SYNOPSIS
Objectives
Risk factors for human immunodeficiency virus (HIV) disease progression among American Indians (AIs) have been poorly characterized. We assessed the impact of socioeconomic factors and use of traditional healing on HIV disease progression in a rural AI community.
Methods
From January 2004 through December 2006, we interviewed 36 HIV-positive AIs regarding their socioeconomic status, incarceration, and use of traditional healing. We also collected chart-abstracted adherence and substance-abuse data. Through bivariate analysis, we compared these factors with the CD4-cell counts and log HIV-1 viral loads (VLs). Using a simple regression model, we assessed interactions between the significant associations and the outcome.
Results
Participant characteristics included being male (58.3%), being transgender (13.9%), having ever been incarcerated (63.9%), having a household income of <$1,000/month (41.7%), being unemployed (61.1%), being diagnosed with alcohol abuse (50.0%), and using traditional medicine (27.8%) in the last 12 months. Higher VLs were associated with recent incarceration (p<0.05), household income of <$1,000/month (p<0.05), and provider-assessed alcohol abuse (p<0.05). We found an interaction between incarceration and alcohol abuse, and alcohol abuse was the factor more strongly associated with higher VLs. A lower CD4 count was associated with recent incarceration (p<0.05) and use of traditional medicine (p<0.05).
Conclusions
Alcohol abuse is an important contributor to HIV disease progression, and participants with lower CD4 counts were more likely to use traditional medicine. HIV care among this rural AI population should focus on addressing alcohol abuse and other socioeconomic risk factors and promote collaboration between Western medical and Navajo traditional practitioners.
The epidemiology of human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) in the American Indian and Alaska Native (AIAN) communities has been poorly characterized. Early reports from 1992 showed a seroprevalence of 1.0/1,000 among third-trimester patients seen in Indian Health Service (IHS) prenatal clinics and a seroprevalence of 4.5/1,000 for AIAN males seeking care for sexually transmitted disease in IHS clinics.1 At the end of 1993, the Centers for Disease Control and Prevention (CDC) reported 818 AIANs with HIV/AIDS.2 Ten years later, in 2003, this number had more than doubled to 1,788.3 The most recent statistics reported by CDC indicate that the rate of HIV/AIDS cases was 10.6 per 100,000 for the AIAN population, compared with 72.8 for African American, 28.5 for Hispanic, 9.0 for white, and 7.6 for Asian/Pacific Islander racial/ethnic groups. The rate of HIV diagnosis among AIAN males (19.5) was slightly higher than the rate among white men, and the diagnosis rate among AIAN females (7.6) was more than twice the rate of white females.4
The actual number of HIV/AIDS cases in the AIAN population is most likely underestimated.5 As a result of misclassification error, as many as 70% of AIANs are incorrectly assigned other races/ethnicities on medical records and in surveillance data.1,2,6,7 Additionally, many AIANs live in rural areas where access to health-care services, including HIV testing, is extremely limited.8 The U.S. Commission on Civil Rights reports data indicating that only 28% of Native Americans have private health insurance, and 55% rely on IHS to provide primary and secondary care.9 The patchwork of resources and lack of funding experienced by most IHS facilities contribute to undercounting, underreporting, and lack of detailed surveillance of the HIV/AIDS epidemic among AIANs.8,10
In the era of highly active antiretroviral therapy (HAART) regimens, HIV is considered a manageable chronic disease. To receive maximum benefit, however, people living with HIV must receive a diagnosis as early in the course of the disease as possible, enter quality HIV care, and remain in care indefinitely.11 For many, access to necessary health and social services is severely restricted by distance to facilities, poverty, unemployment, and inadequate health-care funding. From 2002 to 2004, 24% of AIANs were living in poverty. This was approximately twice the national average (12%).12 In 2003, unemployment rates among AIANs (15%) were more than twice the national average (6%) and three times the rate for the white population (5%).13 Poverty and unemployment limit resources, creating multiple barriers to access and utilization of treatment services. Poor retention in care has been found to be associated with less improvement in CD4-cell counts and less reduction in HIV viral load (VL) levels.14 Whether in or out of care, AIANs experience a shorter survival time than all other racial/ethnic groups except African Americans. Nine years following diagnosis, 67% of AIANs survive, compared with 66% of African Americans, 74% of Hispanic people, 75% of white people, and 81% of the Asian/Pacific Islander population.4
Little is known about the risk factors for progression of HIV/AIDS in AIAN patients. One longitudinal study (1981–2004) conducted by an IHS urban HIV clinic studied the determinants of survival of AIAN patients with HIV/AIDS.15 Use of HAART at baseline was found to be associated with a reduction in the risk of death, and patients with CD4 counts >200 cells/cubic millimeter (mm3) at baseline (i.e., greater than the clinical definition of AIDS) also tended to live longer than those with CD4 counts <200 cells/mm3. This study, however, focused entirely on Western medicine surrogate clinical markers and did not include analyses of cultural factors unique to AIAN communities, such as traditional native healing, or socioeconomic factors that are perhaps more prevalent in AIAN communities, such as alcohol abuse and lower household incomes.
From 1996 to 2006, in a resource-poor, rural frontier location with a high rate of co-occurring disorders, the Four Corners American Indian Circle of Services Collaborative (4CC) partners created integrated medical/mental-health/cultural systems to serve the needs of AIs with HIV/AIDS on the Navajo Nation.16 The 4CC also provided prevention education and increased HIV testing to high-risk groups. This article describes the results of a study that was conducted through the 4CC, whose partners include the Navajo AIDS Network, Inc.; the Navajo Area Indian Health Service; the Navajo Nation's Division of Health; the University of New Mexico; the University of Washington's Indigenous Wellness Research Institute; and the Na'nizhoozhi Center, Inc., an alcohol rehabilitation center for AIs and other clients. The cross-sectional study investigated the association of sociologic and cultural risk factors for HIV disease progression that are likely prevalent in an AIAN community. Specifically, we explored the relationship among VL, CD4 count, and potential explanatory variables, including physician-reported adherence, use of traditional medicine, substance abuse, work status, and important demographic variables (e.g., age and gender) among HIV-positive AIANs.
METHODS
Setting and recruitment
The Navajo Area IHS (NAIHS) has provided HIV care to 240 patients at eight IHS clinics and hospitals spread over the Navajo Nation, an area the size of West Virginia, since the first case was diagnosed in 1987. In 2006, the clinics cared for 100 HIV-positive individuals. This program is supervised by an IHS HIV clinic in Gallup, New Mexico. This clinic provides primary care, HIV consultative care, mental health care, and a pharmacist-run adherence program. The Navajo AIDS Network (NAN), a nongovernmental organization based in the heart of the Navajo Nation, provides a unique form of case management, utilizing the Navajo language and cultural idiom.
Of the 100 HIV-positive clients known to the NAIHS, 53 patients were under NAN case management. Of those under case management, 69.8% (37/53) were enrolled in the study; demographic and socioeconomic data were available for 97.3% (36/37) of participants; and VL and CD4 data were available for 97.2% (35/36) of participants. Ongoing enrollment occurred during monthly case-management visits, and patients were able to opt out of both case management and study enrollment.
Procedures
The NAN case managers conducted face-to-face interviews with participants every six months, from January 27, 2004, to December 14, 2006. Because enrollment was ongoing, participants could have anywhere from one to five interviews; therefore, data for this study were obtained from the most recent completed interview. As most participants had just two or three interviews, the limited number of interviews precluded a longitudinal analysis. We, therefore, used a cross-sectional approach to determine correlates of the dependent variable. We used sociodemographic information, living status, and self-reported medication adherence to assess association with the primary outcomes—HIV ribonucleic acid (RNA) VLs and CD4 counts. The HIV-care provider chart-abstracted VLs, CD4 counts, and history of substance abuse at each of the eight NAIHS units.
Measures
Clinical outcomes.
The primary outcome measures were CD4 counts and HIV RNA levels. Participants provided blood samples for CD4-count determination and VL testing as part of regular care every three months after HIV diagnosis. Blood was analyzed at various commercial laboratories contracted by the IHS. A chart review was conducted to record the most recent VL and CD4-count results closest to the time of the last data-collection interview. For 15 participants, the chart review date and health survey date were more than 12 months apart.
Sociodemographic variables.
We collected data on variables such as gender (male, female, or transgender), age (continuous), living arrangements (currently living with spouse or partner vs. other arrangements), and years of formal education (dichotomized at more than a high school education) via questionnaire.
Economic and living situation.
We also collected economic and living-situation variables via questionnaire. Work status was defined as (1) employed—full or part time, or occasional; (2) unemployed—includes retired; and (3) permanently or temporarily unable to work due to mental or physical disability. For analytical purposes, response categories were dichotomized as “currently employed” or “not employed.” Gross monthly household income from all sources was reported and dichotomized at the $1,000 per month threshold. Participants also reported whether they had ever been incarcerated and whether they had been incarcerated in the last 12 months. We assessed current housing situation as living in a current stable housing situation or not (transient, temporary housing, or homeless).
Traditional healing.
Traditional healing in this region can be divided into three categories: (1) traditional Navajo medicine, (2) peyote ceremonies, and (3) other non-Navajo traditional medicine systems. We assessed use of traditional healing by a single questionnaire item: “In the past 12 months, have you used traditional healing services?”
Medication adherence.
The attending provider (physician or pharmacist) assessed antiretroviral (ARV) medication adherence based on a series of closed- and open-ended questions at the time of each participant's visit. Questions were based on history (“How many doses did you miss in the last two months?”), other individual and social barriers to taking their ARV medications, and a review of pharmacy records to determine the proportion of prescriptions filled to the total prescribed. Based on these data, a health-care provider from each site estimated the level of adherence in one of four categories: refusal to take medication, poor adherence (<80% of doses taken), good adherence (80% to 95% of doses taken) and excellent adherence (>95% of doses taken). For analysis, this measure was dichotomized as excellent adherence or not.
Alcohol or other substance abuse.
The attending physician assessed alcohol abuse and abuse of other substances in the last 12 months via participant self-report and clinical evidence abstracted from each chart review of visits in the last 12 months. Evidence included a history of emergency-room visits for intoxication, detectable blood-alcohol level, a positive urine toxicology screen, and self-report of substance abuse. Data were abstracted from chart review.
Statistical methods
Univariate analysis provides descriptive statistics of the population for all covariates and outcomes. As VL data were not normally distributed, we conducted a log transformation and used the transformed values in all further analyses. CD4 counts were coded as a dichotomized variable at <200 cells/mm3, per CDC case-definition guidelines for descriptive presentation.17 We conducted bivariate analyses between the logarithm of VL and CD4 count as a continuous measure, with each of the predictive variables and demographic covariates. For binary independent variables, we conducted independent sample t-tests; t-statistics are provided, along with the corresponding p-values for these tests. We then compared significant predictors for associations and, if significant, conducted a simple general linear model regression to assess interactions between the significant associations and the outcome. Corresponding beta coefficient (β) and confidence intervals (CIs) are provided, as well as the overall F-statistic. All analyses were conducted using two-sided tests and a significance level of 0.05.
RESULTS
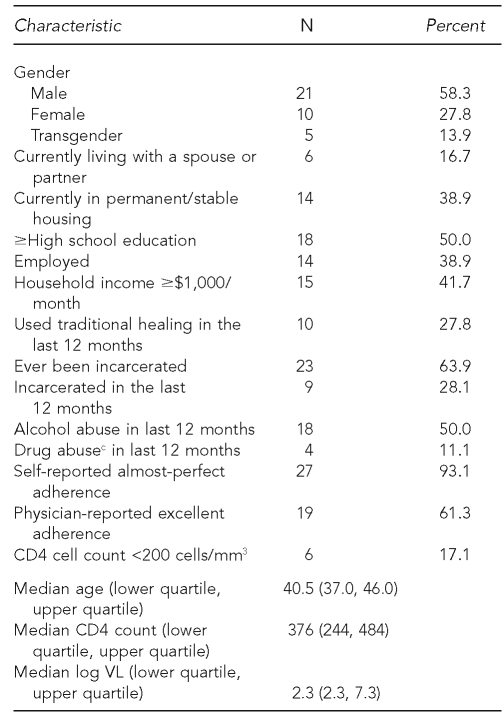
The purpose of the study was to determine risk factors associated with HIV disease progression, defined as an increase in logged detectable VLs and decrease in CD4 counts. Descriptive statistics for sociodemographic and clinical characteristics among 36 HIV-positive Navajos are presented in Table 1. Among the study participants, 13.9% (5/36) of the patients identified themselves as transgender, 38.9% (14/36) were employed, 63.9% (23/36) had ever been incarcerated, 50.0% (18/36) were assessed by providers with alcohol abuse in the last 12 months, and 11.1% (4/36) were assessed by providers with drug abuse in the last 12 months. All four participants diagnosed as abusing drugs also abused alcohol.
Table 1.
Sociodemographic and clinical characteristics among 36 HIV-positive American Indian Navajos interviewed from 2004 to 2006 as part of a study assessing risk factors in HIV disease progressiona,b
aChart-abstracted adherence data were available for 31 participants.
bSelf-reported adherence data were available for 29 participants.
cDrug abusers comprised a subset of alcohol abusers.
HIV = human immunodeficiency virus
mm3 = cubic millimeter
VL = viral load
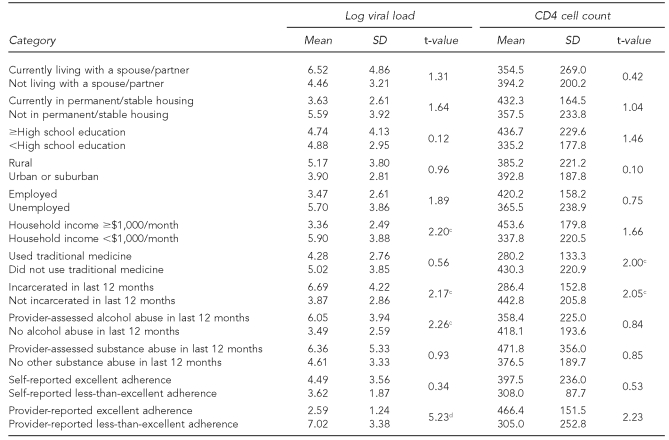
Table 2 shows the important differences between sociodemographic measures and VL and CD4 counts. Specifically, lower mean (M) CD4 counts were associated with being incarcerated in the last 12 months (M=286.4 [standard deviation (SD) = 152.8]) vs. not incarcerated (M=442.8 [SD=205.8], t=2.05, p<0.05) and use of traditional medicine (M=280.2 [SD=133.3]) vs. no reported use of traditional medicine (M=430.3 [SD=220.9], t=2.00, p<0.05). When we assessed the relationship between traditional healing and CD4 count, while stratifying by adherence, we found the use of traditional healing (β= –159.8, CI –306.5, –13.2) as opposed to adherence (β= –306.5, CI –3.9, 277.67) remained a significant predictor of low VL (F=5.32, p<0.01).
Table 2.
Associationa of socioeconomic characteristics with log viral load and CD4 cell count among 35b HIV-positive American Indian Navajos interviewed from 2004 to 2006 as part of a study assessing risk factors in HIV disease progression
aTests of association were conducted using Pearson's Chi-square, with one degree of freedom.
bChart-abstracted adherence data were available for 31 participants.
cp<0.05
dp<0.01
HIV = human immunodeficiency virus
SD = standard deviation
Pertaining to log M VL, higher log VL was associated with household incomes <$1,000/month (M=5.90 [SD=3.88]), vs. >$1,000/month (M=3.36 [SD=2.49], t=2.20, p<0.05); being incarcerated in the last 12 months (M=6.69 [SD=4.22]) vs. no recent incarceration (M=3.87 [SD=2.86], t=2.17, p<0.05); and 12-month provider-assessed alcohol abuse (M=6.05 [SD=3.94]) vs. no alcohol abuse (M=3.49 [SD=2.59], t=2.26, p<0.05). Finally, a provider's assessment of less-than-excellent adherence (M=7.02 [SD=3.38]) vs. excellent adherence (M=2.59 [SD=1.24], t=5.23, p<0.01) was associated with higher VL.
In addition, we found incarceration in the last 12 months was associated with a history of provider-assessed alcohol abuse in the last 12 months (77.8% vs. 39.1%, Pearson's Chi-square [1] = 3.86, p<0.05). When log VL was regressed on 12-month incarceration and alcohol abuse, the incarceration effect fell out of the model and alcohol was the significant predictor (12-month incarceration: β=1.85, CI –0.79, 4.50; 12-month alcohol abuse: β=2.64, CI 0.24, 5.05; F=5.23; p<0.05).
DISCUSSION
In this study, we interviewed and obtained clinical chart data from 36 HIV-positive Navajos receiving HIV care through the NAIHS. To identify factors associated with HIV disease progression, we looked for associations among sociodemographic, economic, and medical characteristics with VLs and CD4 counts. We found important differences with incarceration in the last 12 months, household incomes of <$1,000/month, alcohol abuse, and use of traditional medicine. Most importantly, when VL was assessed with a history of incarceration in the last 12 months and alcohol abuse, alcohol abuse remained the important indicator of poor outcomes.
Incarceration and abuse of alcohol were interrelated. Incarceration among the AI population served in the 4CC project was often linked to alcohol-related offenses, especially driving while intoxicated (DWI) charges. A study of AIs in the northwestern U.S. showed that AIs were overrepresented among individuals arrested for DWI by a factor of 2.5, compared with the overall DWI arrest population.18 Our data suggest that alcohol abuse underlies the statistical significance in VL and CD4 counts, more so than the apparent effect of incarceration.
Another possible factor is lack of access to appropriate HIV medical care and medication while incarcerated. A study conducted in British Columbia, Canada, showed that incarceration in the last six months was connected to a risk of failure to suppress HIV RNA levels in HIV-positive patients on HAART.19 Furthermore, incarceration was associated with increased risk of HIV seropositivity for everyone, regardless of race and gender. HIV/AIDS prevalence in inmate populations is estimated to be four times that in the general population.20 Less conservative estimates place the prevalence among incarcerated populations at five to 10 times that in general populations.20–22 Consistent with the study cited previously, our data showed poor CD4 and VL outcomes for AIs who were incarcerated in the last 12 months. Treatment interruptions during incarceration and post-incarceration living conditions may explain these findings.
Low household incomes may restrict access to health insurance and transportation to care. Although the IHS provides care regardless of income or private and government insurance status, patients seeking care in the HIV clinic in Gallup, New Mexico, have to travel a mean of 90 miles roundtrip to see an HIV specialist, with exceptional cases of 360 miles of roundtrip travel. Lower-income patients might find the cost of gasoline prohibitively expensive and have poorer access to the free health care to which they are entitled.
The most intriguing finding of this study was that use of traditional AI medicine was associated with lower CD4 counts. This effect was unanticipated and surprising to the investigators. Traditional Navajo medicine has as its goal restoring “hozho,” or balance, for the sick person. The sick patient will usually first seek the care of a diagnostician who uses various means, including crystal-gazing, star-gazing, or hand-trembling procedures, to determine the cause of illness. Illness is usually attributed to breaking of taboos/rituals (e.g., contact with snakes and lightning), contact with ghosts, or witchcraft. To restore hozho, the patient is referred by the diagnostician to a Navajo medicine person who performs the appropriate therapeutic ceremony. These ceremonies generally entail prayers, chanting, sand-painting, and therapeutic herbal medications.
There are several possible explanations for this finding between traditional medicine and CD4 counts. One possible explanation, perhaps most obvious to practitioners in the Western medicine scientific model, is a potential drug-drug interaction between traditional herbal medications and ARV therapy. Drug-drug interactions between herbal medicines such as St. John's Wort and the protease inhibitors are well described in the medical literature.23 The rich pharmacopoeia of Navajo traditional medicine has never been studied by Western medicine practitioners, and the possibility of drug-drug interactions has not yet been investigated. However, there have been studies on indigenous medicines used to treat HIV in South Africa that suggest avenues for future research.24
Another possibility is that individuals attending traditional healing ceremonies have suboptimal drug therapy adherence for the duration of the ceremony, though small sample sizes precluded a deeper investigation of this relationship. It is customary for Navajo patients undergoing traditional healing ceremonies to abstain from contact with things foreign, including non-Navajo health-care providers and non-Navajo medicines and procedures. Ceremonies can last as long as five nights and are often followed by other lengthy ceremonies to cure a single illness. The importance of this effect would be very difficult to quantify.
An alternate explanation for the negative correlation between traditional healing and CD4 counts is a historical and cultural one. The Navajo have an ancient traditional medicine system that has served them well for centuries. Contact with Western medical practitioners has only taken place for the last 140 years, and Western medical practitioners and educators on Navajo lands historically attempted to supplant traditional healing, leading to parallel and comparable, rather than integrated, systems.25–28 The NAIHS itself was not established until the mid-1950s. Many Navajos continue to adhere to traditional cultural beliefs and practices, and have much greater faith in the care provided by a medicine man than care provided by Western practitioners. As one Navajo coworker told one of the authors, “Doctor, we Navajos only come to see you IHS doctors as a last resort.” The two systems represent very different world views. Influenced by the historic separation of Western medicine from traditional treatment, the patients in our study who sought traditional treatment may have had lower CD4 counts because they sought Western medical care only after exhausting all possibilities in Navajo traditional treatment. This suggests that Western and traditional health-care providers have to work together to support HIV/AIDS care for their clients.
Limitations
This study had several limitations. First, there was a time lag of slightly more than one year between the final patient interview and the last determination of CD4 count and HIV VL for 15 of the study participants. This could have led to bias in favor of improved CD4 counts and HIV VLs, due to changes in the demographic and socioeconomic study variables. Second, although some participants had longitudinal data, most did not; therefore, we were unable to account for changes in the independent variables over the course of treatment. Third, the physician-/pharmacist-determined adherence measure was an indirect estimate. However, contrary to studies where physician-reported adherence was not correlated with VL,29 we found a significant association. In this study, the triangulation technique of including open-ended items, closed-ended items, and pharmacy records strengthens our physician-reported adherence measure. Finally, as this study was a cross-sectional design, we can only evaluate possible associations and, therefore, cannot state any causal relationship.
CONCLUSIONS
This study identifies recent alcohol abuse, incarceration, and use of traditional medicine as important factors affecting VLs and CD4 counts among AIAN HIV-positive individuals. It is important to identify social determinant of health markers among HIV-positive AIAN individuals for inclusion in future intervention efforts to improve health and well-being among this underserved population. It is imperative that treatment go beyond medical care to look at relevant social, emotional, economic, and cultural issues and needs. One answer may lie in enhanced cultural and medical case management, which would include increased funding for services by culturally competent providers. Many AIANs have expressed a preference for traditional medicines and ceremonies in their treatment regimen; yet, doctors and insurance companies continue to be resistant to anything other than Western medicine.30,31 Western medical providers must be informed about patients' use of traditional Navajo medicine and welcome traditional providers onto the treatment team. Mutual respect and closer working relationships between traditional and Western providers will promote both drug-regimen adherence and increased cultural competency on the part of Western providers. It is our hope that this study will open a dialogue between Native American traditional and Western medical practitioners who care for AIANs who are infected with HIV.
Acknowledgments
The authors thank Michele Peake, PhD, of the University of Washington, Department of Psychology, for assistance with writing and researching references. The authors also acknowledge the contribution of the case managers from the Navajo AIDS Network and the other members of the Four Corners American Indian Circle of Services Collaborative.
Footnotes
This article was supported by a grant from the U.S. Department of Health and Human Services, Health Resources and Services Administration's (HRSA's) Special Projects of National Significance, #5H97HA00254-01-00; the National Center for Minority Health and Health Disparities/Native American Research Centers for Health (NARCH), U26IHS300009A supplement; and the University of California at Los Angeles (UCLA) David Geffen School of Medicine, Department of Family Medicine, Network for Multicultural Research on Health, funded by the Robert Wood Johnson Foundation. The contents of this article are those of the authors and do not necessarily represent the official views of the HRSA, Indian Health Service, Harvard Medical School, University of Washington, UCLA, Navajo AIDS Network, Inc., Network for Multicultural Research on Health, or the Robert Wood Johnson Foundation.
REFERENCES
- 1.Conway GA, Ambrose TJ, Chase E, Hooper EY, Helgerson SD, Johannes P, et al. HIV infection in American Indians and Alaska Natives: surveys in the Indian Health Service. J Acquir Immune Defic Syndr. 1992;5:803–9. [PubMed] [Google Scholar]
- 2.AIDS among racial/ethnic minorities—United States, 1993. MMWR Morb Mortal Wkly Rep. 1994;43(35):644–7. 653–5. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) Vol. 18. Atlanta: CDC; 2008. [cited 2010 Feb 4]. HIV/AIDS surveillance report, 2006. Also available from: URL: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2006report/pdf/2006SurveillanceReport.pdf. [Google Scholar]
- 4.CDC (US) HIV/AIDS among American Indians and Alaska Natives. CDC HIV/AIDS fact sheet. [cited 2007 Jun 20]. Available from: URL: http://www.cdc.gov/hiv/resources/factsheets/PDF/aian.pdf.
- 5.Rowell R, Bouey P. What are American Indian/Alaska Natives' (AI/AN) HIV prevention needs? San Francisco: University of California, San Francisco; 2002. [cited 2010 Feb 4]. Also available from: URL: http://www.caps.ucsf.edu/pubs/FS/pdf/AI-ANFS.pdf. [Google Scholar]
- 6.Lieb LE, Conway GA, Hedderman M, Yao J, Kerndt PR. Racial misclassification of American Indians with AIDS in Los Angeles County. J Acquir Immune Defic Syndr. 1992;5:1137–41. [PubMed] [Google Scholar]
- 7.Thoroughman DA, Frederickson D, Cameron HD, Shelby LK, Cheek JE. Racial misclassification of American Indians in Oklahoma State surveillance data for sexually transmitted diseases. Am J Epidemiol. 2002;155:1137–41. doi: 10.1093/aje/155.12.1137. [DOI] [PubMed] [Google Scholar]
- 8.National Alliance of State and Territorial AIDS Directors. Native Americans and HIV/AIDS: key issues and recommendations for health departments. Washington: NASTAD; 2004. [Google Scholar]
- 9.Commission on Civil Rights (US) A quiet crisis: federal funding and unmet needs in Indian country. [cited 2004 Aug 10]. Available from: URL: http://www.usccr.gov/pubs/na0703/na0731.pdf.
- 10.Satcher D. Surgeon General calls for action on HIV/AIDS: in crisis proportions in American Indian and Alaska Native communities. Indian Country Today 2000 May 17. [cited 2010 Feb 4]. Also available from: URL: http://www.indiancountrytoday.com/archive/28219814.html.
- 11.Janssen RS, Holtgrave DR, Valdiserri RO, Shepherd M, Gayle HD, De Cock KM. The serostatus approach to fighting the HIV epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–24. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeNavas-Walt C, Proctor BD, Lee CH, Census Bureau (US) Income, poverty, and health insurance coverage in the United States: 2004. Washington: Government Printing Office (US); 2005. [cited 2006 Jun 23]. Income, poverty, and health insurance coverage in the United States: 2004. Also available from: URL: http://www.census.gov/prod/2005pubs/p60-229.pdf. [Google Scholar]
- 13.Freeman C, Fox MA, Department of Education (US), National Center for Education Statistics . NCES 2005-108. Washington: Government Printing Office (US); 2005. Status and trends in the education of American Indians and Alaska Natives. [Google Scholar]
- 14.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. [cited 2010 Feb 4];Clin Infect Dis. 2007 44:1493–9. doi: 10.1086/516778. Also available from: URL: http://www.journals.uchicago.edu/doi/full/10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 15.Gorgos L, Avery E, Bletzer K, Wilson C. Determinants of survival for Native American adults with HIV infection. AIDS Patient Care STDS. 2006;20:586–94. doi: 10.1089/apc.2006.20.586. [DOI] [PubMed] [Google Scholar]
- 16.Foley K, Duran B, Morris P, Lucero J, Jiang Y, Baxter B, et al. Using motivational interviewing to promote HIV testing at an American Indian substance abuse treatment facility. J Psychoactive Drugs. 2005;37:321–9. doi: 10.1080/02791072.2005.10400526. [DOI] [PubMed] [Google Scholar]
- 17.Schneider E, Whitmore S, Glynn MK, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1–8. [PubMed] [Google Scholar]
- 18.James WH, Hutchison B, Moore DD, Smith AJ. Predictors of driving while intoxicated (DWI) among American Indians in the northwest. J Drug Educ. 1993;23:317–24. doi: 10.2190/BNU4-LCDM-GL7X-TCHX. [DOI] [PubMed] [Google Scholar]
- 19.Palepu A, Tyndall MW, Li K, Yip B, O'Shaughnessy MV, Schechter MT, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruschak LM. Bureau of Justice Statistics special report (NCJ 205333) Washington: Department of Justice (US); 2004. HIV in prisons and jails, 2002. [Google Scholar]
- 21.Dean-Gaitor HD, Fleming PL. Epidemiology of AIDS in incarcerated persons in the United States, 1994–1996. AIDS. 1999;13:2429–35. doi: 10.1097/00002030-199912030-00015. [DOI] [PubMed] [Google Scholar]
- 22.Laufer FN, Jacob Arriola KR, Dawson-Rose CS, Kumaravelu K, Rapposelli KK. From jail to community: innovative strategies to enhance continuity of HIV/AIDS care. Prison J. 2002;82:84–100. [Google Scholar]
- 23.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet. 2000;355:547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 24.Mills E, Cooper C, Kanfer I. Traditional African medicine in the treatment of HIV. Lancet Infect Dis. 2005;5:465–7. doi: 10.1016/S1473-3099(05)70172-9. [DOI] [PubMed] [Google Scholar]
- 25.Kelm ME. Colonizing bodies: Aboriginal health and healing in British Columbia, 1900–50. Vancouver (BC): University of British Columbia Press; 1998. [Google Scholar]
- 26.Smith LT. Decolonizing methodologies: research and indigenous peoples. New York: Zed Books; 1999. [Google Scholar]
- 27.Waldram JB. Revenge of the windigo: the construction of the mind and mental health of North American Aboriginal peoples. Toronto: University of Toronto Press; 2004. [Google Scholar]
- 28.Middleton AE. Supplanting the medicine man. Mod Hosp. 1922;19:41–4. [Google Scholar]
- 29.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians' attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract. 1995;8:361–6. [PubMed] [Google Scholar]
- 31.Zuckerman S, Haley J, Roubideaux Y, Lillie-Blanton M. Health service access, use, and insurance coverage among American Indians/Alaska Natives and whites: what role does the Indian Health Service play? Am J Public Health. 2004;94:53–9. doi: 10.2105/ajph.94.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]