Abstract
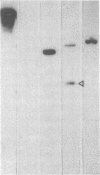
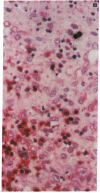
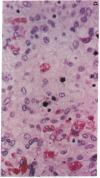
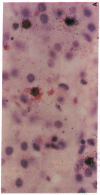
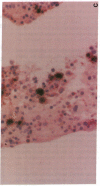
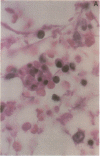
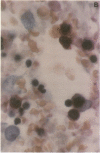
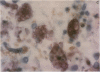
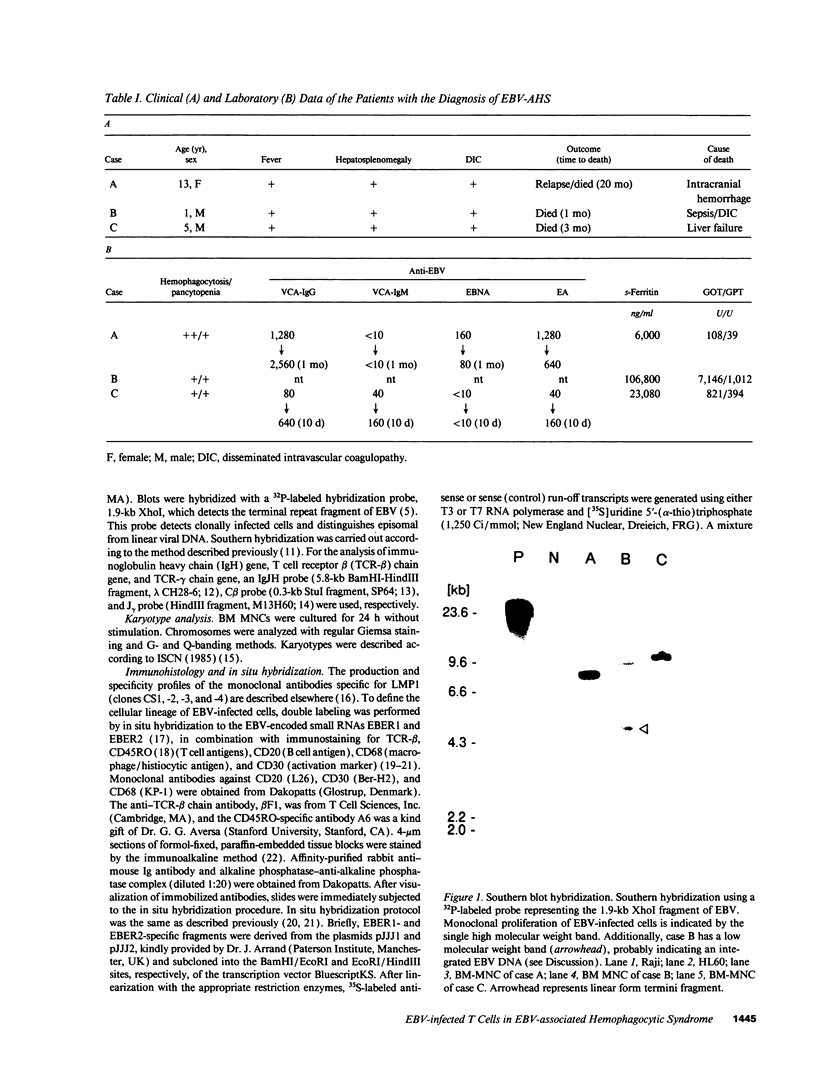
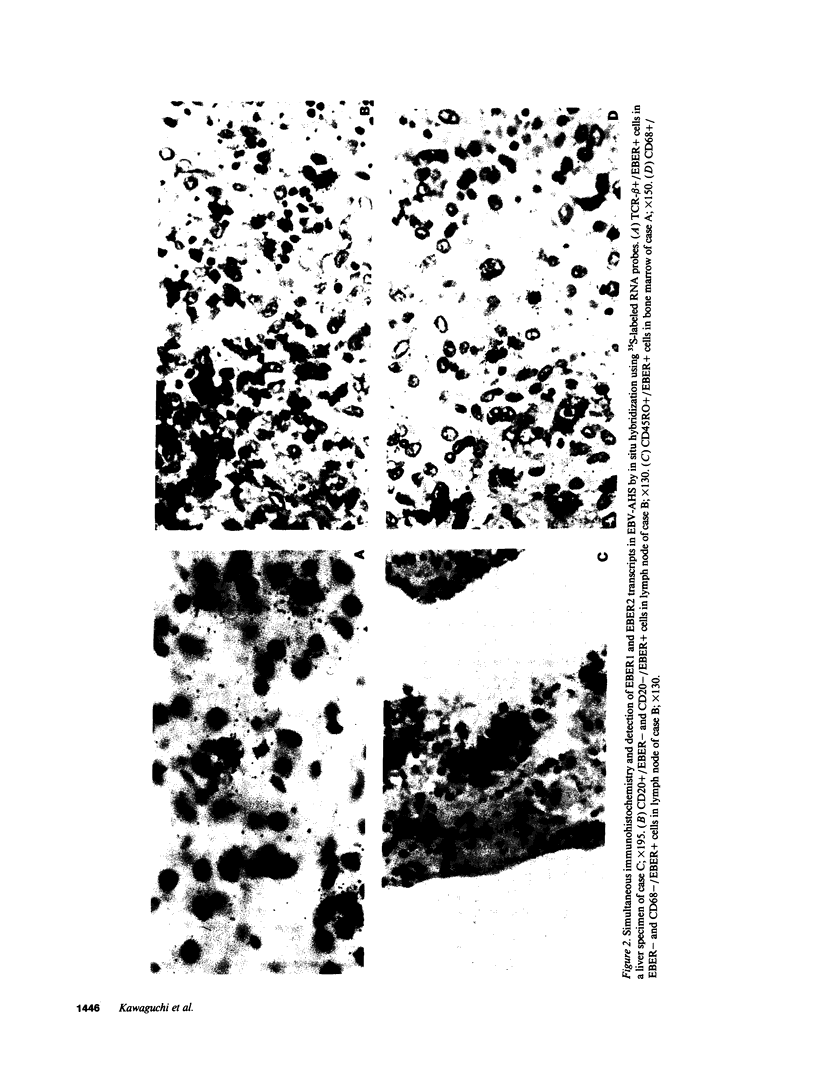
The clonal composition of EBV-infected cells was examined in three cases of EBV-associated hemophagocytic syndrome by analysis of the heterogeneity of terminal repetitive sequences in the EBV genome, indicating monoclonal expansion of EBV-infected cells in all cases. Involvement of T lymphoid cells was determined by in situ hybridization using 35S-labeled RNA probes specific for the small EBV-encoded nuclear RNAs, EBER1 and EBER2, in combination with immunostaining for the TCR-beta chain, CD45RO, CD20, CD30 and CD68 antigens in these three cases. The majority of lymphoid cells showing EBER transcripts were stained by antibodies against CD45RO and T cell receptor-beta. In contrast, EBER-specific signals were not detectable on B cells or hemophagocytic cells. These data support the concept that EBV-associated T cell proliferation is a primary feature of EBV-AHS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berti E., Aversa G. G., Soligo D., Cattoretti G., Delia D., Aiello A., Parravicini C., Hall B. M., Caputo R. A6--a new 45RO monoclonal antibody for immunostaining of paraffin-embedded tissues. Am J Clin Pathol. 1991 Feb;95(2):188–193. doi: 10.1093/ajcp/95.2.188. [DOI] [PubMed] [Google Scholar]
- Boos H., Stoehr M., Sauter M., Mueller-Lantzsch N. Flow cytometric analysis of Epstein-Barr virus (EBV) latent membrane protein expression in EBV-infected Raji cells. J Gen Virol. 1990 Aug;71(Pt 8):1811–1815. doi: 10.1099/0022-1317-71-8-1811. [DOI] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Cleary M. L., Nalesnik M. A., Shearer W. T., Sklar J. Clonal analysis of transplant-associated lymphoproliferations based on the structure of the genomic termini of the Epstein-Barr virus. Blood. 1988 Jul;72(1):349–352. [PubMed] [Google Scholar]
- Craig F. E., Clare C. N., Sklar J. L., Banks P. M. T-cell lymphoma and the virus-associated hemophagocytic syndrome. Am J Clin Pathol. 1992 Feb;97(2):189–194. doi: 10.1093/ajcp/97.2.189. [DOI] [PubMed] [Google Scholar]
- Falini B., Pileri S., De Solas I., Martelli M. F., Mason D. Y., Delsol G., Gatter K. C., Fagioli M. Peripheral T-cell lymphoma associated with hemophagocytic syndrome. Blood. 1990 Jan 15;75(2):434–444. [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y., Yamanaka N., Kataura A., Imai S., Kinoshita T., Mizuno F., Osato T. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990 Jan 20;335(8682):128–130. doi: 10.1016/0140-6736(90)90002-m. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Henter J. I., Elinder G., Söder O., Hansson M., Andersson B., Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991 Dec 1;78(11):2918–2922. [PubMed] [Google Scholar]
- Herbst H., Steinbrecher E., Niedobitek G., Young L. S., Brooks L., Müller-Lantzsch N., Stein H. Distribution and phenotype of Epstein-Barr virus-harboring cells in Hodgkin's disease. Blood. 1992 Jul 15;80(2):484–491. [PubMed] [Google Scholar]
- Horwitz C. A., Henle W., Henle G., Goldfarb M., Kubic P., Gehrz R. C., Balfour H. H., Jr, Fleisher G. R., Krivit W. Clinical and laboratory evaluation of infants and children with Epstein-Barr virus-induced infectious mononucleosis: report of 32 patients (aged 10-48 months). Blood. 1981 May;57(5):933–938. [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Jat P., Arrand J. R. In vitro transcription of two Epstein-Barr virus specified small RNA molecules. Nucleic Acids Res. 1982 Jun 11;10(11):3407–3425. doi: 10.1093/nar/10.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. F., Shurin S., Abramowsky C., Tubbs R. R., Sciotto C. G., Wahl R., Sands J., Gottman D., Katz B. Z., Sklar J. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988 Mar 24;318(12):733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- Katz B. Z., Raab-Traub N., Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J Infect Dis. 1989 Oct;160(4):589–598. doi: 10.1093/infdis/160.4.589. [DOI] [PubMed] [Google Scholar]
- Kikuta H., Taguchi Y., Tomizawa K., Kojima K., Kawamura N., Ishizaka A., Sakiyama Y., Matsumoto S., Imai S., Kinoshita T. Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature. 1988 Jun 2;333(6172):455–457. doi: 10.1038/333455a0. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Forster A., Rabbitts T. H. Genetic polymorphism and exon changes of the constant regions of the human T-cell rearranging gene gamma. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9596–9600. doi: 10.1073/pnas.83.24.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna R. W., Risdall R. J., Brunning R. D. Virus associated hemophagocytic syndrome. Hum Pathol. 1981 May;12(5):395–398. doi: 10.1016/s0046-8177(81)80019-6. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Asada M., Fujimoto J., Inaba T., Takihara Y., Sugita K., Bessho F., Furukawa T., Mizutani S. Clonal analysis of transient myeloproliferative disorder in Down's syndrome. Leukemia. 1991 Jan;5(1):56–59. [PubMed] [Google Scholar]
- Miyashita T., Kawaguchi H., Mizutani S., Tsuchida M. Histiocytic medullary reticulosis, a lethal form of Epstein-Barr-virus-related disorder. Lancet. 1991 Apr 20;337(8747):986–987. doi: 10.1016/0140-6736(91)91630-d. [DOI] [PubMed] [Google Scholar]
- Niedobitek G., Herbst H., Young L. S., Brooks L., Masucci M. G., Crocker J., Rickinson A. B., Stein H. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood. 1992 May 15;79(10):2520–2526. [PubMed] [Google Scholar]
- Pileri S., Falini B., Delsol G., Stein H., Baglioni P., Poggi S., Martelli M. F., Rivano M. T., Mason D. Y., Stansfeld A. G. Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology. 1990 Apr;16(4):383–391. doi: 10.1111/j.1365-2559.1990.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Risdall R. J., McKenna R. W., Nesbit M. E., Krivit W., Balfour H. H., Jr, Simmons R. L., Brunning R. D. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979 Sep;44(3):993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Schwarting R., Gerdes J., Dürkop H., Falini B., Pileri S., Stein H. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood. 1989 Oct;74(5):1678–1689. [PubMed] [Google Scholar]
- Stein H., Gatter K., Asbahr H., Mason D. Y. Use of freeze-dried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest. 1985 Jun;52(6):676–683. [PubMed] [Google Scholar]
- Su I. J., Hsieh H. C., Lin K. H., Uen W. C., Kao C. L., Chen C. J., Cheng A. L., Kadin M. E., Chen J. Y. Aggressive peripheral T-cell lymphomas containing Epstein-Barr viral DNA: a clinicopathologic and molecular analysis. Blood. 1991 Feb 15;77(4):799–808. [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M. CD4+ T cell subsets. Lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J Immunol. 1990 Mar 1;144(5):1788–1799. [PubMed] [Google Scholar]