doi: 10.1093/brain/awm311. *This study demonstrates specificity of brain changes in relation to a restorative therapy.
- 18.Bosnell R, Wegner C, Kincses ZT, Korteweg T, Agosta F, Ciccarelli O, De Stefano N, Gass A, Hirsch J, Johansen-Berg H, et al. Reproducibility of fMRI in the clinical setting: Implications for trial designs. Neuroimage. 2008;42:603–610. doi: 10.1016/j.neuroimage.2008.05.005. *A particularly thoughtful treatment of the issue of reliability of fMRI in its application to studies of patients with neurological disorders.
- 19.Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41:311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Exp Brain Res. 2008;186:183–190. doi: 10.1007/s00221-007-1221-8. [DOI] [PubMed] [Google Scholar]
- 21.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 22.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 23.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 24.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 25.Ward N, Brown M, Thompson A, Frackowiak R. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca MA, Filippi M. Functional MRI in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):36S–41S. doi: 10.1111/j.1552-6569.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 28.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, Moretto G, Bovi P, Gambarin M. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 2007;38:1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 29.Geiger S, Holdenrieder S, Stieber P, Hamann GF, Bruening R, Ma J, Nagel D, Seidel D. Nucleosomes as a new prognostic marker in early cerebral stroke. J Neurol. 2007;254:617–623. doi: 10.1007/s00415-006-0407-5. [DOI] [PubMed] [Google Scholar]
- 30.Brott T, Marler J, Olinger C, Adams H, Tomsick T, Barsan W, Biller J, Eberle R, Hertzberg V, Walker M. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20:871–875. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- 31.Saver J, Johnston K, Homer D, Wityk R, Koroshetz W, Truskowski L, Haley E. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 32.Bocti C, Swartz RH, Gao FQ, Sahlas DJ, Behl P, Black SE. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke. 2005;36:2126–2131. doi: 10.1161/01.STR.0000183615.07936.b6. [DOI] [PubMed] [Google Scholar]
- 33.Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- 34.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. **This landmark paper demonstrates important advances in use of multimodal testing for predicting treatment gains among patients with chronic stroke.
- 36.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Panda S, Davarani SP, Athiraman H, Li Q, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dijkhuizen R, Singhal A, Mandeville J, Wu O, Halpern E, Finklestein S, Rosen B, Lo E. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34:253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. *A clear use of connectivity measures in the assessment of patients with stroke.
- 42.Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke correlates with the degree of cortical reorganization. Clin Neurophysiol. 2004;115:2144–2150. doi: 10.1016/j.clinph.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Zemke A, Heagerty P, Lee C, Cramer S. Motor cortex organization after stroke is related to side of stroke and level of recovery. Stroke. 2003;34:E23–28. doi: 10.1161/01.STR.0000065827.35634.5E. [DOI] [PubMed] [Google Scholar]
- 44.Weiller C, Chollet F, Friston K, Wise R, Frackowiak R. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 45.Turton A, Wroe S, Trepte N, Fraser C, Lemon R. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroenceph clin Neurophys. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 46.Manganotti P, Acler M, Zanette G, Smania N, Fiaschi A. Motor Cortical Disinhibition During Early and Late Recovery After Stroke. Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968307313505. [DOI] [PubMed] [Google Scholar]
- 47.Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 48.Johansen-Berg H, Rushworth M, Bogdanovic M, Kischka U, Wimalaratna S, Matthews P. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaechter J, Kraft E, Hilliard T, Dijkhuizen R, Benner T, Finklestein S, Rosen B, Cramer S. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 50.Luft A, McCombe-Waller S, Whitall J, Forrester L, Macko R, Sorkin J, Schulz J, Goldberg A, Hanley D. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. Jama. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, Demonet JF, Cardebat D. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 52.Cramer S, Nelles G, Benson R, Kaplan J, Parker R, Kwong K, Kennedy D, Finklestein S, Rosen B. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 53.Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34:322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 55.Koski L, Mernar T, Dobkin B. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair. 2004;18:230–249. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- 56.Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F. Prognostic Value of fMRI in Recovery of Hand Function in Subcortical Stroke Patients. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 57.Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, Trouard TP, Squire SW, Weinand ME, Savage CR, et al. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 58.Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil. 2006;87:36–42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Ward N, Brown M, Thompson A, Frackowiak R. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55:829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]