Abstract
A standard oral glucose tolerance test (OGTT) was administered to 28 adults with Williams syndrome (WS). Three quarters of the WS subjects showed abnormal glucose curves, meeting diagnostic criteria for either diabetes or the pre-diabetic state of impaired glucose tolerance. Fasting mean glucose and median insulin levels did not differ significantly in the total WS cohort versus age–gender–BMI matched controls, though the glucose area under the curve was greater in the WS subjects. HbA1c levels were not as reliable as the OGTT in diagnosing the presence of diabetes. Given the high prevalence of impaired glucose regulation, adults with WS should be screened for diabetes, and when present should be treated in accordance with standard medical practice. Hemizygosity for a gene mapping to the Williams syndrome chromosome region (WSCR) is likely the major factor responsible for the high frequency of diabetes in WS. Syntaxin-1A is a prime candidate gene based on its location in the WSCR, its role in insulin release, and the presence of abnormal glucose metabolism in mouse models with aberrantly expressed Stx-1a.
Keywords: diabetes mellitus, impaired glucose tolerance, Williams syndrome chromosome region (WSCR), syntaxin-1A (STX-1A), oral glucose tolerance test
INTRODUCTION
Williams syndrome (WS, OMIM # 194050), originally described as a syndrome with “peculiar” facies, a distinctive cardiovascular lesion, and “mental retardation” [Williams et al., 1961; Beuren et al., 1962], is now known to be a multi-system disorder with potential impact on virtually all organ systems [Morris et al., 1988, 2001; Cherniske et al., 2004]. The features and complications of WS are caused by deletion of 26–28 genes contained in an approximate 1.5–1.8 million basepair Williams–Beuren syndrome chromosome region (WSCR) on chromosome 7q11.23 [Osborne, 1999; Peoples et al., 2000; Antonell et al., 2005]. Intense efforts to draw genotype–phenotype correlations are ongoing [Morris et al., 2003; Tassabehji et al., 2005].
One of the genes mapping to the WSCR is syntaxin-1A (STX-1A) encoding for a protein of the same name [Osborne et al., 1997]. STX-1A plays a role in membrane vesicle fusion and pancreatic beta-cell exocytosis of insulin granules [Bennett et al., 1992]. An overproducing syntaxin-1A transgenic mouse model shows hyperglycemia and reduced insulin secretion following intraperitoneal glucose challenge [Lam et al., 2005]. Findings from this and other models of altered syntaxin-1A stoichiometry [Chan et al., 1999; Nagamatsu et al., 1999], combined with a few clinical reports of diabetes mellitus (DM) in patients with WS, prompted us to study glucose metabolism in WS. We report that 75% of adults with WS show either pre-diabetes (e.g., impaired glucose tolerance) or DM after an oral glucose tolerance challenge. This represents one of the highest frequencies of glucose dysregulation observed in any human population.
METHODS
We recruited 28 adults with WS over the age of 20 for this study. All subjects were examined by one of the authors (BRP), and were offered participation in this research as part of a comprehensive study of adults with WS [Cherniske et al., 2004], or as part of their attendance in genetics clinic. Subjects with either an established diagnosis of DM or a prior history of elevated glucose levels were excluded. Information on past medical history and current pharmacotherapy was collected.
Subjects were studied in the Yale Center for Clinical Investigation—Hospital Research Unit (HRI) at the Yale University School of Medicine. A standard 2-hr oral glucose tolerance test (OGTT) was administered following an overnight fast. Details on the OGGT and biochemical determinations of glucose and insulin levels are provided elsewhere [Sinha et al., 2002]. Each subject’s 2-hr glucose level following ingestion of a 75 g glucose load was used to classify them as having normal glucose tolerance (NGT, glucose at 120 min <140 mg/dl), the pre-diabetic state of impaired glucose tolerance (IGT, glucose at 120 min >140, but <200 mg/dl), or previously unrecognized DM (glucose at 120 min >200 mg/dl) [American Diabetes Association, 2004]. Two WS subjects did not receive an oral glucose load because their fasting glucose levels exceeded 150 mg/dl, thereby categorizing them as having DM. Body mass index (BMI) was calculated as weight in kilograms/[height in meters]2.
Data points are presented either as means ± SD or as medians accompanied by interquartile range (25th centile–75th centile). Matched comparisons were made using McNemar’s test for binary outcomes, and paired t-tests for comparison of means or Wilcoxon Signed Rank tests for comparison of medians. All data were analyzed using SAS version 9.1 (Cary, NC). Insulin sensitivity was measured with whole body insulin sensitivity index (WBISI) using the Matsuda Index [Matsuda and DeFronzo, 1999; Yeckel et al., 2004] and the HOMA-IR [Wallace and Matthews, 2002]. The insulinogenic index (IGI) was calculated as the ratio of the increment in plasma insulin level to that in plasma glucose level during the first 30 min after glucose ingestion [Phillips et al., 1994].
Age–gender–BMI matched controls were selected from a cohort of adults neither with known medical problems nor a family history of diabetes. Matching was successfully accomplished for 17 of the 18 WS subjects diagnosed with NGT or IGT; we were unable to identify a matched control for one 44-year-old WS male with a BMI = 20. Matching was not performed for the WS subjects diagnosed with DM.
The Yale University School of Medicine Human Investigation Committee approved this study on an annual basis. Adults with WS gave their assent to participate; consent was simultaneously obtained from a parent or legal-guardian.
RESULTS
Cohort of WS Subjects
Twenty-eight adults, 10 males and 18 females with a mean age of almost 35 years, participated in this study (Table I). The clinical diagnosis of WS was established in all cases by one of the co-authors (BRP), and confirmed in 25 subjects by FISH or microsatellite marker analysis. The majority of WS subjects were taking at least one medication at the time of OGTT administration; the most common medications were selective serotonin re-uptake inhibitors (SSRIs) and beta-blockers, taken by 50% and 25%, respectively. Additionally, one subject was taking the antipsychotic Olanzapine (Zyprexa), and three subjects were taking thyroid hormone supplementation.
TABLE I.
Characterization of Williams Syndrome (WS) Cohort
| Adults with Williams syndrome (n = 28) | |
|---|---|
| Age (y)a | 34.9 (9.7) |
| Sex | |
| M | 10 |
| F | 18 |
| BMI (kg/m2)a | 26.19 (5.86) |
| Dist. of BMI | |
| <20 | 6 |
| ≥ 20–25 | 4 |
| ≥ 25–30 | 13 |
| >30 | 5 |
| Glucose tolerance statusb | |
| Normal glucose tolerance | 7 (25) |
| Impaired glucose tolerance | 11 (39) |
| Previously unrecognized diabetes | 10 (36) |
| 1st degree relative with diabetes mellitusb,c | |
| Yes | 3 (12) |
| No | 23 (88) |
| Medicationsb,d | |
| Any medications | 21 (75) |
| Beta-blockers | 7 (25) |
| Other anti-HTN | 5 (18) |
| Psychiatric medications | 14e (50) |
| % Total body fata (16 missing) | 29.67 (11.74) |
| Mean HbA1c (%)a | 5.38 (0.56) |
HTN, hypertension; HbA1c, hemoglobin A1C.
Mean (SD).
Frequency (%).
Two patients adopted.
Several patients were taking multiple medications.
The most common medications used to treat psychiatric symptoms were SSRIs; one male patient, taking olanzapine (Zyprexa), was classified as having IGT.
Remarkably, 21 of the 28 WS subjects (75%) had either pre-diabetes with IGT or previously unrecognized DM (Table I). IGT and DM were present in all BMI categories, occurring in 60% of those with BMI’s <25 and in 80% of those with BMI’s >25.
Mean hemoglobin A1c for the entire cohort was normal (5.38%, 4.0–5.9%), but was greater than the upper limit of normal among half the DM group, ranging up to 6.7%.
Comparison of Age–Gender–BMI Matched WS and Control Subjects
After excluding the 10 WS subjects whose OGTT results met criteria for DM, we successfully matched 17 of the remaining 18 subjects to healthy controls (Table II). All controls had NGT, while only 7 of 17 WS subjects demonstrated NGT (P = 0.02). The fasting glucose mean and fasting insulin median did not differ between WS and controls, though levels in the WS cohort encompassed a broader range. Insulin area under the curve (AUC) was comparable between WS subjects and controls. However, the glucose AUC was 24% greater in subjects with WS than their matched controls.
TABLE II.
Non-Diabetic Williams Syndrome Patients Versus Matched Controls
| WS (n = 17) | Matched control (n = 17) | P-value | |
|---|---|---|---|
| Age (y)a | 32.1 (9.1) | 32.9 (9.2) | 0.54 |
| Sex | |||
| M | 7 | 7 | 1.00 |
| F | 10 | 10 | |
| BMI (kg/m2)a | 24.8 (5.5) | 25.7 (4.3) | 0.34 |
| Hypertensionb | |||
| Yes | 8 (47) | 0 (0) | 0.01 |
| No | 9 (53) | 17 (100) | |
| Glucose tolerance statusb,c | |||
| Normal glucose tolerance | 7 (41) | 17 (100) | 0.02 |
| Impaired glucose tolerance | 10 (59) | 0 (0) | |
| Fasting glucose (mg/dl)a | 89.8 (11.7) | 91.4 (6.1) | 0.69 |
| Fasting insulin (median/IQR) | 10 (6–18) | 9 (7–9) | 0.09 |
| HOMA-IR (median/IQR) | 2.23 (1.4–5.4) | 1.89 (1.5–2.4) | 0.74 |
| WBISI (median/IQR) | 4.46 (2.8–7.3) | 5.21 (4.2–6.1) | 0.67 |
| Insulinogenic index (median/IQR) | 0.96 (0.4–1.1) | 1.05 (.6–1.4) | 0.32 |
| AUC glucosea | 144 (31) | 116 (20) | 0.004 |
| AUC insulin (median/IQR) | 46.4 (31.2–77.2) | 41.0 (31.3–51.4) | 0.62 |
AUC, area under curve; BMI, body mass index; F, female; HOMA-IR, homeostasis model assessment–insulin resistance; IQR, interquartile range; M, male; WBISI, whole body insulin sensitivity index; WS, Williams syndrome; y, year.
Mean (SD).
Frequency (%).
WS cases with previously unrecognized diabetes were not matched to healthy controls.
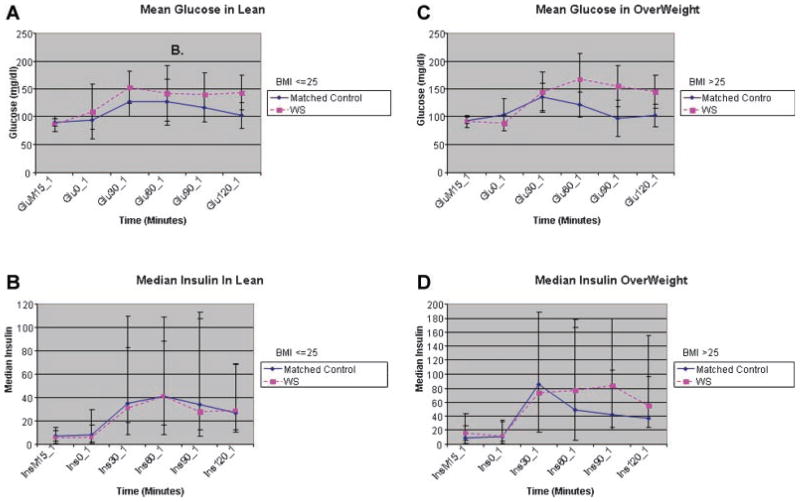
Further analyses were performed to consider the effect of BMI on average glucose and insulin levels (Fig. 1 and Table III). Both the lean and obese WS subjects showed more variability in glucose and insulin levels than did their matched controls.
Figure 1.
Mean (±SD) plasma concentrations of glucose (panel A) and median (±IQR) concentrations of plasma insulin concentrations (panel B) before and during an oral glucose tolerance tests in 8 lean (BMI <25 mg/kg/m2) patients with Williams syndrome and controls. Mean (±SD) plasma concentrations of glucose (panel C) and median (±IQR) concentrations of plasma insulin concentrations (panel D) before and during an oral glucose tolerance tests in 9 overweight (BMI >25 mg/kg/m2) patients with Williams syndrome and controls. IQR, interquartile range; SD, standard deviation.
TABLE III.
Lean WS Patients Versus Matched Controls, and Overweight/Obese WS Patients Versus Matched Controls
| Lean |
Overweight/obese |
|||||
|---|---|---|---|---|---|---|
| WS, N = 8 | Control, N = 8 | P-value | WS, N = 9 | Control, N = 9 | P-value | |
| Agea | 29.9 (8.2) | 32.0 (9.1) | 0.29 | 34.1 (10.0) | 33.7 (9.8) | 0.79 |
| BMIa | 20.1 (2.8) | 21.6 (1.8) | 0.03 | 29.0 (3.5) | 28.6 (2.8) | 0.63 |
| Glucose tolerance statusb,c | ||||||
| Normal glucose tolerance | 4 | 8 | 0.13 | 3 | 9 | 0.04 |
| Impaired glucose tolerance | 4 | 0 | 6 | 0 | ||
| Fasting glucosea | 90.9 (17.6) | 90.6 (6.1) | 0.97 | 88.8 (13.8) | 91.8 (5.7) | 0.42 |
| Fasting insulin (median/IQR) | 5.5 (4.0–18.0) | 7.5 (6.0–8.0) | 0.89 | 14.0 (10.0–22.0) | 9.0 (7.5–10.0) | 0.04 |
| HOMA-IR (median/IQR) | 1.26 (0.71–4.86) | 1.83 (1.36–1.90) | 0.81 | 2.40 (2.09–5.96) | 2.33 (1.57–2.84) | 0.43 |
| WBISI (median/IQR) | 7.62 (4.01–9.10) | 5.24 (5.08–7.43) | 0.69 | 4.29 (2.03–4.50) | 4.90 (4.53–5.37) | 0.07 |
| Insulinogenic index (median/IQR) | 0.61 (0.07–1.19) | 0.83 (0.32–1.13) | 0.58 | 1.00 (0.94–1.08) | 1.13 (1.00–1.69) | 0.43 |
| AUC glucosea | 141.6 (31.4) | 119.5 (23.5) | 0.20 | 145.9 (32.6) | 113.1 (16.1) | 0.006 |
| AUC insulin (median/IQR) | 31.9 (25.1–45.5) | 36.1 (30.7–47.8) | 0.47 | 67.0 (47.4–77.5) | 43.3 (33.9–59.0) | 0.24 |
AUC, area under curve; BMI, body mass index; HOMA-IR, homeostasis model assessment–insulin resistance; IGT, impaired glucose tolerance; IQR, interquartile range; NGT, normal glucose tolerance; WBISI, whole body insulin sensitivity index; WS, Williams syndrome; y, year.
Mean (SD).
Frequency (%).
WS cases with previously unrecognized diabetes were not matched to healthy controls.
Among the lean subjects, the mean fasting glucose levels were comparable between WS cases and the controls; though not statistically significant, all subsequent glucose means were higher in the lean WS subjects than in controls. Median insulin levels, including fasting insulin and 30-min insulin (reflective of 1st phase insulin release), were similar between lean WS and their matched controls.
Among overweight subjects, the WS cases and controls had comparable fasting mean glucose, but not median insulin, levels; the latter averaged higher in the WS cases. Both the absolute glucose and insulin levels trended higher at 60, 90, and 120 min in overweight WS subjects compared to either the matched controls or the lean WS subjects.
DISCUSSION
The medical literature includes mention of overt DM in a few patients with WS [Morris et al., 1988; Lopez-Rangel et al., 1992; Plissart et al., 1994; Imashuku et al., 2000; Nakaji et al., 2001; Cherniske et al., 2004] However, systematic studies of glucose metabolism in this population have not been performed to date. We demonstrate that 21 of 28 (75%) of adults with WS have abnormal glucose tolerance in response to a 2-hr oral glucose challenge. Among WS study participants 34 years of age or older all, except one, have DM (8/17) or IGT (8/17). This extraordinarily high prevalence of glucose dysregulation is similar to that seen in the Pima Indians of Arizona, who are described as having “the highest frequency of type 2 DM of any population in the world” [Prately, 1998]. Comparison of the WS cohort to an age–gender–BMI matched healthy cohort, however, does not demonstrate insulin resistance; that is, individuals with WS do not collectively show elevated fasting insulin levels or an abnormal HOMA-IR.
WS is caused by an ~1.55–1.8 million basepair deletion resulting in the loss of between 26 and 28 genes, depending on the exact point of homologous recombination [Bayes et al., 2003]. We hypothesize that hemizygosity for one or more of these genes contributes to the high frequency of abnormal glucose tolerance seen in our cohort. Additional suggestive evidence comes from OGTT data (not shown) collected in seven 10-to 17-year old WS children and adolescents (mean age = 13.3 years; BMI <20 in four, 20–25 in two, and >30 in one). Even at this young age the pre-diabetic IGT state was found in four subjects, three of whom had a BMI <25. This early onset of pre-diabetes precedes risk factors, such as obesity, medication, and limited physical activity, and points to a genetic pre-disposition to abnormal glucose metabolism.
Hemizygosity for syntaxin-1A (STX-1A), a gene located in the WSCR could be the initiating diabetogenic “hit” in a cascade of events that ultimately result in glucose dysregulation. STX-1A encodes for a protein of the same name, a soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE). This protein contributes to a membrane complex responsible for vesicle docking and fusion, leading to the release of the stored insulin granules that constitute the 1st phase insulin response [Daniel et al., 1999]. Pancreatic beta cells from Stx1a knock-out mice were recently shown to exhibit impaired 1st phase but not 2nd phase insulin secretion [Ohara-Imaizumi et al., 2007]. Rat models of DM, such as Zucker fa/fa and Goto-Kakizaki (GK), demonstrate decreased level of SNARE proteins, including STX-1A [Chan et al., 1999; Nagamatsu et al., 1999]. Transduction of the STX-1A gene into GK pancreatic islets corrected hyperglycemia, though similar manipulation using islets from normal rats resulted in STX-1A over-expression with decreased insulin release [Gaisano et al., 2002]. A transgenic mouse model engineered to modestly over-express STX-1A (35% increase in protein levels) demonstrated fasting hyperglycemia and elevated glucose levels in response to a glucose load [Lam et al., 2005]. Pancreatic beta-cells isolated from these mice also showed reduced Ca2+ ion channel currents and abnormal insulin tolerance.
It appears from these observations that even small fluctuations of STX-1A levels are sufficient to induce profound changes on the function of the components controlling secretion [Lam et al., 2005]. Thus, either excess or deficient STX-1A protein levels can impair insulin release, presumably by disrupting the tightly regulated SNARE complex required for normal vesicle fusion and exocytosis. In WS, one STX-1A allele is deleted, a finding confirmed in 14 of our study subjects (data not shown); this reduction in gene dosage is likely to result in reduced protein levels, though this prediction remains to be experimentally verified. STX-1A has previously been implicated in the etiology or progression of diabetes in two human association studies. Tsunoda and coworkers reported that a single nucleotide polymorphism was associated with earlier age of onset and higher daily insulin requirement in individuals with type 2 diabetes of Asian descent, while a polymorphism in the STX1-A promoter was associated with impaired glucose regulation in overweight Italian subjects [Tsunoda et al., 2001; Romeo et al., 2008].
Hemizygosity for another gene located in the Williams–Beuren syndrome critical region, MLXIPL (formerly WSCR14), which encodes for a basic-helix-loop-helix leucine zipper protein, has also been implicated in abnormal glucose metabolism [Merla et al., 2004]. MLXIPL was recently identified as a locus affecting triglyceride levels in humans [Kathiresan et al., 2008; Kooner et al., 2008; Willer et al., 2008] and a knock-out mouse model showed that MLXIPL is required both for basal and carbohydrate-induced expression of several liver enzymes essential for the coordinated control of glucose metabolism and the synthesis of fatty acids and triglycerides [Iizuka et al., 2004]. Although heterozygous mice were not studied, mice lacking this protein showed somewhat elevated plasma glucose and insulin levels, combined with significant reductions in fatty acid synthesis and fat deposition. The tendency for abnormal fat accumulation seen in some patients with WS [Cherniske et al., 2004] would argue against a similar effect of hemizygosity for MLXIPL, but confirmation awaits a careful analysis of the heterozygous mouse model.
Although we predict that the genetic basis of WS is the major risk for developing diabetes, we cannot exclude other factors. For instance, the increased risk of DM could be non-specific due to gain or loss of genetic material, or a higher prevalence of diabetogenic lifestyle risk factors, rather than caused by deletion of a gene or genes in the WSCR. However, the glucose intolerance of WS seems to be distinct compared to that found in other genetic syndromes, such as Prader–Willi, Turner, and Down syndromes. In these latter disorders, the frequency of abnormal glucose tolerance is lower than that reported in our series. Furthermore, patients with Prader–Willi syndrome primarily have a blunted insulin response, those with Down syndrome typically manifest immune-mediated type I diabetes, while those with Turner syndrome demonstrate a mixed picture of diminished insulin release combined with impaired peripheral glucose utilization [Caprio et al., 1991; Schuster et al., 1996; Anwar et al., 1998; Bakalov et al., 2004; Talebizadeh and Butler, 2004].
There are several limitations of the current study. Our WS sample is small and possibly affected by selection bias. However, we suggest the WS cohort is representative of the general WS population, as study participants were not recruited specifically for an assessment of glucose metabolism [Cherniske et al., 2004]. Despite matching WS subjects to healthy controls of comparable age, gender, and BMI, the two populations differ in several important respects. The majority of WS subjects took one or more prescription medications, while the controls took none. Seven WS patients received beta-blockers for hypertension, and six additional subjects had hypertension; both factors are modest but independent risk factors for diabetes type II [Taylor et al., 2006]. One subject with WS, diagnosed with IGT, was taking the atypical antipsychotic olanzapine (Zyprexa) which has been associated with an excess risk of type II diabetes [American Diabetes Association, 2004; Lamberta et al., 2006]. Finally, many adults with WS engage in limited physical activity. Although we did not administer an objective assessment of this, parents reported activity level in 14 members of our cohort as: not active in 4; moderately active in 8; and very active in 2. We suspect these additional risk factors contribute to the development of diabetes, but are acting in concert with the primary risk factor, deletion of a gene such as STX-1A within the WSCR.
Dissection of the underlying mechanism responsible for abnormal glucose dysregulation will require further studies on more individuals with WS. For the present, WS care-providers should vigilantly monitor for the development of diabetes given the high frequency observed in this cohort. The OGTT should remain the diagnostic standard in WS, since sole reliance on HbA1c levels would have missed the diagnosis of diabetes in half the WS subjects. And finally, we believe it is appropriate to institute lifestyle changes and pharmacotherapy in WS individuals diagnosed with diabetes.
CONCLUSIONS
We report that the majority of individuals with WS over 20 years of age have pre-diabetes/IGT or previously unrecognized DM as defined by a standard oral glucose challenge. We suggest that deletion of a gene in the Williams–Beuren syndrome critical region is the greatest risk factor conferring abnormal glucose metabolism. Future studies will be required to elucidate the gene(s) and mechanism(s) underlying this phenomenon in WS, and whether mutations or polymorphisms in these same genes could contribute to abnormal glucose metabolism in persons in the general population.
Acknowledgments
Grant sponsor: United States Public Health Services; Grant numbers: MO1 RR-00125, RO1 AG-23286.
We thank all the subjects with WS and their families for participation in this study, and the staff of the Yale Center for Clinical Investigation—Hospital Research Unit for their expert technical assistance. This work was supported by grants from the United States Public Health Services MO1 RR-00125, RO1 AG-23286 (KFP), and a Distinguished Clinical Scientist Award from the American Diabetes Association (KFP).
Contributor Information
Barbara R. Pober, Associate Professor of Pediatrics at Harvard Medical School, a member of the Department of Surgery Children’s Hospital of Boston, and a member of the Department of Pediatrics at the MassGeneral Hospital for Children in Boston, Massachusetts. Dr. Pober’s interests include the management and natural history of Williams syndrome, as well as the genetics of congenital diaphragmatic hernia.
Erica T. Wang, Women’s Health Clinical Research Fellow at the University of California, San Francisco. Dr. Wang’s interests include the epidemiology of polycystic ovary syndrome and its associated cardiovascular risk factors
Sonia Caprio, Professor (Section of Endocrinology) in the Department of Pediatrics at Yale University School of Medicine. Dr Caprio’s interests are the metabolic complications of childhood obesity, and type II diabetes
Kitt F. Petersen, Associate Professor (Section of Endocrinology), Department of Internal Medicine at Yale University School of Medicine. Her areas of interest include metabolism and magnetic resonance (MR) spectroscopy
Cynthia Brandt, Associate Professor at the Yale Center for Medical Informatics. Dr. Brandt directs the Clinical Research Informatics Cores for the Yale Center for Investigative Medicine and the Yale Cancer Center.
Takara L. Stanley, Instructor in Pediatrics at Harvard Medical School and a member of the Pediatric Endocrine Unit at the Massachusetts General Hospital for Children. Her research interests include the metabolic and endocrine complications of HIV infection and other conditions associated with abnormal body fat distribution
Lucy R. Osborne, Associate Professor of Medicine and Molecular Genetics at the University of Toronto, Canada. Dr. Osborne’s research includes the molecular basis of Williams syndrome, the genetics of infantile spasms and mouse models of human disease
James Dziura, Research Scientist in the Department of Internal Medicine at the Yale School of Medicine and a Biostatistician for the Yale Center for Clinical Investigation in New Haven, CT. Dr. Dziura’s primary research interests are in obesity and diabetes
Barbara Gulanski, Associate Professor (Section of Endocrinology), Department of Internal Medicine at Yale University School of Medicine. Dr Gulanski’s interests are metabolic bone disease, diabetes, and women’s health
References
- American Academy of Pediatrics. Health care supervision for children with Williams syndrome. Pediatrics. 2001;107:1192–1204. [PubMed] [Google Scholar]
- Antonell A, de Luis O, Domingo-Roura X, Perez-Jurado LA. Evolutionary mechanisms shaping the genomic structure of the Williams-Beuren syndrome chromosomal region at human 7q11.23. Genome Res. 2005;15:1179–1188. doi: 10.1101/gr.3944605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar AJ, Walker JD, Frier BM. Type 1 diabetes mellitus and Down’s syndrome: Prevalence, management, and diabetic complications. Diabet Med. 1998;15:160–163. doi: 10.1002/(SICI)1096-9136(199802)15:2<160::AID-DIA537>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Consensus development conference on antipsychotic drugs, obesity, and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Bakalov VK, Cooley MM, Quon MJ, Luo ML, Yanovslci JA, Nelson LM, Sullivan G, Bondy CA. Impaired insulin secretion in the Turner metabolic syndrome. J Clin Endocrinol Metab. 2004;89:3516–3520. doi: 10.1210/jc.2004-0122. [DOI] [PubMed] [Google Scholar]
- Bayes M, Magano LF, Rivera N, Flores R, Perez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet. 2003;73:131–151. doi: 10.1086/376565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- Caprio S, Boulware S, Diamond M, Sherwin RS, Caipenter TO, Rubin K, Amiel S, Press M, Tamborlanc WV. Insulin resistance: An early metabolic defect of Turner’s syndrome. J Clin Endocrinol Metab. 1991;72:832–836. doi: 10.1210/jcem-72-4-832. [DOI] [PubMed] [Google Scholar]
- Chan CB, MacPhail RM, Sheu L, Wheeler MB, Gaisano HY. Beta-cell hypertrophy in fa/fa rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes. 1999;48:997–1005. doi: 10.2337/diabetes.48.5.997. [DOI] [PubMed] [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet Part A. 2004;131A:255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48:1686–1690. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S. Abnormal expression of pancreatic islet exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology. 2002;143:4218–4226. doi: 10.1210/en.2002-220237. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashuku S, Hayashi S, Kuriyama K, Hibi S, Tabata Y, Todo S. Sudden death of a 21-year-old female with Williams syndrome showing rare complications. Pediatr Int. 2000;42:322–324. doi: 10.1046/j.1442-200x.2000.01213.x. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooner JS, Chambers JC, Aguilar-Salinas CA, Hinds DA, Hyde CL, Warnes GR, Gómez Pérez FJ, Frazer KA, Elliott P, Scott J, Milos PM, Cox DR, Thompson JF. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet. 2008;40:149–151. doi: 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- Lam PP, Leung YM, Sheu L, Ellis J, Tsushima RG, Osborne LR, Gaisano HY. Transgenic mouse overexpressing syntaxin-1A as a diabetes model. Diabetes. 2005;54:2744–2754. doi: 10.2337/diabetes.54.9.2744. [DOI] [PubMed] [Google Scholar]
- Lamberta MT, Copeland LA, Sampson N, Duffy SA. New-onset type-2 diabetes associated with atypical antipsychotic medications. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:919–923. doi: 10.1016/j.pnpbp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Lopez-Rangel E, Maurice M, McGillivray B, Friedman JM. Williams syndrome in adults. Am J Med Genet. 1992;44:720–729. doi: 10.1002/ajmg.1320440605. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Merla G, Howald C, Antonarakis SE, Reymond A. The subcellular localization of the ChoRE-binding protein, encoded by the Williams-Beuren syndrome critical region gene 14, is regulated by 14-3-3. Hum Mol Genet. 2004;13:1505–1514. doi: 10.1093/hmg/ddh163. [DOI] [PubMed] [Google Scholar]
- Morris CA, Demsey SA, Leonard CO, Dilts C, Blackburn BL. Natural history of Williams syndrome: Physical characteristics. J Pediatr. 1988;113:318–326. doi: 10.1016/s0022-3476(88)80272-5. [DOI] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, Sornmer A, Moore CA, Hopkin RJ, Spallone PA, Keating MT, Osborne L, Kimberley KW, Stock AD. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: Genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet Part A. 2003;123A:45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: Restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48:2367–2373. doi: 10.2337/diabetes.48.12.2367. [DOI] [PubMed] [Google Scholar]
- Nakaji A, Kawame Y, Nagai C, Iwata M. [Clinical features of a senior patient with Williams syndrome] Rinsho Shinkeigaku. 2001;41:592–598. [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, Matsushima S, Kawakarni H, Watanabe T, Akagawa K, Nagamatsu S. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LR. Williams-Beuren syndrome: Unraveling the mysteries of a microdeletion disorder. Mol Genet Metab. 1999;67:1–10. doi: 10.1006/mgme.1999.2844. [DOI] [PubMed] [Google Scholar]
- Osborne LR, Soder S, Shi XM, Pober B, Costa T, Scherer SW, Tsui LC. Hemizygous deletion of the syntaxin 1A gene in individuals with Williams syndrome. Am J Hum Genet. 1997;61:449–452. doi: 10.1086/514850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples R, Franke Y, Wang YK, Perez-Jurado L, Paperna T, Cisco M, Francke U. A physical map, including a BAC/PAC clone contig, of the Williams-Beuren syndrome—Deletion region at 7q11.23. Am J Hum Genet. 2000;66:47–68. doi: 10.1086/302722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Plissart L, Borghgraef M, Volcke P, Van den Berghe H, Fryns JP. Adults with Williams-Beuren syndrome: Evaluation of the medical, psychological and behavioral aspects. Clin Genet. 1994;46:161–167. doi: 10.1111/j.1399-0004.1994.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Prately RE. Gene-environment interactions in the pathogenesis of type 2 diabetes mellitus: Lessons learned from the Pima Indians. Proc Nutr Soc. 1998;57:175–181. doi: 10.1079/pns19980029. [DOI] [PubMed] [Google Scholar]
- Romeo S, Sentinelli F, Cavallo MG, Leonetti F, Fallarino M, Mariotti S, Baroni MG. Search for genetic variants of the SYN-TAXIN 1A (STX1A) gene: The -352 A >T variant in the STX1A promoter associates with impaired glucose metabolism in an Italian obese population. Int J Obes (Lond) 2008;32:413–420. doi: 10.1038/sj.ijo.0803743. [DOI] [PubMed] [Google Scholar]
- Schuster DP, Osei K, Zipf WB. Characterization of alternations in glucose and insulin metabolism in Prader-Willi subjects. Metabolism. 1996;45:1514–1520. doi: 10.1016/s0026-0495(96)90181-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- Talebizadeh Z, Butler MG. Insulin resistance and obesity-related factors in Prader-Willi syndrome: Comparison with obese subjects. Clin Genet. 2004;67:230–239. doi: 10.1111/j.1399-0004.2004.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Hammond P, Karmiloff-Smith A, Thompson P, Thorgeirsson SS, Durkin ME, Popescu NC, Hutton T, Metcalfe K, Rucka A, Stewart H, Read AP, Maconochie M, Donnai D. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005;310:1184–1187. doi: 10.1126/science.1116142. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Hu FB, Curhan GC. Anti-hypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065–1070. doi: 10.2337/diacare.2951065. [DOI] [PubMed] [Google Scholar]
- Tsunoda K, Sanke T, Nakagawa T, Furuta H, Nanjo K. Single nucleotide polymorphism (D68D, T to C) in the syntaxin 1A gene correlates to age at onset and insulin requirement in Type II diabetic patients. Diabetologia. 2001;44:2092–2097. doi: 10.1007/s001250100015. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta B, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]