Abstract
Meiosis is a critical stage of gametogenesis in which alignment and synapsis of chromosomal pairs occur, allowing for the recombination of maternal and paternal genomes. Here we show that FK506 binding protein (Fkbp6) localizes to meiotic chromosome cores and regions of homologous chromosome synapsis. Targeted inactivation of Fkbp6 in mice results in aspermic males and the absence of normal pachytene spermatocytes. Moreover, we identified the deletion of Fkbp6 exon 8 as the causative mutation in spontaneously male sterile as/as mutant rats. Loss of Fkbp6 results in abnormal pairing and misalignments between homologous chromosomes, nonhomologous partner switches, and autosynapsis of X chromosome cores in meiotic spermatocytes. Fertility and meiosis are normal in Fkbp6 mutant females. Thus, Fkbp6 is a component of the synaptonemal complex essential for sex-specific fertility and for the fidelity of homologous chromosome pairing in meiosis.
Meiosis is a fundamental process in sexually reproducing species that allows genetic exchange between maternal and paternal genomes (1, 2). Defects in high-fidelity meiotic chromosome alignment or in genome segregation in germ cells result in aneuploidies such as trisomy 21 in Down syndrome. Aneuploidy is a leading cause of spontaneous miscarriage in humans and a hallmark of many human cancer cells (2). Once homologs are paired, the chromosomes are connected by a specific structure: the synaptonemal complex (SC) (3). SCs are zipperlike structures assembled along the paired meiotic chromosomes during the prophase of the first meiotic division (3). Although SCs were first discovered more than 45 years ago (4, 5), only very few structural meiosis-specific components of the SC have been identified in mammals, such as SC proteins 1, 2, and 3 [Scp1 (also known as Syn1/Sycp1), Scp2, and Scp3 (also known as Cor1)] (3). Genetic inactivation of the mouse Scp3 gene results in male infertility due to a failure to form chromosome synapsis in meiotic prophase (6). Female Scp3−/− mice have reduced fertility, and embryos from Scp3−/− mothers have increased incidents of aneuploidy (7). To our knowledge, genetic inactivation of Scp1 or Scp2 has not been reported yet.
FK506 binding protein 6 (Fkbp6) is a member of a gene family that contains a prolyl isomerase/FK506 binding domain and tetratricopeptide protein-protein interaction domains (8). Fkbp6 maps to human chromosome 7q11.23 and is commonly deleted in Williams-Beuren syndrome (8), an autosomal, dominant, contiguous gene deletion disorder that encompasses at least 17 different genes and is characterized by a diverse array of abnormalities (9). Here we show that Fkbp6 is an SC component essential for sex-specific fertility and for the fidelity of homologous chromosome pairing in meiosis.
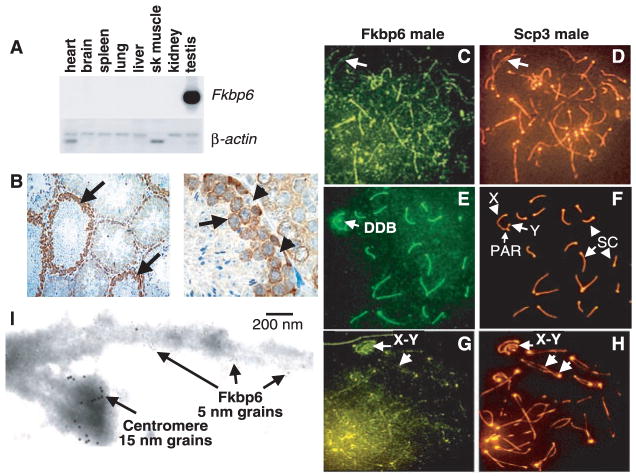
To study the role of Fkbp6 in vivo, we cloned the mouse Fkbp6 homolog by reverse transcription–polymerase chain reaction (RT-PCR) from mouse testis RNA (fig. S1). Mouse Fkbp6 mRNA expression was restricted to the testes (Fig. 1A). Fkbp6 mRNA (fig. S1B) and protein (Fig. 1B) were found in the cytoplasm and nucleus of spermatocytes. Fkbp6 was lost as cells exited prophase I and was not detected in spermatids. In meiotic chromosome spreads, Fkbp6 protein was weakly associated with the chromosome cores before synapsis and SC formation at early prophase (Fig. 1, C and D). At the pachytene stage, in which SCs are fully assembled, Fkbp6 was strongly localized to the SC (Fig. 1, E and F). At the diplotene (late meiotic prophase) stage, in which the homologous chromosomes have initiated repulsion while remaining synapsed, Fkbp6 expression in the chromosome cores was markedly reduced (Fig. 1, G and H). Fkbp6 was also present at the male-specific double dense body (DDB) associated with the X-chromosome core (Fig. 1E). Moreover, at the pachytene and diplotene stages, Fkbp6 colocalized with Scp1 at the synapsed regions of autosomal chromosomes (fig. S2, A to D). As in male germ cells, Fkbp6 was strongly expressed at the synapsed cores during mid-prophase in female meiotic cells, and Fkbp6 expression declined thereafter (fig. S2, E to H). We detected Fkbp6 expression within the SC using immunogold labeling and electron microscopy (EM) of individual prophase chromosomes (Fig. 1I) (10). Thus, Fkbp6 is an SC component.
Fig. 1.
(A) Murine Fkbp6 mRNA expression. β-actin is shown as the control. Sk, skeletal. (B) Immunohistochemistry of Fkbp6 in normal testis. Arrows show Fkbp6 staining in meiotic spermatocytes. Fkbp6 is absent in Sertoli cells (arrowheads). (C to H) Localization of Fkbp6 and Scp3 to the chromosome cores and SCs of mouse meiotic spermatocytes at the [(C) and (D)] zygotene, [(E) and (F)] pachytene, and [(G) and (H)] diplotene stages. The pseudoautosomal regions (PAR), of the X and Y chromosomes are indicated. The male-specific DDB is shown. An anti-centromere antibody was used for counterstaining (orange). Arrows in (C) and (D) indicate the presence of Fkpb6 on unpaired cores. Arrows in (G) and (H) show the X and Y chromosomes and chromosome separation in the diplotene stage. Analysis of meiotic chromosomes from Fkbp6−/− mice revealed that the Fkbp6 antibody is specific (figs. S8 and S9). (I) Demonstration of Fkbp6 as an SC protein in pachytene spermatocytes by EM with a 5-nm immunogold antibody to Fkbp6. The centromere is labeled with antiserum to CREST (15-nm grains).
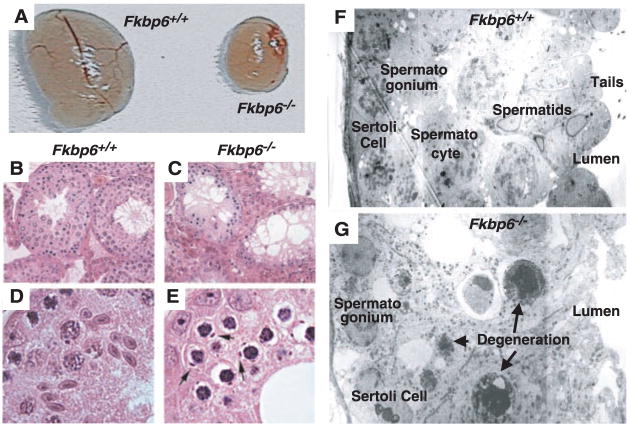
To determine the functional role of Fkbp6 in vivo, we generated mice deficient for Fkbp6 (fig. S3). Both male and female Fkbp6-mutant mice are healthy and have normal life-spans. Extensive analysis showed that no other abnormalities could be detected in any tissues of male Fkbp6-deficient mice. Male, but not female, mice deficient for Fkbp6 were completely sterile. Testes of all Fkbp6−/− males were reduced in size (Fig. 2A). The testis size and fertility of Fkbp6+/− mice resembled that of wild-type littermates. Histological analysis revealed that Fkbp6−/− male mice lacked spermatids, and we did not observe any mature spermatozoa in the caudal epididymis or seminiferous tubules (Fig. 2, B and C). Loss of Fkbp6 expression resulted in abnormal pachytene spermatocytes, defined by the appearance of unusual inclusion bodies and dense compacted nuclei; spermatocytes failed to proceed beyond the pachytene stage (Fig. 2, D and E). No alterations in testosterone, luteinizing hormone, follicle-stimulating hormone, or estradiol were detected, indicating that hormonal imbalances do not contribute to the phenotype. Staining by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL) revealed an increase in apoptosis in spermatocytes of mutant mice (fig. S4, A to D). This increase in cell death was most prevalent at 21 days after birth (fig. S4, C and D), indicating the presence of the spermatogenetic defect during the first wave of meiosis in pubescent animals. Mitotic proliferation of progenitor spermatogonia appeared to proceed normally in Fkbp6−/− testes (fig. S4, E and F). Defective spermatogenesis and the complete absence of spermatids and spermatozoa in Fkbp6−/− males was confirmed with EM (Fig. 2, F and G). The presence of Sertoli cells in the seminiferous tubules suggests that the intratubular environment was normal. Thus, loss of Fkbp6 results in male-specific infertility due to a complete block in spermatogenesis and cell death of meiotic spermatocytes.
Fig. 2.
Complete block in spermatogenesis in Fkbp6−/− mice. (A) Isolated testis from 16-week-old Fkbp6+/+ and Fkbp6−/− mice. (B to E) Hematoxylin and eosin–stained testis sections from 16-week-old mice. [(B) and (D)] Fkbp6+/+ mice. [(C) and (E)] Fkbp6−/− mice. Spermatids are completely absent and unique inclusion bodies are present in abnormal spermatocytes (arrows) in the testes of Fkbp6−/− mice. [(F) and (G)] EM cross sections of (F) an Fkbp6+/+ seminiferous tubule, showing Sertoli cells, spermatogonia, pachytene spermatocytes, spermatids, and spermatozoa tails in the lumen; and (G) an Fkbp6−/− seminiferous tubule with defective germ-cell development. Multiple pachytene spermatocytes display cellular degeneration and there are no postpachytene nuclei. Spermatids and spermatozoa are completely absent.
Various natural aspermic rat and mouse mutants have been described (11, 12). The overall histological phenotypes of natural mutant, aspermic as/as rats are very similar to that observed in our Fkbp6−/− mice, including the presence of the inclusion bodies in spermatocytes (12–14) and apparently abnormal spermatocyte chromosomes (15). The as/as phenotype is controlled by an autosomal recessive allele that maps to rat chromosome 12 in a region syntenic to the human Williams-Beuren critical region (14, 15). Therefore, a mutation in the rat Fkbp6 gene may be the causative mutation of as/as rats.
We amplified the entire Fkbp6 coding region from normal and as/as rats. The 3′ Fkbp6 gene region in the mutant as/as rats was shorter than that of wild types (Fig. 3A). Comparison of the nucleotide sequence revealed that a 93–base pair (bp) region corresponding to exon 8 of the gene was deleted in the mutant rats (Fig. 3B). Fine mapping showed that as/as rats harbored a genomic deletion of a 9357-bp region that includes exon 8 of the Fkbp6 gene (fig. S5). Genomic deletion of exon 8 resulted in Fkbp6 protein expression from as/as testes that was undetectable by Western blot (Fig. 3C) and immunohistochemistry (Fig. 3D). However, the genomic deletion of exon 8 did not result in an apparent alteration in Fkbp6 mRNA expression (Fig. 3E). Whether the genomic deletion results in an unstable Fkbp6 protein and/or expression of a mutant Fkbp6 protein that lacks exon 8 –encoded sequences needs to be determined. These data point to a critical role of the exon 8 –encoded region in the stability and/or function of Fkbp6 in meiotic cells. Thus, a genomic deletion including exon 8 of the rat Fkbp6 gene is causative for the aspermic phenotype in as/as rats.
Fig. 3.
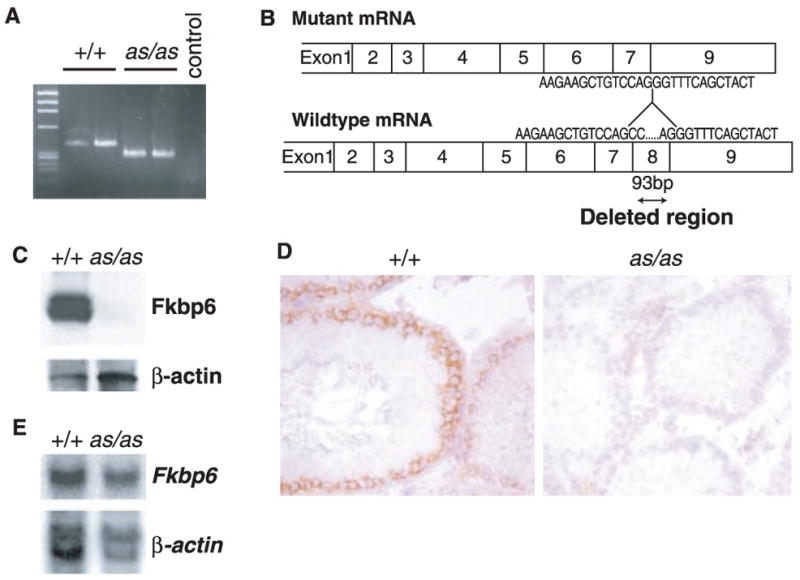
Genomic deletion of Fkbp6-exon 8 in as/as rats. (A) RT-PCR amplification shows a 93-bp reduction of a genomic fragment from as/as rats, compared to wild-type controls. (B) Exon structure and partial nucleic acid sequence of mRNA of wild-type and as/as rats. (C) Western blot analysis of Fkbp6 protein expression in wild-type and as/as testis with an antibody against exon 8–encoded sequences. (D) Immunohistochemistry of Fkbp6 expression in testes of wild-type and as/as rats. (E) Northern blot analysis of Fkbp6 mRNA in wild-type and as/as testis. β-actin controls are shown.
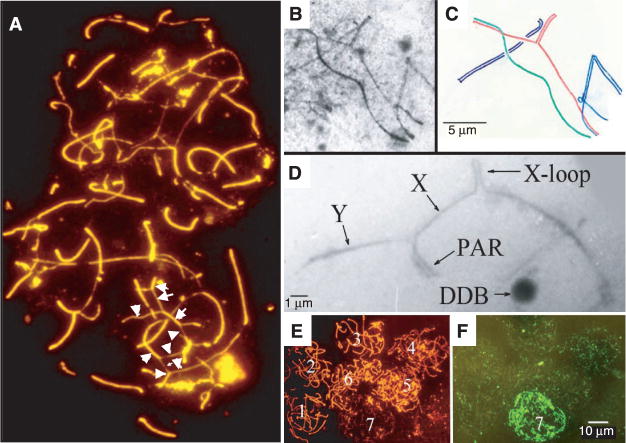
Fkbp6 labels meiotic chromosome cores and, in males, the X chromosome–associated DDB (Fig. 1, C and E). Because loss of Fkbp6 in gene-targeted mice and deletion of Fkbp6 exon 8 in as/as rats resulted in a defect in spermatogenesis, we examined the behavior of meiotic chromosome cores in Fkbp6−/− males. The assembly of chromosome cores and synapsis were similar at early prophase in Fkbp6−/− and control littermates. In Fkbp6−/− pachytene spermatocytes, there were multiple chromosome misalignments and nonhomologous partner switches of the chromosome cores, as demonstrated by fluorescence staining (Fig. 4A) and EM (Fig. 4, B and C). We frequently observed the presence of one or two X-chromosome loops, i.e., autosynapsed regions of the X-chromosome core (Fig. 4D). In meiotic nuclei of Fkbp6−/− cells, the numbers and localization of the Rad51/Dmc1 recombinase complexes appeared normal at the early stages of prophase (Fig. 4, E and F). Fkbp6−/− spermatocytes at later stages of meiosis that displayed chromosomal abnormalities showed massive accumulation of Rad51/Dmc1 expression at the chromosome cores (Fig. 4, E and F), suggesting large numbers of chromosome breaks in Fkbp6−/− spermatocytes. Cells in the later prophase stage of diplotene were completely absent in Fkbp6−/− testes (fig. S6). These synaptic abnormalities in the Fkbp6−/− spermatocytes show that Fkbp6 expression is required for the proper alignment and pairing of chromosome cores at meiotic prophase. Moreover, Fkbp6 may regulate progression and/or maintenance of chromosome synapsis.
Fig. 4.
Defective chromosome synapses in meiotic Fkbp6−/− spermatocytes. (A) Defective chromosome pairing in two Fkbp6−/− spermatocyte nuclei, stained with rhodamine-labeled antibodies to Scp3 (Scp3-rhodamine) and fluorescein isothiocyanate (FITC)–labeled antibodies to Scp1 (orange combined with red in the synapsed segments). Arrows mark partner exchanges and nonhomologous associations. (B) Electron micrograph of an Fkbp6−/− meiotic prophase nucleus. (C) Diagram of (B), illustrating multiple unpaired core segments and nonhomologous pairing between a red and a green core and between a red and a blue core in the Fkbp6−/− nucleus. (D) Electron micrograph of abnormal X-Y chromosome pairing and autosynapsis (an X-loop) in Fkbp6−/− prophase spermatocytes. (E) Seven spermatocyte nuclei in which the center lower nucleus is degenerating and losing Scp3 protein (rhodamine) expression. (F) The nuclei in (E) immunostained for Rad51/Dmc1 protein with FITC.
It has been shown previously that in mutants that exhibit meiotic abnormalities, spermatogenesis frequently appears more severely compromised than oogenesis, suggesting sex-specific checkpoint differences (16). For instance, Scp3 mutant males are infertile because of a block in spermatogenesis at the zygotene stage of meiosis, whereas loss of Scp3 in females results in reduced fertility with reduced litter size and an increased likelihood of aneuploid progeny (6, 7). Because Fkbp6 is an SC protein in oocytes (fig. S2, E to H), we analyzed whether Fkbp6 has a role in fertility and meiosis in females. Fkbp6−/− females were able to breed up to 1 year after birth, and individual Fkbp6−/− female mice carried multiple pregnancies with litter sizes comparable to those of control littermates. Histological analysis showed that ovaries and the differentiation of oocytes were comparable between Fkbp6−/− female and control littermates (fig. S7). Moreover, Fkbp6−/− oocytes progressed normally through all stages of meiosis and continued into late prophase and metaphase I. Alignment of chromosomes, chromosome pairing, Rad51/Dmc1 expression, and chromosome separation in meiotic oocytes of Fkbp6−/− females also appeared normal (Fig. 5, A and B). Male as/as rats are sterile, whereas females are fertile without any detectable abnormality (14). Whether loss of Fkbp6 in females results in aneuploidy in the offspring needs to be studied. Our data indicate that loss of Fkbp6 has no apparent effect on meiotic chromosome pairing and fertility in females.
Fig. 5.
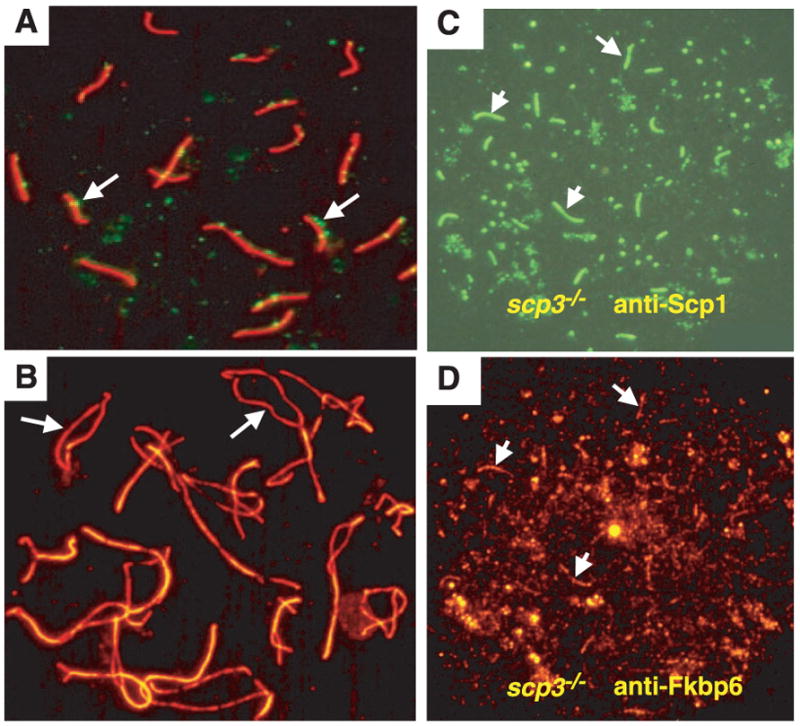
Fkbp6 colocalizes with Scp1 in synapsed chromosomes of Scp3−/− spermatocytes. (A) Normal SC formation in pachytene oocytes stained with Scp3-rhodamine and FITC-labeled antibodies to Rad51 and Dmc1. Rad51 and Dmc1 foci (arrows) define regions of double-strand breaks. (B) Normal chromosome separation (arrows) in diplotene oocytes stained with Scp3-rhodamine, in a 1-day-old Fkbp6−/− female. (C and D) Expression of Fkbp6 and Scp1 in synapsed regions of Scp3−/− zygotene spermatocytes. (C) Short stretches of FITC-labeled Scp1 mark synapsis between the chromosome cores. (D) Fkbp6 (rhodamine) expression at the residual synapsed regions of Scp3−/− zygotene spermatocytes. Arrows in (C) and (D) show Scp1 and Fkbp6 colocalization.
To elucidate a potential mechanism for Fkbp6 function, we speculated that Fkbp6 might bind to one of the known SC proteins. We immunoprecipitated Fkbp6 from primary spermatocytes and analyzed Fkbp6-associated proteins using matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (17). One of the associated proteins was identified as Scp1, and Fkbp6 and Scp1 colocalized in autosomal chromosome synapsis in wild-type spermatocytes (fig. S2, A to D). In Scp3−/− spermatocytes, in which the axial cores are still intact and residual chromosome synapsis occurs in meiotic spermatocytes, Fkbp6 was expressed at the residual chromosome synapsis (Fig. 5, C and D). Fkbp6 expression was also found at the full SCs in Scp3−/− oocytes; Scp1 and Fkbp6 co-localized in these synapses (Fig. 5D). These results show that Fkbp6 associates biochemically with Scp1 in meiotic germ cells and that, even in the absence of Scp3 expression, Fkbp6 colocalizes with Scp1 at the regions of residual chromosome synapses. Thus, Fkbp6 associates with Scp1 and might be able to facilitate chromosome synapsis in the absence of Scp3 expression.
Because chromosome cores initiate synapsis in the early meiotic prophase of Fkbp6−/− mice (18, 19), it is not likely that Fkbp6 functions in synapsis initiation. The synaptic abnormalities at later prophase in Fkbp6−/− mice indicate that Fkbp6 plays a role in monitoring progression and/or maintaining homologous synapsis and that its failure to do so results in the autosynapsis of the X-chromosome core, core misalignment, and nonhomologous partner switches. In contrast, genetic inactivation of murine Scp3 results in defective chromosome synapsis and spermatogenetic arrest at the zygotene stage (6), suggesting that Fkbp6 and Scp3 have different functions in chromosome synapsis in males. Fkbp6 associates with Scp1 and these two proteins colocalize at the sites of residual chromosome synapses that are present in Scp3−/− spermatocytes. The sex-specific differences in male and female fertility and meiosis (16) in both Fkbp6−/− and Scp3−/− mice could be explained by functional redundancy between these two SC components in oocytes. It also will be important to determine if Fkbp6 interacts with other members of the SC such as Scp3 or Scp2. Moreover, the phenotype of Fkbp6−/− males resembles Hsp70-2– (20) and Cyclin A–(21) null mice. In particular, the connection to the HSP70-2 protein needs to be explored, because other members of the Fkbp family have been shown to bind to heat shock proteins associated with steroid receptors (22). Fkbp6 could also be involved in the control of meiotic checkpoints at the pachytene stage (23).
An estimated 15% of couples worldwide remain childless because of infertility (24). Few genetic causes of infertility have been identified in humans. Given our data from mouse mutants and spontaneously mutant rats, it may be interesting to test whether mutations in Fkbp6 account for idiopathic human infertility and whether some Williams-Beuren syndrome patients display infertility due to homozygous disruptions in the Fkbp6 gene. Our genetic data reveal a function for Fkbp-family proteins in the control of meiosis and male-specific fertility. The identification of Fkbp6 as a critical molecule in homologous chromosome pairing in meiosis suggests that other Fkbp6-family proteins might have a role in the control of chromosome pairing and chromosome stability in mitosis and cancer.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/300/5623/1291/DC1
Materials and Methods
Figs. S1 to S9
References and Notes
- 1.Cohen PE, Pollard JW. Bioessays. 2001;23:996. doi: 10.1002/bies.1145. [DOI] [PubMed] [Google Scholar]
- 2.Nasmyth K. Science. 2002;297:559. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 3.Zickler D, Kleckner N. Annu Rev Genet. 1999;33:603. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett DW. J Biophys Biochem Cytol. 1956;2:403. doi: 10.1083/jcb.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moses MJ. J Biophys Biochem Cytol. 1956;2:215. doi: 10.1083/jcb.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan L, et al. Mol Cell. 2000;5:73. doi: 10.1016/s1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- 7.Yuan L, et al. Science. 2002;296:1115. doi: 10.1126/science.1070594. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Lu X, Morris CA, Keating MT. Genomics. 1998;52:130. doi: 10.1006/geno.1998.5412. [DOI] [PubMed] [Google Scholar]
- 9.Osborne LR. Mol Genet Metab. 1999;67:1. doi: 10.1006/mgme.1999.2844. [DOI] [PubMed] [Google Scholar]
- 10.Dresser ME, Moses MJ. Chromosoma. 1980;76:1. doi: 10.1007/BF00292222. [DOI] [PubMed] [Google Scholar]
- 11.Lyon MF, Hawkes SG. Nature. 1970;227:1217. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 12.Ikadai H, Noguchi J, Yoshida M, Imamichi T. J Vet Med Sci. 1992;54:745. doi: 10.1292/jvms.54.745. [DOI] [PubMed] [Google Scholar]
- 13.Atagi Y, Ikadai H, Kurohmaru M, Hayashi Y. J Vet Med Sci. 1993;55:301. doi: 10.1292/jvms.55.301. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi J, et al. Mamm Genome. 1999;10:189. doi: 10.1007/s003359900967. [DOI] [PubMed] [Google Scholar]
- 15.Bayes M, et al. Mol Reprod Dev. 2001;60:414. doi: 10.1002/mrd.1104. [DOI] [PubMed] [Google Scholar]
- 16.Hunt PA, Hassold TJ. Science. 2002;296:2181. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 17.Materials and methods are available as supporting material on Science Online.
- 18.Moens PB, et al. J Cell Sci. 2002;115:1611. doi: 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- 19.Masson JY, West SC. Trends Biochem Sci. 2001;26:131. doi: 10.1016/s0968-0004(00)01742-4. [DOI] [PubMed] [Google Scholar]
- 20.Dix DJ, et al. Development. 1997;124:4595. doi: 10.1242/dev.124.22.4595. [DOI] [PubMed] [Google Scholar]
- 21.Liu D, et al. Nature Genet. 1998;20:377. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 22.Tai PK, et al. Science. 1992;256:1315. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 23.Odorisio T, Rodriguez TA, Evans EP, Clarke AR, Burgoyne PS. Nature Genet. 1998;18:257. doi: 10.1038/ng0398-257. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk MM, Lamb DJ. Nature Cell Biol. 2002;4:41. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 25.This study was partially funded by the Natural Sciences and Engineering Research Council of Canada and IMBA. J. M. P. holds a Canada Research Chair in Cell Biology. M. A. C. was supported in part by a Canadian Institutes of Health Research fellowship. N.K.K. and P.E.C. are supported by AECOM. We would like to thank K. So for expert histology and C. Hoog for Scp3−/− mice.