Abstract
The ability to encode and retrieve our daily personal experiences, called episodic memory, is supported by the circuitry of the medial temporal lobe (MTL), including the hippocampus, which interacts extensively with a number of specific distributed cortical and subcortical structures. In both animals and humans, evidence from anatomical, neuropsychological, and physiological studies indicates that cortical components of this system have key functions in several aspects of perception and cognition, whereas the MTL structures mediate the organization and persistence of the network of memories whose details are stored in those cortical areas. Structures within the MTL, and particularly the hippocampus, have distinct functions in combining information from multiple cortical streams, supporting our ability to encode and retrieve details of events that compose episodic memories. Conversely, selective damage in the hippocampus, MTL, and other structures of the large-scale memory system, or deterioration of these areas in several diseases and disorders, compromises episodic memory. A growing body of evidence is converging on a functional organization of the cortical, subcortical, and MTL structures that support the fundamental features of episodic memory in humans and animals.
Keywords: medial temporal lobe, hippocampus, episodic memory, neuropsychology, Alzheimer's disease
INTRODUCTION
Memory function is critical to daily life, and includes a variety of specific abilities that, at their core, enable information to be stored and retrieved over variable periods, ranging from seconds to days to years. Furthermore, it is now well recognized that there are multiple forms of memory; these are largely divided into kinds of memory that are typically expressed explicitly—that is, by direct conscious access to information (often called declarative memory)—and kinds of memory that are expressed implicitly through changes in behavioral or physiological responses in the absence of conscious access (nondeclarative memory). Nondeclarative memory and some declarative memory abilities are evolutionarily primitive, albeit elaborated at least to some degree in humans. By contrast, one particular form of declarative memory, called episodic memory, may be evolutionarily recent and its existence in animals is debated (Clayton et al, 2003). Here, we will briefly review these different forms of memory, then focus on the cognitive and neurobiological basis of episodic memory and its disorders.
MULTIPLE FORMS OF MEMORY
The domain of implicit or nondeclarative memory includes several forms of learning that occur during the performance of various tasks, and are typically expressed in enhanced or speeded behavioral performance or a change in behavioral choices or values. For example, procedural memory involves learning a sequence of movements or actions, such as swinging a golf club, riding a bicycle, or driving a car. Procedural learning depends on cortico-striatal systems and is compromised in disorders such as Parkinson's disease and Huntington's disease. Emotional memory involves a change in the approach to or avoidance of earlier neutral stimuli as a result of experience, such as in the acquisition of preference for a particular kind of food or aversion to objects with which one had an unpleasant experience. Emotional memory depends on subcortical and cortical interactions with the amygdala and is altered in panic disorders, phobias, and other neuropsychiatric disorders.
Declarative memory is also subdivided into multiple forms. Working memory involves the short-term maintenance of information in mind, and often the manipulation of that information for the purpose of achieving an immediate goal. The classic example is remembering a phone number while picking up and dialing a phone. However, working memory is also important for comprehending long written or spoken sentences, performing calculations, and holding in mind a string of new information or a series of movements. Performing multiple simultaneous tasks also requires working memory.
There are two forms of long-term declarative memory that both involve retrieving earlier stored information: semantic memory and episodic memory. Semantic memory involves memory for factual knowledge that has been learned, but for which specific ‘time and place' information about the source of the original experience is typically not known. Encyclopedic knowledge of information such as the features of objects (eg, apples are usually red), categories (eg, oranges and bananas are both types of fruit), historical events, mathematical tables, cognitive maps, and similar types of information are considered to be stored in semantic memory systems of the brain.
Episodic memory involves the ability to learn, store, and retrieve information about unique personal experiences that occur in daily life. These memories typically include information about the time and place of an event, as well as detailed information about the event itself. The ability to describe the details of a recent holiday gathering or office meeting that took place in the previous weeks or months, for example, depends heavily on intact episodic memory function.
Studies of patients and then animals with focal brain lesions first illuminated distinctions between these multiple forms of memory and the different systems in the brain that subserve them. A brief historical survey will outline several of the early observations regarding amnesic syndromes and their localization.
THE NEUROLOGY AND NEUROPSYCHOLOGY OF EPISODIC MEMORY CIRCUITS
Traditional views of episodic memory circuits emerged largely from human case studies in neurology, neuropsychiatry, and neuropsychology. Animal research and recent human neuroimaging and memory disorders research have led to iterative revisions of these views, but many of these earlier patient-oriented studies provide an important foundation for understanding memory circuits in the brain.
EARLY CLINICAL CASES OF EPISODIC MEMORY LOSS
Although the experimental study of memory can be traced to Hermann Ebbinghaus, William James, and others in the last two decades of the 19th century, these approaches were neglected in patient studies until the famous studies of Brenda Milner and others starting in the 1950s. Before that, though, there were numerous case reports of memory disorders with enough clinical description and accompanying postmortem examination to illuminate some critical elements of the brain's memory networks.
Theodore Ribot, in The Diseases of Memory (1881), initially called attention to the nature and course of amnesia accompanying the dementias. He pointed out that the first characteristic of amnesia in patients with dementia is the loss of memory for recently experienced events with relative preservation of remote events, an observation later termed ‘Ribot's law' in reference to the temporal gradient commonly observed in retrograde amnesias.
In the late 1880s and 1890s, Russian psychiatrist Sergei Korsakoff described a series of patients, mostly alcoholics, with a neuropsychiatric syndrome prominently involving memory loss. The characteristic amnesia, he observed, involved a disturbance of memory for recent events and new material, commonly with remarkably good memory for events occurring long before the illness, and often accompanied by a lack of insight into amnesia with confabulation. Patients often would repeat themselves in conversation, may not recognize the examiner from one encounter to the next, or might reconstruct an encounter with the examiner in a context that never took place.
Simultaneously with but separately from Korsakoff, German psychiatrist and pathologist Carl Wernicke described an acute clinical syndrome involving gait ataxia, opthalmoparesis, and confusion that sometimes evolved into chronic amnesia. Wernicke's encephalopathy and Korsakoff's amnesia eventually came to be recognized as different manifestations of a similar underlying condition. Wernicke's studies of the brains of his patients led to the observation that brainstem pathology was present in the vicinity of the third ventricle, and subsequent investigations by Gudden, Gamper, and others clarified the involvement of the mammillary bodies and dorsomedial nucleus of the thalamus. However, it was also clear from diminished brain weight that more than just those small regions were damaged, leading to hypotheses about cortical involvement.
Other brain regions now known to be importantly involved in episodic memory had roots in early neurologic literature, including Arnold Pick's (early 1900s) and Kahn and Thompson's observations in the 1930s that frontal lobe degeneration led to difficulties accessing ‘memory material that may be there,' and Yakovlev and Locke's observations on the thalamus and cingulate cortex in the 1960s.
In 1907, German psychiatrist and neuropathologist Alois Alzheimer reported the case of a patient who died at age 51 after progressive memory loss and cognitive change, as well as personality and behavioral decline. Application of new histologic techniques identified plaques and tangles, the hallmark pathologic features of Alzheimer's disease (AD). As these pathological features became scrutinized in further studies, it became clear from Fuller's work in the early 1900s and from Henderson and Machlachlan's work in the 1930s, as well as others, that the hippocampal formation was prominently affected.
Although Brown and Schafer had demonstrated experimentally in the 1880s that monkeys with temporal lobe lesions had impaired memory, it was Russian psychiatrist Vladimir Bekhterev who, in 1900, first reported medial temporal lobe (MTL) (uncinate gyrus and hippocampal) softening in a 40-year-old patient who died after exhibiting a profound amnesia. Fifty years later, a handful of other case reports of patients with amnesic syndromes and hippocampal pathology emerged (eg, Glees and Griffith, 1952).
In 1954, Connecticut neurosurgeon William Scoville reported the profound memory loss that occurred in two patients on whom he had performed the same surgical procedure, in one to treat psychosis and in the other to treat a refractory seizure disorder (Scoville, 1954). The surgical procedure involved the bilateral resection of the rostral MTLs, including the hippocampal formation, amygdala, and overlying cortex. Both patients developed profound amnesia, although because of emotional symptoms in the former patient the memory deficit was most obvious in the latter patient, HM.
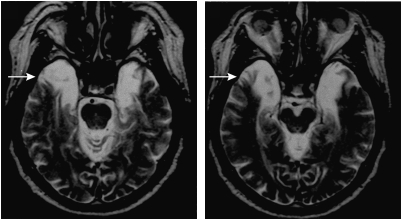
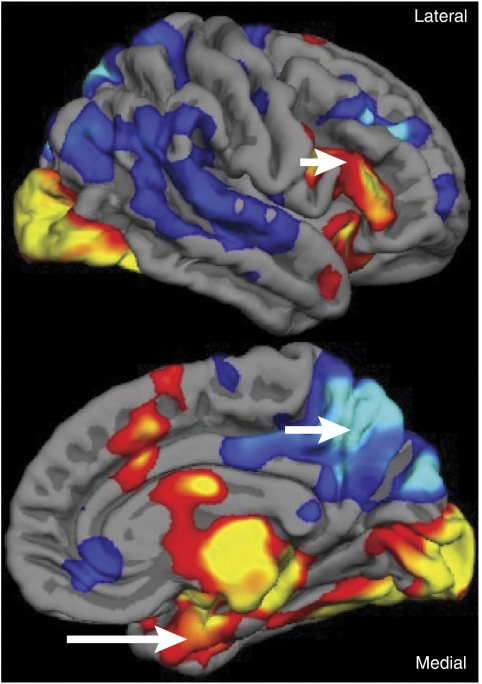
Scoville and Brenda Milner—an English psychologist who had recently completed her training at McGill under Canadian psychologist Donald Hebb—reported in 1957 a series of 10 cases from Scoville's practice of temporal lobe resections (Scoville and Milner, 1957). Of these cases, three were described as having a severe memory deficit—all had undergone bilateral MTL resections including the hippocampal gyrus. They were described as forgetting ‘the incidents of their daily life as fast as they occur.' The striking thing about Case 1, HM, was that his memory deficit was not only profound, including both anterograde and retrograde components with preservation of remote memory, but also isolated in the sense that other aspects of his intellect and personality were spared. Figure 1 shows HM's surgical lesion.
Figure 1.
Two sections from a transverse MRI of HM's brain illustration location of surgical lesion, which involved the excision of the rostral portion of bilateral medial temporal lobes (arrows; areas of bright signal indicate cerebrospinal fluid). Figure courtesy of Professor Suzanne Corkin.
Besides calling prominent attention to the role of the MTL in memory function, it is important to recognize that many critical observations and hypotheses regarding episodic and other forms of memory were generated through studies of a man who became one of the world's most famous neurologic patients, HM, who died on December 4, 2008, at the age of 82 (Squire, 2009). Studies that were initiated in work with him by Brenda Milner, Suzanne Corkin, and many others led to the development of methods for measuring memory dysfunction and to seminal concepts regarding memory as a distinct, separable, localizable ability, and the brain's multiple memory systems, which are summarized extensively elsewhere (Eichenbaum and Cohen, 2001). HM and the other early case studies also provided the initial characterization between forms of declarative memory. Thus, HM, the patients of Bekhterev, those with Korsakoff's amnesia, and patients in the early stage of AD all exhibit severe deficits in episodic memory, that is, greatly diminished ability to remember daily personal events. These findings, and in particular the work with HM that highlighted a critical role for the medial temporal area in episodic memory, provided strong motivation to develop animal models of amnesia that could better identify the critical brain areas within the MTL and the functional circuitry within and outside that area. In the following sections we will review the results of these anatomical studies as well as behavioral and physiological findings from the animal literature, then return to modern studies of the cognitive neuroscience of episodic memory in humans.
NEUROCIRCUITRY OF EPISODIC MEMORY: PERSPECTIVES FROM ANIMAL RESEARCH ANATOMY
Episodic memory is supported by a large network of brain areas, including widespread neocortical association areas and components of the MTL including both the parahippocampal cortical areas and the hippocampus. The anatomical organization of the major pathways of interaction between the neocortex and the medial temporal areas, as well as the organization of medial temporal areas themselves, is also largely conserved across mammalian species from rodents to primates (Amaral and Witter, 1995; Manns and Eichenbaum, 2006; Witter et al, 2000).
The general organization of this system is that virtually all neocortical association areas send projections that converge onto the parahippocampal cortical areas surrounding the hippocampus, which in turn, projects onto each subdivision of the hippocampus. The hippocampal subdivisions are internally connected by a serial, unidirectional path, starting with the dentate gyrus, and continuing through CA3, then CA1, and then the subiculum. The cortical outputs of hippocampal processing, arising in CA1 and subiculum, involve feedback connections back to the parahippocampal region, which projects back to the neocortical association areas from which the inputs to the medial temporal area originated. This organization suggests a central role in organizing or extending the persistence of cortical representations (Eichenbaum, 2000). Neocortical areas contribute to declarative memory through various aspects of cognitive and perceptual processing and memory-associated plasticity in these areas likely constitute the permanent storehouse of declarative memories. The MTL, unlike neocortical areas, has a fully selective function in memory and not other cognitive or perceptual functions. Therefore, we will briefly identify the cortical components of this system across mammalian species then focus on the anatomy and functional organization of the MTL, with particular emphasis on the role of the hippocampus. Figure 2 provides a schematic of the major brain regions described.
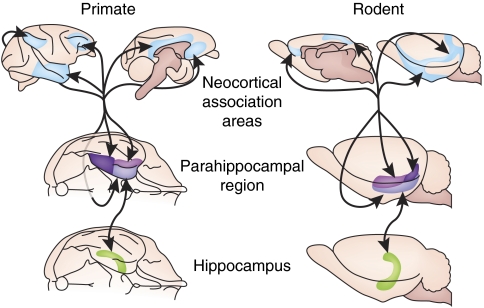
Figure 2.
The anatomy of the medial temporal lobe memory system (Eichenbaum, 2000). In both monkeys and rats the origins of specific information to the hippocampus include virtually every neocortical association area. Each of these neocortical areas (blue) projects to one or more subdivisions of the parahippocampal region, which includes the perirhinal cortex (purple), the parahippocampal (or postrhinal) cortex (dark purple), and the entorhinal cortex (light purple; Burwell et al, 1995; Suzuki, 1996). The subdivisions of the parahippocampal region are interconnected and send major efferents to multiple subdivisions of the hippocampus itself (green), the dentate gyrus, the CA3 and CA1 areas, and the subiculum. Thus, the parahippocampal region serves as a convergence site for cortical input and mediates the distribution of cortical afferents to the hippocampus. Within the hippocampus, there are broadly divergent and convergent connections that could mediate a large network of associations (Amaral and Witter, 1989). The outcomes of hippocampal processing are directed back to the parahippocampal region, and the outputs of that region are directed in turn back to the same areas of the cerebral cortex that were the source of inputs to this region (Burwell et al, 1995; Suzuki, 1996). There are additional structures that have been included as components of this system, including medial diencephalic structures that connect with the hippocampus along with other subcortical areas, through a major fiber bundle called the fornix (Aggleton and Brown, 1999).
Neocortical Areas
The neocortical areas involved in episodic memory include the prefrontal cortex and other areas that mediate working memory, effortful retrieval, source monitoring, and other cognitive processing functions that are essential to conscious recollection (Buckner and Wheeler, 2001; Dobbins et al, 2002; Farovik et al, 2008). In addition, areas of the parietal and temporal cortex are involved in complex perceptual processing essential to contents of information that is the subject of recollection (Uncapher et al, 2006). The architecture and functional organization of neocortical regions involved in episodic memory differ substantially among mammalian species. For example, there are numerous dissimilarities in the neocortex that reflect general differences between small-brained and big-brained mammals, such as cortical size, laminar stratification, and number of polymodal association areas (Krubitzer and Kaas, 2005; Manns and Eichenbaum, 2006). Further, the extent of cortical areas devoted each sensory modality also varies substantially between species. However, despite these substantial distinctions among species, there is remarkable similarity in the pathways and interactions between neocortical association areas and the MTL. Thus, in all mammalian species, projections from all neocortical association areas strongly converge onto the MTL, which also sends strong projections back to the same cortical association areas.
Parahippocampal Region
Outputs of neocortical association areas are funneled into the hippocampus through the parahippocampal region, an interconnected set of medial temporal cortical areas surrounding the hippocampus. These areas include the perirhinal cortex, the parahippocampal cortex (called postrhinal cortex in rodents), and the entorhinal cortex, and the cytoarchitecture and the interconnectivity among these areas is also remarkably similar across mammalian species (Burwell et al, 1995). In addition, parallel pathways arise in the perirhinal and parahippocampal cortices and terminate in the hippocampus similarly through two partially distinct channels (Burwell and Amaral, 1998a; Suzuki and Amaral, 1994). The perirhinal cortex receives inputs from areas concerned with identifying the nonspatial identity of stimuli, whereas the parahippocampal cortex receives inputs from many areas involved in processing the spatial content of sensory information. For example, the monkey perirhinal cortex receives more inputs from areas along the ventral visual pathway thought to be important for object recognition, the parahippocampal cortex receives more inputs from areas along the dorsal visual stream thought to be important for visually guided actions (Suzuki and Amaral, 1994). In rodents, the visual system is not clearly segregated into ventral and dorsal visual streams, yet the perirhinal and postrhinal cortices receive disproportionate nonspatial and spatial information, respectively (Burwell and Amaral, 1998b). For example, the rodent perirhinal cortex receives prominent inputs from the polymodal ventral temporal association area (TEv). In contrast, the postrhinal cortex instead receives prominent spatial inputs from areas such as posterior parietal cortex. The separation of spatial and nonspatial information is partially maintained within the parahippocampal region until it is combined in the hippocampus. In both the rat and the monkey, the perirhinal cortex tends to project more to the lateral entorhinal area (LEA) of the entorhinal cortex, and the parahippocampal cortex tends to project more to the medial entorhinal area (MEA, Witter et al, 2000).
Hippocampus
LEA and MEA send separate projections to the hippocampus. Both pathways project to each of the four hippocampal subregions, but the pattern in which the fibers from both pathways terminate in hippocampal targets differs between those arriving in CA3 and dentate gyrus and those in CA1 and subiculum, such that information passing through LEA and MEA is combined on the same neurons in the dentate gyrus and CA3 but arrives in separate neuronal populations in the subiculum and CA1, an pattern of organization that may support the ability of the hippocampus to both associate and distinguish events and the context in which they appear (Witter et al, 2000).
This anatomical data provide potentially important insights into basic mechanisms that underlie the capacity for episodic memory. Thus, across species, most of the neocortical input to the perirhinal cortex comes from association areas that process unimodal sensory information about qualities of objects (ie, ‘what' information), whereas most of the neocortical input to the parahippocampal cortex comes from areas that process polymodal spatial (‘where') information (Burwell et al, 1995; Suzuki and Amaral, 1994). There are connections between the perirhinal cortex and parahippocampal cortex, but the ‘what' and ‘where' streams of processing remain largely segregated as the perirhinal cortex projects primarily to the LEA whereas the parahippocampal cortex projects mainly to the MEA. Similarly, there are some connections between the entorhinal areas, but the ‘what' and ‘where' information streams mainly converge within the hippocampus. These anatomical considerations suggest a functional organization of the flow of information into and out of the hippocampus, which we will outline next.
EXPERIMENTAL MODELS OF THE FUNCTIONAL ORGANIZATION OF THE EPISODIC MEMORY SYSTEM
The anatomical evidence just described suggests functional distinctions between medial temporal regions that are confirmed by substantial evidence from studies on the effects of selective damage to these areas and by characterizations of the firing properties of neurons in these areas.
Perirhinal and Lateral Entorhinal Cortex
Substantial evidence from studies on animals indicates that neurons in the perirhinal cortex and lateral entorhinal cortex are involved in the representation of and memory for individual perceptual stimuli. Electrophysiological studies on monkeys and rats performing simple recognition tasks have shown that many cells in the perirhinal cortex exhibit enhanced or suppressed responses to stimuli when they re-appear in a recognition test (Suzuki and Eichenbaum, 2000). Complementary studies in animals with damage to the perirhinal cortex indicate that this area may be critical to memory for individual stimuli in the delayed nonmatching to sample task in rats (Otto and Eichenbaum, 1992) and monkeys (Suzuki et al, 1993). These and other data have led several investigators to the view that the perirhinal cortex is specialized for identifying the strength of memories for individual stimuli (Brown and Aggleton, 2001).
Parahippocampal and Medial Entorhinal Cortex
The parahippocampal cortex and MEA may be specialized for processing spatial context. Although perirhinal and lateral entorhinal neurons have poor spatial coding properties, parahippocampal and medial entorhinal neurons show strong spatial coding (Hargreaves et al, 2005). Correspondingly, though object recognition is impaired after perirhinal damage, object-location recognition is deficient after parahippocampal cortex damage in rats (Gaffan et al, 2004) and monkeys (Alvarado and Bachevalier, 2005). Similarly, perirhinal cortex damage results in greater impairment in memory for object pairings whereas parahippocampal cortex lesions result in greater impairment in memory for the context in which an object was presented (Alvarado and Bachevalier, 2005).
Hippocampus
Recent studies using sophisticated signal detection analysis have shown that, as in humans, in rodents recognition memory is supported by a combination of familiarity for previously experienced stimuli and recollection of specific episodes involving those stimuli, and the recollective component of recognition memory depends on the hippocampus (Fortin et al, 2004; Sauvage et al, 2008). In addition, corresponding to the commonly held view that episodic recollection involves memory for the spatial and temporal context of specific experiences, several investigators have argued that animals are indeed capable of remembering the context in which they experienced specific stimuli, and that this capacity also depends on the hippocampus (Clayton and Dickinson, 1998; Day et al, 2003). For example, Ergorul and Eichenbaum (2004) developed a task that assesses memory for events that involve the combination of an odor (‘what'), the place in which it was experienced (‘where'), and the order in which the presentations occurred (‘when'). On each of a series of events, rats sampled an odor in a unique place along the periphery of a large open field. Then, memory for when those events occurred was tested by presenting a choice between an arbitrarily selected pair of the odor cups in their original locations. Analyses of the data on these choices plus other probe tests showed that rats normally use a combination of ‘where' and ‘what' information to judge ‘when' the events occurred whereas rats with hippocampal damage cannot effectively combine ‘what', ‘when', and ‘where' qualities of each experience to compose the retrieved memory. Figure 3 provides an illustration of this model.
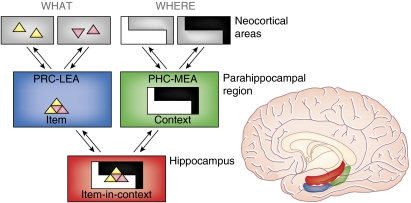
Figure 3.
A proposed functional organization of the medial temporal lobe memory system (Eichenbaum et al, 2007). Neocortical input regarding the object features (‘what') converges in the perirhinal cortex (PRC) and lateral entorhinal area (LEA), whereas details about the location (‘where') of objects converge in the parahippocampal cortex (PHC) and medial entorhinal area (MEA). These streams converge in the hippocampus, which represents items in the context in which they were experienced. Reverse projections follow the same pathways back to the parahippocampal and neocortical regions. Back projections to the PHC–MEA may support recall or context, whereas back projections to the PHC–LEA may support recall of item associations.
Consistent with these findings, many studies have shown that hippocampal neurons encode many features of events and the places where they occur (Eichenbaum, 2004). For example, in one study, rats performed a task in which they had to recognize any of nine olfactory cues placed in any of nine locations (Wood et al, 1999). As the location of the discriminative stimuli was varied systematically, cellular activity related to the stimuli and behavior could be dissociated from that related to the animal's location. Some hippocampal cells encoded particular odor stimuli, others were activated when the rat sampled any odor at a particular place, and yet others fired associated with whether the odor matched or differed from the earlier cue. However, the largest subset of hippocampal neurons fired only associated with a particular combination of the odor, the place where it was sampled, and the match–nonmatch status of the odor. Another study examined the firing properties of hippocampal neurons in monkeys performing a task in which they rapidly learned new scene–location associations (Wirth et al, 2003). Just as the monkeys acquired a new response to a location in the scene, neurons in the hippocampus changed their firing patterns to become selective to particular scenes.
The combination of findings from the anatomy and functional characterizations in animal models are consistent with the anatomically guided hypothesis about the functional organization of the hippocampal system and suggest mechanisms by which the anatomical components of this system interact in support of the phenomenology of episodic recollection. After experience with a stimulus, the perirhinal and LEAs may match a memory cue to a stored template of the stimulus, reflected in suppressed activation that may signal the familiarity of previously experienced stimuli but does not provide information about where or when it was experienced. Outputs from perirhinal and LEAs back to neocortical areas may be sufficient to generate the sense of familiarity without participation of the hippocampus. In addition, during the initial experience, information about the to-be-remembered stimulus, processed by the perirhinal and LEAs, and about the spatial and possibly nonspatial context of the stimulus, is processed by the parahippocampal and MEAs, converge in the hippocampus. During subsequent retrieval, presentation of the cue may drive the recovery of object-context representations in the hippocampus that, through back projections, regenerates a representation of the contextual associations in parahippocampal and MEAs, which cascades that information back to neocortical areas that originally processed the item and contextual information. This processing pathway may constitute a principal mechanism for episodic recollection of unique events across species (Eichenbaum et al, 2007). Notably, there are also direct connections between perirhinal and parahippocampal cortex and between zones of the entorhinal cortex (Burwell et al, 1995; Suzuki and Amaral, 1994). One possibility is that these connections are strengthened over time after learning through reactivations that involve loops through the hippocampus, and these connections within cortical areas might support a gradual consolidation of memories in those cortical areas (Eichenbaum et al, 1999); this mechanism could explain why retrograde amnesia reaches farther back in time when damage to the MTL includes cortical areas in addition to the hippocampus (Rempel-Clower et al, 1996).
As described below, studies on humans have shown that a specific set of neocortical areas beyond the MTL also has important functions in episodic memory. Studies of the role in episodic memory of neocortical areas are far less developed in animals. However, recent evidence suggests that at least some cortical areas may also have a critical function in episodic memory in animals. In one such study, rats with damage to the prefrontal cortex were tested on recognition memory using signal detection analysis methods (Farovik et al, 2008). The results showed that damage to the prefrontal cortex results in a selective deficit in recollection with spared familiarity, similar to the effects of damage to the hippocampus. However, the detailed pattern of performance characterized in the signal detection analyses showed that the nature of the recollection impairment after prefrontal damage was different from that after hippocampal damage. Specifically, damage to the hippocampus resulted in forgetting of items previously seen in the study phase, whereas damage to the prefrontal cortex resulted in false-positive responses to items that were not seen in the study phase but were experienced in prior study lists. Thus, as in humans, the prefrontal cortex may have a selective function in episodic memory by monitoring and selecting retrieved memories.
AGING
Cognitive aging involves the deterioration of higher order intellectual capacities associated with deterioration of brain function. In humans, cognitive aging most prominently involves a decline in episodic memory, similar to what one might expect of a mild form of damage to the MTL area. The development of animal models of cognitive aging are based on studies of functions of the MTL structures and, in particular, the hippocampus. These studies have indeed shown that aged rats are impaired on tests of hippocampal spatial memory function, and that this cognitive impairment can be related to deterioration of neurobiologial markers of function within the hippocampus (Gallagher and Rapp, 1997; Wilson et al, 2006).
In addition, a recent study has have offered an approach to specifically assess episodic recollection in aging animals, using a signal detection analysis of recognition memory that has distinguished age-associated deficits in episodic recollection vs relatively intact familiarity in humans (Robitsek et al, 2008). This study used a combination of behavioral assessments in the Morris water maze task and signal detection analyses of recognition memory to show several parallels between cognitive aging in rodents and humans that strengthen the animal model. First, aged rats showed a considerable variability in the severity of their spatial and recognition memory impairments. Second, the age-associated deficit in recognition memory was isolated to impairment in episodic recollection and not familiarity. Third, some aged rats compensated for the deficit in episodic recollection by using a compensatory strategy of using intact familiarity as a basis for recognition. All of these findings support the hypothesis that cognitive aging has a common basis across species, providing a valid platform for exploring interventions that might alleviate age-associated memory disorder.
Additional neurophysiological studies on the firing patterns of hippocampal neurons suggest a network information processing failure that may underlie the deficit in recollection. These studies focus on hippocampal ‘place cells', principal neurons in the hippocampus, which fire reliably when a rat is in a particular location in a familiar environment. When young rats subsequently explore a novel environment, the patterns of spatial firing of individual hippocampal neurons typically change, consistent with a distinct representation for the novel experience. However, place cells in aged rats often fail to change when the animal is exposed to a novel environment and instead often show the same spatial firing pattern as they did in the familiar environment, and this rigidity of spatial representation predicts the spatial memory impairment in the water maze (Wilson et al, 2006). This abnormality has been characterized as a failure in pattern separation of the contextual stimuli from the familiar and novel environments and a consequent tendency toward pattern completion of the representation of the familiar spatial context. Notably, this abnormality was selective to area CA3, the subfield of the hippocampus, which normally is inclined to strong pattern completion or separation (known as attractor dynamics) (Guzowski et al, 2004). One interpretation of these findings is that such a failure in pattern separation could also underlie the deficit in the episodic recollection component of recognition memory. Thus, failure to pattern separate distinct experiences would be expected to compromise the animal's ability to distinguish specific experiences with stimuli in the current list from that of prior appearances of the same stimuli in earlier lists. The result would be a catastrophic pattern of interference for the hippocampus and a consequent inability to recollect the experience of being presented with a specific item in this study list. This argument is speculative, but is consistent with the common observation of inappropriate intrusions of old memories associated with cognitive aging (Kahana et al, 2005). The extension of the rodent model into aspects of odor recognition memory allows for further examination of this and other hypotheses about the neurobiological bases of cognitive aging.
NEUROCIRCUITRY OF EPISODIC MEMORY: PERSPECTIVES FROM HUMAN NEUROIMAGING
The rapid development of neuroimaging techniques over the past couple decades has led to an explosion of research on episodic memory in humans. As many of these techniques are relatively noninvasive and widely available, investigators have been able to combine advanced anatomic and functional neuroimaging and other brain mapping methods with sophisticated experimental cognitive paradigms. Some of this work has confirmed and extended knowledge derived from animal research of the organization and roles of nodes of episodic memory neurocircuitry, whereas other work has probed memory-related abilities that are difficult to study in animals or thought to be uniquely human. In this section, human functional neuroimaging will be reviewed with a focus on the MTL memory system, the isocortical memory system, and recent work attempting to explicitly investigate large-scale episodic memory networks. Relatively little functional neuroimaging work (with important exceptions, Shohamy and Wagner, 2008) has been performed on the contributions of thalamic, basal forebrain, and other subcortical regions to human episodic memory.
The Human Medial Temporal Lobe Episodic Memory System
Since the 1990s, positron emission tomography and functional magnetic resonance imaging (fMRI) experiments have demonstrated the ‘activation' of the hippocampal formation and other MTL regions during the performance of episodic memory tasks. It is important to point out that the activation of a brain region as detected with functional neuroimaging methods is relative, as functional neuroimaging measures obtained during task performance are typically compared with measures obtained during a control condition, thus identifying relatively greater activity in one condition than another. Furthermore, co-registration techniques that are usually required to align data from multiple subjects to each other are imperfect but continue to be improved (Miller et al, 2005). From a conceptual standpoint, given the variability of MTL subregion size and shape between subjects, some investigators prefer to localize fMRI data at the individual subject level first and then perform statistical analyses, rather than trying to localize activity at the group level after aligning subjects to a template.
An important technical and experimental design development in the late 1990s, ‘event-related' fMRI (drawn from event-related potential electroencephalographic methodology), enabled the detection of relatively greater hippocampal and other MTL activation during the encoding of stimuli that were subsequently remembered as compared with those that were not remembered (Burock et al, 1998). In the decade since the first papers showing relatively greater MTL activity during subsequently remembered items (Brewer et al, 1998; Wagner et al, 1998b), many clever experiments have built on this approach to elucidate specific functional neuroanatomic properties of MTL and other brain regions. Figure 4 highlights the data supporting these observations.
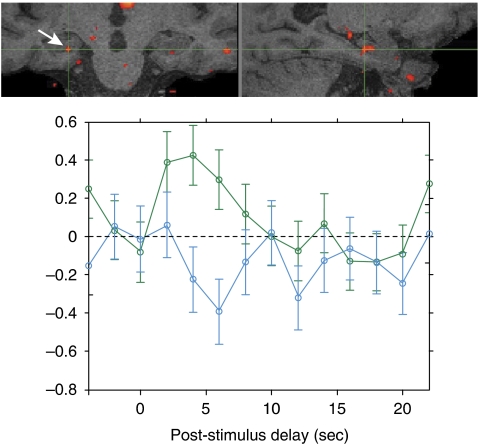
Figure 4.
Functional MRI of 70-year-old individual during the encoding of new face-name pairs. Brain image shows localization of activation, which includes hippocampus (arrow and crosshair) and other medial temporal lobe regions. Crosshair shows localization of the area from which time course of blood-oxygen-level-dependent activity is derived, showing the signal for items that are subsequently correctly recognized (green, hits) vs items that are not correctly recognized (blue, misses).
One issue that has been investigated since the early days of functional neuroimaging is the differential activation of the MTL during encoding as opposed to retrieval. Partly because of the large number of variables that often differ between experiments, there is still not consensus regarding whether there is a specific topographic organization in the MTL that differentiates the functional neuroanatomy of encoding or retrieval. Some investigators find that the rostral hippocampal formation is relatively more active during encoding whereas the caudal hippocampus is relatively more active during retrieval (Zeineh et al, 2003), but the opposite findings have also been observed (Gabrieli et al, 1997). Within the canonical MTL subregions, some investigators have found encoding-related activity in hippocampus-proper CA fields with retrieval-related activity in subiculum (Zeineh et al, 2003), whereas others have found a pattern that differs (Pihlajamaki et al, 2003).
A major challenge when trying to connect human with animal data regarding the MTL is the gap in resolution. The human functional neuroimaging technique with the highest spatial resolution, fMRI, is still striving to obtain resolution for differential effects on the order of 1 mm, with most studies at advanced centers still working in the 1.5–2 mm range. Furthermore, the signal-to-noise ratio of blood-oxygen-level-dependent (BOLD) fMRI in the MTL is notoriously poor. Technical advances being worked out at some centers are still badly needed for further advances that would bring human MTL imaging closer to animal data.
Questions related to the specificity of MTL regions for memory processes have been the subject of a number of studies, including associative vs item-based memory and the dual process model of episodic memory (recollection vs familiarity). Some evidence supports the concept that the hippocampal formation is selectively activated for recollection or for associative memories, and the perirhinal or entorhinal cortex is activated for familiarity or for item-based nonassociative memories (Davachi et al, 2003; Eichenbaum et al, 2007; Squire et al, 2004). However, other studies of these same topics have produced conflicting results (Squire et al, 2007).
The specificity of MTL regions for the particular content of episodic memory has received some attention. Successful memory for faces tends to be associated with activity in perirhinal and rostral hippocampal regions, whereas objects tend to be associated with somewhat more caudal activity (Preston et al, 2009). Memory for indoor or outdoor scenes or other spatial context information is fairly reliably associated with posterior parahippocampal activity (Maguire, 1997; Stern et al, 1996). Surprisingly, little work has been performed on object-in-context associative memory performance, although one study distinguished activation of the hippocampus and posterior parahippocampal region associated with memory for objects in imagined contextual scenes from activation of the perirhinal cortex associated with memory for the objects alone (Davachi et al, 2003).
Neuroimaging investigations have recently turned to the remarkably fine-grained ability of components of the MTL memory system to form associations even in the absence of complete information, while at the same time distinguishing related but distinct objects or contexts from each other—pattern completion vs pattern separation, as described above. Similarly to rodent studies, initial experiments suggest that CA3 or dentate gyrus has an important function in pattern separation within the hippocampal formation (Bakker et al, 2008).
Recent neuroimaging research has begun to provide evidence convergent with animal data that the MTL may have specific functions in the flexible use of episodic memory trace information for processes that probably subserve inferential reasoning and generalization. Transitive inference, the ability to use previously learned associative relationships between items to draw inferences about probable associations that have never been specifically learned, seems to depend on the hippocampal formation (Dusek and Eichenbaum, 1997). Functional neuroimaging evidence also supports the specific role of the hippocampal formation in transitive inference in humans (Heckers et al, 2004).
The Human Isocortical Episodic Memory System
Although tremendous attention has been focused in human functional neuroimaging episodic memory experiments on the MTL memory system, the fundamental involvement of isocortical brain regions has been obvious since the first studies. It was initially not clear the extent to which ventrolateral temporal, medial and lateral parietal, and medial and lateral frontal regions supported activities necessary but not sufficient for the performance of encoding and retrieval tasks, including perceptual, lexical, semantic, attentional, control, or decision making activities. Many of these isocortical regions were commonly activated in memory imaging experiments. With the advent of subsequent memory paradigms, it became clear that a number of these areas seem to be active contributors to the process of learning and memory itself, as they are relatively more active during successful encoding and retrieval than during unsuccessful attempts. Human cortical regions engaged during memory encoding are shown in Figure 5.
Figure 5.
Functional MRI of a group of young human subjects during the encoding of novel pictures that are subsequently recalled, showing cortical regions involved in the large-scale episodic memory network and related areas. Red/yellow regions are activated (including ventrolateral prefrontal cortex (top image, arrow) and medial temporal cortex (bottom image, long arrow)) during the encoding of new pictures of objects, whereas blue regions are deactivated (including posterior cingulate/precuneus (bottom image, short arrow) below baseline.
Regions in the temporal lobe, which are outside the MTL, can be functionally engaged in subsequent memory paradigms. Although some of these activations may reflect material-specific or process-specific aspects of stimulus perception, reports of subsequent memory effects in some regions suggest that there may be specific roles in episodic memory. The ventral temporal cortex, including fusiform gyrus, is commonly engaged when pictures of visual objects are presented, and the lateral temporal cortex including superior temporal gyrus is typically engaged during the encoding of auditory information. Furthermore, consistent with hypotheses regarding re-activation of sensory cortex during memory retrieval, imaging experiments have demonstrated that subregions within sensory-specific temporal isocortical brain regions, which are engaged during encoding, are re-engaged during retrieval of sensory-specific information (Wheeler et al, 2000). In addition, the degree to which subjects report that they use strategies emphasizing visual features or visual relationships among items (as opposed to verbal strategies) to perform a memory task relates to the level of engagement of visual regions in the occipitotemporal cortex during encoding (Kirchhoff and Buckner, 2006).
Although early patient studies led some clinicians to posit that frontal lesions impair memory performance purely in a secondary fashion through effects on attention, organization, and motivation (Hecaen and Albert, 1978; Luria, 1973), others have emphasized the primacy of the frontal lobes for memory, including delayed response, associative learning, and memory for temporal order (Milner, 1971; Milner et al, 1985; Petrides, 1985; Stuss et al, 1994). More recent clinical neuropsychological studies of memory function have shown multiple dissociations in memory impairment in patients with frontal lesions compared with MTL amnesics, including temporal order and source memory (Janowsky et al, 1989; Shimamura et al, 1990). Neuroimaging data have largely provided support for ideas initially developed in work with patients, indicating that frontal systems are important for the spontaneous use of strategies during encoding, the tagging of events with temporal and other contextual information, and search, monitoring, and other controlled processes during retrieval (Davidson et al, 2006).
The ventrolateral prefrontal cortex is uniformly involved in the encoding of information, with particular contribution of the left ventrolateral prefrontal cortex for semantic processing of information being encoded (Fletcher and Henson, 2001). The prominent leftward lateralization of encoding processes led to the generation of the ‘HERA' model of memory, or ‘hemispheric encoding/retrieval asymmetry' (Habib et al, 2003; Tulving et al, 1994a), which puts forth a process-specific outline accounting for the common findings of leftward activation during encoding and rightward activation during retrieval. Other accounts emphasize content-specific aspects of prefrontal function and memory, with left-lateralized ventrolateral activity for verbal material and right-lateralized activity for visual material (Wagner et al, 1998a).
Several prefrontal regions in both hemispheres have been shown in neuroimaging experiments to make distinct contributions to episodic memory retrieval. It is important to consider the variety of cognitive processes that have been proposed as relevant for retrieval (Rugg et al, 2002; Rugg and Wilding, 2000). First, preretrieval processing is thought to support the attempt to retrieve information in response to a cue, and theoretically including retrieval mode (the cognitive state or set subserving attempted retrieval), retrieval effort (level of difficulty involved in the task), and retrieval orientation (qualitatively different forms of processing of retrieval cues, such as recognition vs source judgements). Neuroimaging studies manipulating retrieval effort and orientation generally show modulation of anterior and ventrolateral regions of the left prefrontal cortex, whereas studies of retrieval mode have suggested the involvement of frontopolar cortex, possibly in a right-lateralized manner.
Postretrieval processing operates on the product of the retrieval attempt, and involves the monitoring and evaluation of retrieved information, ultimately resulting in a retrieval decision (Stevens and Grady, 2007). Right dorsolateral prefrontal cortex seems to be consistently involved in postretrieval processing. Finally, recently developed event-related fMRI experimental designs have begun to illuminate differential transient vs sustained contributions to memory retrieval, with evidence supporting the involvement of the right frontopolar cortex in retrieval mode and left dorsolateral prefrontal cortex in individual retrieval attempts (Velanova et al, 2003).
The roles of the posterior lateral and medial parietal cortices in episodic memory have received greater attention partly as a result of functional neuroimaging studies, which have consistently shown a variety of memory-related effects in these regions (Wagner et al, 2005). In the medial parietal regions, including posterior cingulate, precuneus, and retrosplenial cortex, and in the left lateral posterior parietal cortex, including inferior parietal lobule and to a lesser extent superior parietal lobule, there is a consistent increase in activity during the recognition of old, previously encountered items as compared with new items (Tulving et al, 1994b). This ‘old–new' effect is also present for items falsely thought to be old (Kahn et al, 2004; Wheeler and Buckner, 2003), presumably reflecting the subjective experience of having previously encountered an object. Recent functional neuroimaging data are beginning to indicate that there may be functionally distinct subregions within the lateral parietal cortex, including possibly a region within the intraparietal sulcus that may reflect familiarity effects and an inferior parietal lobule region that may reflect detailed recollection (Wagner et al, 2005). Although it is clear that there are important primary and secondary afferent and efferent connections between parietal cortical regions and the MTL (Kobayashi and Amaral, 2003, 2007; Mesulam et al, 1977; Van Hoesen et al, 1972), increasing experimental attention is being devoted to the specific functional roles of parietal cortices in episodic memory and frontoparietal and parietotemporal interactions.
The Large-Scale Distributed Episodic Memory Network
Lesion and neuroimaging data are often focused on the contributions of one or more specific regions to task performance, although it is widely acknowledged that most abilities depend on large-scaled distributed brain networks (Mesulam, 1998). Prefrontal–temporal interactions have been a particularly attractive starting point for theoretical work in this area on episodic memory (Simons and Spiers, 2003). Although lesion analysis studies in monkeys and humans and other data have been used to develop hypotheses about large-scale distributed episodic memory networks, human neuroimaging data inherently provide fruitful data for analysis at the systems level. Besides the co-activation of brain regions during the performance of episodic encoding and retrieval tasks, as described above, new systems-level analytic approaches to functional neuroimaging data have begun to show the interactions between brain regions that make up the large-scale episodic memory network.
So-called functional connectivity neuroimaging data analytic approaches have existed for nearly a decade, and have been applied in a few studies to questions related to the interaction between brain regions engaged in memory tasks. These approaches enable the detection of correlated activity increases between spatially distributed brain regions, and causal modeling using activity increases in one region to predict activity increases in another. These types of analyses have provided evidence for prefrontal–medial temporal–ventrolateral temporal interactions in episodic encoding that leads to successful subsequent free recall, for example (Dickerson et al, 2007).
A prominent line of functional neuroimaging research has identified a set of brain regions that are relatively more active during undirected epochs of time during experiments than during epochs involving the directed performance of a variety of tasks, including sensorimotor, attentional, language, and other types of tasks. These brain regions include posterior cingulate/precuneus, lateral inferior parietal lobule, lateral and medial temporal, and medial prefrontal cortices, and have been called the ‘default mode' network (Buckner et al, 2008; Raichle et al, 2001). Many of these brain regions are activated during various processes involved in specific memory encoding or retrieval tasks, as described above using data from task-related functional neuroimaging experiments. This observation has led to the hypothesis that one activity that may be prominently occurring during these ‘rest' epochs is thinking about the past or future, or ‘mental time travel,' which engages episodic memory systems of the brain.
The convergence of thinking about functional brain systems and observations derived from studies of undirected ‘rest' epochs of experiments has led to an explosion of studies using ‘resting-state' functional connectivity MRI (fc-MRI), which is an exciting new neuroimaging technique for investigating large-scale functional-anatomic systems. Spontaneous fluctuations in BOLD signal can be readily detected in subjects whether or not a task is being performed. The degree to which these spontaneous fluctuations are correlated between different brain regions can be interrogated using a systems perspective. The initial experiments in this field have validated findings using this technique against findings using the same technique in anesthetized monkeys, and have demonstrated that functional brain networks identified using fc-MRI correspond to networks identified using traditional tract tracing methods (Vincent et al, 2007). These techniques have already been used to show the presence of a hippocampal–parietal network (Vincent et al, 2006), and the distinction between subnetworks within the MTL memory system, including the rostral hippocampal–perirhinal network vs the caudal hippocampal—parahippocampal–retrosplenial network (Kahn et al, 2008). This approach will clearly provide valuable novel contributions to knowledge about human memory networks in the brain, and will likely help provide further links between human and animal data. Figure 6 illustrates the spatial localization of these brain regions.
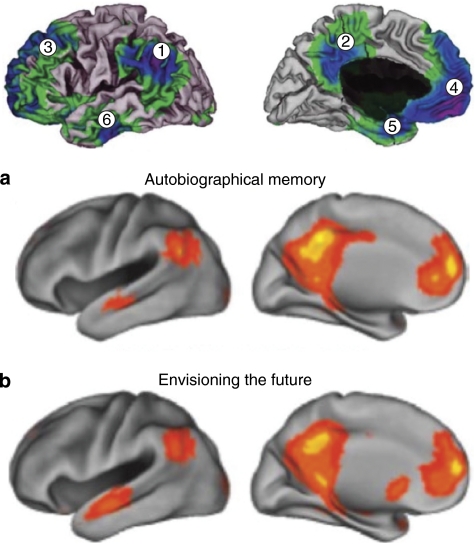
Figure 6.
Cortical areas of the ‘default mode' network, which is a set of brain regions that are deactivated below baseline level during the performance of most types of tasks (top, including inferolateral parietal (1), posterior cingulate/precuneus (2), dorsolateral prefrontal (3), medial prefrontal (4), medial temporal (5), and rostrolateral temporal (6)). These same regions are activated above baseline during the retrieval of autobiographical memories (a) and during the envisioning of specific potential future events (b).
HUMAN MEMORY DISORDERS
A host of disorders can lead to amnesic syndromes in humans, including prominent deficits in episodic memory. Systematic studies of syndromes in which amnesia is the core symptom can provide valuable insights into the functional neuroanatomy and neuropsychology of human memory function. New insights into a few of these syndromes are highlighted here. Some neuropsychiatric disorders, including depression (Drevets et al, 2008 # 4760; Neumeister et al, 2005 # 4773), schizophrenia (Drevets et al, 2008; Neumeister et al, 2005), and posttraumatic stress disorder (Shin et al, 2004), seem to affect memory systems (particularly the MTL) in important ways but the core clinical phenotype involves affective-cognitive dysfunction beyond episodic memory, so they will not be reviewed here.
As many investigative groups tend to focus on one or a few of these disorders, the methods used to study these various forms of human amnesia have often been heterogeneous, hindering the development of generalizable conclusions across etiologies of amnesia. It would be beneficial for investigators to consider harmonizing, as best as possible, methods between human and animal studies, as well as between human cognitive neuroscience and patient-oriented neuropsychological studies of human amnesias of different etiologies.
Alzheimer's Disease
AD is the most common clinical amnesic syndrome, although it is important to keep in mind that by definition its diagnosis involves the presence of more than pure memory loss—the dementia of AD is a multidomain disorder, typically including executive dysfunction and varying degrees of visuospatial and language deficits. The prodromal phase of AD before dementia, which may last for a decade or more, is referred to as mild cognitive impairment (MCI), the prototypical form of which is amnesic. Amnesic MCI has received extensive investigative attention in the last decade (Frank and Petersen, 2008), with recent efforts illuminating functional and structural abnormalities in the MTL (Dickerson and Sperling, 2008).
The anatomy of AD not only involves prominent MTL pathology very early in the course of the disease (Gomez-Isla et al, 1996), but also pathologic involvement of lateral temporoparietal and medial parietal cortex, as well as a lesser (and more variable) degree of pathology in lateral and medial prefrontal cortex. Although the involvement of these non-MTL cortical regions has been long known from studies of postmortem tissue (Arnold et al, 1991; Tomlinson et al, 1970), their early involvement has been clarified with modern in vivo neuroimaging studies (Buckner et al, 2005; Dickerson et al, 2009; Klunk et al, 2004). Figure 7 shows MTL atrophy in a patient with mild AD.
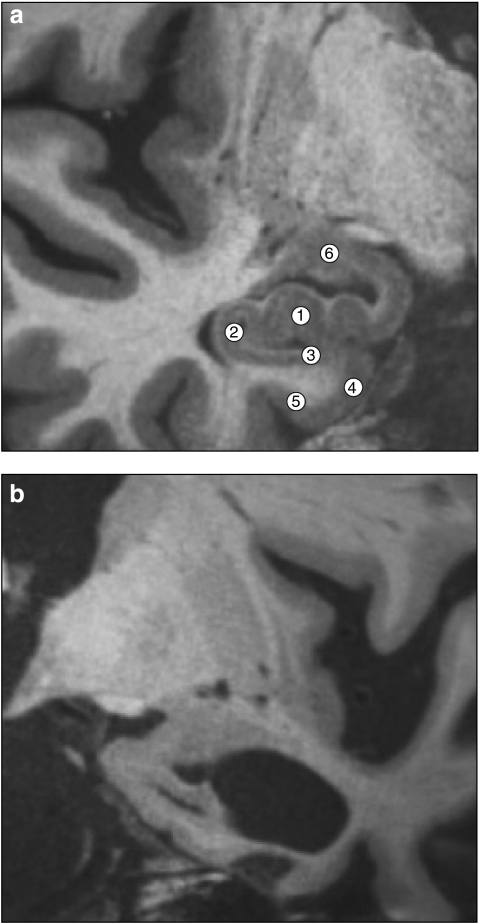
Figure 7.
Ultrahigh-resolution (380 μm in-plane voxel size) structural MRI images of the human medial temporal lobe in a 24-year-old neurologically intact individual (a) and in a 72-year-old patient with mild Alzheimer's disease (b). In the young individual, a variety of MTL subregions can be seen, including CA3/dentate gyrus (1), CA1 (2), subiculum (3), entorhinal cortex (4), perirhinal cortex (5), and amygdala (6). Hippocampal formation and other medial temporal lobe structures are atrophic in Alzheimer patient.
Structural neuroimaging has shown the atrophy of regions within the MTL memory system in AD (Jack et al, 1997), as well as cortical regions that include important hubs of the episodic memory system (Dickerson and Sperling, 2008). Figure 8 highlights cortical regions that undergo atrophy in AD. The degree of atrophy of some of these regions relates to the level of specific types of memory impairment in AD (de Toledo-Morrell et al, 2000). Beyond structural measures of regional brain atrophy, functional neuroimaging has shown that dysfunction of these regions is present in patients with AD and that the level of dysfunction relates to the severity of memory impairment (Chetelat et al, 2003; De Santi et al, 2001; Mosconi et al, 2008). Recently, revolutionary new imaging technology using molecular ligands that bind to pathologic protein forms that accumulate in the AD brain is illuminating the localization and severity of pathology in various brain regions in living patients (Klunk et al, 2004; Small et al, 2006). Investigators have begun to combine these various imaging modalities to highlight the important observation that the molecular pathology of AD is localized in and is associated with dysfunction and atrophy of brain areas that include the episodic memory network (Buckner et al, 2005; Mormino et al, 2009). Further work using these techniques promises to build important bridges spanning the gap between postmortem histology and in vivo imaging measures of brain-behavior changes in patients with AD.
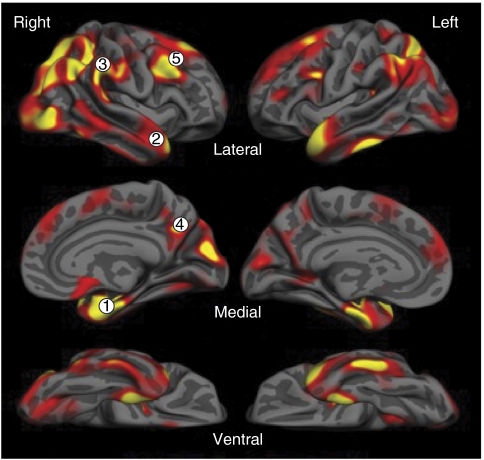
Figure 8.
The cortical signature of regional thinning in Alzheimer's disease. Brain regions highlighted in red/yellow are thinner than age-matched cognitively intact controls in mild AD. The episodic memory network is prominently affected (including the medial temporal lobe (1), parts of the lateral parietal cortex (3), and posterior cingulate/precuneus (4)), as are nodes of several other networks (including the parts of the lateral parietal cortex (3), temporal pole (2), and dorsolateral prefrontal cortex (5)) subserving cognitive and behavioral function with relative sparing of sensorimotor regions.
The memory deficit of AD is classically conceptualized as a dysfunction of consolidation or ‘storage' (Salmon, 2008). This is widely measured in the clinic using tests of delayed free verbal recall, which show the patient's inability to spontaneously retrieve words that were encoded 10–20 min or so previously. Retention or ‘savings' measures are also heavily used, which explicitly provide a measure indicting the percentage of information that was initially recalled during learning that is still able to be recalled without cueing after a delay. Other hallmarks of this amnesic syndrome include intrusions from interference materials and discriminability deficits on recognition measures.
It is important to recognize that delayed free recall of recently learned information is dependent on a variety of processes subserved by the large-scale memory network as outlined above, including executive control processes involved in retrieval effort and search as well as monitoring and decision making, which are likely subserved by systems outside the MTL, in addition to the primary memory processes supported by the MTL itself. It may be that this form of memory test is sensitive to early AD because it draws on so many components of the system, yet this also makes it relatively nonspecific.
A number of recent investigations using methods drawn from cognitive neuroscience have highlighted other abnormalities of episodic memory performance in patients with AD. Studies of the dual process recollection and familiarity model initially suggested that familiarity was preserved in AD but recollection was not (Dalla Barba, 1997), but more recent investigations suggest that both processes may be impaired (Ally et al, 2009a). Regardless of their precise mechanisms, impairments in the ability to discriminate old from new, along from a more liberal response bias (Budson et al, 2006), seem to lead to an increase in false memory in AD (Budson et al, 2006). This may be one reason for the increased frequency of confabulation in AD (Attali et al, 2009).
Recent studies of prospective memory in AD suggest that there are prominent impairments in this form of memory, possibly even of greater severity than the retrospective deficit (Blanco-Campal et al, 2009).
Finally, the finding that the picture superiority effect (pictures of objects are better remembered than words representing the same objects) is preserved in AD (Ally et al, 2009b) highlights the fact that clinical approaches to memory assessment in AD often make little use of observations from basic cognitive neuroscience. That is, on verbal memory testing, which is the standard approach to testing that is widely used in AD clinical research and drug trials, patients with AD often reach floor performance, making it difficult to detect effects of interest, whereas if pictorial stimuli were used there might still be enough variance to enable the detection of effects of interest.
Hippocampal Sclerosis
Hippocampal sclerosis is a pathologic diagnosis involving neuronal loss and gliosis of the hippocampal formation, preferentially affecting the CA1 region and subiculum (Dickson et al, 1994; Lippa and Dickson, 2004). Although it has received relatively little investigation, it seems to be a relatively common pathologic finding in elderly patients with dementia, particularly in individuals greater than 80-years old. It is distinct from hippocampal or mesial temporal sclerosis associated with epilepsy. A recent community-based study of the neuropathology of amnesic MCI identified hippocampal sclerosis in 3 of 15 patients who died (Petersen et al, 2006). Detailed neuropathologic investigation of a series of 10 cases showed the presence of τ-positive lesions and the accompaniment of other non-Alzheimer pathologies, frequently a form of frontotemporal lobar degenerative pathology (Probst et al, 2007). The authors also reported frequent asymmetric hippocampal and ipsilateral mammillary body atrophy, which they proposed could be used as an imaging marker potentially helpful for diagnosis. Although there are currently relatively little data about hippocampal sclerosis as human amnesic syndrome and clinical diagnostic criteria have yet to be developed, it seems that the frequency and specificity of this etiology of late-life amnesia warrants further investigation.
Amnesias Associated with Focal Lesions
Focal lesions of hippocampal subregions (particularly CA1, Sommer's sector) and other portions of the MTL can be produced by hypoxic-ischemic injury (eg, because of cardiac arrest, asphyxiation, carbon monoxide poisoning), infectious processes (eg, herpes encephalitis, which often affects temporal isocortex as well as MTL), inflammatory processes (eg, forms of autoimmune limbic encephalitis), and other disorders. Ischemic stroke (particularly involving the posterior cerebral artery) can cause MTL damage, and can also affect other regions of the large-scale memory system, including thalamus; hemorrhagic stroke can affect basal forebrain and neighboring regions.
Developmental amnesia is a condition typically caused by perinatal hypoxic-ischemic injury, with resultant emergence of the memory deficit during development typically after the toddler years (Vargha-Khadem et al, 1997, 2001). In recent years, studies have begun to elucidate the normal development of recognition memory in first few days of infancy with recognition memory being present over longer delays as development progresses in the first year of life, followed by the development of semantic memory later in the first year of life, followed finally by the gradual development and elaboration of forms of free recall, source memory, and flexible memory over the course of childhood (de Haan et al, 2006). This normal trajectory can be profoundly derailed as a result of a variety of rare disorders early in life. It seems that some individuals with primarily hippocampal developmental damage may be predominantly impaired in free recall and recollective aspects of recognition, with relative preservation of familiarity-based recognition memory (Baddeley et al, 2001; Brandt et al, 2008). Furthermore, semantic memory can be partially or even quite well preserved, including the acquisition of new semantic knowledge (Gardiner et al, 2008). Although neuroimaging data indicate that structures outside the MTL can be damaged in hypoxic-ischemic amnesia, including regions of the thalamus, cases of developmental amnesia that have been studied in detail are associated with at least 20–30% volume loss bilaterally (Isaacs et al, 2003).
A body of cognitive neuroscience research with focal MTL lesion patients has centered on the hotly debated issues regarding dual process theories of memory and, for proponents of such models, the relative importance of the hippocampal formation as opposed to medial temporal cortex for recollection vs familiarity (Aggleton and Brown, 1999; Eichenbaum et al, 1994). Evidence exists in support of the contention that patients with damage thought to be fairly restricted to the hippocampal formation have memory deficits predominantly involving recollection with relative sparing of familiarity, whereas those with larger lesions including MTL cortex have both recollection and familiarity deficits (Eichenbaum et al, 2007). However, there are many contentious issues in this debate (Squire et al, 2004), one of the most important of which is that it is very difficult to be confident in our knowledge of the precise localization of the lesions in vivo.
Going back to studies with HM, there has been debate about the degree to which the acquisition of new semantic information can be performed in patients with MTL lesions. This is of interest in part because of questions regarding whether facts (semantic memory) are initially learned in a manner similar to events (episodic memory). Recent studies of patients with MTL lesions continue to provide evidence that it is possible for humans with significant MTL damage to learn some new facts, albeit with significant difficulty (Bayley et al, 2008).
Retrograde amnesia has received increasing attention recently, with a growing number of debates emerging about the types, relative severity (particularly in relation to anterograde amnesia), and temporal gradient of remote memory impairment (Kopelman and Kapur, 2001; Squire and Bayley, 2007). More sophisticated methods for testing the challenging domain of autobiographical memory in patients with focal amnesias have demonstrated intact remote memory in patients with damage restricted to hippocampal formation (Kirwan et al, 2008). Creative single-case studies, including a behavioral and functional MRI study of a patient with apparent isolated retrograde amnesia (Levine et al, 2009), have begun to suggest that dysfunction in medial prefrontal and parietal cortical regions impedes the first person re-experiencing of past events.
Although investigations are increasingly being performed of patients with amnesic syndromes characterized and localized primarily on the basis of neuroimaging data, it is extremely important to continue to perform postmortem examinations of as many of these individuals as possible. The neuropathology of a handful of patients who have been studied in detail during life has been described, supporting the notion that focal hippocampal pathology can result in a dense anterograde as well as graded retrograde amnesia (Rempel-Clower et al, 1996; Zola-Morgan et al, 1986). A recent series shows similar amnesic syndromes in a patient with focal hippocampal pathology as well as two patients with thalamic pathology (one with infarcts and one with Korsakoff's syndrome), which the authors interpret to indicate that damage to the MTL or diencephalic memory system (including dorsomedial nucleus, adjacent internal medullary lamina, mammilary bodies, mammilothalamic tract, or anterior nuclei) can produce similar memory disorders (Gold and Squire, 2006).
The thalamic components of the episodic memory system are receiving increasing attention in functional neuroanatomic studies as well as behavioral investigations (Van der Werf et al, 2003). A detailed report including imaging (DWI tractography of white matter and anatomical imaging of gray matter) of thalamic nuclei and mammilothalamic tracts indicated that, although the two patients in the report exhibited anterograde amnesia, their performance did not fit as well as predicted with the roles of thalamic components of the system in the dual process model of recognition memory (Cipolotti et al, 2008).
Although the role of the prefrontal cortex in confabulation has been hypothesized on the basis of single case or small group studies (Benson et al, 1996), there have been only a few investigations of large samples of patients with prefrontal lesions. Confabulation, defined as a ‘false' memory that the patient believes to be true, often involving a statement about a personal autobiographical event that the patient never in fact experienced, has been thought to result from a memory deficit with superimposed executive dysfunction that leads to impairment in monitoring. A recent investigation of 38 patients with frontal lesions and 12 patients with temporoparietal lesions indicated that confabulation was strongly associated with orbital and medial prefrontal (particularly anterior cingulate) lesions (Turner et al, 2008). As some patients with confabulation did not show executive deficits, the authors interpret their data to indicate that confabulation may be a result of a specific executive deficit not tapped by traditional tests or of a specific episodic memory monitoring or control deficit localized to ventromedial prefrontal cortex.
Episodic memory performance in patients with focal parietal lesions has received very little attention. An initial study of source memory in patients with lateral parietal lesions indicated that no impairment was present in these patients (Simons et al, 2008). Although an fMRI experiment had been used in this study to localize the posterolateral parietal activation associated with a source memory judgment and the patients' lesions included this area, the authors ended by concluding that lateral parietal cortex may participate in—but does not seem to be necessary for—this form of recollection.
Epilepsy
It has long been appreciated that many patients with epilepsy (and seizure disorders more generally) exhibit memory impairment. This is particularly true among patients with temporal lobe epilepsy (TLE), the most common form of adult-onset epilepsy, in which epileptiform activity commonly originates in or involves the hippocampal formation and neighboring MTL cortical regions. Starting with the pioneering work by Milner and colleagues at the Montreal Neurological Institute, neuropsychological performance data have suggested a material-specific memory disorder in patients with TLE, which is thought to typically begin as a lateralized disorder that produces verbal memory impairment when localized to the dominant hemisphere and visual or visuospatial memory impairment when localized to the nondominant hemisphere (Milner, 1972).
Recent investigations motivated in part by astute clinical observations indicate that there are other forms of amnesic disorders in patients with epilepsy. Transient epileptic amnesia (TEA) (Kapur, 1993) is a form of anterograde and/or retrograde amnesia that occurs as brief, usually recurrent, episodes often in middle-aged or elderly patients with epilepsy. The pathophysiology of the disorder is thought to involve an ictal or postictal form of epileptic MTL disturbance. In addition to these brief episodic periods of amnesia, TEA may also be accompanied by two other forms of persistent memory loss: accelerated long-term forgetting and remote memory impairment (Bell and Giovagnoli, 2007; Butler et al, 2007). Accelerated long-term forgetting describes the apparent normal initial learning and recall of information over minutes to hours followed by an unusually rapid loss of information over days to weeks (original ref). Remote memory impairment involves the relatively prominent impairment of remote autobiographical or semantic memory compared with performance on tests of recent memory (Kapur et al, 1989). Both of these latter forms of memory impairment have been reported in single case or small group studies, but generally have not been very heavily investigated.
Further studies of these forms of epileptic amnesia will likely illuminate important outstanding questions in the field of human memory disorders. TEA often occurs in individuals of similar age to those that experience transient global amnesia (TGA), although the duration of the episode of amnesia is usually shorter in TEA (30–60 min) than in TGA (4–10 h). A better understanding of these disorders could provide insights into the vulnerability of the normal dynamics of MTL function, and may also provide opportunities for the investigation of the behavioral effects of transient amnesia, as experimental methods to transiently inactivate (eg, transcranial magnetic stimulation) MTL have proven elusive to date. As TEA has been commonly reported to occur on awakening, further studies may shed additional light on the interactions between sleep and memory (Stickgold and Walker, 2007). Finally, accelerated long-term forgetting and remote memory impairment will likely provide valuable information relevant to theories of memory consolidation (Alvarez and Squire, 1994; Moscovitch and Nadel, 1998).
Transient Global Amnesia
TGA is a striking clinical syndrome involving the acute onset of anterograde and temporally graded retrograde amnesia with otherwise normal neuropsychiatric function (Fisher and Adams, 1964; Hodges and Warlow, 1990). It usually lasts less than 1 day and resolves completely, except for the loss of memory for experience during the episode. A wide range of pathophysiologic mechanisms have been postulated, including migraine pathophysiology (spreading neurophysiologic depression), ischemia, epileptiform activity, and others (Sander and Sander, 2005).
Several recent findings have led to the revision of pathophysiologic hypotheses. Observations that patients are often engaged in stressful or physically vigorous activities before TGA led Lewis (1998) to propose that the combination of a valsalva-inducing process in combination with jugular venous insufficiency would lead to a state of venous stasis that may preferentially affect the MTL. Although this proposal is controversial, there is a substantially higher rate of jugular venous insufficiency in TGA patients than in controls (Sander and Sander, 2005). The apparent presence of phobic personality traits in many TGA patients (Inzitari et al, 1997) has led to the suggestion that hyperventilation may predispose to cerebral vasoconstriction and hypoperfusion (Pantoni et al, 2000).
Advances in diffusion-weighted MRI have enabled the detection of punctate lesions with restricted diffusion in some patients with TGA, which usually (but not always) indicates ischemia. In a recent study that used DWI, a large majority of patients were found to have DWI abnormalities in the hippocampal formation, usually unilaterally but in some cases bilaterally (Sedlaczek et al, 2004). The authors proposed that the vascular anatomy of the hippocampal formation, with the dorsolateral aspect being a watershed zone, may predispose to the vulnerability of regions in which the lesions were frequently detected, and that more sensitive imaging methods may enable the more routine detection of such subtle abnormalities.
CONCLUSIONS AND FUTURE DIRECTIONS
This review suggests several areas of convergence in studies on the anatomy of the MTL memory system and the larger-scale distributed memory system including brain regions outside the MTL, as well as neuropsychological and neurophysiological studies on episodic memory function in animals and humans. First, there is growing convergence of evidence that the hippocampus serves a fundamental and selective role in episodic memory across mammalian species. In animals, experimental lesions restricted to the hippocampus result in relatively selective loss of features of episodic memory. Similarly, studies on memory disorders in humans show that compromise of hippocampal function across a broad variety of disorders results in deficits in episodic memory with variable sparing of other aspects of declarative memory. Harmonization of behavioral and anatomic methods within and across species, to the degree possible, should provide further evidence of the specificity of hippocampal lesions on forms of episodic memory and related abilities. Second, and correspondingly, physiological studies, involving single neurons recordings in animals and functional imaging studies in humans, support the view that the hippocampus is involved in episodic memory, and may be particularly involved in encoding conjunctions of important events with where and when they occur. Third, additional studies have shown the cortical areas adjacent to the hippocampus (perhirhinal, parahippocampal, and entorhinal cortex) also have a function in episodic memory across species. Additional work on the detailed anatomy and connectivity of MTL regions, behavioral paradigms, and important technical advances in neurophysiology and functional neuroimaging data acquisition and analysis will likely contribute in valuable ways toward our understanding of the functional neuroanatomy of this system. Finally, substantial evidence from studies on humans, and some evidence on animals, show a large-scale distributed network of cortical areas that participate in important ways in episodic memory; particularly, the prefrontal cortex in humans and animals has a critical function in executive functions that monitor and manage episodic memory. Further studies in multiple animal species as well as in humans will shed light on the fundamental roles of these brain regions and the ways in which humans differ from even our closest primate relatives.
There are also two major areas in which information is sparse, and future studies could elucidate how the episodic memory system works and how its disorders might be better understood and, hopefully, ameliorated. First, further work is needed to identify the distinct roles of anatomical components of the cortical–hippocampal system, including both the isocortical areas that are directly connected with the MTL and the allocortical and periallocortical areas immediately surrounding and intimately connected with the hippocampus. In addition to further characterizing their individual roles, we need to understand how these areas interact with each other in support of the encoding, organizing, and retrieval of episodic memories. Studies in both animals and humans, which involve simultaneous monitoring of different components of this system, using multiple single recordings in animals and functional connectivity analyses in humans, may show how the system components normally interact and how the system processing breaks down in memory disorders. Second, we need to understand how the detailed circuitry within subdivisions of the hippocampal formation—that is, Ammon's horn, the dentate gyrus, and subiculum, and also the circuitry along the rostrocaudal axis of the hippocampus—supports computations that underlie features of episodic memory. For example, the findings that describe the marked attractor dynamics (pattern completion and pattern separation) of area CA3 and the dentate gyrus in both animals and humans may provide a window in the computational circuitry on which features of episodic memory are based, and those computations might be the functions that are fundamentally compromised in memory disorders (for reviews, see Colgin et al, 2008; Guzowski et al, 2004). Similarly, understanding how potentially distinctive representations along the rostrocaudal axis of the hippocampus are integrated might provide clues about a functional topography in this system. Greater understanding of functional neuroanatomy and computational processing of episodic memory systems, and how they can be modulated pharmacologically, should pave the way toward new treatments for memory disorders.
Acknowledgments
This work was generously supported by the National Institute on Aging (R01-AG29411, R21-AG29840) and the Alzheimer's Association.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- Aggleton JP, Brown MW.1999Episodic memory, amnesia, and the hippocampal-anterior thalamic axis Behav Brain Sci 22425–444.discussion 444-89. [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 2009a;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. The picture superiority effect in patients with Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2009b;47:595–598. doi: 10.1016/j.neuropsychologia.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP.1995Hippocampal formationIn: Paxinos G (ed).The Rat Nervous System2nd ednAcademic Press: San Diego, CA; 443–493. [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW.1991The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease Cereb Cortex 1103–116.One of the first systematic investigations of the localization and severity of Alzheimer neuropathologic abnormalities in the cerebral cortex. [DOI] [PubMed] [Google Scholar]
- Attali E, De Anna F, Dubois B, Dalla Barba G. Confabulation in Alzheimer's disease: poor encoding and retrieval of over-learned information. Brain. 2009;132:204–212. doi: 10.1093/brain/awn241. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: implications for the acquisition of semantic memory. J Cogn Neurosci. 2001;13:357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, O'Reilly RC, Curran T, Squire LR. New semantic learning in patients with large medial temporal lobe lesions. Hippocampus. 2008;18:575–583. doi: 10.1002/hipo.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Giovagnoli AR. Recent innovative studies of memory in temporal lobe epilepsy. Neuropsychol Rev. 2007;17:455–476. doi: 10.1007/s11065-007-9049-3. [DOI] [PubMed] [Google Scholar]
- Benson DF, Djenderedjian A, Miller BL, Pachana NA, Chang L, Itti L, et al. Neural basis of confabulation. Neurology. 1996;46:1239–1243. doi: 10.1212/wnl.46.5.1239. [DOI] [PubMed] [Google Scholar]
- Blanco-Campal A, Coen RF, Lawlor BA, Walsh JB, Burke TE. Detection of prospective memory deficits in mild cognitive impairment of suspected Alzheimer's disease etiology using a novel event-based prospective memory task. J Int Neuropsychol Soc. 2009;15:154–159. doi: 10.1017/S1355617708090127. [DOI] [PubMed] [Google Scholar]
- Brandt KR, Gardiner JM, Vargha-Khadem F, Baddeley AD, Mishkin M. Impairment of recollection but not familiarity in a case of developmental amnesia. Neurocase. 2008;15:60–65. doi: 10.1080/13554790802613025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD.1998Making memories: brain activity that predicts how well visual experience will be remembered Science 2811185–1187.One of a pair of important papers demonstrating the level of reigonal brain activation in the medial temporal lobe and other areas associated specifically with successful memory encoding in living humans. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus. Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. 2005Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory J Neurosci 257709–7717.Demonstration of the convergence of imaging measures of regional brain pathology, dysfunction, and atrophy on the episodic memory network in living patients with Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG.1998aCortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat J Comp Neurol 398179–205.Demonstration that parahippocampal cortical areas in rats distinguish between the processing of unimodal perceptual information about objects in perirhinal cortex and lateral entorhinal cortex contrasted with polymodal spatial information processing in the parahippocampal cortex and medial entorhinal cortex. See for similar results in monkeys. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZ. The syndrome of transient epileptic amnesia. Ann Neurol. 2007;61:587–598. doi: 10.1002/ana.21111. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Husain M, Crinion J, Bird CM, Khan SS, Losseff N, et al. The role of the thalamus in amnesia: a tractography, high-resolution MRI and neuropsychological study. Neuropsychologia. 2008;46:2745–2758. doi: 10.1016/j.neuropsychologia.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future. Nat Rev Neurosci. 2003;4:685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A.1998Episodic-like memory during cache recovery by scrub jays Nature 395272–274.Evidence that animals possess capacities akin to episodic memory. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD.2003Multiple routes to memory: distinct medial temporal lobe processes build item and source memories Proc Natl Acad Sci USA 1002157–2162.Evidence supporting the proposal of a functional organization of the medial temporal lobe memory system in which distinct ‘what' and ‘where' pathways converging through the parahippocampal region into the hippocampus where items and events are represented in the context where they were experienced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: linking basic research with clinical evaluation and remediation. J Int Neuropsychol Soc. 2006;12:210–223. doi: 10.1017/S1355617706060334. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, Morris RG.2003Glutamate-receptor-mediated encoding and retrieval of paired-associate learning Nature 424205–209.Evidence that plasticity in hippocampal circuitry is critical to remembering which food was consumed where in a large open field. [DOI] [PubMed] [Google Scholar]
- de Haan M, Mishkin M, Baldeweg T, Vargha-Khadem F. Human memory development and its dysfunction after early hippocampal injury. Trends Neurosci. 2006;29:374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus. 2000;10:136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, et al. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: an event-related functional-anatomic MRI study. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, et al. 1994Hippocampal sclerosis: a common pathological feature of dementia in very old (> or=80 years of age) humans Acta Neuropathol 88212–221.An important study calling attention to a previously neglected form of pathology underlying a human memory disorder, which may be relatively common. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the brain. Oxford University Press: OUP New York, NY; 2001. [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:499–517. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for ‘what,' ‘where,' and ‘when'. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Arce M, Eichenbaum H. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J Neurosci. 2008;28:13428–13434. doi: 10.1523/JNEUROSCI.3662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM, Adams RD. Transient Global Amnesia. Acta Neurol Scand Suppl. 1964;40 Suppl 9:1–83. [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H.2004Recollection-like memory retrieval in rats is dependent on the hippocampus Nature 431188–191.Using a signal detection analysis of recognition memory, this investigation identified recollection-like memory dependent on the hippocampus and familiarity-like memory independent of the hippocampus in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AR, Petersen RC. Mild cognitive impairment. Handb Clin Neurol. 2008;89:217–221. doi: 10.1016/S0072-9752(07)01220-1. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Healey AN, Eacott MJ. Objects and positions in visual scenes: effects of perirhinal and postrhinal cortex lesions in the rat. Behav Neurosci. 2004;118:992–1010. doi: 10.1037/0735-7044.118.5.992. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Brandt KR, Baddeley AD, Vargha-Khadem F, Mishkin M. Charting the acquisition of semantic knowledge in a case of developmental amnesia. Neuropsychologia. 2008;46:2865–2868. doi: 10.1016/j.neuropsychologia.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glees P, Griffith HB. Bilateral destruction of the hippocampus (cornu ammonis) in a case of dementia. Monatsschr Psychiatr Neurol. 1952;123:193–204. doi: 10.1159/000140010. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. The anatomy of amnesia: neurohistological analysis of three new cases. Learn Mem. 2006;13:699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW., Jr, Morris JC, Growdon JH, Hyman BT.1996Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease J Neurosci 164491–4500.Quantitative demonstration of the loss of neurons at the origin of the perforant pathway in humans with Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 2003;7:241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ.2005Major dissociation between medial and lateral entorhinal input to dorsal hippocampus Science 3081792–1794.Demonstration of differences in the coding properties of neurons in different regions of the entorhinal cortex, showing that medial entorhinal neurons develop spatial representations of the environment whereas lateral entorhinal neurons do not. [DOI] [PubMed] [Google Scholar]
- Hecaen H, Albert ML. Human Neuropsychology. John Wiley & Sons. Wiley: Hoboken, NJ; 1978. [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Warlow CP. The aetiology of transient global amnesia. A case-control study of 114 cases with prospective follow-up. Brain. 1990;113 (Pt 3:639–657. doi: 10.1093/brain/113.3.639. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Pantoni L, Lamassa M, Pallanti S, Pracucci G, Marini P. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54:866–873. doi: 10.1001/archneur.1997.00550190056015. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Vargha-Khadem F, Watkins KE, Lucas A, Mishkin M, Gadian DG. Developmental amnesia and its relationship to degree of hippocampal atrophy. Proc Natl Acad Sci USA. 2003;100:13060–13063. doi: 10.1073/pnas.1233825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR., Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Dolan ED, Sauder CL, Wingfield A. Intrusions in episodic recall: age differences in editing of overt responses. J Gerontol B Psychol Sci Soc Sci. 2005;60:P92–P97. doi: 10.1093/geronb/60.2.p92. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL.2008Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity J Neurophysiol 100129–139.Epub 2008 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N. Transient epileptic amnesia—a clinical update and a reformulation. J Neurol Neurosurg Psychiatry. 1993;56:1184–1190. doi: 10.1136/jnnp.56.11.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Young A, Bateman D, Kennedy P. Focal retrograde amnesia: a long term clinical and neuropsychological follow-up. Cortex. 1989;25:387–402. doi: 10.1016/s0010-9452(89)80053-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Bayley PJ, Galvan VV, Squire LR. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proc Natl Acad Sci USA. 2008;105:2676–2680. doi: 10.1073/pnas.0712155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. 2004Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B Ann Neurol 55306–319.Demonstration of important new molecular imaging method for detecting amyloid plaque pathology in living humans with Alzheimer's disease. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III Cortical efferents. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Kapur N. The loss of episodic memories in retrograde amnesia: single-case and group studies. Philos Trans R Soc Lond B Biol Sci. 2001;356:1409–1421. doi: 10.1098/rstb.2001.0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Kaas J. The evolution of the neocortex in mammals: how is phenotypic diversity generated. Curr Opin Neurobiol. 2005;15:444–453. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Turner GR, Mandic M, Mackey A.2009Behavioral and functional neuroanatomical correlates of anterograde autobiographical memory in isolated retrograde amnesic patient M L Neuropsychologia 472188–2196.E-pub 2008 Dec 30. [DOI] [PubMed] [Google Scholar]
- Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352:397–399. doi: 10.1016/S0140-6736(98)01442-1. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Dickson DW. Hippocampal sclerosis dementia: expanding the phenotypes of frontotemporal dementias. Neurology. 2004;63:414–415. doi: 10.1212/01.wnl.0000136241.71716.72. [DOI] [PubMed] [Google Scholar]
- Luria AR. The Working Brain. Penguin Books. Penguin, NY, NY; 1973. [Google Scholar]
- Maguire EA. Hippocampal involvement in human topographical memory: evidence from functional imaging. Philos Trans R Soc Lond B Biol Sci. 1997;352:1475–1480. doi: 10.1098/rstb.1997.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H.2006Evolution of the hippocampusIn: Kaas JH (ed)Evolution of the Nervous System Academic Press: Oxford; 465–490. [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121 ( Pt 6:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci USA. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M, Smith ML.1985Frontal lobes and the temporal organization of memory Hum Neurobiol 4137–142.Early modern study of the importance of prefrontal brain regions for episodic memory. [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: in defense of the multiple-trace model. Curr Opin Neurobiol. 1998;8:297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol Scand. 2000;102:275–283. doi: 10.1034/j.1600-0404.2000.102005275.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V, et al. Encoding of novel picture pairs activates the perirhinal cortex: an fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD.2009High-resolution fMRI of Content-sensitive Subsequent Memory Responses in Human Medial Temporal Lobe J Cogn Neurosci2009 Jan 13. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol. 2007;114:335–345. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG.1996Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation J Neurosci 165233–5255.Detailed investigation of the histologic basis of episodic memory loss in amnesic patients who had undergone extensive behavioral characterization during life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H.2008Cognitive aging: a common decline of episodic recollection and spatial memory in rats J Neurosci 288945–8954.This study used signal detection analysis in recognition memory to establish a rodent model of the selective loss of episodic recollection associated with aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends Cogn Sci. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Salmon DP. Chapter 5 Neuropsychology of aging and dementia. Handb Clin Neurol. 2008;88:113–135. doi: 10.1016/S0072-9752(07)88005-5. [DOI] [PubMed] [Google Scholar]
- Sander K, Sander D. New insights into transient global amnesia: recent imaging and clinical findings. Lancet Neurol. 2005;4:437–444. doi: 10.1016/S1474-4422(05)70121-6. [DOI] [PubMed] [Google Scholar]
- Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci. 2008;11:16–18. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB. The limbic lobe in man. J Neurosurg. 1954;11:64–66. doi: 10.3171/jns.1954.11.1.0064. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B.1957Loss of recent memory after bilateral hippocampal lesions J Neurol Neurosurg Psychiatry 2011–21.The seminal study of H.M. and other amnesic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlaczek O, Hirsch JG, Grips E, Peters CN, Gass A, Wohrle J, et al. Detection of delayed focal MR changes in the lateral hippocampus in transient global amnesia. Neurology. 2004;62:2165–2170. doi: 10.1212/01.wnl.0000130504.88404.c9. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans. Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Squire LR.2009The legacy of patient H M. for neuroscience Neuron 616–9.A valuable perspective on the contributions to memory research of H.M. and the teams of scientists who studied him. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Grady CL.2007Insight into frontal lobe function from functional neuroimaging studies of episodic memoryIn: Miller BL, Cummings JL (eds).The Human Frontal Lobes: Function and Disorders,2nd ednGuilford Press: New York; 207–226. [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo C, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury on word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal, and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin Neurosci. 1996;8:3–12. [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994a;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci USA. 1994b;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MS, Cipolotti L, Yousry TA, Shallice T. Confabulation: damage to a specific inferior medial prefrontal system. Cortex. 2008;44:637–648. doi: 10.1016/j.cortex.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N.1972Cortical afferents to the entorhinal cortex of the Rhesus monkey Science 1751471–1473.An early investigation of the connectional topography of the primate entorhinal cortex, highlighting its ‘bottleneck' position in bringing information into the medial temporal lobe memory system. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Mishkin M. Dissociations in cognitive memory: the syndrome of developmental amnesia. Philos Trans R Soc Lond B Biol Sci. 2001;356:1435–1440. doi: 10.1098/rstb.2001.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. 2007Intrinsic functional architecture in the anaesthetized monkey brain Nature 44783–86.An important validation of new imaging technology for investigating large-scale connectional brain systems in living primates. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998a;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998b;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA.2003Single neurons in the monkey hippocampus and learning of new associations Science 3001578–1581.This paper indentified the development of robust associative representations in hippocampal neurons during learning in monkeys. See for similar results in rats. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]