Abstract
The sympathetic nervous system is involved in regulating various cardiovascular parameters including heart rate (HR) and HR variability. Aberrant sympathetic nervous system expression may result in elevated HR or decreased HR variability, and both are independent risk factors for development of cardiovascular disease, including heart failure, myocardial infarction, and hypertension. Epidemiologic studies have established that impaired HR control is linked to increased cardiovascular morbidity and mortality. One successful way of decreasing HR and cardiovascular mortality has been by utilizing β-blockers, because their ability to alter cell signaling at the receptor level has been shown to mitigate the pathogenic effects of sympathetic nervous system hyperactivation. Numerous clinical studies have demonstrated that β-blocker-mediated HR control improvements are associated with decreased mortality in postinfarct and heart failure patients. Although improved HR control benefits have yet to be established in hypertension, both traditional and vasodilating β-blockers exert positive HR control effects in this patient population. However, differences exist between traditional and vasodilating β-blockers; the latter reduce peripheral vascular resistance and exert neutral or positive effects on important metabolic parameters. Clinical evidence suggests that attainment of HR control is an important treatment objective for patients with cardiovascular conditions, and vasodilating β-blocker efficacy may aid in accomplishing improved outcomes.
Keywords: adrenergic beta-antagonists, heart failure, hypertension, myocardial infarction
Introduction
Numerous studies have reported that increased heart rate (HR) is a predictor of cardiovascular mortality in healthy people, those who have had a myocardial infarction (MI), and in patients with heart failure (HF).1 Increased HR is recognized as a negative prognostic factor independent of other clinical parameters, including left ventricular function.1 The increased mortality observed with an increased HR may be a consequence of the deleterious sympathovagal imbalance that can be characterized by sympathetic nervous system predominance, vagal depression, or the combined impact of this dysregulation on cardiovascular function.1,2 Elevated HR increases cardiac output (short term) and myocardial oxygen consumption, while simultaneously reducing time of diastole and myocardial blood supply, conditions that favor the development of myocardial ischemia and arrhythmias in ischemic areas.1
Blockade of β-adrenergic receptors is part of the combined medical prevention of cardiovascular disease.3,4 β-Blockers have been efficacious and beneficial in the treatment of various cardiovascular disease states, including angina, HF, MI, and ventricular arrhythmias.5 Randomized controlled clinical studies consistently demonstrate that β-blockers reduce sudden cardiac death by 30% to 50% in patients with coronary artery disease and HF.6 The clinical benefits of β-blockers have been attributed to their ability to antagonize β-adrenergic receptors in the heart and the periphery.7 Traditional β-blockers (eg, atenolol and propranolol), which target either β1- (cardioselective) or β1- and β2-adrenergic receptors (nonselective), decrease BP primarily via a reduction in HR and cardiac output, but do not appreciably affect peripheral vascular resistance.5 Acutely, uptitration of β-blockers can decrease cardiac output and increase vascular tone, which may exert a detrimental effect on renal perfusion and decrease patient drug tolerability, while exacerbating glucose and lipid metabolism.8,9 In addition, these metabolic perturbations may lead to further vascular complications by adversely affecting endothelial function and promoting the development or progression of diabetes.9,10
Vasodilatory β-blockers (eg, carvedilol, labetalol, and nebivolol) and those that provide more complete adrenergic blockade may, in part, mediate vasodilation via blockade of α1-adrenergic receptors or increased endothelium-derived nitric oxide release, which may lead to a reduction in total peripheral vascular resistance.9 This review will examine the data, including recent analyses from the large cardiovascular trials, related to adrenergic blockade, HR control, and its impact on outcomes across the cardiovascular disease spectrum (ie, patients who have had a MI or who have HF or hypertension).
Heart rate and heart rate variability
Heart rate is not a static hemodynamic parameter but instead changes over time in response to physical and mental demands. Heart rate is normally determined by spontaneous and periodic depolarizations of the sinoatrial node, the frequency of which is modulated by the sympathetic and parasympathetic divisions of the autonomic nervous system, the intrinsic cardiac nervous system, reflexes, and respiration. These neural systems also partially control cardiac contractility and conduction of electrical activity through the heart. As a result, HR (chronotropism), contractility (inotropism), and conduction (dromotropism) are adjusted to meet the changing needs of the body. Aberrant sympathetic activation has been implicated as part of the sequelae consistent with the development of HF, MI, and hypertension.11,12 Profoundly elevated sympathetic activity for an extended period accompanied by parasympathetic withdrawal may result in chronically elevated HR, as well as neurohormonal stimulation, and is associated with a decreased threshold for ventricular fibrillation. This upregulation of the sympathetic nervous system and increased adrenergic activation is also associated with pathologic remodeling, myocyte apoptosis, and a dysregulation of calcium handling that leads to myocardial ischemia, a decrement in contractile function, and an increased risk of ventricular arrhythmias.13,14 Due to the correlative linkage of HR and sympathetic nervous system outflow, HR control may be used as a surrogate for sympathetic nervous system activity.
Optimal heart rate
Heart rate varies between individuals, and in a resting individual HR may vary according to time of day, physical conditioning, environmental influences, and sympathetic nervous system vagal tone. However, recent reports suggest that HR should generally be maintained substantially below the traditionally defined tachycardia threshold of 90 to 100 beats/minute.15 A continuous linear increase in cardiovascular risk has been noted in patients whose HR exceeds 60 beats/minute.16,17 Results from the Global Utilization of Streptokinase and TPA for Occluded Arteries (GUSTO-1) study suggest that an increased risk of cardiovascular disease may even exist when HR is <60 beats/minute.18 Increases in HR exceeding 10 beats/minute are associated with a 14% increase in cardiovascular mortality and a 20% increase in total mortality in patients with hypertension.19 In the general population, the mortality risk is increased three-fold in individuals with a HR of 90 to 99 beats/minute compared with individuals with a HR ≤ 60 beats/minute.20
Heart rate and mortality
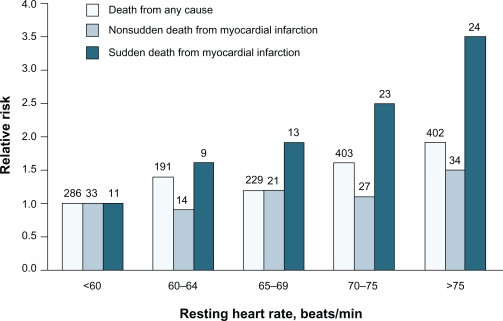
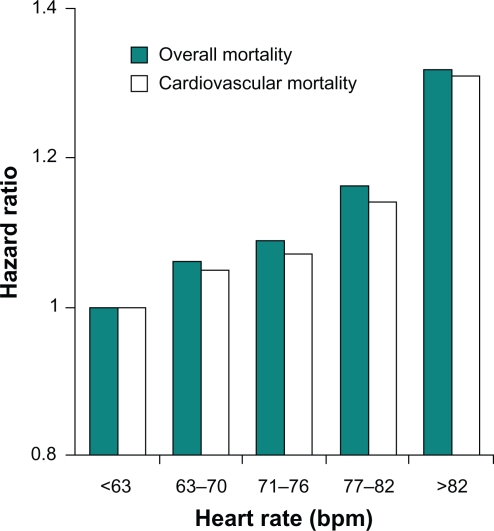
Numerous studies report that a significant association exists between resting HR and cardiovascular and all-cause mortality in the general population, as well as among patients with cardiovascular disease. Epidemiologic studies involving approximately 30,000 individuals over a period of five to 36 years revealed an inverse relationship between HR and survival in the general population.20–25 The risk of coronary artery disease, stroke, death due to noncardiovascular diseases, and total mortality increased with higher HR. Among 5713 healthy male volunteers without known or suspected cardiovascular disease who were observed for an average of 23 years, cardiovascular and all-cause mortality from acute MI increased with progressive elevations in resting HR.17 This independent variable remained significant after adjustment for exercise capacity, age, diabetes, systolic arterial pressure, body mass index, level of physical activity, and other factors. The relationship was most apparent for sudden death (Figure 1).17 In a study of 10,267 patients with acute coronary syndromes, mortality at 30 days and 10 months progressively increased with increasing HR (P < 0.001).26 Similarly, all-cause and cardiovascular mortality were directly related to resting HR at study entry in 24,913 patients with suspected or proven coronary artery disease who participated in the Coronary Artery Surgery Study (CASS) registry for a period of 15 years (P < 0.0001; Figure 2).15,16 The predictive capacity of HR was independent of concomitant hypertension, diabetes, smoking, left ventricular ejection fraction, and number of diseased coronary vessels.
Figure 1.
Heart rate and mortality in healthy individuals: Relative risk of death from any cause, nonsudden death from myocardial infarction (MI), and sudden death from MI in 5713 people without known or suspected heart disease. Differences among quintiles with respect to risk of death from any cause, P < 0.001; nonsudden death from cardiac causes, P = 0.02; sudden death from cardiac causes, P < 0.001. Copyright @ 2005. Massachusetts Medical Society. All rights reserved. Reprinted with permission from Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958.
Figure 2.
Heart rate and mortality in coronary artery disease: Relationship between resting heart rate and all-cause and cardiovascular mortality in 24,913 patients with suspected or proven coronary artery disease. Based on data from Diaz et al.16 Copyright © 2007. Reprinted with permission from Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830.
Abbreviation: bpm, beats per minute.
Because the association between HR and mortality is well known, resting HR is currently included in risk assessment indices for patients with acute coronary syndromes (eg, the Global Registry of Acute Coronary Events risk prediction score)27 and acute MI (eg, the Gruppo Italiano per lo Studio della Sopravivenza nell’Infarto Miocardico Prevenzione risk assessment model28 and the Thrombolysis in Myocardial Infarction Risk Score29). However, HR is not included in some of the more widely used indices for cardiovascular risk assessment, including the Copenhagen Risk Score30 and the European SCORE project,31 which indicates that HR is not universally accepted as a prognostic factor and a potential therapeutic target in patients with cardiovascular disease.
Heart rate variability
Heart rate variability (HRV) refers to the beat-to-beat difference or R-R interval change in the intrinsic rhythm of the heart.32 Assessment of HRV may provide a surrogate measure of cardiac health, as defined by the degree of equilibrium between sympathetic and parasympathetic (vagus nerve) activity.33 HRV can be assessed by time or frequency domain indices.32 Time domain measures are based on the amount of time in milliseconds in the beat-to-beat intervals of the heart or on the differences between the normal beat-to-beat intervals.33 Frequency domain measures of HRV provide information about the frequency distribution of the components of HRV using power spectral density analysis.33 Nonlinear dynamic analysis (eg, Poincaré plots) may also be used to quantify HRV, but the clinical utility of this method has not been fully established.32 As discussed in subsequent sections, numerous studies have demonstrated the positive prognostic power of reduced HRV to predict all-cause mortality, sudden cardiac death, and cardiac events in patients who have experienced an MI, as well as in patients who have HF or hypertension.
Heart rate control in heart failure
HF is frequently associated with a decreased threshold for ventricular fibrillation, as well as an increased risk of other malignant arrhythmias and sudden cardiac death. Increased HR (eg, atrial fibrillation with a rapid ventricular rate or multiple premature contractions) may contribute to the development of HF and is also associated with a poor prognosis in patients with HF.34 In the normal heart, a stepwise increase in contractility develops as HR increases. Ninety percent of patients with HF die of cardiovascular causes.35 Approximately half of these patients die from progressive, advanced disease and the remaining patients die suddenly, most frequently because of arrhythmia despite a perceivably stable clinical condition. Sudden cardiac death occurs most frequently in patients in New York Heart Association Functional Class II or III. Risk factors for sudden cardiac death include elevated resting HR, and reduced HRV and left ventricular ejection fraction.36
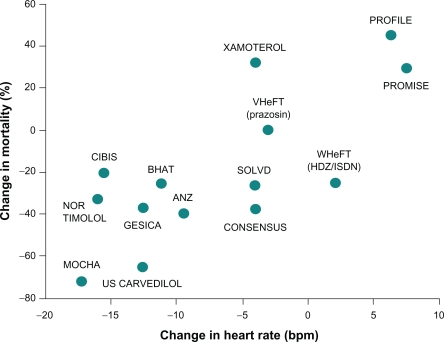
Numerous studies have established a relationship between reduction in HR and improved survival of patients with HF who are receiving β-blocker therapy (Figure 3).37 In a recent meta-analysis of 35 studies of patients with chronic systolic HF (n = 22,926), a strong correlation was observed between HR and annualized all-cause mortality (P = 0.004) and between change in HR and change in left ventricular ejection fraction (P < 0.001).38 As a result, it was suggested that the HR-lowering effect of β-blockers was a major contributor to the clinical benefit associated with these agents. In a study of 152 patients with HF who were receiving β-blocker therapy, greater reductions in HR were associated with better clinical outcomes for patients overall, and higher β-blocker doses provided additional clinical benefits among patients with persistently elevated HR.39 These results suggest that the magnitude of reduction in HR may be more important than achieving the target dose of β-blocker therapy in patients with HF.38,39 In the Cardiac Insufficiency BIsoprolol Study (CIBIS), treatment of 557 patients with bisoprolol reduced HR by approximately 15 beats/minute relative to placebo (P < 0.001), and HR change was the most powerful predictor of survival (P < 0.01).40 In the larger CIBIS II study (n = 2539), baseline HR and HR change were both significant predictors of mortality (P ≤ 0.005).41 The most favorable prognosis occurred in patients with the lowest baseline HR and with the greatest HR reduction, conditions which were encountered more frequently in the bisoprolol group than in the placebo group.
Figure 3.
Heart rate reduction and mortality in heart failure: Relationship between change in heart rate and mean change in mortality among patients with chronic heart failure who received β-blocker therapy. Copytright © 1999. Reprinted with permission from Kjekshus Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Suppl. 1999;1(Suppl H):H64–H69.
The Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial evaluated patients with left ventricular dysfunction or HF after a myocardial infarction (n = 1959),42 whereas the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial enrolled only patients with severe HF (n = 2289).43 The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) enrolled patients with New York Heart Association Class II–IV HF with an ejection fraction <40% (n = 3991).44 These trials each independently demonstrated benefits of β-blockade in patients with HF throughout a large spectrum of disease. Additionally, the Carvedilol or Metoprolol (tartrate) European Trial (COMET; n = 3029) may suggest that nonselective neurohumoral blockade has an additional benefit compared with selective β1-blockade.45 Consequently, adrenergic-receptor pathophysiology and thereafter specific signal transduction pathways may underlie the benefit of using specific β-blockers.46 Although studies using one of the three β-blockers approved for HF in the US (carvedilol, metoprolol succinate, or bisoprolol) have demonstrated benefit in patients with HF, nebivolol has also received approval in Europe for the treatment of mild-to-moderate HF in patients ≥70 years of age.47 Nebivolol is a β1-selective β-blocker without α1-adrenergic receptor blocking activity. Nebivolol, which is approved for the treatment of hypertension in the US, has a neutral effect on metabolic parameters in patients with hypertension.48 Nebivolol has been shown to reduce BP and HR to a similar extent as atenolol at one-tenth of the dose.49 More importantly, the hemodynamic effect observed with nebivolol treatment better preserved cardiac output by decreasing peripheral vascular resistance and increasing stroke volume compared with atenolol.50
Comparison of bisoprolol, carvedilol, and nebivolol in patients with HF demonstrated that each agent decreased HR to a similar extent.51 Additionally, exercise capacity increases during β-blocker therapy. For example, among 16 healthy male volunteers, HR during exercise decreased by 14% in the bisoprolol group, 15% in the carvedilol group, and 13% in the nebivolol group (P < 0.05).51 Additionally, the effect of carvedilol and nebivolol on exercise capacity were compared in a 12-month study of patients with nonischemic dilated cardiomyopathy.52 Exercise duration improved significantly in both groups of patients (P = 0.01), although patients treated with nebivolol experienced an initial decrease in exercise capacity over the first three months.52 In patients with HF, reduction in peak VO2 is associated with left ventricular systolic dysfunction and increased neurohormonal response. Treatment with carvedilol improved left ventricular systolic function, exercise tolerance (at 12 months, exercise was prolonged by 143.9 sec; P = 0.001), and peak oxygen consumption as well as significant reductions in brain natriuretic peptide, endothelin-1, and associated cytokines (ie, interleukin-6 and tumor necrosis factor-α).53 In a recent analysis of 47 randomized studies of HF, a significant increase in the six-minute walk test was observed in three of 17 studies that involved β-blocker therapy.54 Similar to the results of exercise treadmill tests, patients who received β-blocker therapy for more severe HF experienced greater improvements in the six-minute walk test compared with those having milder HF. Therefore, administration of β-blocker therapy to patients with HF is associated with improved HR control, improvement in clinical functioning, and reduction in mortality and hospitalization risk. The benefits of β-blocker therapy are clear, ie, mortality and HF hospital admissions are reduced by approximately one-third when eligible patients receive β-blocker therapy.55,56
Chronotropic incompetence in heart failure
Patients with HF experience severe chronic exercise intolerance. Although the pathophysiology of exercise intolerance is not completely understood, chronotropic incompetence, defined as an impaired capacity to increase HR during exercise, and diastolic dysfunction are important determinants of this condition.57 Chronotropic incompetence occurs in >70% of patients with advanced HF and is believed to arise as a result of β-receptor desensitization and impaired norepinephrine release.58 Risk factors for the development of chronotropic incompetence include increased left ventricular mass, enlarged cavity size, and depressed systolic function.59 Chronotropic incompetence is predictive of mortality and coronary artery disease risk, even after adjusting for age, physical fitness, and other standard cardiovascular risk factors.60 Although some studies have reported that chronotropic incompetence was more common in patients taking β-blockers,61 β-blockade has been reported to have a minimal effect on the association between chronotropic incompetence and cardiovascular mortality.62,63 At doses <10 mg, nebivolol did not attenuate the exercise-induced increase in HR, thereby suggesting that nebivolol may mitigate the risk of chronotropic incompetence suggested to occur with β-blockade.64 Similarly, carvedilol dose did not affect the HR dynamics during treadmill exercise testing among patients with HF who were stratified by resting HR ≤ (60 beats/minute or >60 beats/minute).65 In a trial comparing the β1 effects of metoprolol succinate and carvedilol, carvedilol did not attenuate exercise-induced HR to the same degree as metoprolol.66 Cardiac pacing may be required to restore chronotropic competence and exercise capacity in patients with persistent bradycardia, as well as allowing for continued β-blocker therapy.67 There is a developing body of literature regarding the treatment of diastolic HF and chronotropic incompetence, but the clinical relevance of this information has yet to be determined.
Heart rate control after myocardial infarction
Increased HR in patients with atherosclerosis may impair the stability of coronary plaques because of repetitive changes in BP that induce mechanical stress. Increased HR (> 80 beats/minute) is associated with more plaque ruptures compared with lower HR in patients with coronary artery disease (n = 106).68 HRs in patients following MI are higher than in patients who have not experienced an acute event.69 Consequently, HR has been identified as an independent risk factor for the development of plaque rupture. In addition, HR but not HRV was identified as an independent prognostic indicator of mortality in a study of 366 patients after MI (P < 0.001).70 However, a subsequent study reported that decreased HRV and increased randomness of HR shortly after MI are independent risk factors for mortality in this patient population.71 Similar to HF, increased HR or reduced HRV are associated with increased cardiovascular mortality in patients after MI.32 A meta-analysis of the GISSI-2 and GISSI-3 trials that included approximately 20,000 patients demonstrated that inhospital mortality rates after MI increased from 3% for patients with HR <60 beats/minute on admission to 10% for patients with HR >100 beats/minute on admission.72 Furthermore, higher HR at hospital discharge correlated with increased mortality rates after one year.69
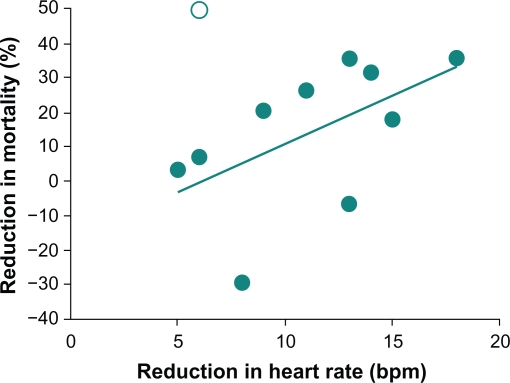
Traditional β-blockers exert beneficial effects on HR and HRV and improve mortality rates in patients who have experienced an MI. Administration of β-blockers to 1427 patients within six hours of the onset of MI symptoms resulted in a mean reduction in infarct size that was directly proportional to the mean reduction in HR (P < 0.001).73 Furthermore, a significant association was reported between reduction in HR and reduction in mortality in 11 long-term β-blocker studies that involved more than 16,000 patients (Figure 4; r = 0.60; P < 0.05).73 The Norwegian Timolol study reported similar results in that β-blocker-mediated HR reductions in patients who had experienced an MI were a significant predictor of overall mortality.74 Compared with placebo, timolol treatment was associated with a 42% reduction in overall mortality compared with placebo (P < 0.001); in logistic regression analysis, HR during follow-up remained predictive but treatment did not, suggesting that the beneficial effect of timolol on mortality could be ascribed to its effect on HR. HRV was also significantly improved among 28 patients who were treated with atenolol or metoprolol tartrate for six weeks after an acute MI (P ≤ 0.01); trends toward lower HR were also observed in both treatment groups.2 Similarly, treatment of 30 patients who were stable following an MI with atenolol or metoprolol controlled-release (succinate) for six weeks decreased HR (P < 0.001) and increased HRV.75 Propranolol treatment was also associated with significantly greater improvements in HRV after an acute MI compared with placebo (P <0.05; n =184). 76
Figure 4.
Heart rate reduction and mortality after myocardial infarction: Relationship between mean reduction in heart rate and mean change in mortality relative to placebo in randomized, placebo-controlled studies of β-blockers after myocardial infarction (r = 0.60; P < 0.05). Copyright © 1986. Reprinted with permission from Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials.Am J Cardiol. 1986;57(12):43F–49F.
Similar to the traditional β-blockers, vasodilating β-blockers exert beneficial effects on HR and HRV in patients who have experienced an MI. Carvedilol produced reductions in HR relative to placebo in 151 patients with an acute MI (P < 0.0001).77 Labetalol, a nonselective β-blocker that targets α1-, β1-, and β2-adrenergic receptors, is used for the treatment of hypertension of all severities and during hypertensive emergencies.78,79 When given acutely, labetalol decreases peripheral vascular resistance and BP but may have limited effects on HR and cardiac output.80 In another study, administration of labetalol to 32 patients with sustained elevations in systemic arterial pressure after a recent MI resulted in significant reductions in HR relative to pretreatment levels (P < 0.01).81 Nebivolol and atenolol both decreased HR in patients who had ischemic heart disease and a previous MI (n = 40); however, nebivolol maintained cardiac output and improved ejection fraction (P < 0.05).82 The relationship between improved HR control and decreased mortality has not been assessed among patients who have been treated with vasodilating β-blockers after MI.
Heart rate control in hypertension
In patients with hypertension, sympathetic nervous system overactivity increases HR, contributing to cardiac output and raised BP. The association between increased HR and the development of hypertension was demonstrated in the HARVEST (Hypertension and Ambulatory Recording VEnetia STudy) trial, which revealed a strong linkage between elevated HR and increases in BP among patients with Stage 1 hypertension.83 Patients whose HR was persistently elevated during the six-year study period had a two-fold higher risk of developing hypertension compared with patients with normal HR (n = 796). In patients with hypertension, normal sinus rhythm, and cardiovascular risk factors (n = 18,900), increasing HR from 81 to 119 beats/minute was associated with an increasing proportion of patients with microalbuminuria (63% to 69%, respectively; P < 0.0001).84 Elevated HR is also an independent predictor of microalbuminuria, a predictor for cardiovascular events, and an indicator of renal impairment in patients with hypertension (n = 18,900).84 In addition, greater impairment of HRV responsiveness to autonomic challenge was observed in patients with hypertension compared with a normotensive group (n = 40).85 Increased HR generally results in a poor prognosis for patients with hypertension. The rate of complications caused by cardiovascular disease as well as total mortality in patients with hypertension increased two-fold when HR increased by 40 beats/minute (n =4530). 86
The importance of lowering systemic vascular resistance and increasing tissue perfusion in patients with hypertension is well recognized, given that clinical evidence has established an association between impairment of microcirculation and development of end organ damage.87,88 Consequently, a goal of hypertension management is effective BP reduction while maintaining tissue perfusion. Traditional β-blockers reduce BP via decreased cardiac output but do not directly affect central aortic pressure or peripheral resistance, although a slight compensatory increase in peripheral resistance may occur.9 Administration of the traditional β-blocker, atenolol, to patients with hypertension significantly reduced HRV compared with placebo or losartan (P < 0.05).89,90 HR and BP at rest and during exercise was decreased in 10 patients with mild to moderate hypertension who received atenolol therapy for five years; however, systemic vascular resistance was elevated and cardiac output remained depressed compared with pretreatment levels.91 Similar results have also been reported using 10 different traditional β-blockers.9 Therefore, although traditional β-blockers lower BP, they do not appear to normalize cardiac hemodynamics in patients with hypertension. In addition, traditional β-blockers are associated with an increased risk for the development of abnormalities in metabolic parameters (eg, diabetes or endothelial dysfunction) or stroke compared with other antihypertensive agents.92–94
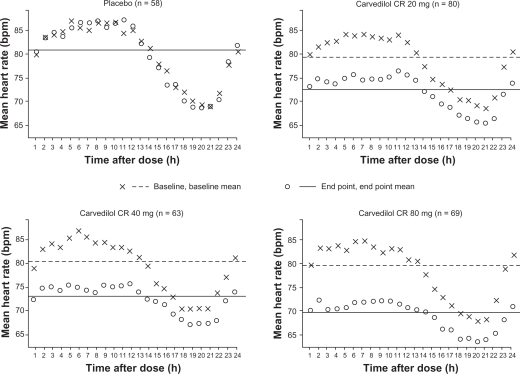
Vasodilatory β-blockers reduce BP via the lowering of peripheral vascular resistance and only slightly decreased in cardiac output; decreases in central aortic pressure have also been observed with vasodilatory β-blockers.79,95 In contrast with traditional β-blockers, carvedilol was shown to maintain cardiac output, decrease vascular resistance, and decrease HR to a lesser extent.96 A once-daily formulation of the vasodilatory β-blocker carvedilol controlled-release was administered to 320 patients with hypertension, resulting in greater reductions in HR (Figure 5) and 24-hour diastolic BP compared with placebo (P ≤ 0.001).97 In the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) study of 1235 patients with hypertension and Type 2 diabetes mellitus, reductions in diastolic BP were similar in the carvedilol and metoprolol tartrate treatment groups (10.0 ± 0.4 and 10.3 ± 0.3 mmHg, respectively). At mean doses required to achieve target BP (carvedilol 35 mg/day; metoprolol 256 mg/day), both agents effectively reduced HR, although decreases were less among carvedilol- versus metoprolol-treated patients (6.7 ± 0.4 versus 8.3 ± 0.4 beats/minute, respectively; P < 0.001).98 Of clinical importance, carvedilol demonstrated neutral or positive effects on glycemic control and lipid metabolism in analyses of the GEMINI study. After six weeks of treatment, once-daily nebivolol reduced HR by 10.6 ± 10.3 beats/minute, systolic BP by 29 ± 17 mmHg, and diastolic BP by 16 ± 10 mmHg in an observational study of 6376 patients with hypertension.99 Patients with higher initial values experienced greater reductions in HR and BP compared with patients having moderately elevated initial values.99 In other clinical trials, nebivolol reduced vascular resistance and improved endothelial function in patients with hypertension, and also lowered the levels of the inflammatory marker, high-sensitivity C-reactive protein, in healthy volunteers who smoked cigarettes.100,101
Figure 5.
Twenty-four-hour heart rate in patients with hypertension at baseline and after six weeks of treatment with carvedilol controlled-release (CR) or placebo. Copyright © 2006. Reprinted with permission from Weber MA, Bakris GL, Tarka EA, Iyengar M, Fleck R, Sica DA. Efficacy of a once-daily formulation of carvedilol for the treatment of hypertension. J Clin Hypertens (Greenwich). 2006;8(12):840–849.
The clinical relevance of β-blocker-mediated HR control to long-term clinical outcomes is less clear in hypertension than in HF or after MI. However, it is clear that reducing peripheral vascular resistance with a vasodilatory β-blocker is a beneficial mechanism to patients with hypertension, ie, a state of inherently elevated peripheral vascular resistance. In addition, vasodilating β-blockers maintain cardiac output but decrease peripheral vascular resistance, which improves peripheral blood flow. Improved blood flow is a major contributing factor to the more favorable tolerability and metabolic profiles of vasodilating β-blockers compared with traditional β-blockers.48,98
Recently, the use of β-blockade in essential hypertension has been called into question. Bangalore et al report increased cardiac events in hypertensive patients being treated with β-blockade.102 A lower HR achieved from β-blockade compared with other antihypertensives or placebo in a meta-analysis of over 34,000 patients with hypertension was associated with an increase in all-cause mortality, cardiovascular mortality, MI, stroke, and HF. One caveat to this study, however, is that 78% of those studied were prescribed atenolol, and it has been suggested that atenolol, and not β-blockade itself, was the cause. The results were certainly provocative, and clearly additional testing needs to be conducted to determine whether this is a class effect or an effect based on receptor specificity.46
Conclusion
An elevated or invariant HR is associated with the development of complications or various cardiovascular diseases including HF, MI, and hypertension. Patients with impaired HR control are at increased risk for all-cause and cardiovascular mortality, especially sudden cardiac death. As a result, HR should be included among the major risk factors for cardiovascular disease and should be used to establish individual cardiovascular risk profiles. Epidemiologic studies have demonstrated that β-blockers improve HR control and decrease mortality in patients with cardiovascular disease. Clinical evidence has established a clear relationship between improved HR control and decreased mortality in patients who have had an MI or who have HF. Although HR is an important contributor to the development of hypertension, a definite association between improved HR control and decreased mortality has yet to be established in this patient population. However, the importance of decreased peripheral vascular resistance while maintaining tissue perfusion is well recognized in patients with certain cardiovascular conditions, such as hypertension. Traditional β-blockers do not decrease but may in fact increase peripheral vascular resistance during long-term treatment. In contrast, vasodilating β-blockers reduce peripheral vascular resistance and maintain cardiac output. Consequently, vasodilating β-blockers are an appropriate treatment option for patients with cardiovascular disease who are at high risk of sudden cardiac death, HF, or coronary artery disease, and for those with concordant comorbidities, including diabetes and peripheral vascular disease.
Acknowledgments
ProEd Communications provided editorial assistance with this paper and was supported by GlaxoSmithKline, Philadelphia, Pennsylvania.
Footnotes
Disclosures
DF has received grant support from NIH Grant HL084498, NIH Grant HD 058997, the Heart Failure Society of America, the GlaxoSmithKline Research Fund, and the Medtronic Research Fund. TE has received grant support from NIH Grant HL 48848 and NIH Grant HD-58997. RW has received grant support from the Pfizer Fellowship in Health Disparities, the American Academy of Family Physicians Foundation, Cardiomems DSMB, and CVRx Research. DM declares no conflicts of interest in this work.
References
- 1.Lanza GA, Fox K, Crea F. Heart rate: A risk factor for cardiac diseases and outcomes? Pathophysiology of cardiac diseases and the potential role of heart rate slowing. Adv Cardiol. 2006;43:1–16. doi: 10.1159/000095401. [DOI] [PubMed] [Google Scholar]
- 2.Lurje L, Wennerblom B, Tygesen H, Karlsson T, Hjalmarson A. Heart rate variability after acute myocardial infarction in patients treated with atenolol and metoprolol. Int J Cardiol. 1997;60(2):157–164. doi: 10.1016/s0167-5273(97)00104-6. [DOI] [PubMed] [Google Scholar]
- 3.Lonn E, Grewal J. Drug therapies in the secondary prevention of cardiovascular diseases: Successes, shortcomings and future directions. Curr Vasc Pharmacol. 2006;4(3):253–268. doi: 10.2174/157016106777698360. [DOI] [PubMed] [Google Scholar]
- 4.Poulter NR, Dobson JE, Sever PS, Dahlof B, Wedel H, Campbell NR. Baseline heart rate, antihypertensive treatment, and prevention of cardiovascular outcomes in ASCOT (Anglo – Scandinavian Cardiac Outcomes Trial) J Am Coll Cardiol. 2009;54(13):1154–1161. doi: 10.1016/j.jacc.2009.04.087. [DOI] [PubMed] [Google Scholar]
- 5.Frishman WH. A historical perspective on the development of β-adrenergic blockers. J Clin Hypertens. 2007;9(4 Suppl 3):19–27. [Google Scholar]
- 6.Egan BM, Basile J, Chilton RJ, Cohen JD. Cardioprotection: The role of beta-blocker therapy. J Clin Hypertens (Greenwich) 2005;7(7):409–416. doi: 10.1111/j.1524-6175.2005.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25(15):1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006;70(11):1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 9.Messerli FH, Grossman E. Beta-blockers in hypertension: Is carvedilol different? Am J Cardiol. 2004;93(9A):7B–12B. doi: 10.1016/j.amjcard.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Brocq ML, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: From molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10(9):1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- 11.Krum H. Sympathetic activation and the role of beta-blockers in chronic heart failure. Aust N Z J Med. 1999;29(3):418–427. doi: 10.1111/j.1445-5994.1999.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34(4 Pt 2):724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 13.De Jong MJ, Randall DC.Heart rate variability analysis in the assessment of autonomic function in heart failure J Cardiovasc Nurs 2005203186–195.quiz 196–197 [DOI] [PubMed] [Google Scholar]
- 14.Feldman DS, Elton TS, Sun B, Martin MM, Ziolo MT. Mechanisms of disease: Detrimental adrenergic signaling in acute decompensated heart failure. Nat Clin Pract Cardiovasc Med. 2008;5(4):208–218. doi: 10.1038/ncpcardio1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 16.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 17.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 18.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91(6):1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB. Risk stratification in hypertension: New insights from the Framingham Study. Am J Hypertens. 2000;13(1 Pt 2):3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelmsen L, Berglund G, Elmfeldt D, et al. The multifactor primary prevention trial in Goteborg, Sweden. Eur Heart J. 1986;7(4):279–288. doi: 10.1093/oxfordjournals.eurheartj.a062065. [DOI] [PubMed] [Google Scholar]
- 21.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112(6):736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 22.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: The NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121(1 Pt 1):172–177. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg RJ, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women. The Framingham Study. Arch Intern Med. 1996;156(5):505–509. [PubMed] [Google Scholar]
- 24.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J. 1985;109(4):876–885. doi: 10.1016/0002-8703(85)90653-2. [DOI] [PubMed] [Google Scholar]
- 25.Singh BN. Morbidity and mortality in cardiovascular disorders: Impact of reduced heart rate. J Cardiovasc Pharmacol Ther. 2001;6(4):313–331. doi: 10.1177/107424840100600401. [DOI] [PubMed] [Google Scholar]
- 26.Kovar D, Cannon CP, Bentley JH, Charlesworth A, Rogers WJ. Does initial and delayed heart rate predict mortality in patients with acute coronary syndromes? Clin Cardiol. 2004;27(2):80–86. doi: 10.1002/clc.4960270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 28.Marchioli R, Avanzini F, Barzi F, et al. Assessment of absolute risk of death after myocardial infarction by use of multiple-risk-factor assessment equations: GISSI-Prevenzione mortality risk chart. Eur Heart J. 2001;22(22):2085–2103. doi: 10.1053/euhj.2000.2544. [DOI] [PubMed] [Google Scholar]
- 29.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen TF, Davidsen M, Ibsen H, Jorgensen T, Jensen G, Borch-Johnsen K. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials: PRE-CARD and the Copenhagen Risk Score. J Cardiovasc Risk. 2001;8(5):291–297. doi: 10.1177/174182670100800508. [DOI] [PubMed] [Google Scholar]
- 31.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 32.Chattipakorn N, Incharoen T, Kanlop N, Chattipakorn S. Heart rate variability in myocardial infarction and heart failure. Int J Cardiol. 2007;120(3):289–296. doi: 10.1016/j.ijcard.2006.11.221. [DOI] [PubMed] [Google Scholar]
- 33.Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 34.Reil JC, Bohm M. The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol. 2007;96(9):585–592. doi: 10.1007/s00392-007-0537-5. [DOI] [PubMed] [Google Scholar]
- 35.Young JB. Sudden cardiac death syndrome and pump dysfunction: The link. J Heart Lung Transplant. 2000;19(Suppl 8):S27–S31. doi: 10.1016/s1053-2498(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 36.Nessler J, Nessler B, Kitlinski M, et al. Sudden cardiac death risk factors in patients with heart failure treated with carvedilol Kardiol Pol 200765121417–1422.discussion 1423–1424 [PubMed] [Google Scholar]
- 37.Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Suppl. 1999;1(Suppl H):H64–H69. [Google Scholar]
- 38.Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101(6):865–869. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Huang RL, Listerman J, Goring J, Giesberg C, Nading MA, Butler J.Beta-blocker therapy for heart failure: Should the therapeutic target be dose or heart rate reduction? Congest Heart Fail 2006124206–10.quiz 211–212 [DOI] [PubMed] [Google Scholar]
- 40.Lechat P, Escolano S, Golmard JL, et al. Prognostic value of bisoprolol-induced hemodynamic effects in heart failure during the Cardiac Insufficiency BIsoprolol Study (CIBIS) Circulation. 1997;96(7):2197–2205. doi: 10.1161/01.cir.96.7.2197. [DOI] [PubMed] [Google Scholar]
- 41.Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103(10):1428–1433. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 42.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 43.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 44.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 45.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 46.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: Beta-adrenergic receptors – alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2(9):475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 47.Veteran’s Health Administration Pharmacy Benefits Management Services and the Medical Advisory Panel National PBM drug monograph: Nebivolol (Bystolic) 2008. Jun, www.pbm.va.gov/monograph/Nebivolol.doc. Accessed Feb 18, 2009.
- 48.Agabiti Rosei E, Rizzoni D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs. 2007;67(8):1097–1107. doi: 10.2165/00003495-200767080-00001. [DOI] [PubMed] [Google Scholar]
- 49.Simon G, Johnson ML. Comparison of antihypertensive and beta 1-adrenoceptor antagonist effect of nebivolol and atenolol in essential hypertension. Clin Exp Hypertens. 1993;15(3):501–509. doi: 10.3109/10641969309041625. [DOI] [PubMed] [Google Scholar]
- 50.Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92(3):344–348. doi: 10.1016/s0002-9149(03)00645-3. [DOI] [PubMed] [Google Scholar]
- 51.Stoschitzky K, Stoschitzky G, Brussee H, Bonelli C, Dobnig H. Comparing beta-blocking effects of bisoprolol, carvedilol and nebivolol. Cardiology. 2006;106(4):199–206. doi: 10.1159/000093060. [DOI] [PubMed] [Google Scholar]
- 52.Patrianakos AP, Parthenakis FI, Mavrakis HE, Diakakis GF, Chlouverakis GI, Vardas PE. Comparative efficacy of nebivolol versus carvedilol on left ventricular function and exercise capacity in patients with nonischemic dilated cardiomyopathy. A 12-month study. Am Heart J. 2005;150(5):985. doi: 10.1016/j.ahj.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Nessler J, Nessler B, Kitlinski M, Gackowski A, Piwowarska W, Stepniewski M.Concentration of BNP, endothelin 1, pro-inflammatory cytokines (TNF-alpha, IL-6) and exercise capacity in patients with heart failure treated with carvedilol Kardiol Pol 2008662144–151.discussion 152–153 [PubMed] [Google Scholar]
- 54.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: A systematic review. Eur Heart J. 2005;26(8):778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 55.Jafri SM. The effects of beta blockers on morbidity and mortality in heart failure. Heart Fail Rev. 2004;9(2):115–121. doi: 10.1023/B:HREV.0000046366.31764.ca. [DOI] [PubMed] [Google Scholar]
- 56.Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail. 2001;3(3):351–357. doi: 10.1016/s1388-9842(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 57.Kitzman DW. Exercise intolerance. Prog Cardiovasc Dis. 2005;47(6):367–379. doi: 10.1016/j.pcad.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Jorde UP, Vittorio TJ, Kasper ME, et al. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: Time to pace? Eur J Heart Fail. 2008;10(1):96–101. doi: 10.1016/j.ejheart.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Lauer MS, Larson MG, Evans JC, Levy D. Association of left ventricular dilatation and hypertrophy with chronotropic incompetence in the Framingham Heart Study. Am Heart J. 1999;137(5):903–909. doi: 10.1016/s0002-8703(99)70415-1. [DOI] [PubMed] [Google Scholar]
- 60.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93(8):1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 61.Witte KK, Clark AL.Chronotropic incompetence in heart failure J Am Coll Cardiol 2006483595author reply 595–596 [DOI] [PubMed] [Google Scholar]
- 62.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96(9):1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 63.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14(2):215–221. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 64.Weiss R. Nebivolol: A novel beta-blocker with nitric oxide-induced vasodilatation. Vasc Health Risk Manag. 2006;2(3):303–308. doi: 10.2147/vhrm.2006.2.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carvalho VO, Rodrigues Alves RX, Bocchi EA, Guimaraes GV.Heart rate dynamic during an exercise test in heart failure patients with different sensibilities of the carvedilol therapy Heart rate dynamic during exercise test Int J Cardiol 2009January18[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Vittorio TJ, Zolty R, Kasper ME, et al. Differential effects of carvedilol and metoprolol succinate on plasma norepinephrine release and peak exercise heart rate in subjects with chronic heart failure. J Cardiovasc Pharmacol Ther. 2008;13(1):51–57. doi: 10.1177/1074248407312629. [DOI] [PubMed] [Google Scholar]
- 67.Stecker EC, Fendrick AM, Knight BP, Aaronson KD. Prophylactic pacemaker use to allow beta-blocker therapy in patients with chronic heart failure with bradycardia. Am Heart J. 2006;151(4):820–828. doi: 10.1016/j.ahj.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104(13):1477–1482. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 69.Hjalmarson A, Gilpin EA, Kjekshus J, et al. Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol. 1990;65(9):547–553. doi: 10.1016/0002-9149(90)91029-6. [DOI] [PubMed] [Google Scholar]
- 70.Abildstrom SZ, Jensen BT, Agner E, et al. Heart rate versus heart rate variability in risk prediction after myocardial infarction. J Cardiovasc Electrophysiol. 2003;14(2):168–173. doi: 10.1046/j.1540-8167.2003.02367.x. [DOI] [PubMed] [Google Scholar]
- 71.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16(1):13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 72.Zuanetti G, Hernndes-Bernal F, Rossi A, Comerio G, Paolucci G, Maggioni AP. Relevance of heart rate as a prognostic factor in myocardial infarction: The GISSI experience. Eur Heart J Suppl. 1999;1(Suppl H):H52–H57. [Google Scholar]
- 73.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57(12):43F–49F. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 74.Gundersen T, Grottum P, Pedersen T, Kjekshus JK. Effect of timolol on mortality and reinfarction after acute myocardial infarction: Prognostic importance of heart rate at rest. Am J Cardiol. 1986;58(1):20–24. doi: 10.1016/0002-9149(86)90234-1. [DOI] [PubMed] [Google Scholar]
- 75.Tuininga YS, Crijns HJ, Brouwer J, et al. Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients. Circulation. 1995;92(12):3415–3423. doi: 10.1161/01.cir.92.12.3415. [DOI] [PubMed] [Google Scholar]
- 76.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91(2):137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 77.Basu S, Senior R, Raval U, van der Does R, Bruckner T, Lahiri A. Beneficial effects of intravenous and oral carvedilol treatment in acute myocardial infarction. A placebo-controlled, randomized trial. Circulation. 1997;96(1):183–191. doi: 10.1161/01.cir.96.1.183. [DOI] [PubMed] [Google Scholar]
- 78.Louis WJ, McNeil JJ, Drummer OH. Pharmacology of combined alpha-beta-blockade. I. Drugs. 1984;28(Suppl 2):16–34. doi: 10.2165/00003495-198400282-00003. [DOI] [PubMed] [Google Scholar]
- 79.Pedersen ME, Cockcroft JR. The vasodilatory beta-blockers. Curr Hypertens Rep. 2007;9(4):269–277. doi: 10.1007/s11906-007-0050-2. [DOI] [PubMed] [Google Scholar]
- 80.MacCarthy EP, Bloomfield SS. Labetalol: A review of its pharmacology, pharmacokinetics, clinical uses and adverse effects. Pharmacotherapy. 1983;3(4):193–219. doi: 10.1002/j.1875-9114.1983.tb03252.x. [DOI] [PubMed] [Google Scholar]
- 81.Verma SP, Silke B, Hussain M, et al. Sympathetic (alpha-beta) or calcium channel blockade for hypertensive myocardial infarction? A haemodynamic comparison of labetalol and nifedipine. J Hypertens. 1988;6(11):897–904. doi: 10.1097/00004872-198811000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Stoleru L, Wijns W, van Eyll C, Bouvy T, Van Nueten L, Pouleur H. Effects of D-nebivolol and L-nebivolol on left ventricular systolic and diastolic function: Comparison with D-L-nebivolol and atenolol. J Cardiovasc Pharmacol. 1993;22(2):183–190. doi: 10.1097/00005344-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Palatini P, Graniero GR, Mormino P, et al. Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Circulation. 1994;90(6):2870–2876. doi: 10.1161/01.cir.90.6.2870. [DOI] [PubMed] [Google Scholar]
- 84.Bohm M, Reil JC, Danchin N, Thoenes M, Bramlage P, Volpe M. Association of heart rate with microalbuminuria in cardiovascular risk patients: Data from I-SEARCH. J Hypertens. 2008;26(1):18–25. doi: 10.1097/HJH.0b013e3282f05c8a. [DOI] [PubMed] [Google Scholar]
- 85.Madsen LB, Rasmussen JK, Moller DS, Nyvad O, Pedersen EB. Heart rate variability in white-coat hypertension. Blood Press Monit. 2008;13(2):65–71. doi: 10.1097/MBP.0b013e3282f13f5b. [DOI] [PubMed] [Google Scholar]
- 86.Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am Heart J. 1993;125(4):1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 87.Levy B. Importance of improving tissue perfusion during treatment of hypertension. J Hypertens Suppl. 2006;24(5):S6–S9. doi: 10.1097/01.hjh.0000240040.05097.5e. [DOI] [PubMed] [Google Scholar]
- 88.Levy BI. [The importance of microcirculation and tissue perfusion in hypertension] Curr Med Res Opin. 2005;21(Suppl 5):S1–S6. doi: 10.1185/030079905X56411. [DOI] [PubMed] [Google Scholar]
- 89.Campelo M, Polonia J, Serrao P, Cerqueira-Gomes M. Evaluation of the sympathetic nervous system using heart rate variability and plasma hormones in hypertensive patients treated with cilazapril and atenolol. Cardiology. 1996;87(5):402–408. doi: 10.1159/000177128. [DOI] [PubMed] [Google Scholar]
- 90.Chern CM, Hsu HY, Hu HH, Chen YY, Hsu LC, Chao AC. Effects of atenolol and losartan on baroreflex sensitivity and heart rate variability in uncomplicated essential hypertension. J Cardiovasc Pharmacol. 2006;47(2):169–174. doi: 10.1097/01.fjc.0000199225.17928.f5. [DOI] [PubMed] [Google Scholar]
- 91.Lund-Johansen P. Hemodynamic consequences of long-term beta-blocker therapy: A 5-year follow-up study of atenolol. J Cardiovasc Pharmacol. 1979;1(5):487–495. doi: 10.1097/00005344-197909000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007;100(8):1254–1262. doi: 10.1016/j.amjcard.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 93.Bradley HA, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens. 2006;24(11):2131–2141. doi: 10.1097/01.hjh.0000249685.58370.28. [DOI] [PubMed] [Google Scholar]
- 94.Cruickshank JM. Are we misunderstanding beta-blockers. Int J Cardiol. 2007;120(1):10–27. doi: 10.1016/j.ijcard.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 95.Sarafidis PA, Bakris GL.Do the metabolic effects of beta blockers make them leading or supporting antihypertensive agents in the treatment of hypertension? J Clin Hypertens (Greenwich) 200685351–356.quiz 357–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stafylas PC, Sarafidis PA. Carvedilol in hypertension treatment. Vasc Health Risk Manag. 2008;4(1):23–30. doi: 10.2147/vhrm.2008.04.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber MA, Bakris GL, Tarka EA, Iyengar M, Fleck R, Sica DA. Efficacy of a once-daily formulation of carvedilol for the treatment of hypertension. J Clin Hypertens (Greenwich) 2006;8(12):840–849. doi: 10.1111/j.1524-6175.2006.05696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: A randomized controlled trial. JAMA. 2004;292(18):2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 99.von Fallois J, Faulhaber HD. [Nebivolol, a third generation beta blocker: the modern treatment of hypertension. Results of a multicentric observational study] Fortschr Med Orig. 2000;118(Suppl 2):77–82. [PubMed] [Google Scholar]
- 100.Munzel T, Gori T. Nebivolol: The somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54(16):1491–1499. doi: 10.1016/j.jacc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt AC, Flick B, Jahn E, Bramlage P. Effects of the vasodilating beta-blocker nebivolol on smoking-induced endothelial dysfunction in young healthy volunteers. Vasc Health Risk Manag. 2008;4(4):909–915. [PMC free article] [PubMed] [Google Scholar]
- 102.Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52(18):1482–1489. doi: 10.1016/j.jacc.2008.06.048. [DOI] [PubMed] [Google Scholar]