Abstract
Social deprivation in early life disrupts emotionality and attentional processes in humans. Rearing rats in isolation reproduces some of these abnormalities, which are attenuated by daily handling. However, the neurochemical mechanisms underlying these responses remain poorly understood. We hypothesized that post-weaning social isolation alters the endocannabinoid system, a neuromodulatory system that controls emotional responding. We characterized behavioral consequences of social isolation and evaluated whether handling would reverse social isolation-induced alterations in behavioral reactivity to context and the endocannabinoid system. At weaning, pups were single or group housed and concomitantly handled or not handled daily until adulthood. Rats were tested in emotionality- and attentional-sensitive behavioral assays (open field, elevated plus maze, startle and prepulse inhibition). Cannabinoid receptor densities and endocannabinoid levels were quantified in a separate group of rats. Social isolation negatively altered behavioral responding. Socially-isolated rats that were handled showed less deficits in the open field, elevated plus maze, and prepulse inhibition tests. Social isolation produced site-specific alterations (supraoptic nucleus, ventrolateral thalamus, rostral striatum) in cannabinoid receptor densities compared to group rearing. Handling altered the endocannabinoid system in neural circuitry controlling emotional expression. Handling altered endocannabinoid content (prefrontal and piriform cortices, nucleus accumbens) and cannabinoid receptor densities (lateral globus pallidus, cingulate and piriform cortices, hippocampus) in a region-specific manner. Some effects of social isolation on the endocannabinoid system were moderated by handling. Isolates were unresponsive to handling-induced increases in cannabinoid receptor densities (caudal striatum, anterior thalamus), but were sensitive to handling-induced increases in endocannabinoid content (piriform cortex), compared to group-reared rats. Our findings suggest alterations in the endocannabinoid system may contribute to the abnormal isolate phenotype. Handling modifies the endocannabinoid system and behavioral reactivity to context, but surmounts only some effects of social isolation. These data implicate a pivotal role for the endocannabinoid system in stress adaptation and emotionality-related disturbances.
Keywords: anandamide, 2-arachidonoylglycerol (2-AG), early-life stress, emotionality, handling, schizophrenia
Rearing rats in isolation post-weaning is an animal model of social deprivation that recapitulates features of limbic-based psychopathology in humans. Social isolation models aspects of anxiety disorders (Haller and Halasz, 1999, Lukkes et al., 2009), substance abuse (Hall et al., 1997, Howes et al., 2000, Advani et al., 2007), and schizophrenia (Varty et al., 1999a, Schubert et al., 2009). Rodents reared in deprivation of social contact exhibit an abnormal behavioral phenotype that includes hyperlocomotion in response to a novel environment (Sahakian et al., 1982, Hall et al., 1998), altered habituation (Einon and Morgan, 1976, Gentsch et al., 1982), and disrupted exploratory behaviors (Paulus et al., 2000, Varty et al., 2000). Several lines of evidence suggest social isolation increases the likelihood of psychiatric-like features (for review see Fone and Porkess, 2008).
Social isolation produces schizophrenia-like deficits in perceptual processes, including deficits in sensorimotor gating (Geyer et al., 1993, Powell et al., 2002). Social isolation also increases aggression (Wongwitdecha and Marsden, 1996, Toth et al., 2008) and avoidance (Petkov and Rousseva, 1984, Del Arco et al., 2004). Brains derived from schizophrenic patients show altered cytoarchitectural and volumetric changes in the hippocampus and prefrontal cortex (for review see McGlashan and Hoffman, 2000), cortical regions that regulate social cognition (Gur et al., 2000, Venkatasubramanian et al., 2008). These anatomical changes are mirrored in isolation-reared rats. Brains derived from socially-isolated animals exhibit reductions in dendritic spine densities in the prefrontal cortex and hippocampus (Silva-Gomez et al., 2003). Isolates also exhibit lower levels of the synaptic marker synaptophysin in the hippocampal dentate gyrus (Varty et al., 1999b) and reduced brain volume in the prefrontal cortex (Schubert et al., 2009). Isolation-reared rats exhibit increased dopamine D2 receptor binding in rat striatum (Guisado et al., 1980, King et al., 2009), nucleus accumbens, amygdala, and substantia nigra pars compacta (Djouma et al., 2006). These observations are in line with increased in vivo occupancy of striatal D2 receptors by dopamine in schizophrenic patients (Abi-Dargham et al., 2000).
In socially-deprived rodents, chronic experimenter handling attenuates anxiety-like behavior (Gentsch et al., 1988, Vinod et al., 2008, Haller et al., 2009) and induces stress-protective effects (Plotsky and Meaney, 1993, Krebs-Thomson et al., 2001). Handling also reverses negative effects induced in other animal models of early-life stress and schizophrenia (Francis et al., 2002, Tejedor-Real et al., 2007). Handling has, consequently, been viewed as a reciprocal treatment for early-life stress (for review see Laviola et al., 2008). Handling produces behavioral and anatomical changes that parallel environmental enrichment (Szeligo and Leblond, 1977, Escorihuela et al., 1994), suggesting that handling acts like another environmental intervention as a reciprocal treatment for early-life stress. However, whether handling has the ability to mobilize endocannabinoids or alter cannabinoid receptor densities is unknown.
Endocannabinoids are lipid-derived neuromodulatory substances that regulate signaling at the interface between dopaminergic, glutamatergic, and gamma-aminobutyric acid (GABA)-ergic transmission (for review see Katona and Freund, 2008). The endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG) bind to presynaptic cannabinoid CB1 receptors to control synaptic strength. CB1 receptors modulate synaptic transmission by curbing GABA and glutamate release (Misner and Sullivan, 1999, Ohno-Shosaku et al., 2001, Straiker and Mackie, 2005). The endocannabinoid signaling system thus regulates dopamine transmission both indirectly, by preventing glutamate and GABA release onto dopaminergic neurons, and directly by potentially forming CB1-D2 receptor heteromers (for review see Ferre et al., 2009).
Dysregulation of the endocannabinoid signaling system – comprised of cannabinoid receptors, their endogenous ligands, and endocannabinoid-metabolizing enzymes is – implicated in disturbances of emotion and stressor responsiveness (Hill et al., 2005, Eisenstein et al., 2009). Schizophrenic patients exhibit increased cannabinoid receptor densities in the dorsolateral prefrontal cortex (Dean et al., 2001) and anterior and posterior cingulate gyrus (Zavitsanou et al., 2004, Newell et al., 2006), but not in the superior temporal gyrus (Deng et al., 2007). Furthermore, acute schizophrenic patients demonstrate elevated anandamide levels in cerebrospinal fluid (Leweke et al., 1999, Giuffrida et al., 2004) and blood (De Marchi et al., 2003). Cannabinoid receptor activation induces behavioral deficits in sensorimotor gating as well as deficits in medial prefrontal cortex and hippocampal function (Fernandez-Espejo and Galan-Rodriguez, 2004, Ballmaier et al., 2007, Dissanayake et al., 2008); these effects are reversed by CB1 receptor antagonists (see also Martin et al., 2003, Malone et al., 2004). Thus, an emerging body of literature suggests the endocannabinoid system is perturbed in schizophrenia and other affect-related disturbances, including anxiety and mood disorders (Cohen et al., 2008, Leweke and Koethe, 2008).
Social isolation may produce long-term alterations in the endocannabinoid system (Malone et al., 2008). Social isolation increased immunoreactivity for the anandamide-hydrolyzing enzyme fatty-acid amide hydrolase (FAAH) in the caudate putamen and nucleus accumbens (Malone et al., 2008). Social isolation also decreased CB1 receptor immunoreactivity in the caudate putamen and amygdala (Malone et al., 2008). Notwithstanding, effects of social isolation on the endocannabinoid system remain poorly understood and the functional consequences of such changes are unknown. Moreover, to our knowledge, environmental treatments to reverse endocannabinoid system alterations induced in models of early-life stress and schizophrenia are yet to be elucidated.
In the present study, we characterized the impact of post-weaning social isolation on behavioral reactivity to context in emotionality and attentional dependent tests. We evaluated whether chronic experimenter handling would reverse the abnormal behavioral phenotype of isolates. Further, we tested the hypothesis that key components of the endocannabinoid system undergo alterations in the brains of adult rats subjected to social isolation. We examined the novel possibility that chronic handling would reverse alterations in the endocannabinoid system produced by social isolation.
METHODS
Animals and breeding procedures
Subjects were male Sprague Dawley rats derived from 21 timed pregnant female Sprague Dawley rats (Charles River Laboratories, INC, Wilmington, MA) received from the supplier at 14 days gestation. A third of the selected dams were used for neuroanatomical and biochemical experiments and the remainder were used for behavioral experiments. Dams were monitored daily to determine the date of parturition. All dams selected for use in the present experiments gave birth to a litter containing up to five male pups. Pups remained undisturbed and co-housed with their dam and siblings from birth until weaning. A total of 80 rats were used in behavioral experiments and 32 rats were used in neuroanatomical and biochemical experiments. All experimental groups consisted of pups derived from a minimum of two separate litters. Rats used in the same experiment were held in a shared holding room maintained under a 12:12 light:dark cycle and temperature of 23 ± 1 °C to ensure similar sensory experience (i.e., shared visual, auditory, and some olfactory cues). Rats were given ad libitum access to food and water. All procedures were carried out in accordance with the appropriate national guidelines for the Care and Use of Laboratory Animals and formal approval to conduct the experiments was obtained from the institutional animal subjects review board. All efforts were made to minimize the number of animals used and their suffering.
Post-weaning social isolation
At weaning, male pups within a litter were equally distributed to experimental groups; male offspring from the same litter were uniformly assigned to single (1 rat/42 × 22 × 20 cm cage) or group (5 rats/52 × 28 × 20 cm cage) polycarbonate cages and remained in these conditions until behavioral evaluations were performed (postnatal day 70 or 73) or brains were harvested for quantification of cannabinoid receptor density and endocannabinoid content (postnatal day 70). Rearing manipulations occurred continuously throughout adolescence, a critical period of development (Einon and Morgan, 1977) and lasted 7 weeks (for review see Lapiz et al., 2003), as robust changes in behavior may require continuous isolation rearing (Bakshi and Geyer, 1999, Varty et al., 1999a). Cages were cleaned and refilled with sawdust bedding weekly for single-housed and biweekly for group-housed rats. Single housing prevented, whereas group housing permitted, cohort-induced tactile stimulation.
Chronic Handling
Single- and group-reared rats were randomly assigned to concomitantly experience (or not experience) experimenter-induced tactile stimulation by repeated exposure to 5 min of handling on postnatal days 21–70. Handling was performed during the light phase of the light:dark cycle between 2:00 to 5:30 PM on each weekday, except weekends. Handling consisted of caressing the fur of the neck and the dorsal surface of the animal with one hand, while leaving a hand in contact with the vibrissae and snout, to facilitate olfactory recognition. During handling, the rat was placed in the lap of the experimenter or on a flat surface. If the rat did not display signs of discomfort (e.g., defecation/urination, tail rigidity, intense grooming, or startle responses), the handler proceeded to pet the rat. Handled and non-handled rats were kept on opposite sides of a shared holding room and, within each side, were further arranged by rearing condition.
Behavioral testing
The day before behavioral testing, rats were brought into the testing room for 30 min within their own cages to habituate them to the testing room. Behavioral tests were conducted during the light phase of the light:dark cycle between 10:00 AM to 4:00 PM. Between tests, behavioral equipment was wiped cleaned with a 5% acetic acid solution that was allowed to dry before reusing equipment.
Open field
Socially isolated or group reared rats that were concomitantly handled or non-handled (N = 32; n = 7–9/group) were tested in the open field on postnatal day 70. The open field was a Plexiglas square grey arena (80 × 80 × 80 cm) surrounded by 4 black walls. On the floor, two zones of equivalent areas were defined: a central square compartment of 56 cm/side and a concentric peripheral frame including the area within 24 cm from the walls. Rats were placed in the central zone and behavior was monitored for 5 min. Room light and background noise was kept at 15 lux and 65 dB, respectively. Locomotor activity was measured as the number of times the two front paws of a rat crossed a 4 × 4 grid superimposed onto a video recorded image of the open field. The duration of time spent in the central compartment of the open field and number of fecal boli excreted during this test was also assessed.
Elevated plus maze
The same rats (N = 32) tested in the open field were retested 3 days later in the elevated plus maze on postnatal day 73. Rats were placed in the central platform of the test apparatus and video-recorded for 5 min in a dimly lit (15 lux), sound-attenuated (60 dB) environment, as previously described (Pellow et al., 1985). The maze was made of Plexiglas (gray floor, black walls) and included two open (50 × 10 cm) and two closed arms (50 × 10 × 40 cm) extending from a central platform (10 × 10 cm). The apparatus was elevated 60 cm above the floor. Behavioral measures included the time spent in open and closed arms and frequency of head dips and stretched-attend postures, as described by Griebel et al., (2002).
Startle reflex and prepulse inhibition
A separate set of rats (N = 48) that were either socially isolated or group reared and either concomitantly handled or non-handled (n = 12/group) were tested on postnatal day 70 in the acoustic startle and prepulse inhibition (PPI) test, as previously described (Bortolato et al., 2005). Rats were placed in a startle reflex apparatus (Med Associates, St Albans, VT, USA) for a 5 min-acclimation period with a 70 dB background noise that continued during the entire session. Each rat was exposed to a session consisting of 3 consecutive trial sequences. In sequence 1 and 3, five pulse-alone trials of 115 dB were presented. During sequence 2, 12 pulse-alone trials, 30 trials of pulse preceded by 73, 76, or 82 dB prepulse tones (10 for each level of prepulse intensity), and 8 no-stimulus trials (only background noise) were delivered in pseudo-random order. Pulse and prepulse tone duration was 80 and 40 ms, respectively. The prepulse-pulse delay was 100 ms. Inter-trial-intervals were randomly selected to occur within a 10 to 15 s range. Percent PPI was calculated with the following formula: 100-[(mean startle amplitude for prepulse-pulse trials/mean startle amplitude for pulse-alone trials) × 100]. As no interaction between prepulse levels and treatment were found in the statistical analysis, % PPI values were collapsed across prepulse intensity to represent average % PPI.
Tissue preparation
A separate set of rats (N = 32) that were either socially isolated or group reared and concomitantly handled or non-handled (n = 8/group) were decapitated on postnatal day 70. Brains were rapidly dissected, and snap frozen in precooled isopentane (−30°C). Brains were stored at low temperature (−30°C and −80°C) until use. The right hemisphere of the rat brain was used to measure cannabinoid receptor densities and distribution using [3H]CP55,940 binding and quantitative autoradiography. The left hemisphere was used to obtain tissue punches for quantification of endocannabinoid content using liquid chromatography/mass spectrometry (LC/MS).
Receptor binding and autoradiography
Coronal brain sections (14 μM thickness) were cryostat cut and mounted 4 sections/slide. Cannabinoid receptor binding was performed using [3H]CP55,940 (specific activity 139.6 Ci/mmol; Research Triangle Institute, Research Triangle Park, NC, USA), as described previously (Herkenham et al., 1991, Hohmann and Herkenham, 1998, Hohmann et al., 1999). Nonspecific binding was determined in the presence of 10 μM CP55,940. Briefly, binding was performed in cytomailers (3 h at 37 °C) in 50 mM Tris-HCl (pH 7.4) containing 5% bovine serum albumin and either 4.6 or 3.3 nM [3H]CP55,940. Binding assays were performed by neuroanatomical level of section, so that brains from rats in all experimental groups were processed concurrently in the same assay. All slides were washed (4 h at 0 °C) in a buffer containing 1% bovine serum albumin, fixed in 0.5% formalin in 50 mM Tris-HCl (pH 7.4 at 25 °C) and blown dry. Sections were apposed to [3H]-sensitive film (Amersham Hyperfilm, GE Healthcare LifeSciences, Piscataway, NJ) with [3H] standards ([3H] microscales, Amersham, Arlington Heights, IL, USA) for 8 weeks for levels incubated in 4.6 nM [3H]CP55,940 and 9 weeks for levels incubated in 3.3 nM [3H]CP55,940. Films were developed in D19 developer (Carestream Health, Rochester, NY, USA) and air-dried.
Densitometry
Film images were scanned (ScanMaker 9800XL, Microtek, Cerritos, CA, USA) and densitometry performed using NIH Image software (U.S. National Institutes of Health, http://rsb.info.nih.gov/nih-image/). Mean binding densities in select brain regions (see Table 1) were calculated and converted to nCi/mg tissue weight based on a best-fit 3rd degree polynomial formula that incorporates tissue equivalent values provided by Amersham. Brain areas were outlined using a rat brain atlas (Paxinos and Watson, 1998). Densitometric measurements were obtained from 3–4 near adjacent sections to measure total and nonspecific binding for each structure within each rat. Nonspecific binding (determined in sections adjacent to total binding sections) was subtracted from total binding to obtain specific binding values used in data analysis. Densitometry was performed on original images that were in no way digitally manipulated. Example photomicrographs were uniformly transformed across groups to a color scale using NIH Image J.
Table 1.
[3H]CP55,940 binding to cannabinoid receptors in the adult rat brain after post-weaning social isolation and handling treatment
| Brain Region | Group Reared Non-Handled | Group Reared Handled | Socially Isolated Non-Handled | Socially Isolated Handled |
|---|---|---|---|---|
| Basal Ganglia/Striatum | ||||
| Caudal caudate putamen (CPu) | 13.71 ± 0.87++ | 22.68 ± 1.90 | 15.73 ± 1.44+ | 15.99 ± 1.66+ |
| Caudal dorsal CPu | 12.83 ± 0.80+ | 19.64 ± 2.21 | 14.27 ± 0.77++ | 15.08 ± 0.97 |
| Caudal ventral CPu | 13.06 ± 0.87 | 21.13 ± 2.24 | 16.10 ± 2.28 | 16.40 ± 3.18 |
| Rostral CPu | 16.28 ± 0.91 | 12.63 ± 1.91 | 18.42 ± 2.63 | 17.48 ± 1.42 |
| Rostral dorsolateral CPu | 19.35 ± 1.17 | 15.34 ± 2.36 | 21.20 ± 3.04 | 20.64 ± 1.75 |
| Rostral dorsomedial CPu | 14.10 ± 0.69 | 10.60 ± 1.50 | 16.01 ± 2.15* | 14.63 ± 0.99* |
| Rostral ventrolateral CPu | 17.32 ± 0.93 | 13.93 ± 2.48 | 20.42 ± 3.26* | 20.67 ± 2.31* |
| Rostral ventromedial CPu | 14.56 ± 0.71 | 11.12 ± 1.65 | 16.48 ± 2.62 | 15.64 ± 1.53 |
| Lateral globus pallidus | 34.72 ± 4.24 | 48.72 ± 2.32 | 35.78 ± 4.25* | 39.95 ± 2.20* |
| Nucleus accumbens core | 12.16 ± 0.72 | 9.13 ± 1.25 | 13.54 ± 2.49 | 13.11 ± 1.38 |
| Nucleus accumbens shell | 11.39 ± 0.74 | 8.59 ± 1.39 | 12.21 ± 2.19 | 12.65 ± 1.41 |
| Olfactory tubercle | 7.57 ± 0.41 | 5.20 ± 0.71 | 8.17 ± 1.67 | 8.48 ± 1.00 |
| Cerebral Cortex | ||||
| Cingulate cortex | 9.00 ± 0.58 | 13.09 ± 1.38## | 9.01 ± 0.47 | 10.74 ± 0.88## |
| Motor cortex | 9.76 ± 0.85 | 7.55 ± 0.95 | 10.30 ± 1.58 | 10.13 ± 0.92 |
| Piriform cortex | 6.95 ± 0.47 | 9.57 ± 1.16# | 7.51 ± 0.49 | 8.62 ± 1.08# |
| Septum | ||||
| Lateral septum | 12.47 ± 1.04 | 9.30 ± 1.56 | 10.27 ± 1.54 | 13.48 ± 1.68 |
| Limbic diagonal band nuclei | 13.66 ± 0.95 | 10.56 ± 1.13 | 12.71 ± 1.23 | 11.63 ± 1.51 |
| Vertical limbic diagonal band | 16.08 ± 0.89 | 12.67 ± 1.60 | 14.21 ± 1.52 | 12.57 ± 1.60 |
| Amygdala | ||||
| Basolateral amygdala nuclei | 9.08 ± 0.70 | 9.93 ± 1.02 | 9.28 ± 1.29 | 8.12 ± 0.88 |
| Central amygdaloid nuclei | 12.04 ± 0.60 | 11.11 ± 1.19 | 10.29 ± 1.06 | 10.07 ± 1.10 |
| Hypothalamus & Thalamus | ||||
| Anterior thalamic nuclei | 4.19 ± 0.17++ | 5.66 ± .047 | 4.34 ± 0.22+ | 4.20 ± 0.23++ |
| Arcuate nuclei | 5.92 ± 0.47 | 6.63 ± 1.15 | 6.01 ± 0.78 | 6.33 ± 1.67 |
| Medial preoptic area | 6.99 ± 0.60 | 8.44 ± 0.92 | 7.15 ± 0.87 | 7.42 ± 0.56 |
| Superoptic nucleus | 8.80 ± 0.66 | 11.25 ± 1.31 | 7.26 ± 1.02* | 7.86 ± 0.77* |
| Ventrolateral thalamic nuclei | 7.16 ± 0.40 | 6.70 ± 0.58 | 6.14 ± 0.56* | 5.53 ± 0.47* |
| Hippocampal Formation | ||||
| CA1 – CA3 & dentate gyrus | 13.74 ± 0.70 | 10.85 ± 1.02# | 12.31 ± 0.74 | 11.95 ± 0.66# |
| CA1 – CA3 | 14.82 ± 0.66 | 11.67 ± 1.02# | 14.07 ± 0.91 | 13.58 ± 0.96# |
| CA1 | 17.39 ± 1.05 | 12.59 ± 1.54 | 16.80 ± 1.47 | 16.76 ± 1.33 |
| CA2 | 17.91 ± 0.82 | 13.00 ± 1.85# | 15.95 ± 1.21 | 15.14 ± 0.62# |
| CA3 | 18.50 ± 0.79 | 13.61 ± 2.18 | 16.37 ± 1.10 | 17.02 ± 1.45 |
| Dentate gyrus | 16.50 ± 0.85 | 13.50 ± 1.32 | 13.73 ± 1.25 | 13.77 ± 0.91 |
Data are mean ± S.E.M. (n = 8 per group);
P < 0.05 vs. Group-Reared;
P < 0.01,
P < 0.05 vs. Non-Handled;
P < 0.01,
P < 0.05 vs. Group-Reared/Handled.
Lipid extractions
Punches derived from single-hemisphere frozen brains were homogenized in methanol (0.3 mL) containing [2H4]-anandamide and [2H8]-2-AG (Cayman Chemicals, Ann Arbor, MI, USA) as internal standards. Protein concentration was determined in the homogenate to normalize samples using the bicinchinonic acid protein assay (Pierce, Rockford, IL). Tissue was collected using the rat brain atlas of Paxinos and Watson (1998) as a guide. The coordinates (relative to bregma) and dimensions of punches collected for selected structures of interest were: prefrontal cortex (+1.7 mm anterior-posterior (AP), +0.5 mm medial-lateral (ML),−3 mm dorsal-ventral (DV); 2 mm × 2 mm; adapted from Marsicano et al. (2002)), piriform cortex (+1.7 mm AP, +4.5 mm ML, −7 mm DV; 2 mm × 1 mm), nucleus accumbens (+1.7 mm AP, +1 mm ML, −3 mm DV; 2 mm × 2 mm), and hippocampus (−2.3 mm AP, +1 mm ML,−4 mm DV; 2 mm × 2 mm). Protein content in punches from the hippocampus, prefrontal cortex, and nucleus accumbens averaged 25–30 μg/sample. Punches from the piriform cortex averaged 10–15 μg protein/sample. Lipids were extracted with chloroform (2 vol) and washed with water (1 vol). Endocannabinoids were fractionated by open-bed silica gel column chromatography, as previously described (Moise et al., 2008, Astarita and Piomelli, 2009). Lipids were reconstituted in chloroform, loaded onto small glass columns packed with Silica Gel G (60-Å 230–400 Mesh ASTM; Whatman, Clifton, NJ, USA), and washed with 2 ml of chloroform. Anandamide and 2-AG were eluted with 1 ml of chloroform/methanol (9:1, vol/vol). Eluates were dried under N2 and reconstituted in 50 μL of methanol for LC/MS analyses.
LC/MS analyses
An 1100-LC system coupled to a 1946A-MS detector (Agilent Technologies, Inc., Palo Alto, CA, USA) equipped with an electrospray ionization interface was used to measure anandamide and 2-AG levels in each tissue punch. Lipids were separated using a XDB Eclipse C18 column (50 × 4.6 mm i.d., 1.8 μm, Zorbax), eluted with a gradient of methanol in water (from 75% to 85% in 2.5 min, to 90% in 7.5 min, to 100% in 14 min, and to 75% in 20 min) at a flow rate of 1.0 mL/min. Column temperature was kept at 40 C. MS detection was in the positive ionization mode, capillary voltage was at 3 kV, and fragmentor voltage varied from 120V. N2 was used as drying gas at a flow rate of 13 L/min and temperature of 350 °C. Nebulizer pressure was set at 60 PSI. Quantifications were conducted using an isotope-dilution method (Moise et al., 2008, Astarita and Piomelli, 2009) by monitoring Na+ adducts of the molecular ions ([M+Na]+) in the selected ion-monitoring mode. Quantification limits were 0.08 pmol for anandamide and 0.4 pmol for 2-AG.
Statistical Analysis
Homogeneity of variance and group normality were validated using the Levene and Kolmogorov-Smirnov statistics, respectively. A separate two-way (Rearing × Handling) independent analysis of variance was performed for each behavior and each structure for LC/MS and densitometry measures. Tukey post hocs were performed to identify the source of significant interactions. Endocannabinoid levels and elevated plus maze behavior (% open arm time as a function of total maze time) were additionally analyzed using planned comparisons (two-tailed, independent samples t-tests) between rearing conditions, performed separately in non-handled and handled rats. Planned comparisons were performed because handling has been reported to alter anxiolytic-like effects of the fatty-acid amide hydrolase (FAAH) inhibitor URB597 in the elevated plus maze (Haller et al., 2009). Thus, planned comparisons offered statistical sensitivity (decreased likelihood of making a type II error) for examination of the effects of rearing by removing variance due to handling, which could otherwise mask detection of endocannabinoid-dependent effects in a 2 × 2 ANOVA.
Comparisons that did not meet the equal variance assumption were corrected by fractional adjustment of degrees of freedom. Classic eta squared (η2) effect size calculations were performed to gauge the amount of variance our manipulations accounted for in the dependent measures evaluated. Using Cohen’s standards, eta squared values above 0.0099, 0.058, and 0.1379 can be considered small, medium, and large effects, respectively (Cohen, 1998), although limitations of these stated criteria (e.g., overestimation of population association, dependence upon sample size) must be acknowledged (Levine and Hullett, 2002, Pierce et al., 2004). Eta squared was calculated by dividing SSFactor by SSCorrected Total. All other analyses were performed using SPSS statistical software (version 16.0; SPSS Incorporated, Chicago, IL, USA).
RESULTS
Behavioral effects of social isolation
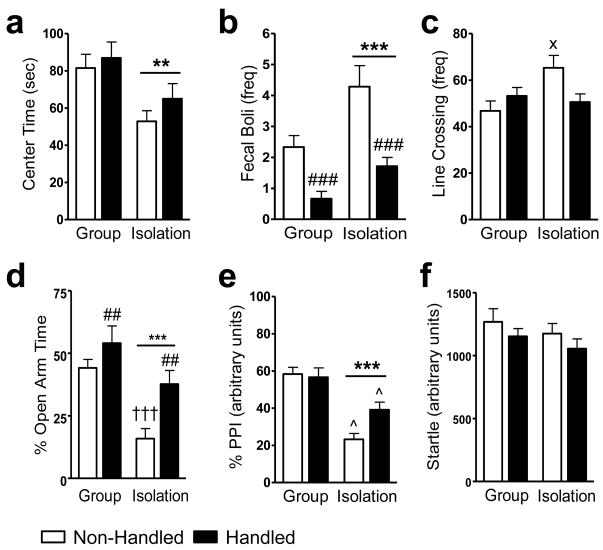
In the open field, socially-isolated rats spent less time in the center of the open field relative to group-reared rats (Rearing main effect: F1,28 = 10.56, P = 0.003; η2 = 0.265; Fig. 1a). Isolates excreted more fecal boli in the open field compared to group-reared rats (Rearing main effect: F1,28 = 13.46, P = 0.001; η2 = 0.196; Fig. 1b). In the elevated plus maze, isolates exhibited a lower % time in the open arms (Rearing main effect: F1,28 = 17.87, P = 0.000; η2 = 0.322; Fig. 1d) and a greater % time in the closed arms (Rearing main effect: F1,28 = 14.42, P = 0.001; η2 = 0.303; Data not shown), compared to group-reared rats. The number of closed arm entries was unaltered in isolates relative to socially-reared rats (P = 0.003; Data not shown). In the elevated plus maze, isolates also engaged in fewer stretch-attend postures (Rearing main effect: F1,28 = 105.33, P = 0.000; η2 = 0.769; Data not shown) and head dips (Rearing main effect: F1,28 = 18.40, P = 0.000; η2 = 0.412; Data not shown). In the prepulse inhibition test, isolates exhibited a lower % PPI relative to group-reared rats (Rearing main effect: F1,28 = 42.53, P = 0.000; η2 = 0.451; Fig. 1e). The deficit in % PPI observed in isolates was not global, as there was no difference in acoustic startle amplitude between groups (P > 0.05; Fig. 1f).
Figure 1.
Social isolation alters behavioral responsiveness to context in the (a - c) open field, (d) elevated plus maze and (e) prepulse inhibition test without altering (f) acoustic startle amplitude. Handling altered behavioral responsiveness to context in the (b) open field and (d) elevated plus maze in a manner opposite to that of isolates. Isolates deprived of handling exhibit hyperlocomotor activity measured by (c) line crossings in the open field and a decreased time spent in the (d) open arms of the elevated plus maze compared to group-reared rats; these differences were not present in isolates subjected to handling. Socially-isolated rats that were handled showed a lessened deficit in (e) % PPI compared to their non-handled counterparts, but were less able to inhibit responding to a prepulse compared to group-reared rats. Data are mean ± S.E.M. ***P < 0.001, **P < 0.01 vs. Group Reared (ANOVA); ###P < 0.001, ##P < 0.01 vs. Non-handled (ANOVA); xP < 0.05 vs. Group Reared/Non-Handled (ANOVA, Tukey post hoc); †††P < 0.001 vs. Group Reared/Non-Handled (t-test, two-tailed); ^P < 0.05 vs. all other groups (ANOVA, Tukey post hoc).
Behavioral effects of chronic handling
In the open field, handled rats excreted fewer fecal boli than non-handled rats (Handling main effect: F1,28 = 26.86, P = 0.000; η2 = 0.392; Fig. 1b). In the elevated plus maze, handling increased % open arm time (Handling main effect: F1,28 = 8.99, P = 0.006; η2 = 0.162; Fig. 1d), but did not alter % closed arm time (P > 0.05; Data not shown), compared to non-handling. Closed arm entries, stretch-attend postures, and head dips in the elevated plus maze were unaffected by handling manipulation (P > 0.05; Data not shown).
Behavioral effects of social isolation in handled and non-handled rats
In the open field, social isolation altered locomotor activity depending on handling condition, as assessed by the number of line crossings (Interaction effect: F1,28 = 6.21, P = 0.019; η2 = 0.163; Fig. 1c). In non-handled rats, isolates exhibited more line crossings than group-reared rats (P < 0.05; Fig. 1c); this effect was absent in handled isolates (P > 0.05; Fig. 1c). In the elevated plus maze, non-handled isolates exhibited lower % open arm time compared to group-reared rats (t14 = 5.41, P = 0.000; Fig. 1d), but handled isolates were no different from group-reared rats on these measures (P > 0.05; Fig. 1d). In the prepulse inhibition test, social isolation altered % PPI in a manner dependent upon handling treatment (Interaction effect: F1,28 = 4.77, P = 0.034; η2 = 0.051; Fig. 1e). Non-handled isolates exhibited the greatest reduction in % PPI compared to all other groups (P < 0.05; Fig. 1e). Handling isolates slightly elevated, but did not restore % PPI to levels observed in group-reared rats (P > 0.05; Fig. 1e).
Effects of social isolation on cannabinoid receptor densities
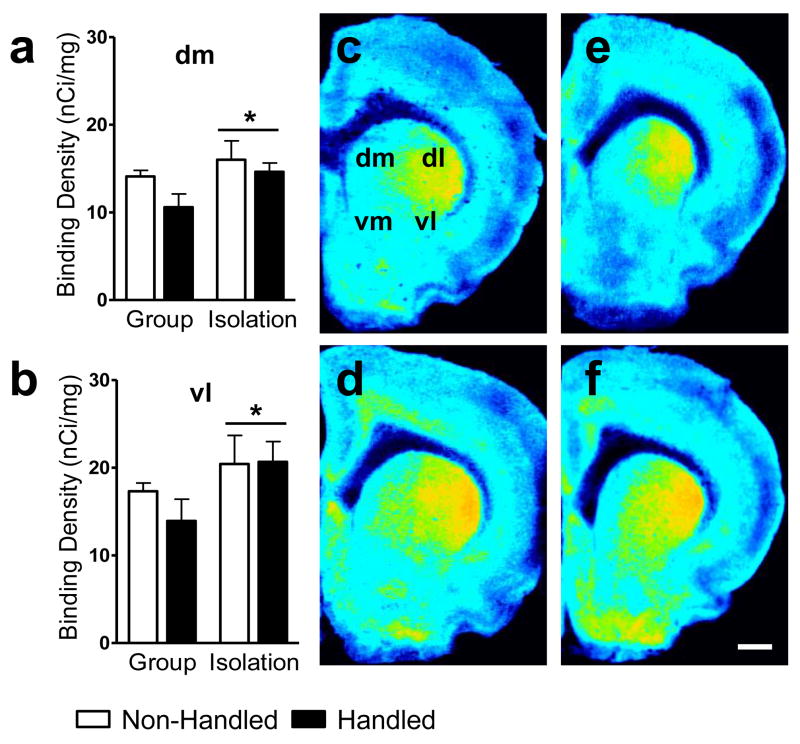
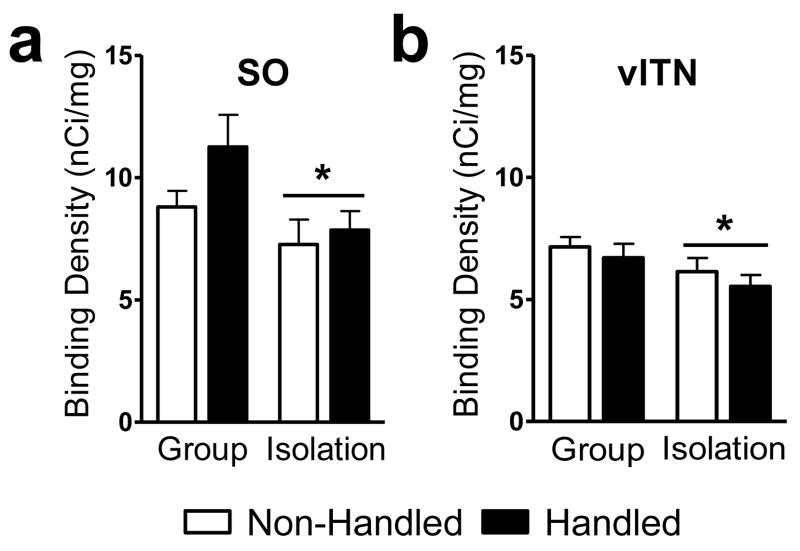
Percent specific binding was 94% (S.D. ± 1.35) averaged across films, documenting high sensitivity of binding and autoradiographic methods employed here. Social isolation increased cannabinoid receptor densities in the rostral dorsomedial (Rearing main effect: F1,28 = 4.24, P = 0.049; η2 = 0.119; Fig. 2a) and ventrolateral caudate putamen (Rearing main effect: F1,28 = 4.21, P = 0.05; η2 = 0.127; Fig. 2b) compared to group rearing (see also Fig. 2c–f). However, social isolation decreased cannabinoid receptor densities in the supraoptic hypothalamic nucleus (Rearing main effect: F1,21 = 5.89, P = 0.024; η2 = 0.194; Fig. 3a) and ventrolateral thalamic nuclei (Rearing main effect: F1,24 = 4.56, P = 0.043; η2 = 0.154; Fig. 3b) compared to group rearing (see also Fig. 4–5).
Figure 2.
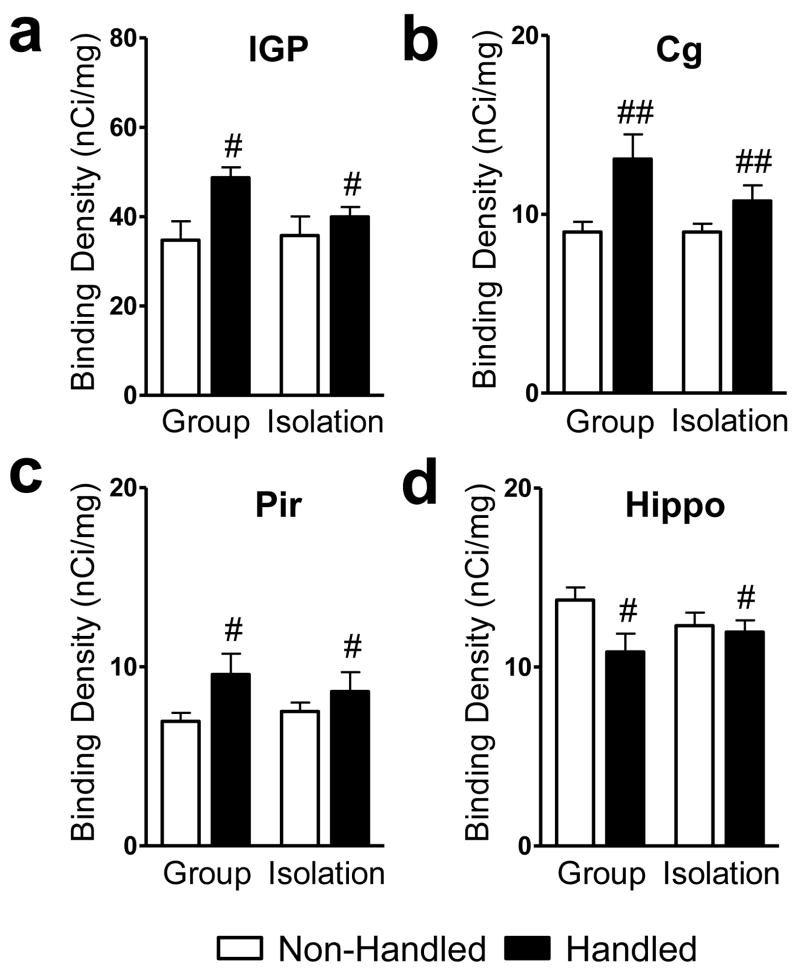
Socially-isolated rats show increased cannabinoid receptor density in the (a) dorsomedial and (b) ventrolateral caudate putamen compared to group-reared rats. Representative photomicrographs show [3H]CP55,940 binding in brains of adult rats that were either (top: c, e) group or (bottom: d, f) isolation reared and concomitantly (right: e, f) handled or (left: c, d) not handled post-weaning. Sections were collected +1.70 mm from bregma. The rostral caudate putamen was divided into quadrants as previously reported (Hohmann and Herkenham, 2000). dm, dorsomedial; dl, dorsolateral; vm, ventromedial; vl, ventrolateral. Scale bar equals 1 mm. Data are mean ± S.E.M. *P < 0.05 vs. Group Reared (ANOVA).
Figure 3.
Socially-isolated rats show decreased cannabinoid receptor density in the (a) supraoptic nucleus of the hypothalamus and (b) ventrolateral thalamus compared to group-reared rats. Data are mean ± S.E.M. *P < 0.05 vs. Group Reared (ANOVA). Representative photomicrographs are shown in Fig. 4–5. SO, supraoptic nucleus; vlTN, ventrolateral thalamic nuclei.
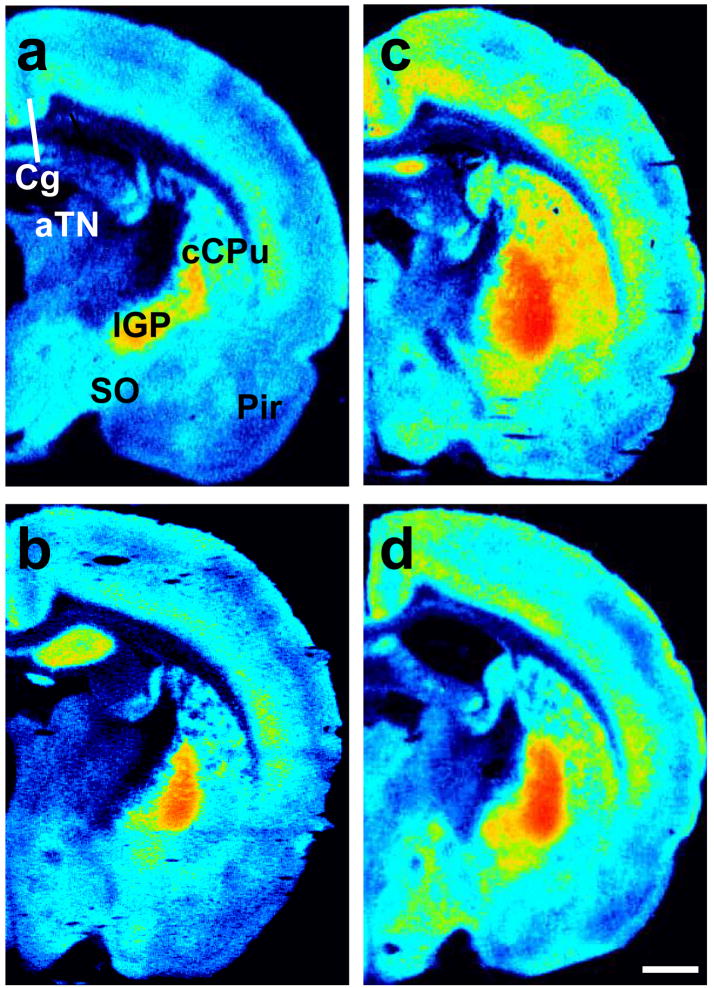
Figure 4.
Representative photomicrographs show [3H]CP55,940 binding in brains from adult rats that were either (top: a, c) group or (bottom: b, d) isolation reared and concomitantly (right: c, d) handled or (left: a, b) not handled post-weaning. Sections were collected −1.30 mm from bregma. aTN, anterior thalamic nuclei; Cg, cingulate cortex; lGP, lateral globus pallidus; Pir, piriform cortex; cCPu, caudal caudate putamen; SO, supraoptic nucleus. Scale bar equals 1mm.
Figure 5.
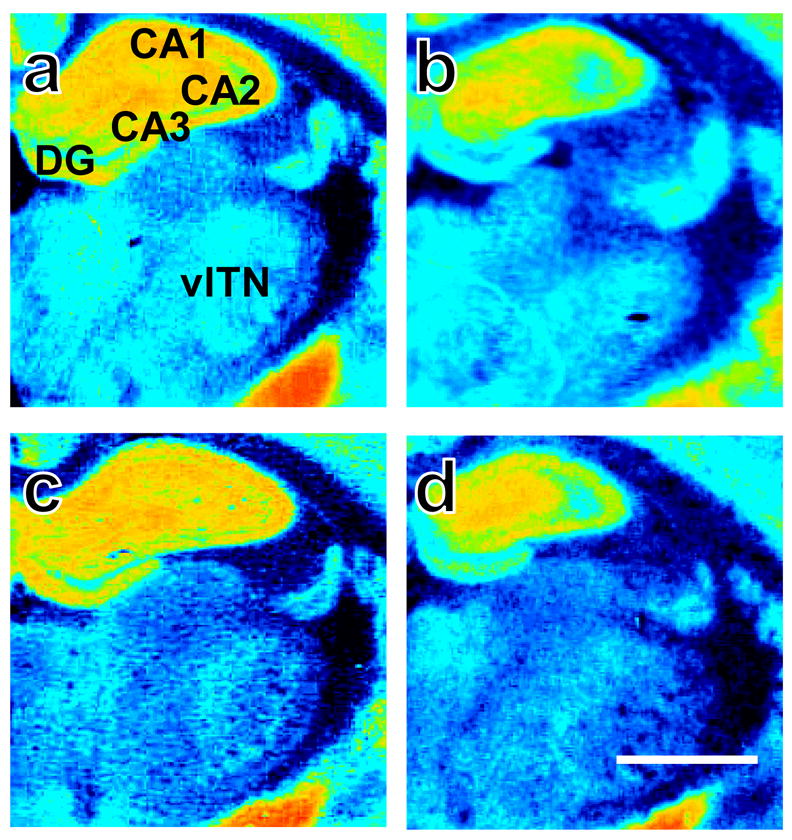
Representative photomicrographs show [3H]CP55,940 binding in brains derived from adult rats that were either (top: a, b) group or (bottom: c, d) isolation reared and concomitantly (right: b, d) handled or (left: a, c) not handled post-weaning. Sections were collected −2.30 mm from bregma. CA 1–3, molecular layers of hippocampus CA 1–3; DG, dentate gyrus; vlTN, ventrolateral thalamic nuclei. Scale bar equals 1mm.
Effects of chronic handling on cannabinoid receptor densities
Handling altered cannabinoid receptor densities within the limbic input-output loop of the basal ganglia (see Fig. 4–5). Handled rats exhibited increased cannabinoid receptor densities in the lateral globus pallidus (Handling main effect: F1,23 = 6.57, P = 0.017; η2 = 0.197; Fig. 7a), cingulate cortex (Handling main effect: F1,23 = 10.06, P = 0.004; η2 = 0.272; Fig. 7b), and piriform cortex (Handling main effect: F1,23 = 4.79, P = 0.039; η2 = 0.165; Fig. 7c) relative to non-handled rats. By contrast, handling decreased cannabinoid receptor densities in CA1–3 and the dentate gyrus of the hippocampus compared to non-handling (Handling main effect: F1,24 = 4.38, P = 0.047; η2 = 0.143; Fig. 7d). Subsequent analysis revealed that handled rats exhibited decreased cannabinoid receptor densities in CA2 (Handling main effect: F1,24 = 5.95, P = 0.022; η2 = 0.183; Data not shown) and, to a lesser extent, in CA1–3 (Handling main effect: F1,24 = 4.10, P = 0.054; η2 = 0.136; Data not shown), compared to non-handled rats.
Figure 7.
Handled rats show altered cannabinoid receptor densities in the limbic loop of the basal ganglia. Handled rats exhibit increased cannabinoid receptor densities in the (a) lateral globus pallidus, (b) cingulate cortex, and (c) piriform cortex, but decreased cannabinoid receptor density in the (d) hippocampus, relative to non-handled rats. Data are mean ± S.E.M. ##P < 0.01, #P < 0.05 vs. Non-Handled (ANOVA). Representative photomicrographs are shown in Fig. 4–5. Cg, cingulate cortex; Hippo, hippocampus CA 1–3 and dentate gyrus; lGP, lateral globus pallidus; Pir, piriform cortex.
Effects of social isolation on cannabinoid receptor densities in handled and non-handled rats
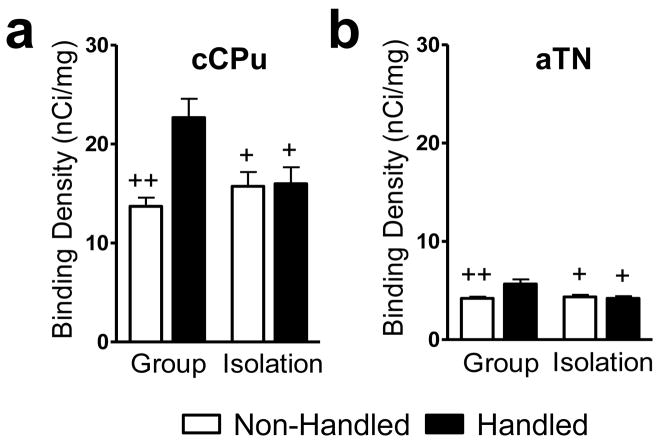
Handling altered cannabinoid receptor binding densities at caudal levels of the caudate putamen (Interaction effect: F1,23 = 8.59, P = 0.008; η2 = 0.189; Fig. 8a), specifically in the dorsal caudate putamen (Interaction effect: F1,23 = 4.80, P = 0.039; η2 = 0.126; Data not shown), and anterior thalamus (Interaction effect: F1,23 = 7.15, P = 0.014; η2 = 0.177; Fig. 8b) in a manner that was dependent on rearing conditions. Handling increased cannabinoid receptor densities in these regions in group- (P < 0.05), but not isolation-reared rats (P > 0.05 for all comparisons; Fig. 8a–b). No changes in cannabinoid receptor densities were found in any other structure examined (P > 0.05; see Table 1).
Figure 8.
Socially-isolated rats fail to exhibit handling-induced increases in cannabinoid receptor density exhibited by their group-reared counterparts in the (a) caudal caudate putamen and (b) anterior thalamic nuclei. Data are mean ± S.E.M. ++P < 0.01, +P < 0.05 vs. Group Reared/Handled (ANOVA). Representative photomicrographs are shown in Fig. 4–5. aTN, anterior thalamic nuclei; cCPu, caudal caudate putamen.
Effects of social isolation on endocannabinoid content
A 2 × 2 ANOVA failed to detect a reliable main effect of isolation rearing on endocannabinoid content. However, social isolation differentially altered endocannabinoid levels based on handling condition in the prefrontal and piriform cortices (see Fig. 6a–l).
Figure 6.
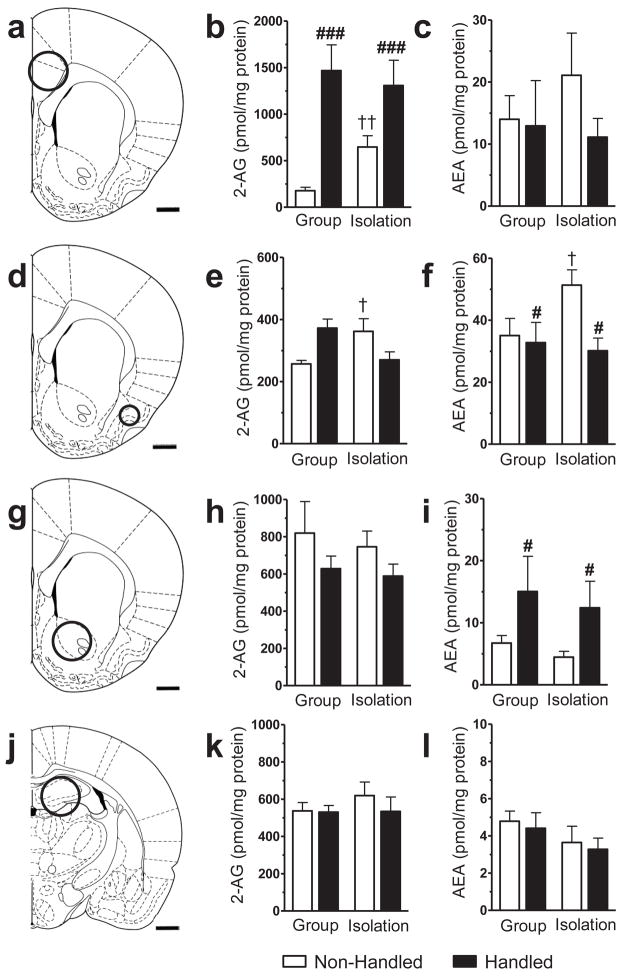
Endocannabinoid content in brain punches derived from adult rats with manipulated rearing and handling histories post-weaning. Single hemisphere punches were obtained at the level of the (a – c) prefrontal cortex, (d – f) piriform cortex, (g – i) nucleus accumbens, and (j – l) hippocampus as outlined (a, d, g, j) and assayed for 2-arachidonoylglycerol (2-AG) and anandamide (AEA). Social isolation modified endocannabinoid content in rats that were non-handled; social isolation increased (b) 2-AG in the prefrontal cortex, without altering (c) AEA levels, and increased both (e) 2-AG and (f) AEA content in the piriform cortex, relative to group rearing. Handling altered endocannabinoid content in both a ligand and brain region specific manner. In the prefrontal cortex, handling selectively increased (b) 2-AG but not (c) AEA levels compared to non-handling. In the piriform cortex, handling decreased (f) AEA, but unaltered (e) 2-AG, relative to non-handling. In the nucleus accumbens, handling increased (i) AEA but not (h) 2-AG compared to non-handling. (k, l) Endocannabinoid content was unchanged in the hippocampus. Data are mean ± S.E.M. ###P < 0.01, #P < 0.05 vs. Non-handled (ANOVA); ††P < 0.01, †P < 0.05 vs. Group Reared/Non-Handled (t-test, two-tailed). Scale bar equals 1 mm.
Effects of chronic handling on endocannabinoid content
In the prefrontal cortex, handling increased 2-AG (Handling main effect: F1,26 = 22.51, P = 0.000; η2 = 0.445; Fig. 6b), but not anandamide (P > 0.05; Fig. 6c) levels relative to non-handling. In the piriform cortex, handling decreased anandamide (Handling main effect: F1,28 = 4.83, P = 0.036; η2 = 0.129; Fig. 6f), but not 2-AG (P > 0.05; Fig. 6e) levels relative to non-handling. In the nucleus accumbens, handling increased anandamide (Handling main effect: F1,28 = 5.03, P = 0.033; η2 = 0.150; Fig. 6i) levels, without altering levels of 2-AG (P = 0.109; Fig. 6h), relative to non-handling. In the hippocampus, endocannabinoid levels were not affected by handling (P > 0.05; Fig. 6j–l).
Effects of social isolation on endocannabinoid content in handled and non-handled rats
Two-way ANOVA failed to reveal a reliable rearing X handling interaction (P > 0.05). A priori comparisons were performed separately in non-handled and handled rats on this measure (see Methods). In the prefrontal cortex, isolation rearing of non handled rats increased 2-AG levels (t8.24 = −3.71, P = 0.006; Fig. 6b) relative to their group-reared counterparts (P > 0.05). By contrast, 2-AG levels in the prefrontal cortex were similar in isolation- and group-reared rats subjected to handling. In this same structure, anandamide levels were not differentially altered by handling in isolates (P > 0.05; 6c). In the piriform cortex, isolation rearing increased both 2-AG (t8.06 = −2.48, P = 0.038; Fig. 6e) and anandamide (t14 = −2.20, P = 0.045; Fig. 6f) levels in non-handled rats compared to their group reared counterparts. These latter effects of isolation rearing were absent in handled rats (P > 0.05).
DISCUSSION
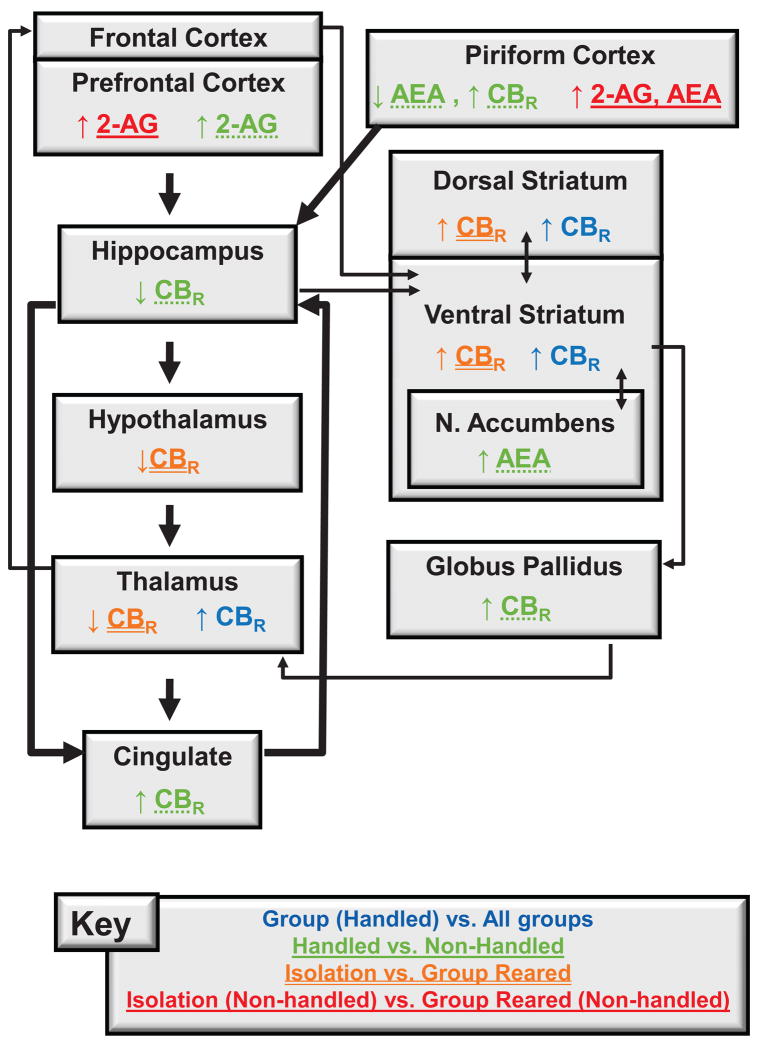
Early-life experience with impoverished or enriching environmental factors can affect later life. In our study, environmental manipulations (social isolation and chronic handling), performed from weaning to adulthood, spanned the critical developmental period of adolescence in rodents. During adolescence, key neurotransmitter systems (e.g., endocannabinoid, glutamate, dopamine) undergo maturation (Spear, 2000, Galve-Roperh et al., 2009) and positive events may either facilitate future adaptations (Boyce and Ellis, 2005) or counteract consequences of negative events (Laviola et al., 2008). The efficacy of the manipulations used here to alter both behavioral reactivity to context (see Fig. 1) and the endocannabinoid system (for summary see Fig. 9) may be attributed to the elevated susceptibility of rodents to environmental manipulations occurring during this developmental window.
Figure 9.
Neuroanatomical circuitry altered by post-weaning rearing and handling manipulations alone and in interaction. Thick arrows connect structures of Papez circuitry whereas thin arrows connect input-output loops through the basal ganglia. Key indicates treatment groups that are both color and underline coded to summarize changes in the endocannabinoid system. The key corresponds to the following group comparisons: Group Reared (Handled) vs. All other groups, Handled vs. Non-Handled, Isolation vs. Group Reared, and Isolation (Non-Handled) vs. Group Reared (Non-Handled). AEA, anandamide; 2-AG, 2-arachidonoylglycerol; CBR, cannabinoid receptor.
Social isolation negatively alters, whereas chronic handling positively alters behavioral reactivity to context
We verified that social isolation perturbs behavior in emotionality- and attentional-sensitive measures (e.g., open field, elevated plus maze, and prepulse inhibition test) (Tanaka et al., Hall et al., 1998, Powell et al., 2002). We also verified that handling produced beneficial behavioral consequences in these same measures (Fernandez-Teruel et al., 1990, Fernandez-Teruel et al., 1992, Krebs-Thomson et al., 2001). Consistent with previous reports, handling attenuates many isolation-induced behaviors, including hyperlocomotor activity, anxiety-like behavior (Present report; Gentsch et al., 1988, Gariépy et al., 2002) and deficits in sensorimotor gating (Present report; Krebs-Thomson et al., 2001, Rosa et al., 2005). Thus, social isolation produces relatively stable behavioral effects across laboratories.
The heightened behavioral responding exhibited by isolates in the elevated plus maze (reduced risk assessment, avoidance of the aversive open arms) probably enhanced their sensitivity to the anxiolytic-like properties of handling. Individual housing and a lack of either handling or habituation all increase responding to anxiolytics in the elevated plus maze (for review see Hogg, 1996). Of course, the elevated plus maze measures only a facet of the multidimensional construct of emotionality (Ramos, 2008). The open field may measure aspects of anxiety that are both shared and distinct from the elevated plus maze. In the open field, two negatively related measures of emotionality (Hall, 1934) – novelty-induced ambulation and defecation – were exacerbated in isolates. Here, the use of multiple measures strengthened our understanding of the emotional consequences underlying both adverse and beneficial early-life manipulations. Results from these measures provide converging lines of evidence to conclude that isolates have an anxiogenic-, whereas handled rats have an anxiolytic-like behavioral profile.
As expected, isolates were less able to gate sensory stimuli in the prepulse inhibition test compared to socially-reared rats. Prepulse inhibition is an adaptive mechanism that permits identification of potentially distracting/unimportant stimuli so that responding is averted to those with more relevance (for review see Koch and Schnitzler, 1997). Handling isolates partially ameliorated the deficit in prepulse inhibition exhibited by isolates, but did not fully compensate for it. In line with our data, different strains of rats with dissimilar anxiety-like traits respond uniquely in the prepulse inhibition test (van den Buuse, 2003). Even though the acoustic startle response and prepulse inhibition are intertwined processes, the neurobiology mediating the two phenomena are suggested to be distinct (Li et al., 2009). We found the overall pre-attentional responsiveness of isolates to the startle stimulus was unaltered. Together, these data suggest pre-attentional deficits of isolates are somewhat reversible through environmental manipulations.
Social isolation alters the endocannabinoid system in a region and ligand specific manner
In isolates, deprivation of olfactory cues from littermates produces conditioned odor deficits (Zimmerberg et al., 2009). Moreover, social isolation alters several neurotransmitter systems in brain regions involved in olfaction; social isolation increases 2-AG and anandamide levels in the piriform cortex (present report) and increases cholinergic and serotonergic fiber densities in the olfactory bulb and piriform cortex (Lehmann and Lehmann, 2007). Deficits in olfactory-related behaviors and circuitry may worsen the impaired interaction isolates have with their environment (for review see Fone and Porkess, 2008). Along the same line, we show social isolation alters the endocannabinoid system in the supraoptic nucleus of the hypothalamus. The supraoptic nucleus, a brain region implicated in social behaviors, is part of the magnocellular neurosecretory system of the hypothalamus (Martin, 2003). Input to oxytocin synthesizing neurons of the supraoptic nucleus is modulated by endocannabinoids that act at CB1 receptors (McDonald et al., 2008). It is interesting to speculate that downregulation of cannabinoid receptors observed here in socially-isolated rats is associated with increased endocannabinoid signaling; such changes would be expected to modify GABA-ergic and glutamatergic inputs to oxytocin neurons in the supraoptic nucleus and, ultimately, regulate oxytocin release. Long-lasting dysregulation in the endocannabinoid system in the supraoptic nucleus may, therefore, contribute to the perturbed social behavior of isolates (if the loss of these receptors are on inhibitory neurons) or represent a compensatory mechanism (if the loss of these receptors are on excitatory neurons) exhibited in the brains of isolates.
In the caudate putamen, social isolation increased cannabinoid receptor densities relative to group rearing. Likewise, cannabinoid receptor densities were increased in the caudate putamen in a model of schizophrenia induced by neonatal basolateral amygdala lesions (Bouwmeester et al., 2007). However, Malone and colleagues (2008) recently reported decreases in CB1 immunoreactivity in the caudate putamen of socially-isolated rats using immunohistochemical methods. Several explanations may account for the discrepancy between our study and that of Malone et al. (2008). First, differences in techniques used to measure receptors (immunofluorescence vs. binding density) exist between the two studies. In our work, [3H]CP55,940 binding would be expected to label all populations of cannabinoid receptors, whereas the C-terminal antibody used by Malone et al. (2008) may preferentially label specific subpopulations of cannabinoid receptors. Malone et al. (2008) used a CB1 antibody, raised in goat, that was directed against residues 401 – 473 of the C-terminal of CB1. This antibody is thought to preferentially label CB1 receptors on GABAergic, but not glutamatergic, neurons (Katona et al., 2006, Kawamura et al., 2006, Nyilas et al., 2009). By contrast, a highly sensitive later-generation CB1 antibody, raised in guinea pig, detects CB1 receptors on glutamatergic axons in the hippocampus and spinal cord (Katona et al., 2006, Kawamura et al., 2006, Nyilas et al., 2009) that were previously unrecognized by earlier generations of CB1 antibodies (Katona et al., 1999, Egertova and Elphick, 2000, Farquhar-Smith et al., 2000, Salio et al., 2002). Second, immunoreactive labeling by C-terminal antibodies may be masked by the presence of C-terminal interacting proteins (e.g. CRIP1a) that modulate CB1 receptor activity (Niehaus et al., 2007). Third, sensitivity of immunostaining may vary with the level of tissue fixation and receptor internalization (Hohmann, 2002). Fourth, the anatomical divisions of the caudate putamen evaluated also differed between the two studies. One or all of these factors may contribute to differences observed between the previous (Malone et al., 2008) and present report.
The striatum may be a unique region where isolation rearing alters D2 and CB1 receptor monomer expression (Bean and Lee, 1991, Malone et al., 2008, King et al., 2009). In striatal membranes, antagonistic intramembrane interaction occurred whereby CB1 receptor stimulation reduced D2 receptor affinity (Marcellino et al., 2008). Increases in CB1 receptor densities in isolates may produce altered D2 receptor dependent neurotransmission in the striatum. Given the presence of D2-CB1 receptor heterodimers in the striatum (for review see Ferre et al., 2009), social isolation may also alter D2-CB1 receptor heterodimer expression. At present, no selective pharmacological agent for a D2-CB1 receptor heterodimer is commercially available to directly test this hypothesis. Notwithstanding, future studies could extend the work of Malone et al. (2008) to obtain a description of D2-CB1 receptor colocalization after social isolation.
We found, as did others, that cannabinoid receptor densities did not change in cingulate and prefrontal cortices after social isolation (Malone et al., 2008). In contrast, schizophrenic patients exhibit increased cannabinoid receptor densities in these cortices compared to controls (Dean et al., 2001, Zavitsanou et al., 2004, Newell et al., 2006). Chronic-intermittent phencyclidine treated rats exhibit schizophrenia-like cognitive deficits and reductions in cannabinoid agonist-stimulated [35S]GTPγS binding in the prefrontal cortex compared to controls (Vigano et al., 2009). It is possible that similar changes also resulted from social isolation, but normalized before receptor densities were measured in adulthood. Experiments that elucidate the time course of changes in CB1 receptor densities and endocannabinoid content during the developmental window corresponding to adolescence (i.e., the social isolation manipulation window) are, therefore, warranted. It is particularly noteworthy that social isolation throughout adolescence in rats (present report), chronic-intermittent phencyclidine treatment in adolescent rats (Vigano et al., 2009), and exposure to repeated restraint stress in adolescent mice (Patel et al., 2005) all increased 2-AG, but not anandamide, in the prefrontal cortex (see also Seillier et al., 2009). Generalizations across models may provide important information about the role of the endocannabinoid system in response to chronic stressors (Hohmann et al., 2005, Rademacher et al., 2008, Rossi et al., 2008).
More work is necessary to determine whether chronic anxiolytic, antidepressant, antipsychotic, or glucocorticoid receptor antagonist (e.g., RU-486) treatments prevent the effects of post-weaning social isolation in rodents on the endocannabinoid system. To our knowledge, in otherwise naïve rodents, effects of chronic RU-486 treatment on the endocannabinoid system are unknown and effects of chronic anxiolytic, antidepressant and antipsychotic treatments are yet to be fully characterized. Chronic treatment with anxiolytics and antidepressants (Hill et al., 2006, Hesketh et al., 2008, Hill et al., 2008) and subchronic and chronic administration of antipsychotics (Andersson et al., 2005, Sundram et al., 2005, Wiley et al., 2008, Secher et al., 2009) all alter the endocannabinoid system. Thus, the therapeutic efficacy of these pharmacological agents may result, at least in part, from actions on the endocannabinoid system. Although chronic anxiolytics, antidepressants, antipsychotics, and even handling share some similar treatment outcomes and alter the endocannabinoid system, the underlying effects of these treatments on the endocannabinoid system are likely distinct.
Chronic handling alters the endocannabinoid system in a region and ligand specific manner
Chronic handling produced stable changes in the endocannabinoid signaling system in both Papez circuitry and input-output loops of the basal ganglia (see Figure 9). Here, we demonstrate for the first time that chronic handling alters both cannabinoid receptor densities and endocannabinoid content within brain structures that control emotional expression (i.e., lateral globus pallidus, prefrontal, piriform, and cingulate cortices, hippocampus, nucleus accumbens). In the prefrontal cortex, handling increased 2-AG without reliably altering anandamide levels, compared to non-handling. Handling accounted for about half of the variance in 2-AG content in the prefrontal cortex, as assessed by effect size calculations (Cohen, 1998). To a lesser extent, in the nucleus accumbens, handling increased anandamide levels without altering levels of 2-AG, compared to non-handling. Further, the reliable main effect of handling we observed in the piriform cortex for anandamide content may potentially be attributable to increased anandamide levels observed in non-handled isolates. Our data are consistent with the observation that handling decreased the anxiolytic effect of the FAAH inhibitor URB597 (Haller et al., 2009). Handling and URB597 may rely on similar endocannabinoid system-dependent mechanisms. Chronic handling may also facilitate habituation to stressors in an endocannabinoid-dependent manner, as handling itself eventually loses aversive quality after chronic exposure. Our data support the use of chronic handling, as a research tool, to manipulate the endocannabinoid system.
Handling increased cannabinoid receptor densities in the limbic loop of the basal ganglia, including basal ganglia output structures (lateral globus pallidus) and allocortical areas (cingulate and piriform cortex), but decreased cannabinoid receptor densities in archicortex (hippocampus), relative to non-handling. Handling, like subchronic treatment with the antipsychotic haloperidol (Andersson et al., 2005), increased [3H]CP55,940 binding to cannabinoid receptors in the globus pallidus. In the piriform cortex, handling altered both endocannabinoid content and cannabinoid receptor densities in a manner consistent with a causal relationship between these factors. Handling-induced reductions in anandamide levels may produce the observed upregulation of cannabinoid receptors in this region. In archicortex, chronic handling decreased cannabinoid receptor densities relative to non-handling. If chronic handling decreases the number of cannabinoid receptors available to inhibit calcium influx into glutamatergic neurons, it is reasonable to speculate handling might also enhance long-term potentiation. Supporting this hypothesis, both acute handling (Korz and Frey, 2003) and social isolation (Lu et al., 2003) reverse long-term potentiation generated by tetanic stimulation in the hippocampus. In contrast, male rats exposed to early maternal deprivation – a model of early-life stress and schizophrenia – exhibit decreases in CB1 (probably on GABA neurons) receptor immunoreactivity in regions of CA1 and CA3 of the hippocampus, compared to controls on postnatal day 13 (Suarez et al., 2009). Together, these data implicate a role for the endocannabinoid system in the ability of handling to alter behavior and neuronal physiology.
In socially isolated rats, chronic experimenter handling was not sufficient to modify cannabinoid receptor densities in the caudate putamen and thalamus; handling increased cannabinoid receptor densities in these same structures in group-, but not isolation-reared rats. Handling has been shown to preferentially reverse corticosterone levels in mice bred for low aggressiveness, but failed to reverse corticosterone levels in mice bred for high aggressiveness (Gariépy et al., 2002). Thus, it is, perhaps, not surprising that chronic handling did not reverse the array of changes induced by social isolation on the endocannabinoid system. Overall, we interpret our data to suggest that handling alone produces regulatory changes in the endocannabinoid system, but the effectiveness of this manipulation on the endocannabinoid system is diminished in rats reared in social deprivation.
CONCLUSION
The present report demonstrates that post-weaning social isolation alters principal components of the endocannabinoid system in limbic brain regions. The observed dysregulation of the endocannabinoid system may, therefore, contribute to the abnormal behavior of isolates. Chronic experimenter handling both reduces and treats the perturbed behavior of isolates in emotionality-and attentional sensitive assays. Handling also specifically modulates the endocannabinoid system. However, chronic handling alone is not sufficient to reverse the abundance of social deprivation-induced changes in cannabinoid receptor densities or endocannabinoid levels. These observations support a pivotal role for the endocannabinoid signaling system in the adaptation to stressful life events (for review see Finn, 2009, Rossi et al., 2009) as well as in emotionality-related disturbances (Leweke and Koethe, 2008, Lutz, 2009).
Acknowledgments
This research was supported by DA022702 and DA021644 (to DP and AGH). NRS is supported by a NIDA Diversity Supplement to DA021644.
ABBREVIATIONS
- AP
Anterior-posterior
- 2-AG
2-arachidonoylglycerol
- DV
dorsal-ventral
- GABA
gamma-aminobutyric acid
- LC/MS
liquid chromatography/mass spectrometry
- ML
medial-lateral
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10:595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Andersson M, Terasmaa A, Fuxe K, Stromberg I. Subchronic haloperidol increases CB(1) receptor binding and G protein coupling in discrete regions of the basal ganglia. J Neurosci Res. 2005;82:264–272. doi: 10.1002/jnr.20630. [DOI] [PubMed] [Google Scholar]
- Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2755–2767. doi: 10.1016/j.jchromb.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav. 1999;67:385–392. doi: 10.1016/s0031-9384(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Bortolato M, Rizzetti C, Zoli M, Gessa G, Heinz A, Spano P. Cannabinoid receptor antagonists counteract sensorimotor gating deficits in the phencyclidine model of psychosis. Neuropsychopharmacology. 2007;32:2098–2107. doi: 10.1038/sj.npp.1301344. [DOI] [PubMed] [Google Scholar]
- Bean G, Lee T. Social isolation and cohabitation with haloperidol-treated partners: effect on density of striatal dopamine D2 receptors in the developing rat brain. Psychiatry Res. 1991;36:307–317. doi: 10.1016/0165-1781(91)90029-o. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Frau R, Orru M, Fa M, Manunta M, Puddu M, Mereu G, Gessa GL. Kappa opioid receptor activation disrupts prepulse inhibition of the acoustic startle in rats. Biol Psychiatry. 2005;57:1550–1558. doi: 10.1016/j.biopsych.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Gerrits MA, Roozemond JG, Snapper J, Ronken E, Kruse CG, Westenberg HG, van Ree JM. Neonatal basolateral amygdala lesions affect monoamine and cannabinoid brain systems in adult rats. Int J Neuropsychopharmacol. 2007;10:727–739. doi: 10.1017/S1461145706007346. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. New York: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust N Z J Psychiatry. 2008;42:357–368. doi: 10.1080/00048670801961156. [DOI] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Zhu S, Terasmaa A, Mohammed AH, Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology (Berl) 2004;171:148–155. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- Deng C, Han M, Huang XF. No changes in densities of cannabinoid receptors in the superior temporal gyrus in schizophrenia. Neurosci Bull. 2007;23:341–347. doi: 10.1007/s12264-007-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake DW, Zachariou M, Marsden CA, Mason R. Auditory gating in rat hippocampus and medial prefrontal cortex: effect of the cannabinoid agonist WIN55,212-2. Neuropharmacology. 2008;55:1397–1404. doi: 10.1016/j.neuropharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci. 2006;23:3319–3327. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Einon D, Morgan M. Habituation of object contact in socially-reared and isolated rats (Rattus norvegicus) Animal Behaviour. 1976;24:415–420. [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV, Hohmann AG. Endocannabinoid modulation of amphetamine sensitization is disrupted in a rodent model of lesion-induced dopamine dysregulation. Synapse. 2009;63:941–950. doi: 10.1002/syn.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorihuela RM, Tobena A, Fernandez-Teruel A. Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behav Brain Res. 1994;61:169–173. doi: 10.1016/0166-4328(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Galan-Rodriguez B. Sensorimotor gating in mice is disrupted after AM404, an anandamide reuptake and degradation inhibitor. Psychopharmacology (Berl) 2004;175:220–224. doi: 10.1007/s00213-004-1851-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Jimenez P, Tobena A. Infantile stimulation and perinatal administration of Ro 15-1788: additive anxiety-reducing effects in rats. Eur J Pharmacol. 1990;191:111–114. doi: 10.1016/0014-2999(90)94104-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Nunez JF, Goma M, Driscoll P, Tobena A. Early stimulation effects on novelty-induced behavior in two psychogenetically-selected rat lines with divergent emotionality profiles. Neurosci Lett. 1992;137:185–188. doi: 10.1016/0304-3940(92)90400-2. [DOI] [PubMed] [Google Scholar]
- Ferre S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP. Endocannabinoid-mediated modulation of stress responses: Physiological and pathophysiological significance. Immunobiology. 2009 doi: 10.1016/j.imbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I, Palazuelos J, Aguado T, Guzman M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2009;259:371–382. doi: 10.1007/s00406-009-0028-y. [DOI] [PubMed] [Google Scholar]
- Gariépy J-L, Rodriguiz RM, Jones BC. Handling, genetic and housing effects on the mouse stress system, dopamine function, and behavior. Pharmacology Biochemistry and Behavior. 2002;73:7–17. doi: 10.1016/s0091-3057(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Behavioural comparisons between individually- and group-housed male rats: effects of novel environments and diurnal rhythm. Behav Brain Res. 1982;6:93–100. doi: 10.1016/0166-4328(82)90084-5. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialization. Physiology & Behavior. 1988;43:13–16. doi: 10.1016/0031-9384(88)90091-1. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado E, Fernandez-Tome P, Garzon J, Del Rio J. Increased dopamine receptor binding in the striatum of rats after long-term isolation. Eur J Pharmacol. 1980;65:463–464. doi: 10.1016/0014-2999(80)90359-3. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. Journal of Comparative Psychology. 1934;18:385–403. [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on sucrose consumption in rats. Physiol Behav. 1997;62:291–297. doi: 10.1016/s0031-9384(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Halasz J. Mild social stress abolishes the effects of isolation on anxiety and chlordiazepoxide reactivity. Psychopharmacology (Berl) 1999;144:311–315. doi: 10.1007/s002130051012. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh SA, Brennan AK, Jessop DS, Finn DP. Effects of chronic treatment with citalopram on cannabinoid and opioid receptor-mediated G-protein coupling in discrete rat brain regions. Psychopharmacology (Berl) 2008;198:29–36. doi: 10.1007/s00213-007-1033-3. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Hillard CJ, Gorzalka BB. Differential effects of the antidepressants tranylcypromine and fluoxetine on limbic cannabinoid receptor binding and endocannabinoid contents. J Neural Transm. 2008;115:1673–1679. doi: 10.1007/s00702-008-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006;31:2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63:476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Thomson K, Giracello D, Solis A, Geyer MA. Post-weaning handling attenuates isolation-rearing induced disruptions of prepulse inhibition in rats. Behavioural Brain Research. 2001;120:221–224. doi: 10.1016/s0166-4328(00)00374-0. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Lehmann D. Transmitter balances in the olfactory cortex: adaptations to early methamphetamine trauma and rearing environment. Brain Res. 2007;1141:37–47. doi: 10.1016/j.brainres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci. 2009;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152:265–272. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Long LE, Taylor DA. The effect of SR 141716 and apomorphine on sensorimotor gating in Swiss mice. Pharmacol Biochem Behav. 2004;77:839–845. doi: 10.1016/j.pbb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Muller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Martin JH. The limbic system and cerebral circuits for emotions, learning, and memory. In: Foltin J, et al., editors. Neuroanatomy: textbook and atlas. New York: McGraw-Hill Companies; 2003. pp. 377–406. [Google Scholar]
- Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, Lowe DA. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology (Berl) 2003;165:128–135. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- McDonald NA, Kuzmiski JB, Naderi N, Schwab Y, Pittman QJ. Endogenous modulators of synaptic transmission: cannabinoid regulation in the supraoptic nucleus. Prog Brain Res. 2008;170:129–136. doi: 10.1016/S0079-6123(08)00412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise AM, Eisenstein SA, Astarita G, Piomelli D, Hohmann AG. An endocannabinoid signaling system modulates anxiety-like behavior in male Syrian hamsters. Psychopharmacology (Berl) 2008;200:333–346. doi: 10.1007/s00213-008-1209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Liu Y, Wallis KT, Egertova M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, Elphick MR, Lewis DL. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964–1978. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Varty GB, Geyer MA. The genetic liability to stress and postweaning isolation have a competitive influence on behavioral organization in rats. Physiol Behav. 2000;68:389–394. doi: 10.1016/s0031-9384(99)00193-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Petkov VV, Rousseva S. Effects of caffeine on aggressive behavior and avoidance learning of rats with isolation syndrome. Methods Find Exp Clin Pharmacol. 1984;6:433–436. [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Powell SB, Swerdlow NR, Pitcher LK, Geyer MA. Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav. 2002;77:55–64. doi: 10.1016/s0031-9384(02)00817-x. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Rosa ML, Silva RC, Moura-de-Carvalho FT, Brandao ML, Guimaraes FS, Del Bel EA. Routine post-weaning handling of rats prevents isolation rearing-induced deficit in prepulse inhibition. Braz J Med Biol Res. 2005;38:1691–1696. doi: 10.1590/s0100-879x2005001100018. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, Usiello A, Centonze D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Bernardi G, Usiello A, Centonze D. Adaptations of striatal endocannabinoid system during stress. Mol Neurobiol. 2009;39:178–184. doi: 10.1007/s12035-009-8061-4. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Burdess C, Luckhurst H, Trayhurn P. Hyperactivity and obesity: the interaction of social isolation and cafeteria feeding. Physiol Behav. 1982;28:117–124. doi: 10.1016/0031-9384(82)90112-3. [DOI] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Conrath M. Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience. 2002;110:755–764. doi: 10.1016/s0306-4522(01)00584-x. [DOI] [PubMed] [Google Scholar]
- Schubert MI, Porkess MV, Dashdorj N, Fone KC, Auer DP. Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience. 2009;159:21–30. doi: 10.1016/j.neuroscience.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Secher A, Husum H, Holst B, Egerod KL, Mellerup E. Risperidone Treatment Increases CB(1) Receptor Binding in Rat Brain. Neuroendocrinology. 2009 doi: 10.1159/000245220. [DOI] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2009:1–14. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J, Llorente R, Romero-Zerbo SY, Mateos B, Bermudez-Silva FJ, de Fonseca FR, Viveros MP. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus. 2009;19:623–632. doi: 10.1002/hipo.20537. [DOI] [PubMed] [Google Scholar]
- Sundram S, Copolov D, Dean B. Clozapine decreases [3H] CP 55940 binding to the cannabinoid 1 receptor in the rat nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:428–433. doi: 10.1007/s00210-005-1074-2. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, Yuri K. Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience. doi: 10.1016/j.neuroscience.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Sahagun M, Biguet NF, Mallet J. Neonatal handling prevents the effects of phencyclidine in an animal model of negative symptoms of schizophrenia. Biol Psychiatry. 2007;61:865–872. doi: 10.1016/j.biopsych.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Toth M, Halasz J, Mikics E, Barsy B, Haller J. Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav Neurosci. 2008;122:849–854. doi: 10.1037/0735-7044.122.4.849. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Deficient prepulse inhibition of acoustic startle in Hooded-Wistar rats compared with Sprague-Dawley rats. Clin Exp Pharmacol Physiol. 2003;30:254–261. doi: 10.1046/j.1440-1681.2003.03823.x. [DOI] [PubMed] [Google Scholar]
- Varty GB, Braff DL, Geyer MA. Is there a critical developmental ‘window’ for isolation rearing-induced changes in prepulse inhibition of the acoustic startle response? Behav Brain Res. 1999a;100:177–183. doi: 10.1016/s0166-4328(98)00129-6. [DOI] [PubMed] [Google Scholar]
- Varty GB, Marsden CA, Higgins GA. Reduced synaptophysin immunoreactivity in the dentate gyrus of prepulse inhibition-impaired isolation-reared rats. Brain Res. 1999b;824:197–203. doi: 10.1016/s0006-8993(99)01173-7. [DOI] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117:420–431. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Vigano D, Guidali C, Petrosino S, Realini N, Rubino T, Di Marzo V, Parolaro D. Involvement of the endocannabinoid system in phencyclidine-induced cognitive deficits modelling schizophrenia. Int J Neuropsychopharmacol. 2009;12:599–614. doi: 10.1017/S1461145708009371. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Kendler SH, Burston JJ, Howard DR, Selley DE, Sim-Selley LJ. Antipsychotic-induced alterations in CB1 receptor-mediated G-protein signaling and in vivo pharmacology in rats. Neuropharmacology. 2008;55:1183–1190. doi: 10.1016/j.neuropharm.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Foote HE, Van Kempen TA. Olfactory association learning and brain-derived neurotrophic factor in an animal model of early deprivation. Dev Psychobiol. 2009;51:333–344. doi: 10.1002/dev.20373. [DOI] [PubMed] [Google Scholar]