Abstract
Background
We previously showed that the burden of Chlamydia pneumoniae in carotid plaques was significantly associated with plaque interleukin (IL)-6, and serum IL-6 and C-reactive protein (CRP), suggesting that infected plaques contribute to systemic inflammatory markers in patients with stroke risk. Since lipoprotein-associated phospholipase A2 (Lp-PLA2) mediates inflammation in atherosclerosis, we hypothesized that serum Lp-PLA2 mass and activity levels and plaque Lp-PLA2 may be influenced by plaque C. pneumoniae infection.
Methodology/Principal Findings
Forty-two patients underwent elective carotid endarterectomy. Tissue obtained at surgery was stained by immunohistochemistry for Lp-PLA2 grade, macrophages, IL-6, C. pneumoniae and CD4+ and CD8+ cells. Serum Lp-PLA2 activity and mass were measured using the colorimetric activity method (CAM™) and ELISA, respectively. Serum homocysteine levels were measured by HPLC. Eleven (26.2%) patients were symptomatic with transient ischemic attacks. There was no correlation between patient risk factors (smoking, coronary artery disease, elevated cholesterol, diabetes, obesity, hypertension and family history of genetic disorders) for atherosclerosis and serum levels or plaque grade for Lp-PLA2. Plaque Lp-PLA2 correlated with serum homocysteine levels (p = 0.013), plaque macrophages (p<0.01), and plaque C. pneumoniae (p<0.001), which predominantly infected macrophages, co-localizing with Lp-PLA2.
Conclusions
The significant association of plaque Lp-PLA2 with plaque macrophages and C. pneumoniae suggests an interactive role in accelerating inflammation in atherosclerosis. A possible mechanism for C. pneumoniae in the atherogenic process may involve infection of macrophages that induce Lp-PLA2 production leading to upregulation of inflammatory mediators in plaque tissue. Additional in vitro and in vivo research will be needed to advance our understanding of specific C. pneumoniae and Lp-PLA2 interactions in atherosclerosis.
Introduction
Carotid atherosclerosis is a major risk factor for an ischemic stroke [1]. While lipid metabolism and inflammation have been the major focus of atherosclerosis research for many years, there has been a growing interest in lipoprotein-associated phospholipase A2 (Lp-PLA2) because it is a key enzyme both in lipid metabolism and in stimulating inflammation [2].
Lp-PLA2 is a calcium-independent member of the phospholipase A2 enzyme family. Monocytes, macrophages, T-lymphocytes, mast cells and liver cells are the main sources for Lp-PLA2 [3], [4]. It is carried primarily by low-density lipoprotein (LDL). Lp-PLA2 catalyzes the hydrolysis of oxidized LDL, which produces proinflammatory mediators lysophosphatidylcholine (LysoPC) and oxidized fatty acid (oxFA) [5].
Many clinical studies have found an association between increasing serum levels of Lp-PLA2 mass and/or activity at the time of a cardiovascular incident in addition to an elevated risk of mortality and morbidity over time [6], [7], [8]. One study showed that Lp-PLA2 mRNA and protein levels were six times higher in atherosclerotic lesions compared to normal tissue samples [9].
Cumulative evidence suggests that C. pneumoniae also plays an important role in atherosclerosis [10], [11], [12], [13], [14]. The organism is thought to infect pulmonary monocytes that are then transported via the vasculature to localize in arteries where infection can spread [15]. C. pneumoniae is a ubiquitous pathogen that frequently causes upper and lower respiratory tract infections worldwide [16]. More than half of the patients with atherosclerosis have evidence for C. pneumoniae infection based on a variety of studies using detection methods such as immunohistochemistry (IHC) and electron microscopy of plaques [17], [18], PCR or real time RT-PCR of DNA/RNA extracted from plaques [18], [19], [21], and seroepidemiologic analyses among different populations [20], [21]. Other studies have shown viable organisms in the carotid arteries of stroke patients [19], [22] and patients with CAD [11], [23]. Furthermore, recent studies in murine and rabbit models suggest that C. pneumoniae can target the vasculature, induce inflammation and initiate or promote the development of atherosclerosis [14], [24], [25]. In the same models, C. pneumoniae accelerated atherosclerotic development, while treatment with azithromycin prevented the disease [12], [14]. However, treatment did not have the same effect on chronically infected mice [26], where organism persistence may have contributed to resistance to therapy. Recent in vitro studies also strongly suggest a role for C. pneumoniae in the genesis and progression of atherosclerosis [27], [28]
More recently, we have shown that the burden of C. pneumoniae infection was significantly associated with up-regulation of plaque interleukin (IL)-6 expression, which correlated with elevated serum levels of IL-6 and C-reactive protein (CRP) [18]. IL-6 stimulates liver CRP production, an acute phase reactant associated with risk of myocardial infarction (MI) and stroke. IL-6 secretion in C. pneumoniae-infected plaques could explain elevated systemic markers of inflammation among individuals at risk for vascular events.
There is currently no research, to our knowledge, correlating serum Lp-PLA2 mass and activity levels with plaque Lp-PLA2 or the interaction of C. pneumoniae infection and Lp-PLA2 on arterial disease and inflammation. We hypothesized that serum Lp-PLA2 mass and activity levels as well as plaque Lp-PLA2 would be significantly elevated in the presence of plaque C. pneumoniae infection, suggesting an interactive role in accelerating inflammation in atherosclerosis.
Materials and Methods
Ethics Statement
The University of California at San Francisco (UCSF) and Children's Hospital Oakland Research Institute (CHRCO) Institutional Review Board committees approved the study. Informed written consent was obtained for all study subjects. The study was conducted according to the principles of the Declaration of Helsinki.
Study subjects
In this cross-sectional study, subjects underwent elective carotid endarterectomy at UCSF, as described previously [19]. The treated carotid artery was associated with signs and/or symptoms of neurologic disease.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) detection by immunohistochemistry (IHC) in carotid artery tissue
Lp-PLA2 was detected by IHC using three, five-micron sections per carotid plaque in optimal cutting temperature (OCT) medium. The carotid plaque tissue was stored at −80°C in OCT until sectioning. Briefly, each section was blocked with casein (Biocare Medical, Concord, CA) and stained with anti-Lp-PLA2 monoclonal antibody (diaDexus, Inc., South San Francisco, CA) diluted 1∶400 in diluent (Biocare). Samples were washed with TBS, blocked with avidin (Biocare), washed again and blocked with biotin (Biocare) prior to applying goat, anti-mouse IgG antibody (Biocare). Streptavidin was applied followed by alkaline phosphatase chromagen-fast red (Biocare). The section was counterstained with hematoxylin to detect each cell. In independent experiments, excess primary antibody and, separately, excess secondary antibody was used on adjacent sections to ensure no non-specific staining of either antibody for Lp-PLA2. In addition, a mouse non-immune IgG (Biocare) was used as a final negative control.
Using light microscopy at 400×, samples were read independently by two individuals who were blinded to all patient data. Samples were graded based on percentage of the tissue staining for Lp-PLA2 for the entire plaque section. We used 1, 2 or 3+ for the entire carotid section for each patient sample (3 sections per patient carotid sample) where a grade of ≥1 was considered positive for Lp-PLA2; 1, 1–25% of the tissue; 2, 26–50% of the tissue; 3, >50% of the tissue. The three sections from each carotid sample were used to determine the within-sample variation, and the average of the three was used for analysis. Because of the ease of visualization of the staining for Lp-PLA2 (see Fig 1A), software was not required for quantitation.
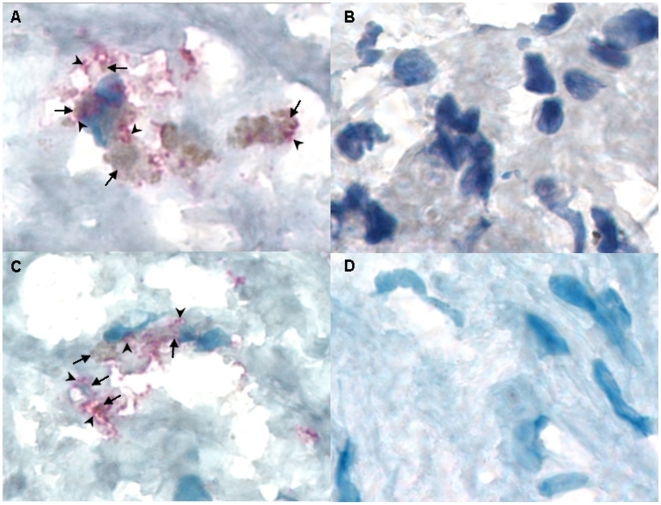
Figure 1. Carotid plaque sections showing co-localization of Lp-PLA2 and C. pneumoniae, and macrophages and C. pneumoniae.
A) Lipoprotein-associated phospholipase A2 (Lp-PLA2) was detected by Immunohistochemistry (IHC) using anti- Lp-PLA2 specific monoclonal antibody (diaDexus) and chromagen fast red (arrowheads); C. pneumoniae was detected by IHC using a CHsp60-specific MAb and chromagen DAB (arrows); ×1000. B) Negative control of a positive carotid plaque section (same patient as in A); ×1000. C) Carotid plaque section showing co-localization of C. pneumoniae (DAB; arrows) and macrophages detected by IHC using CD68 macrophage-specific monoclonal antibody and chromagen fast red (arrowheads); ×1000. D) Negative control of a positive carotid plaque section (same patient as in C).
All sections that stained positive for Lp-PLA2 were probed with chlamydial-specific heat shock protein 60 (CHsp60) MAb (Affinity Bioreagents) to determine the precise co-localization of Lp-PLA2 and infection using the methods as described above except that a horseradish peroxidase–conjugated secondary antibody with chromogen diaminobenzidine (DAB; Biocare) was used to detect chlamydiae. Positive and negative controls were as described previously [18], [19]. All three sections for each patient were read in entirety and analyzed as described for Lp-PLA2 except that the cells were counted to determine the number with Lp-PLA2 alone, C. pneumoniae alone, and the number with both for each section as quantitative measures.
Two additional adjacent sections for each patient sample were used to co-localize C. pneumoniae with macrophages. The sections were stained for C. pneumoniae as above, and macrophages were stained with fast red as for Lp-PLA2 except that the primary monoclonal antibody was macrophage specific (CD68; Biocare).
While other infectious agents may also be involved in atherosclerosis, the evaluation of these pathogens was beyond the scope of this study.
Prior data on the patient population used for analyses
In our prior studies, plaque tissues were noted to have a high grade of atherosclerosis [18], [19]. Data on the same population from our previous publications were also used for analyses [18], [19]. These included IHC to detect macrophages, CD4+ cells, CD8+ cells, IL-6, and C. pneumoniae in the adjacent sections of the same block of carotid tissue used for Lp-PLA2 IHC above. In addition, we previously determined plaque IL-6 gene expression by quantitative (q)RT-PCR, plaque C. pneumoniae burden by qRT-PCR, serum C-reactive protein (CRP) levels, and IL-6 serum protein levels, the methods of which are described in detail in our references [18], [19].
Measurement of serum mass and activity levels for lipoprotein-associated phospholipase A2 (Lp-PLA2) and homocysteine levels
All serum biochemical analyses were performed on serum from blood or blood plasma obtained at the time of carotid endarterectomy.
Lp-PLA2 mass was determined by ELISA (PLAC® Test; diaDexus) in serum according to the manufacturers instructions using two specific monoclonal antibodies in a 96-well format. Quantitation was calibrated to a recombinant Lp-PLA2 antigen standard. The lower detection limit was 2 ng/mL; interassay coefficient of variation (CV) was between 6% and 7%. Lp-PLA2 activity levels were measured by CAM™ assay (diaDexus) in serum according to the manufacturers instructions. Samples were analyzed in a 96-well microplate with a colorimetric substrate converted on hydrolysis by phospholipase enzyme. Briefly, 25 µL of sample, standard, or control was added per well, followed by addition of assay buffer plus substrate. Change in absorbance was measured at 405 nm. Lp-PLA2 activity in nmol/min/mL was calculated from the slope, based on a standard conversion factor from a p-Nitrophenol calibration curve. Activity levels between 13.5–46.1 were considered as quartile-1, 46.2–69 as quartile-2, 69.1–89.2 as quartile-3, and 89.3–143.2 as quartile-4.
Homocysteine levels in serum (the preferred sample type) were measured by fluorometric high-performance liquid chromatography (HPLC; Quest Diagnostics).
Statistical analysis
Clinical and laboratory characteristics of patients were compared by serum Lp-PLA2 mass and activity levels and Lp-PLA2 plaque grade. Before comparing continuous variables for Lp-PLA2 plaque grades, the normality assumption was checked by Shapiro-Wilk test and the distributional diagnostic plots for these variables: age, Lp-PLA2 serum activity, and homocysteine levels. All except homocysteine had a normal distribution. Square root transformation of homocysteine levels was performed to achieve the normality assumption.
Student t-test was used to compare plaque Lp-PLA2 positive vs. negative groups for continuous variables with normal distribution. Pearson chi-square test was used to compare plaque Lp-PLA2 positive vs. negative groups for binomial variables: history of smoking, coronary artery disease, elevated cholesterol, diabetes, obesity, hypertension and family history of genetic disorders, elevated serum CRP, serum IL-6, C. pneumoniae by qRT-PCR and plaque IL-6, CD4+, CD8+, macrophages, and C. pneumoniae. Multiple logistic regression was also performed for these comparisons, including all variables associated with Lp-PLA2 (at p<0.20) with a stepwise removal of any that did not contribute (at p>0.10). Since Lp-PLA2 serum activity results were categorized into four quartiles, Kruskal-Wallis test was used for comparing serum Lp-PLA2 quartiles for clinical characteristics. Nonparametric Spearman Rank test was used to calculate correlation coefficients between variables with Bonferroni adjustment. A P value of <0.05 was considered statistically significant. STATA version-9 (College Station, TX) was used for all analyses.
Results
Patient characteristics and association with serum Lp-PLA2 activity and plaque Lp-PLA2
Characteristics of the 42 study patients are shown in Tables 1 and 2 stratified by Lp-PLA2 plaque status and Lp-PLA2 serum activity, respectively. We considered the 42 patients to be a representative cohort of patients with neurologic signs and/or symptoms consistent with carotid vascular disease in addition to the fact that they were enrolled consecutively after informed consent from the pre-operative evaluation clinic at UCSF as previously described [18], [19]. None of the patients had stroke but all had neurologic symptoms, indicating carotid ischemia: 30 (71.4%) had symptoms on the left side, 11 (26.2%) on the right, and 1 (2.4%) bilaterally. It should be noted that the treated carotid artery was associated with symptoms on the ipsilateral side. There were no significant correlations of risk factors (smoking, coronary artery disease, elevated cholesterol, diabetes, obesity, hypertension and family history of genetic disorders) or clinical characteristics with Lp-PLA2 serum levels or tissue Lp-PLA2 grade ≥1 (Tables 1 and 2) or with C. pneumoniae infection as defined by qRT-PCR or IHC as described previously [18], [19] (Tables 3 and 4).
Table 1. Clinical characteristics of study population by plaque Lp-PLA2.a .
| Characteristics | Total (n = 42) | Plaque Lp-PLA2 negative (n = 23) | Plaque Lp-PLA2 positive (n = 19) | P Valueb |
| Age (mean +/− s.d.) | 72 (9.4) | 72.4 (+/−10.6) | 72.3 (+/−8) | 0.987 |
| Sex: | 0.327 | |||
| Male | 30 (71.4%) | 15 (65.2%) | 15 (78.9%) | |
| Female | 12 (28.6%) | 8 (34.8%) | 4 (21.1%) | |
| Smoker | 33 (78.6%) | 17 (73.9%) | 16 (84.2%) | 0.418 |
| CAD | 23 (54.8%) | 11 (47.8%) | 12 (63.2%) | 0.320 |
| Hypertension history | 36 (85.7%) | 20 (87%) | 16 (84.2%) | 0.800 |
| High cholesterol | 27 (64.3%) | 14 (60.8%) | 13 (68.4%) | 0.611 |
| Diabetes mellitus | 9 (21.4%) | 4 (17.4%) | 5 (26.3%) | 0.483 |
| Symptomatic | 0.264 | |||
| Left side | 30 (71.4%) | 18 (78.3%) | 12 (63.2%) | |
| Right side | 11 (26.2%) | 4 (17.4%) | 7 (63.7%) | |
| Both | 1 (2.4%) | 1 (4.3%) | 0 | |
Abbreviations: Lp-PLA2, Lipoprotein-associated phospholipase A2; CAD, Coronary Artery Disease.
Values expressed are for plaque Lp-PLA2 grade of ≥1 as the results were the same for any grade ≥1.
P values were generated by chi-square test except for age, where t-test was used for comparison.
Table 2. Clinical characteristics of study population by serum Lp-PLA2 activity.a .
| Characteristics | Lp-PLA2 Activity 1st quartile (n = 10)c | Lp-PLA2 Activity 2nd quartile (n = 10)c | Lp-PLA2 Activity 3rd quartile (n = 10)c | Lp-PLA2 Activity 4th quartile (n = 11)c | P Valueb |
| Age (mean +/− s.d.) | 67.9 (+/−10.1) | 71 (+/−10.1) | 75.9 (+/−7.8) | 73.7(+/−8.5) | 0.519 |
| Sex: | 0.403 | ||||
| Male (n = 29b) | 8 (27.6%) | 5 (17.2%) | 8 (27.6%) | 8 (27.6%) | |
| Female (n = 12b) | 2 (17%) | 5 (41%) | 2 (17%) | 3 (25%) | |
| Smoker | 8 (25%) | 7 (21.9%) | 6 (25%) | 11 (28.1%) | 0.916 |
| CAD | 2 (9.1%) | 7 (31.8%) | 6 (27.3%) | 7 (31.8%) | 0.101 |
| Hypertension | 7 (20%) | 9 (25.7%) | 8 (22.9%) | 11 (31.4%) | 0.243 |
| High cholesterol | 4 (15.4%) | 8 (30.8%) | 5 (19.2%) | 9 (34.6%) | 0.115 |
| Diabetes mellitus | 3 (33.3%) | 1 (11.1%) | 1 (11.1%) | 4 (44.5%) | 0.337 |
| Symptomatic: | 0.095 | ||||
| Right side | 9 (31%) | 7 (24.2%) | 4 (13.8%) | 9 (31%) | |
| Left side | 1 (9.1%) | 2 (18.2%) | 6 (54.6%) | 2 (18.2%) | |
| Both sides | 0 | 1 (100%) | 0 | 0 | |
Abbreviations: Lp-PLA2, Lipoprotein-associated phospholipase A2; Lp-PLA2.
Activity, range measured in nmol/min/mL; CAD, Coronary Artery Disease.
Lp-PLA2 Activity range measured in nmol/min/mL.
P values were generated by chi-square test except for age, where t-test was used for comparison.
Serum Lp-PLA2 activity information was missing for one person.
Table 3. Correlations among plaque characteristics and serum levels of inflammatory markers.
| Carotid Plaque Lp-PLA2 | Serum Lp-PLA2 mass (ng/mL) | Serum Lp-PLA2 activity | |
| Carotid Plaque | |||
| Cpn by qRT-PCR | 0.21 | −0.28 | −0.23 |
| Cpn by IHC | 0.39a | −0.24 | −0.08 |
| IL-6 expression | 0.23 | −0.31d | −0.22 |
| IL-6 by IHC | 0.11 | −0.34d | −0.32d |
| Macrophages | 0.37b | 0.06 | 0.11 |
| CD4+ | 0.17 | −0.04 | −0.13 |
| CD8+ | −0.02 | −0.10 | −0.13 |
| B-cell | −0.11 | −0.22 | −0.32 |
| Lp-PLA2 | 1 | 0.14 | 0.19 |
| Serum | |||
| Lp-PLA2 mass (ng/mL) | 0.14 | 1 | 0.76a |
| Lp-PLA2 activity | 0.19 | 0.76a | 1 |
| CRP | 0.18 | −0.25 | −0.21 |
| IL-6 | 0.08 | −0.27 | −0.19 |
| Homocysteine | 0.38c | −0.006 | 0.12 |
Abbreviations: Lp-PLA2, Lipoprotein-associated phospholipase A2; Cpn, C. pneumoniae; qRT-PCR, quantitative real-time reverse transcription PCR; IL-6, interleukin-6; IHC, immunohistochemistry; CRP, C-reactive protein.
p<0.001.
p<0.01.
p<0.013.
p<0.05.
Table 4. Correlations between serum Lp-PLA2 activity, and plaque and serum Lp-PLA2 mass.
| Serum Lp-PLA2 activity (range in nmol/min/mL) | Plaque Lp-PLA2 a | Serum Lp-PLA2 mass (ng/mL) |
| 1st quartile (13–46.1) | 0.04 (p = 0.822) | −0.47 (p< = 0.001) |
| 2nd quartile (46.2–69) | −0.14 (p = 0.375) | −0.31 (p = 0.043) |
| 3rd quartile (69.1–89.2) | 0.02 (p = 0.987) | 0.37 (p = 0.015) |
| 4th quartile (89.3–143.2) | 0.13 (p = 0.417) | 0.48 (p = 0.001) |
Abbreviations: Lp-PLA2, Lipoprotein-associated phospholipase A2.
Values expressed are for plaque Lp-PLA2 grade of ≥1 as there were no significant correlations with grades >1.
Correlation among carotid plaque characteristics and serum levels of inflammatory markers
Serum Lp-PLA2 mass and activity levels were significantly correlated (r = 0.76, p = 0.001, Table 3). High Lp-PLA2 activity was also correlated with Lp-PLA2 mass (r3 = 0.37 and r4 = 0.48, p3 = 0.015 and p4 = 0.001, respectively, Table 4).
Interestingly, 94.7% (18/19) of patients who had plaque Lp-PLA2 also had plaque C. pneumoniae. Plaque Lp-PLA2 presence (for all quantitative grades above 1) was significantly correlated with C. pneumoniae (r = 0.39, p = 0.001) and macrophages (r = 0.37, p = 0.01, Table 3), and with higher serum homocysteine levels (r = 0.38, p = 0.013, Table 3). Plaque Lp-PLA2 co-localized with C. pneumoniae, macrophages and CD4+ lymphocytes by IHC in the shoulder and necrotic core of the plaques as has been noted by others [9]. We found that 52% of cells showed evidence for Lp-PLA2 protein and infection with C. pneumoniae (Figure 1A). In addition, 39% of macrophages were infected with C. pneumoniae (Figure 1B).
In Table 3, the correlation between carotid plaque Lp-PLA2 and plaque IL-6 expression, IL-6 detected by IHC, serum IL-6, and CRP was statistically insignificant for all plaque Lp-PLA2 grades. Serum Lp-PLA2 mass levels were negatively correlated with plaque IL-6 expression and IL-6 detected by IHC (r = −0.31, p = 0.048; r = −0.34, p = 0.03, respectively), and not correlated with serum IL-6 or CRP. Serum Lp-PLA2 activity levels were negatively correlated with IL-6 detected by IHC (r = −0.32, p = 0.04) and not correlated with plaque IL-6 expression, serum IL-6 or CRP.
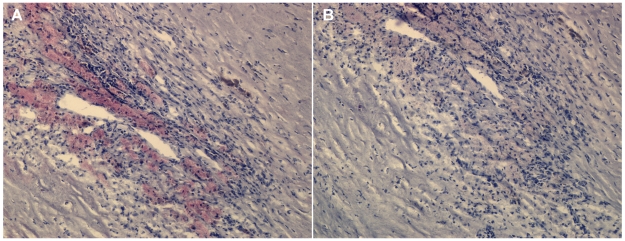
Figure 2A shows staining of Lp-PLA2 (red) in the perivascular necrotic area of carotid plaque. This region was rich in macrophages in addition to C. pneumoniae infected macrophages. Figure 2B shows the adjacent section stained with secondary antibody and omission of primary antibody against Lp-PLA2 as a control for specificity. There were similar results for staining with the control mouse non-immune IgG antibody (data not shown).
Figure 2. Carotid plaque section from a patient with atherosclerosis.
A) Lipoprotein-associated phospholipase A2 (Lp-PLA2) was detected by Immunohistochemistry (IHC) using anti- Lp-PLA2 specific monoclonal antibody (diaDexus) and chromagen fast red; 400×. B) The five micron adjacent carotid plaque section from the same patient was stained as in A but the primary antibody was omitted as a control for specificity; 400×.
Discussion
This is the first study, to our knowledge, that evaluates the correlation between Lp-PLA2 serum mass and activity levels with presence of Lp-PLA2 in carotid plaques, and the association of these indicators with plaque C. pneumoniae and other inflammatory mediators. Lp-PLA2 serum mass and activity levels correlated well with one another but not with plaque Lp-PLA2. However, plaque Lp-PLA2 was significantly correlated with plaque C. pneumoniae infection, macrophages and serum homocysteine levels. A high percentage of macrophages were infected, and many cells showed co-localization of Lp-PLA2 with C. pneumoniae. Thus, a possible mechanism for C. pneumoniae in the atherogenic process may involve infection of macrophages that induce Lp-PLA2 production leading to upregulation of inflammatory mediators in plaque tissue.
We found no significant correlation between patient risk factors for atherosclerosis and serum levels or plaque grade for Lp-PLA2 in our study. Our findings are similar to those of others [29] but in contrast to some publications that reported a correlation between clinical characteristics or risk factors and serum Lp-PLA2 mass or activity levels [30], [31]. Earlier publications initially found strong correlations between serum Lp-PLA2 levels and clinical characteristics, which decreased significantly after adjustment for measures of atherosclerosis [32], [33]. In our study, the lack of correlation between either clinical characteristics or risk factors and Lp-PLA2 might be explained by the small sample size. However, serum Lp-PLA2 mass and activity levels may not be consistently reliable risk markers for atherosclerosis.
There is prior evidence that both serum Lp-PLA2 mass and activity levels are influenced by infection such as hepatitis C, malaria and influenza [34], [35], [36]. For example, malaria researchers have shown a positive correlation between circulating levels of Lp-PLA2, parasitemia and severity of disease [35]. Studies of the interrelationship of influenza with inflammatory responses and atherosclerosis were initiated based on the observation of a strong association between acute respiratory infections, acute MI and sudden death in winter [36]. In a murine model of influenza, Lp-PLA2 activity in high density lipoproteins (HDL) was found to decrease two days after inoculation of influenza, reaching the lowest levels within a week, while Lp-PLA2 modification of LDL and lipid peroxide products increased as monocyte migration was induced [36].
In our study, only plaque Lp-PLA2, but not serum Lp-PLA2 mass or activity levels, was significantly associated with the presence of C. pneumoniae. Prior studies have shown that persistent C. pneumoniae infection, characterized by up-regulation of chlamydial heat shock protein 60 expression, induced LDL oxidation that leads to macrophage activation [37]. It is well known that oxidized LDL is also a substrate of Lp-PLA2 catalyzed reactions, resulting in LysoPC and oxFA [5]. LysoPC induces proinflammatory cytokines and chemokines, such as IL-1β, IL-6, TNF-α, and monocyte chemoattractant protein 1 (MCP-1) [38]. IL-1β, IL-6 and TNF-α trigger atherogenesis by sensitizing vascular smooth muscle cells [39] and inducing secretion of cellular adhesion molecules [40] and matrix metalloproteinase (MMP) by monocytes during later stages of atherosclerosis [41]. MCP-1 recruits T cells and monocytes, inhibits endothelial nitric oxide (causing endothelial dysfunction), induces monocyte-macrophage colony-stimulating factor (M-CSF) secretion by smooth muscle cells and stimulates macrophage proliferation [42], [43], [44]. In our study, we found that plaque Lp-PLA2 was significantly correlated with plaque macrophages, which is consistent with these studies.
Several studies have shown that C. pneumoniae activated macrophages induce pro-inflammatory cytokine/chemokines, such as IL-6, IL-8 and MCP-1 [18], [45], [46]. In our previous evaluation of the same tissue samples as in the present study, we found that macrophages in the carotid plaques co-localized with CD4+ lymphocytes [18], which can secrete pro-inflammatory cytokines and further fuel the atherogenic process. Both CD4+ cells and macrophages release interferon gamma (IFN-γ), which can resolve chlamydial infection or stimulate a non-replicative persistent state that can result in chronic infection that is likely resistant to antimicrobial treatment.
IL-6 is an acute phase reactant secreted by activated macrophages, Th2 cells and B cells. We previously showed that quantitatively higher levels of carotid plaque C. pneumoniae measured by qRT-PCR and semi-quantitative IHC was associated with higher IL-6 expression in both plaques and serum [18]. Subsequent studies have also shown that atherosclerotic progression, based on intima-media wall thickness, was associated with higher serum IL-6 levels among C. pneumoniae patients [47]. In in vitro studies, C. pneumoniae induces the production of IL-6 in peripheral monocytes and smooth muscle cells [45]. Neither serum nor plaque IL-6 correlated with serum Lp-PLA2 activity or mass levels or with plaque Lp-PLA2 grade in our study. However, one other study also failed to show a correlation between serum IL-6 and Lp-PLA2 activity [48]. This might be due to the indirect pathways induced by Lp-PLA2 where the temporal influence of Lp-PLA2 and up-regulation of serum IL-6 are missed because only a single serum sample is obtained at the time of endartectomy. Similarly, given that we found a lack of association of serum Lp-PLA2 mass or activity levels with plaque Lp-PLA2 and with plaque C. pneumoniae, it is possible that either the timing of sample collection yields a false negative result or that what is occurring locally in the tissue does not always reflect the circulating systemic levels of Lp-PLA2. Thus, some patients may not express elevated serum Lp-PLA2 levels in association with risk factors or disease [32], [33] or with infection.
There was a significant correlation of plaque Lp-PLA2 with serum homocysteine levels. Homocysteine exerts an independent effect on vascular smooth muscle cell proliferation, although the mechanism(s) is not well understood [49]. It is unclear from our data whether there is a direct interaction between homocysteine and plaque Lp-PLA2 that may accelerate atherosclerotic progression.
Overall, we found that macrophages, many of which were infected with C. pneumoniae, co-localized with Lp-PLA2. A high percentage of cells demonstrated co-localization of Lp-PLA2 and C. pneumoniae. These findings suggest that macrophages may be activated by C. pneumoniae infection, inducing Lp-PLA2 production and subsequent proinflammatory mediators, and, under the influence of Lp-PLA2 byproducts, result in macrophage proliferation that in turn release inflammatory mediators. This scenario indicates a possible indirect mechanism for C. pneumoniae involvement in the atherogenic process. However, additional research focused on in vitro cell and in vivo animal models will be needed to advance our understanding of the interaction of C. pneumoniae infection with Lp-PLA2 in inflammation and atherosclerotic disease.
Acknowledgments
We would like to thank the nurses and doctors in the surgery department for their assistance with this study, and the excellent technical expertise of Catherine McDonough.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Pacific Vascular Research Foundation (www.pvrf.org), the National Institutes of Health, R01 AI039499 (to DD) in addition to reagents from diaDexus (www.diadexus.com). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 2.Mohler ER, 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, et al. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 3.Asano K, Okamoto S, Fukunaga K, Shiomi T, Mori T, et al. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999;261:511–514. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 4.Tarbet EB, Stafforini DM, Elstad MR, Zimmerman GA, McIntyre TM, et al. Liver cells secrete the plasma form of platelet-activating factor acetylhydrolase. J Biol Chem. 1991;266:16667–16673. [PubMed] [Google Scholar]
- 5.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338 ( Pt 2):479–487. [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–1593. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 8.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 9.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 10.Saikku P. The epidemiology and significance of Chlamydia pneumoniae. J Infect. 1992;25(Suppl 1):27–34. doi: 10.1016/0163-4453(92)91913-v. [DOI] [PubMed] [Google Scholar]
- 11.Kuo CC, Grayston JT, Campbell LA, Goo YA, Wissler RW, et al. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old). Proc Natl Acad Sci U S A. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhlestein JB. Animal models of chlamydia and atherosclerosis. Am Heart J. 1999;138:S514–515. doi: 10.1016/s0002-8703(99)70289-9. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez JA. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sessa R, Nicoletti M, Di Pietro M, Schiavoni G, Santino I, et al. Chlamydia pneumoniae and atherosclerosis: current state and future prospectives. Int J Immunopathol Pharmacol. 2009;22:9–14. doi: 10.1177/039463200902200102. [DOI] [PubMed] [Google Scholar]
- 16.Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181(Suppl 3):S402–410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 17.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SC, Zhang H, Messina LM, Lawton MT, Dean D. Chlamydia pneumoniae burden in carotid arteries is associated with upregulation of plaque interleukin-6 and elevated C-reactive protein in serum. Arterioscler Thromb Vasc Biol. 2005;25:2648–2653. doi: 10.1161/01.ATV.0000189157.88630.d1. [DOI] [PubMed] [Google Scholar]
- 19.Johnston SC, Messina LM, Browner WS, Lawton MT, Morris C, et al. C-reactive protein levels and viable Chlamydia pneumoniae in carotid artery atherosclerosis. Stroke. 2001;32:2748–2752. doi: 10.1161/hs1201.099631. [DOI] [PubMed] [Google Scholar]
- 20.Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–839. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- 21.LaBiche R, Koziol D, Quinn TC, Gaydos C, Azhar S, et al. Presence of Chlamydia pneumoniae in human symptomatic and asymptomatic carotid atherosclerotic plaque. Stroke. 2001;32:855–860. doi: 10.1161/01.str.32.4.855. [DOI] [PubMed] [Google Scholar]
- 22.Apfalter P, Loidl M, Nadrchal R, Makristathis A, Rotter M, et al. Isolation and continuous growth of Chlamydia pneumoniae from arterectomy specimens. Eur J Clin Microbiol Infect Dis. 2000;19:305–308. doi: 10.1007/s100960050481. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez JA, Ahkee S, Ganzel B, Ogend L, Gaydos CA, et al. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Hauer AD, de Vos P, Peterse N, ten Cate H, van Berkel TJ, et al. Delivery of Chlamydia pneumoniae to the vessel wall aggravates atherosclerosis in LDLr−/− mice. Cardiovasc Res. 2006;69:280–288. doi: 10.1016/j.cardiores.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 25.de Kruif MD, van Gorp EC, Keller TT, Ossewaarde JM, ten Cate H. Chlamydia pneumoniae infections in mouse models: relevance for atherosclerosis research. Cardiovasc Res. 2005;65:317–327. doi: 10.1016/j.cardiores.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Blessing E, Campbell LA, Rosenfeld ME, Chesebro B, Kuo CC. A 6 week course of azithromycin treatment has no beneficial effect on atherosclerotic lesion development in apolipoprotein E-deficient mice chronically infected with Chlamydia pneumoniae. J Antimicrob Chemother. 2005;55:1037–1040. doi: 10.1093/jac/dki128. [DOI] [PubMed] [Google Scholar]
- 27.Bunk S, Susnea I, Rupp J, Summersgill JT, Maass M, et al. Immunoproteomic identification and serological responses to novel Chlamydia pneumoniae antigens that are associated with persistent C. pneumoniae infections. J Immunol. 2008;180:5490–5498. doi: 10.4049/jimmunol.180.8.5490. [DOI] [PubMed] [Google Scholar]
- 28.Gieffers J, Solbach W, Maass M. In vitro susceptibility and eradication of Chlamydia pneumoniae cardiovascular strains from coronary artery endothelium and smooth muscle cells. Cardiovasc Drugs Ther. 2001;15:259–262. doi: 10.1023/a:1011972424529. [DOI] [PubMed] [Google Scholar]
- 29.Oldgren J, James SK, Siegbahn A, Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J. 2007;28:699–704. doi: 10.1093/eurheartj/ehl565. [DOI] [PubMed] [Google Scholar]
- 30.Tsimikas S, Willeit J, Knoflach M, Mayr M, Egger G, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J. 2009;30:107–115. doi: 10.1093/eurheartj/ehn502. [DOI] [PubMed] [Google Scholar]
- 31.Persson M, Berglund G, Nelson JJ, Hedblad B. Lp-PLA2 activity and mass are associated with increased incidence of ischemic stroke: a population-based cohort study from Malmo, Sweden. Atherosclerosis. 2008;200:191–198. doi: 10.1016/j.atherosclerosis.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Kardys I, Oei HH, van der Meer IM, Hofman A, Breteler MM, et al. Lipoprotein-associated phospholipase A2 and measures of extracoronary atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2006;26:631–636. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 33.Kiortsis DN, Tsouli S, Lourida ES, Xydis V, Argyropoulou MI, et al. Lack of association between carotid intima-media thickness and PAF-acetylhydrolase mass and activity in patients with primary hyperlipidemia. Angiology. 2005;56:451–458. doi: 10.1177/000331970505600413. [DOI] [PubMed] [Google Scholar]
- 34.Caini P, Guerra CT, Giannini C, Giannelli F, Gragnani L, et al. Modifications of plasma platelet-activating factor (PAF)-acetylhydrolase/PAF system activity in patients with chronic hepatitis C virus infection. J Viral Hepat. 2007;14:22–28. doi: 10.1111/j.1365-2893.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 35.Vadas P, Taylor TE, Chimsuku L, Goldring D, Stefanski E, et al. Increased serum phospholipase A2 activity in Malawian children with falciparum malaria. Am J Trop Med Hyg. 1993;49:455–459. doi: 10.4269/ajtmh.1993.49.455. [DOI] [PubMed] [Google Scholar]
- 36.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 37.Kalayoglu MV, Hoerneman B, LaVerda D, Morrison SG, Morrison RP, et al. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J Infect Dis. 1999;180:780–790. doi: 10.1086/314931. [DOI] [PubMed] [Google Scholar]
- 38.Carlquist JF, Muhlestein JB, Anderson JL. Lipoprotein-associated phospholipase A2: a new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn. 2007;7:511–517. doi: 10.1586/14737159.7.5.511. [DOI] [PubMed] [Google Scholar]
- 39.Jaulmes A, Thierry S, Janvier B, Raymondjean M, Marechal V. Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. FASEB J. 2006;20:1727–1729. doi: 10.1096/fj.05-5514fje. [DOI] [PubMed] [Google Scholar]
- 40.Amberger A, Maczek C, Jurgens G, Michaelis D, Schett G, et al. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:ceoive>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajavashisth TB, Xu XP, Jovinge S, Meisel S, Xu XO, et al. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99:3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 42.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang MY, Tsoi C, Wight TN, Chait A. Lysophosphatidylcholine regulates synthesis of biglycan and the proteoglycan form of macrophage colony stimulating factor. Arterioscler Thromb Vasc Biol. 2003;23:809–815. doi: 10.1161/01.ATV.0000069208.20268.D0. [DOI] [PubMed] [Google Scholar]
- 45.Rodel J, Woytas M, Groh A, Schmidt KH, Hartmann M, et al. Production of basic fibroblast growth factor and interleukin 6 by human smooth muscle cells following infection with Chlamydia pneumoniae. Infect Immun. 2000;68:3635–3641. doi: 10.1128/iai.68.6.3635-3641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Högdahl M, Söderlund G, Kihlström E. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS. 2008;116:1082–1088. doi: 10.1111/j.1600-0463.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 47.Jitsuiki K, Yamane K, Nakajima M, Nakanishi S, Tasaki N, et al. Association of Chlamydia pneumoniae infection and carotid intima-media wall thickness in Japanese Americans. Circ J. 2006;70:815–819. doi: 10.1253/circj.70.815. [DOI] [PubMed] [Google Scholar]
- 48.Furberg CD, Nelson JJ, Solomon C, Cushman M, Jenny NS, et al. Distribution and correlates of lipoprotein-associated phospholipase A2 in an elderly cohort: The Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:792–799. doi: 10.1111/j.1532-5415.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 49.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]