Abstract
Noise can induce excitable systems to make transient transitions between quiescent and active states. Here we investigate the possibility that these transitions occur locally in a spatially-extended medium, leading to the occurrence of spatiotemporal patches of activation. We show that this can in fact occur in a parameter range such that there exist (in general unstable) localized solutions of the governing deterministic reaction-diffusion equations. Our work is motivated by a recent biological example showing transiently excited cell membrane regions.
Localized patterns arise in situations where the coupling between nonlinear reactions and diffusive transport can allow for an active region to co-exist with surrounding quiescent media [1]. These patterns can propagate, as in the classic cases of solitons in the nonlinear Schrodinger equation [2], or can be stationary, as initially discussed by Koga and Kuramoto for chemical systems and further studied by others [3{5]. In the chemical context, there has been much theoretical and experimental work on localized structures, the former focusing on two component reaction-diffusion models [6] and the latter enabled by the invention of continuously fed unstirred reactors [7]. Aside from these, examples of localized structures have been observed in many other experimental systems, ranging from fluid mechanics [8] to optics [9] to granular media [10] to vegetation [11]. However, in all the above mentioned scenarios the localized structures, once formed, are either oscillatory or stationary in time, namely the established pattern is stable and the system does not spontaneously return to the homogeneous quiescent state.
In this paper we focus on the idea of generating finite-lifetime localized structures that we will refer to as ‘patches’. Our work is motivated by a specific biological example to be discussed shortly, but is meant to investigate this general issue using a generic model rather than one specifically aimed at any particular experimental realization. It is well known that excitable dynamical systems can be induced to make temporally localized excursions to the excited state by the addition of noise [12], and we here ask the same type of question in a spatially- extended excitable medium: can noise induce a spatio-temporally localized excursion to the excited state? We show that this is indeed possible, as long as the system supports a (usually unstable) localized solution of the deterministic limit of the equations.
First, we present the biological motivation. In the cellular slime mold Dictyostelium discodium, it has been shown that various proteins leave the cytoplasm and attach to the cell membrane, upon stimulation with the chemical chemoattractant cAMP [13]. In particular, Van Haastert and co-workers have shown [14] that uniform application of cAMP leads, after some delay, to the spontaneous emergence of localized regions of high protein concentration, visualized as fluorescent patches along the membrane (Fig. 1). The patches, of sizes around 9 μm (on the two-dimensional plane image of the cell), are closely related to the subsequent formation of pseudopods (cell extensions). A typical patch has a one minute lifetime, after which it is degraded and eventually reappears elsewhere. When the cell is exposed to a cAMP gradient, a single patch will typically appear, with average size and life time which are similar to the uniform stimulus case. Additional and more upstream effectors were also shown to have a similar dynamics of appearing, disappearing and re-appearing[15]. It was hypothesized [16] that this dynamics has a crucial role in determining the observed cell shape and motion.
FIG. 1.

(color online) (A) Cell at different times after stimulation with 1 uM cAMP. (B) PHCrac-GFP at the boundary, presented as the difference of fluorescence intensity between the boundary and the cytosol, color-coded as shown below panel A. The ordinate refers to the position along the cell with 0 indicated by the arrow in the cell image. This figure is taken with permission from [14]
Previous works attempted to explain the spontaneous formation of membrane-bound patches, based on an assumed Turing instability of the uniform state [17]. This explanation does not account for the observed finite lifetime - once a patch is formed it is stationary and does not spontaneously vanish. While this could be accommodated by introducing “self-poisoning” (i.e. the patch creates another chemical species that inexorably destroys it [18]), the question remains whether intrinsically transient structures are possible. An alternate possibility is that these patches are nucleated by random fluctuations acting on an excitable system; this hypothesis forms the motivation for the study presented here. As we show below, a spatially extended system can still be excitable and excursions around an excited state can lead to the formation of finite-time localized patterns.
To study this question we introduce a standard two-component FitzHugh-Nagumo model consisting of coupled equations for an activator u and an inhibitor v:
| (1) |
We will assume that β < 1. Without diffusion in either species, this two-component system has three fixed points for all values of the parameters α and β. The quiescent fixed point at u = −1, is always stable; the system switches from being excitable to bistable as α increases through unity due to a change of stability of the fixed point with largest u; this fixed point is either at or at u = 1, depending on whether α is larger or smaller than 1 + β. The unstable fixed points in this system are typically saddles. In all subsequent simulations, Δx is chosen to be and Δt is chosen to be small enough for numerical stability.
We will investigate this system in one spatial dimension. We chose to focus on the one-dimensional case because of the experimental motivation: In most of the relevant experiments, cells are attened (using agar or two- dimensional microfluidic devices) so that the cell membrane is practically one-dimensional. We believe that also in higher dimensions, qualitatively similar results will be obtained, due to the interplay between the local excitation and the long-range inhibition.
For the standard case of more rapidly diffusing activator Du > Dv, a localized excitation will spread as a counter-propagating pair of pulses. We are interested here in the opposite situation, where the inhibitor spreads more rapidly. In this case, one might expect the system to be able to support localized excitations in which the creation of inhibitor by the localized activated region prevents propagation. We note that this scenario is common in the context of the studied biological system. Dictyostelium cells polarize (i.e. form well distinguished front and back) in the presence of a cAMP gradient as shallow as 5%. Most of the suggested models that try to explain this phenomenon are based on local excitation and global (or long-ranged) inhibition [13, 19]. The model we propose here is therefore compatible with other models in the field and describes a plausible biological mechanism.
To see if fluctuations can generate localized and transient structures, we add noise to the u equation. As already mentioned, noise can generate excursions to the excitable state in non-spatially-extended systems and in one dimension, noise can also generate counter-propagating pulses [20]. In order to generate non-trivial responses, the noise magnitude needs to exceed some threshold, below which no excursion will occur. But spatially coupling many points with high noise magnitude leads to an overall very noisy behavior, that prevents the formation of any coherent structures. Also, it is extremely unlikely that the noise in a real biological system would be Gaussian and white. Instead, we assume that the noise also arises from a (local) excitable system and hence is spatially sparse and uncorrelated and lasts for a fixed time. Such punctuate patterns are in fact seen in nonlinear excitable processes (see [21] for an example in a model of genetic networks). Specifically, only a small fraction of the points (0.001–0.01%), randomly selected, are given a non-zero noise term. The non-zero noise terms are uniformly distributed in the range [−1,1], and have a correlation time of 50 Δt, which is much smaller than the patch life time.
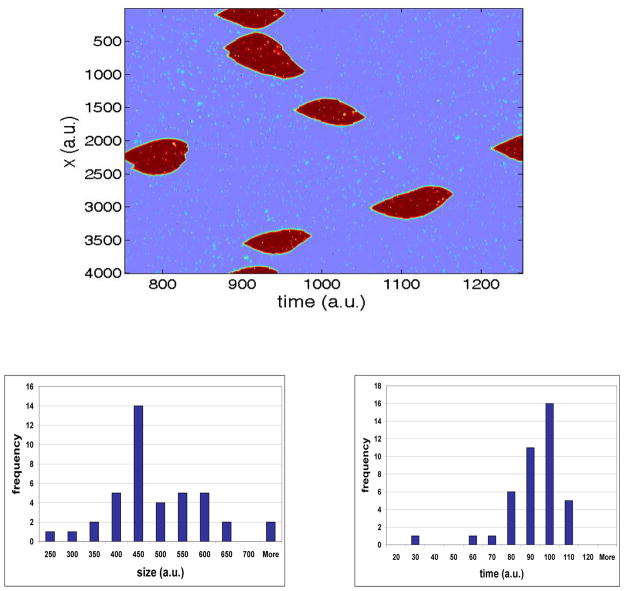
Fig 2a shows that our stochastic excitable system can indeed generate patches (defined to be regions of high u) which are localized in time and space. These patches have a typical width and life time, as can be seen in Figs 2b and 2c, respectively, but the individual patch sizes are distributed around this characteristic average size. In the experimental work described above, PH-domain patches were found to have a variability in size of about 35% (standard-deviation over mean) in the case of uniform stimulation. For the model case presented in Fig. 2 the size variability of the patches is 30% and for the case of β = 0.6 a variability of 40% was obtained (data not shown). Hence, the experimental data is consistent with this type of stochastic process. For some cases, patches are nucleated close enough to each other such that their inhibitory v fields overlap. In parameter ranges where this occurs, patch sizes are affected by the noise frequency, as it affects the density of nucleated patches. For example, if the noise frequency is set to be larger than the one used for Fig 2a, the patches become significantly smaller due to this mutual inhibition and vanish slightly faster. However, for a given set of parameters patches are narrowly distributed so that typical time and space scales can be defined.
FIG. 2.
(color online) a. Randomly created localized patches, obtained by adding temporally-correlated noise with uniformly distributed noise to a small number of the points (as described in the text). The patches have a typical size and lifetime that depend on their density. In this system α = 0.5, β = 0.7, ε = 0.01, Du = 1,Dv = 5 and noise frequency is 0.002%. b–c. Patch width (b) and lifetime (c) distribution (system parameters as in a).
Given the existence in an excitable system of stationary solutions, it is natural to inquire about their connection to the patches we have seen in simulations. We therefore construct the localized solution for the deterministic set of equations (1). We start from the approximate analytical solution for small ε, wherein u switches from u = 1 to u = −1 over a spatial scale of order over which v is essentially constant. The detailed solution takes the form [22]
| (2) |
where
and the full width Λ is determined by
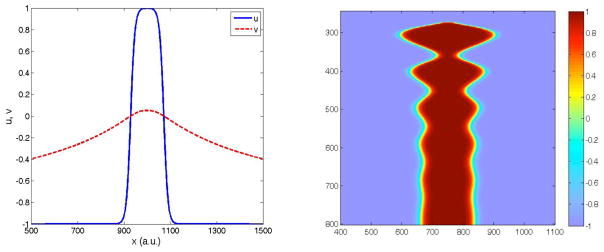
These solutions can be numerically extended to finite values of ε, as can be seen in Fig. 3a for the same set of parameters as in Fig. 2a.
FIG. 3.
(color online) a. Stationary pulse solution for the system of eqs. (1). u(x) (solid blue line) and v(x) (dashed red line) System parameters as in Fig. 2. b. Deterministic simulation (no noise). The stationary pulse may be stable or unstable, as seen here for α = 0.5, β = 0.6, ε = 0.01,Du = 1,Dv = 6. The stationary pulse eventually vanishes via an oscillatory instability.
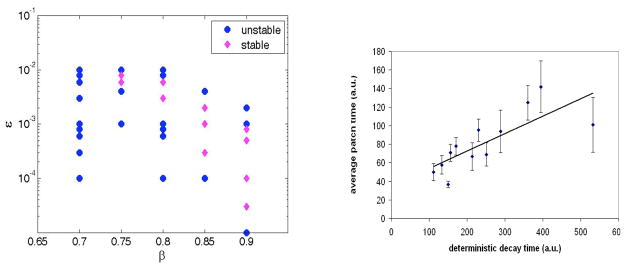
The pulse solutions have a limited range of stability in ε, as can be seen by direct numerical simulation of the noise-free system. For ε above and below certain limits, the solutions exhibit a Hopf instability. For example, For the parameter α = 0.5, β= 0.7, ε = 0.01, Du = 1, Dv = 5 used in Figs 2a and 3a the pulse solution is stable, whereas in Fig 3b (for β = 0.6) the pulse solution exhibits a growing oscillation which eventually destroys the excited state. The instability for small ε can be understood from the analytic solution [23]. The large ε instability on the other hand is not present in the sharp front solution. A β − ε plane phase-diagram is presented in Fig. 4a.
FIG. 4.
(color online)a. Phase space for the parameters β and ε, obtained by deterministic simulations. The simulations were typically run 108 iterations before stability was asserted. Du = 0.2, Dv = 0.5, a = 0.5. dx and dt were chosen as described in the text. b. The lifetime of the randomly created patches is related to the decay time of the unstable pulse solution.
It is clear that these localized pulse solutions are not precisely connected to the patches we have seen. Most importantly, the qualitative dynamics of the patches is the same on both sides of the stability boundary of the pulse solutions. Also, the pulse solution and the stochastically-created patches show a significant size difference: The smallest patches in the stochastic simulation (Fig. 2a) were of size 250 (with the average being higher yet), while the width of the corresponding pulse solution for this set of parameters is roughly 200, as can be seen in Fig. 3a. Dynamically, this occurs because the u field in a nucleated patch expands rapidly but is eventually stopped by the faster spreading v concentration once its kinetics kicks in. This over-expanded u field then collapses back to the quiescent state. In the localized solution, there is a very carefully tuned balance between these two fields, a balance that the dynamics apparently cannot converge to from generic initial conditions. We have indeed verified that the basin of attraction of a stable localized state is extremely small (data not shown). Therefore, a randomly nucleated patch cannot converge to the pulse solution, but will rather grow too much and then vanish when the inhibitor v kicks in. This guarantees robustness of the transient patches phenomenon: even when the system supports a stable pulse solution, a stochastically nucleated patch will always be transient. Nevertheless, patches are only seen for parameters such that an isolated pulse exists, since outside this regime, the system gives rise to travelling waves.
The robust transient nature of the patches might be important in the biological context. The fact that the membrane bound effectors show a consistent pattern of appearing and disappearing implies that this might be beneficial to the cell, for example by letting the cell re- orient and quickly respond to a change in the external signal. On the other hand, a very stable patch on the membrane might be harmful. From the stability diagram in Fig. 4a it seems that a small change in one of the parameters would result in switching the pulse solution’s stability; however, as already mentioned it turns out this change of stability has little influence on the characteristics of the stochastic patches.
Changing parameters allows us to tune not only the size of the patches but also their typical duration. The parameter that has the largest influence on the stochastic patch is β. Increasing β clearly makes the localized solutions more stable, but the reduced excitability makes it much harder (for the same noise level) to successfully create a patch. There is at least a phenomenological correlation between the decay time of the deterministic pulse solution (when it is unstable) and the lifetime of the spontaneously created patches, as can be seen in Fig. 4b. We do not have an analytic handle on this correlation.
As mentioned above, when the cell is subjected to a chemical gradient only one patch is typically seen, now on the part of the membrane with the highest concentration, i.e. in the direction of the chemical’s source. Our model can explain such a state quite naturally by assuming that the excitability of the system varies in space as a response to an external cue, controlled for example by the parameter β. The system becomes less and less excitable as β reaches unity and varying β in space will lead to patch formation only in a designated area in which β is sufficiently small. Ref. [24] gives one example of how the cell could use cell receptor data [25] input to a gradient sensing model to output a spatially varying activation field. The output of this model can also be used as an input for a locomotion model [16].
To summarize, the model system we study in this work exhibits the formation of random localized patches and their spontaneous degradation after a typical lifetime. This offers a possible starting point for new mechanistic approaches to similar patterns recently seen in cells responding to chemotactic chemical stimuli. The patches are loosely related to stationary solutions of the deterministic equations dynamics of the system. The biological system described here was used as a motivation to this study, and while this simple model cannot account for the significant cell complexity it provides a new frame- work for thinking and studying the formation of transient localized patters.
Acknowledgments
We thank W. J. Rappel, W. Loomis and E. Ben-Jacob for comments and stimulating discussions. The work was supported by the National Institutes of Health Grant P01 GM078586. DK acknowledges support from CTBP (NSF PHY-0822283) during a recent visit.
References
- 1.Vanag VK, Epstein IR. Chaos. 2007;17:037110. doi: 10.1063/1.2752494. [DOI] [PubMed] [Google Scholar]
- 2.Scott A. Nonlinear Science: Emergence and Dynamics of Coherent Structures. Oxford Univ. Press; 2003. [Google Scholar]
- 3.Koga S, Kuramoto Y. Prog Theor Phys. 1980;63:106. [Google Scholar]
- 4.Ito A, Ohta T. Phys Rev A. 1992;45:8374. doi: 10.1103/physreva.45.8374. [DOI] [PubMed] [Google Scholar]
- 5.Schimansky-Geier L, et al. Z Phys B. 1995;96:417. [Google Scholar]
- 6.Muratov CB, Osipov VV. Phys Rev E. 1996;54:4860. doi: 10.1103/physreve.54.4860. [DOI] [PubMed] [Google Scholar]
- 7.Vigil RD, Ouyang Q, Swinney HL. Physica A. 1992;188:17. [Google Scholar]
- 8.Arbell H, Fineberg J. Phys Rev Lett. 2000;85:756. doi: 10.1103/PhysRevLett.85.756. [DOI] [PubMed] [Google Scholar]
- 9.Gomila D, Matias MA, Colet P. Phys Rev Lett. 2005;94:063905. doi: 10.1103/PhysRevLett.94.063905. [DOI] [PubMed] [Google Scholar]
- 10.Umbanhowar PB, Melo F, Swinney HL. Nature. 1996;382:793. [Google Scholar]
- 11.Meron E, Yizhaq H, Gilad E. Chaos. 2007;17:037109. doi: 10.1063/1.2767246. [DOI] [PubMed] [Google Scholar]
- 12.Pikovsky A, Kurths J. Phys Rev Lett. 1997;78:775. [Google Scholar]
- 13.Parent CA, Devreotes PN. Science. 1999;284:765. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 14.Postma M, et al. Mol Biol Cell. 2003;14:5019. doi: 10.1091/mbc.E03-08-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki AT, Chun C, Takeda K, Firtel RA. J Cell Biol. 2004;167:505. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]; Firtel R. private communication.
- 16.Hecht I. in preparation. [Google Scholar]
- 17.Levine H, Rappel WJ. Phys Rev E. 2005;72:061912. doi: 10.1103/PhysRevE.72.061912. [DOI] [PubMed] [Google Scholar]
- 18.Meinhardt H. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 19.Levchenko A, Iglesias PA. Biophysical Journal. 2002;82:50. doi: 10.1016/S0006-3495(02)75373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry H, Levine H. Phys Rev E. 2003;68:031914. doi: 10.1103/PhysRevE.68.031914. [DOI] [PubMed] [Google Scholar]
- 21.Suel GM, Garcia-Ojalvo J, Lieberman LM, Elowitz MB. Nature. 2006;440:545. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Jacob E, Cohen I, Levine H. Adv Phys. 2000;49:395. [Google Scholar]
- 23.Kessler DA, Levine H. unpublished. [Google Scholar]
- 24.Levine H, Kessler DA, Rappel WJ. Proc Natl Acad Sci U S A. 2006;103:976. doi: 10.1073/pnas.0601302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rappel WJ, Levine H. Phys Rev Lett. 2008;100:228101. doi: 10.1103/PhysRevLett.100.228101. [DOI] [PMC free article] [PubMed] [Google Scholar]