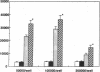
Abstract
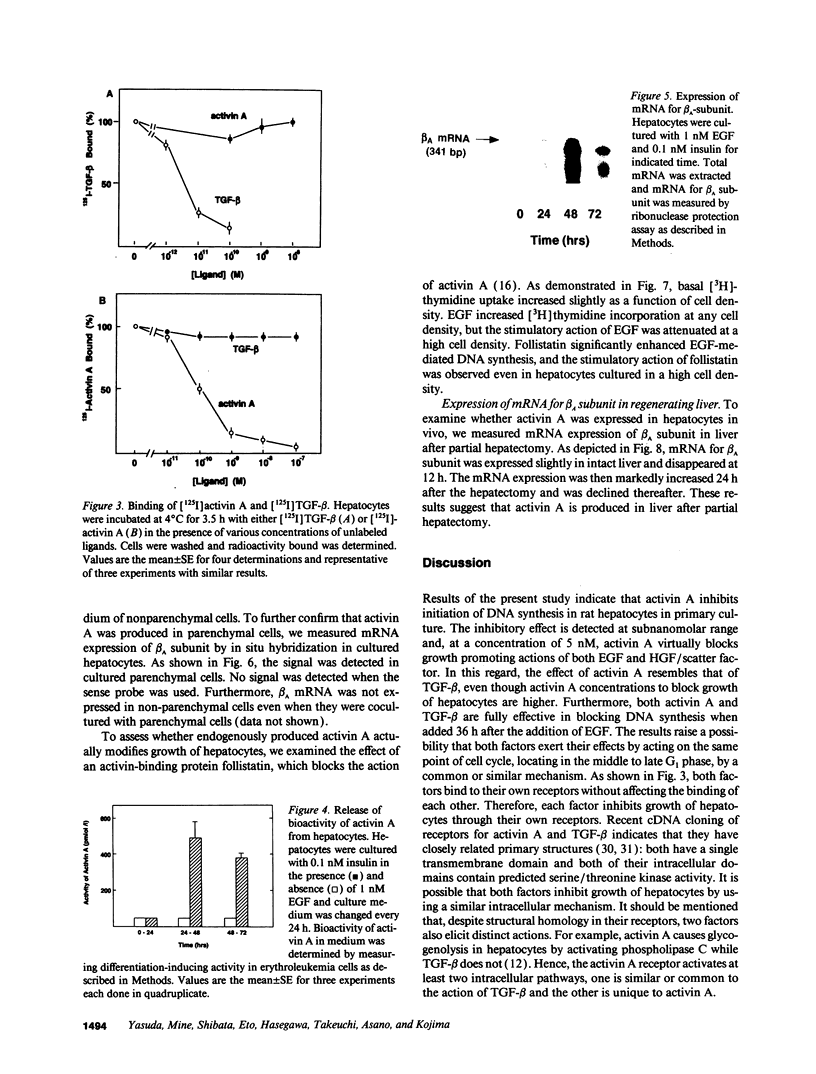
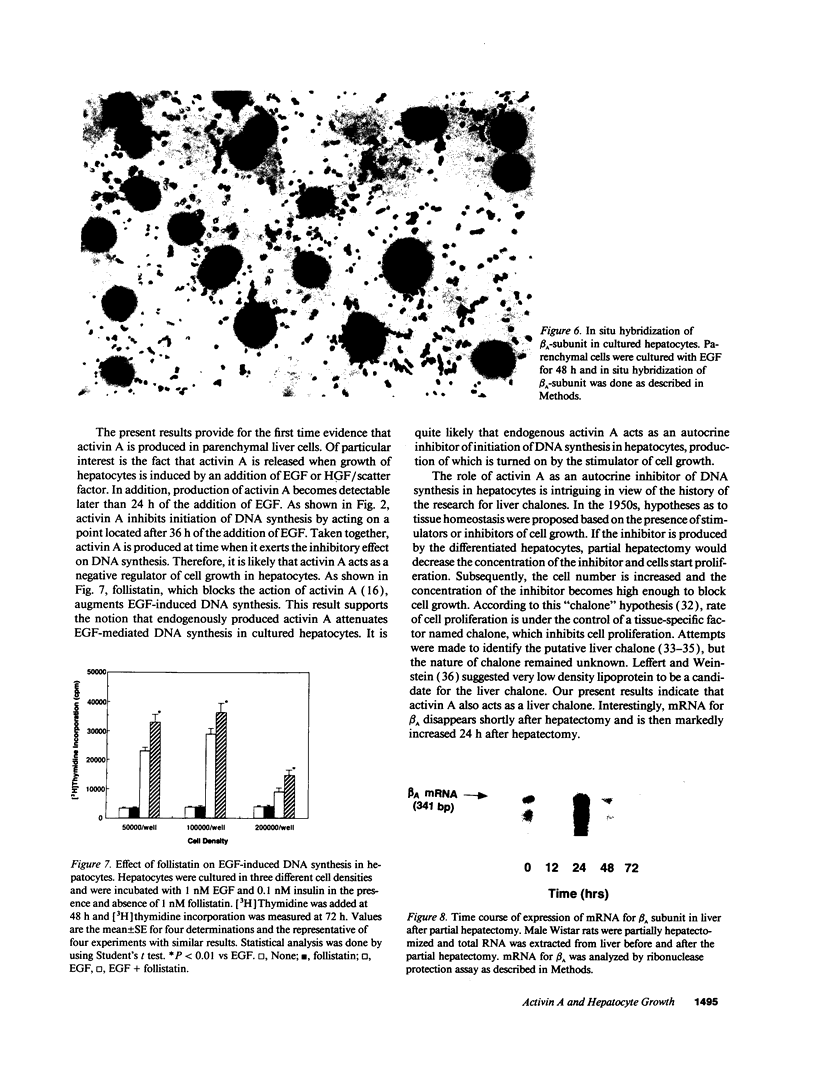
The present study was conducted to examine the effect of activin A on growth of rat hepatocytes. EGF induced a 10-fold increase in DNA synthesis as assessed by [3H]thymidine incorporation in cultured hepatocytes. When activin A was added together with EGF, DNA synthesis induced by EGF was markedly inhibited. Inhibition was detected at a concentration of 10(-10) M, and 5 x 10(-9) M activin A almost completely blocked EGF-mediated DNA synthesis. Similarly, activin A completely blocked DNA synthesis induced by hepatocyte growth factor/scatter factor. Activin A was capable of inhibiting EGF-mediated DNA synthesis, even when added 36 h after the addition of EGF. With the same time interval, TGF-beta also blocked EGF-induced DNA synthesis. Although both activin A and TGF-beta inhibited growth of hepatocytes in a similar manner, either activin A or TGF-beta did not compete with each other in their binding when assessed by competitive binding using an iodinated ligand. When hepatocytes were incubated with EGF, release of bioactivity of activin A into culture medium was detected after 48 h or later. Activity of activin A was released from parenchymal cells but not from nonparenchymal cells. mRNA for beta A subunit of activin was detected only slightly in unstimulated hepatocytes, but markedly increased at 48 h after the addition of EGF. To determine whether endogenously produced activin A affects DNA synthesis, we examined the effect of follistatin, an activin-binding protein that blocks the action of activin A. An addition of follistatin significantly enhanced EGF-induced DNA synthesis. Finally, in partial hepatectomized rat, expression of mRNA for beta A subunit in liver was markedly increased 24 h after the partial hepatectomy. These results indicate that activin A inhibits initiation of DNA synthesis in hepatocytes by acting on its own receptor and that activin A acts as an autocrine inhibitor of DNA synthesis in rat hepatocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOUGH W. S. The control of mitotic activity in adult mammalian tissues. Biol Rev Camb Philos Soc. 1962 Aug;37:307–342. doi: 10.1111/j.1469-185x.1962.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corrigan A. Z., Bilezikjian L. M., Carroll R. S., Bald L. N., Schmelzer C. H., Fendly B. M., Mason A. J., Chin W. W., Schwall R. H., Vale W. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology. 1991 Mar;128(3):1682–1684. doi: 10.1210/endo-128-3-1682. [DOI] [PubMed] [Google Scholar]
- Deschamps Y., Verly W. G. The hepatic chalone. II. Chemical and biological properties of the rabbit liver chalone. Biomedicine. 1975 May;22(3):195–208. [PubMed] [Google Scholar]
- Esch F. S., Shimasaki S., Cooksey K., Mercado M., Mason A. J., Ying S. Y., Ueno N., Ling N. Complementary deoxyribonucleic acid (cDNA) cloning and DNA sequence analysis of rat ovarian inhibins. Mol Endocrinol. 1987 May;1(5):388–396. doi: 10.1210/mend-1-5-388. [DOI] [PubMed] [Google Scholar]
- Eto Y., Tsuji T., Takezawa M., Takano S., Yokogawa Y., Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Miyamoto K., Hasegawa Y., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of bovine follicular fluid inhibin of about 32 kDa. Mol Cell Endocrinol. 1986 Jan;44(1):55–60. doi: 10.1016/0303-7207(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990 Sep 27;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Kondo S., Sakurai T., Etoh Y., Shibai H., Muramatsu M. Activin/EDF as an inhibitor of neural differentiation. Biochem Biophys Res Commun. 1990 Nov 30;173(1):193–200. doi: 10.1016/s0006-291x(05)81040-x. [DOI] [PubMed] [Google Scholar]
- Hillier S. G. Regulatory functions for inhibin and activin in human ovaries. J Endocrinol. 1991 Nov;131(2):171–175. doi: 10.1677/joe.0.1310171. [DOI] [PubMed] [Google Scholar]
- Jirtle R. L., Carr B. I., Scott C. D. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration. J Biol Chem. 1991 Nov 25;266(33):22444–22450. [PubMed] [Google Scholar]
- Katayama T., Shiota K., Takahashi M. Activin A increases the number of follicle-stimulating hormone cells in anterior pituitary cultures. Mol Cell Endocrinol. 1990 Mar 5;69(2-3):179–185. doi: 10.1016/0303-7207(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Kuribayashi K., Hikata M., Hiraoka O., Miyamoto C., Furuichi Y. A rapid and efficient purification of poly(A)-mRNA by oligo(dT)30-Latex. Nucleic Acids Symp Ser. 1988;(19):61–64. [PubMed] [Google Scholar]
- Leffert H. L., Weinstein D. B. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture. IX. Specific inhibition of DNA synthesis initiation by very low density lipoprotein and possible significance to the problem of liver regeneration. J Cell Biol. 1976 Jul;70(1):20–32. doi: 10.1083/jcb.70.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- McMahon J. B., Farrelly J. G., Iype P. T. Purification and properties of a rat liver protein that specifically inhibits the proliferation of nonmalignant epithelial cells from rat liver. Proc Natl Acad Sci U S A. 1982 Jan;79(2):456–460. doi: 10.1073/pnas.79.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., McKenna M. P., Taylor D. L. A transient rise in cytosolic calcium follows stimulation of quiescent cells with growth factors and is inhibitable with phorbol myristate acetate. J Cell Biol. 1985 Aug;101(2):372–379. doi: 10.1083/jcb.101.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine T., Kojima I., Ogata E. Stimulation of glucose production by activin-A in isolated rat hepatocytes. Endocrinology. 1989 Aug;125(2):586–591. doi: 10.1210/endo-125-2-586. [DOI] [PubMed] [Google Scholar]
- Murata M., Onomichi K., Eto Y., Shibai H., Muramatsu M. Expression of erythroid differentiation factor (EDF) in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1988 Feb 29;151(1):230–235. doi: 10.1016/0006-291x(88)90583-9. [DOI] [PubMed] [Google Scholar]
- Nadal C. Control of liver growth by growth inhibitors (chalones). Arch Toxicol Suppl. 1979;(2):131–142. doi: 10.1007/978-3-642-67265-1_11. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takio K., Eto Y., Shibai H., Titani K., Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990 Feb 16;247(4944):836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kaku K., Azuno Y., Okafuji K., Etoh Y., Shiozaki M., Sasaki H., Inoue T., Kaneko T. Effect of erythroid differentiation factor on megakaryocytic differentiation of L8057, a murine megakaryoblastic leukemia cell line. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1042–1047. doi: 10.1016/0006-291x(91)92042-i. [DOI] [PubMed] [Google Scholar]
- Noji S., Tashiro K., Koyama E., Nohno T., Ohyama K., Taniguchi S., Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990 Nov 30;173(1):42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Kimura H., LaCorbiere M., Vaughan J., Karr D., Fischer W. H. Activin is a nerve cell survival molecule. Nature. 1990 Apr 26;344(6269):868–870. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- Shimaoka S., Nakamura T., Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res. 1987 Sep;172(1):228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Sokol S., Melton D. A. Pre-existent pattern in Xenopus animal pole cells revealed by induction with activin. Nature. 1991 May 30;351(6325):409–411. doi: 10.1038/351409a0. [DOI] [PubMed] [Google Scholar]
- Sugino H., Nakamura T., Hasegawa Y., Miyamoto K., Abe Y., Igarashi M., Eto Y., Shibai H., Titani K. Erythroid differentiation factor can modulate follicular granulosa cell functions. Biochem Biophys Res Commun. 1988 May 31;153(1):281–288. doi: 10.1016/s0006-291x(88)81219-1. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Xiao S., Findlay J. K., Robertson D. M. The effect of bovine activin and follicle-stimulating hormone (FSH) suppressing protein/follistatin on FSH-induced differentiation of rat granulosa cells in vitro. Mol Cell Endocrinol. 1990 Feb 12;69(1):1–8. doi: 10.1016/0303-7207(90)90082-j. [DOI] [PubMed] [Google Scholar]