Abstract
Background
Transgenic rats with inducible expression of the mouse Ren2 renin gene [strain name: TGR(Cyp1a1Ren2)] allow induction of various degrees of ANG II-dependent hypertension. Dietary administration of the aryl hydrocarbon indole-3-carbinol (I3C) at a dose of 0.15% induces a slowly developing form of ANG II-dependent hypertension whereas dietary administration of a higher dose (0.3%) of I3C results in the development of ANG II-dependent malignant hypertension. Cessation of administration of 0.15% I3C results in the normalization of blood pressure, indicating the reversibility of hypertension induced by this dose of I3C. The present study was performed to determine if ANG II-dependent malignant hypertension is similarly reversible following cessation of dietary administration of 0.3% I3C.
Methods
Cyp1a1-Ren2 rats (n=6) were fed a normal diet containing 0.3% I3C for 11 days to induce malignant hypertension.
Results
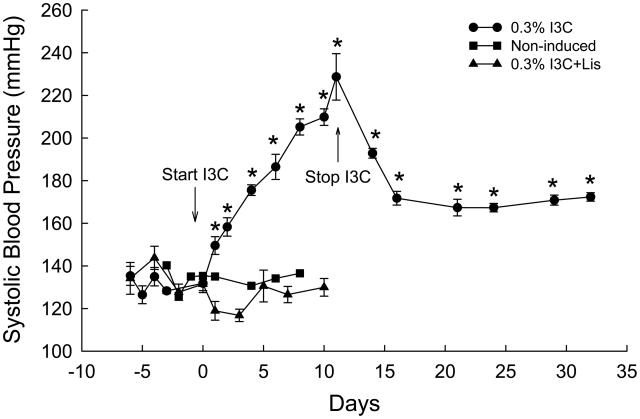
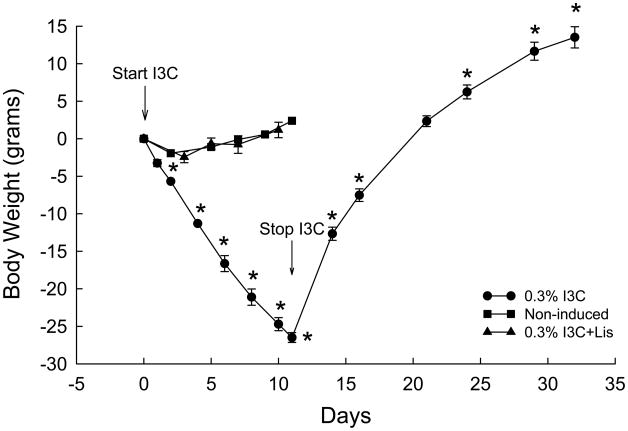
Cyp1a1-Ren2 rats induced with I3C exhibited pronounced increases in systolic blood pressure (SBP) (132±3 to 229±11 mmHg, P<0.001) and marked decreases in body weight (303±4 to 222±2 g, P<0.001). When I3C administration was terminated, SBP decreased to 167±4 mmHg (P<0.01) and body weight increased to normal levels (309±2 g, P<0.01) within 12 days. However, SBP remained significantly elevated (172±1 mmHg, P<0.01) for up to 3 weeks following termination of dietary administration of 0.3% I3C. In addition, the magnitude of the blood pressure response to intravenous bolus administration of 50 ng of ANG II (50 μl in volume) 3 weeks following cessation of dietary I3C administration was substantially higher than that observed in normotensive control rats (134±1 mmHg, n=6) not previously induced with 0.3% I3C (53±2 vs. 38±3 mmHg, P<0.05).
Conclusions
The present findings demonstrate that transient induction of ANG II-dependent malignant hypertension results in prolonged elevations of arterial blood pressure and marked augmentation of the magnitude of the pressor response to ANG II in Cyp1a1-Ren2 transgenic rats.
Keywords: kidney, renin-angiotensin system, malignant hypertension, AT1 receptors, renal hemodynamics
Introduction
The transgenic rat line with inducible expression of the mouse Ren2 renin gene [strain name: TGR(Cyp1a1Ren2)] was created by inserting the mouse Ren2 renin gene, fused to an 11.5 kb fragment of the cytochrome P450 1a1 (Cyp1a1) promoter, into the genome of the Fischer 344 rat.1 Cyp1a1, which catalyzes the oxidation of a wide range of endogenous lipophilic compounds and xenobiotics2–4, is not constitutively expressed, but is highly inducible upon exposure to various aryl hydrocarbons such as indole-3-carbinol (I3C).2–8 Induction of Cyp1a1 is mediated by the aryl hydrocarbon receptor, which is a basic helix-loop-helix-transcription factor that binds to specific DNA elements in the Cyp1a1 promoter.2,4,9 Rats transgenic for the Cyp1a1-Ren2 construct do not constitutively express the Ren2 renin gene. Rather, the Ren2 gene is expressed primarily in the liver, only upon induction of the Cyp1a1 promoter by aryl hydrocarbons such as I3C.1 In this transgenic rat line, induction of the Cyp1a1 promoter by dietary administration of I3C results in a fixed level of expression of the Ren2 renin gene and in the development of ANG II-dependent hypertension.1,10–17 Dietary administration of I3C at a dose of 0.15% induces a slowly developing form of ANG II-dependent hypertension whereas dietary administration of a higher dose (0.3%) of I3C results in the development of ANG II-dependent malignant hypertension.10–17 Cessation of administration of 0.15% I3C results in the normalization of blood pressure, indicating the reversibility of hypertension induced by this dose of I3C.10 The present study was performed to determine if ANG II-dependent malignant hypertension is similarly reversible following cessation of dietary administration of 0.3% I3C. In the present study, transient induction of malignant hypertension induced prolonged elevations of systolic blood pressure (SBP) following cessation of dietary I3C administration. Accordingly, an additional objective was to determine the ANG II-dependence of the sustained elevation of arterial blood pressure following cessation of dietary administration of 0.3% I3C.
Methods
The experimental procedures in this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center. Experiments were performed on adult male transgenic rats [TGR(Cyp1a1Ren2)] with inducible expression of the mouse Ren2 renin gene.1 All transgenic rats used in the present study were bred at Tulane University School of Medicine from stock animals supplied by Harlan UK Limited, Bicester, UK. The experimental animals were divided into three groups. Group 1 (Non-induced; n=6) transgene-negative Fischer 344 rats were maintained on a normal rat diet containing I3C at a dose of 0.3% (wt/wt; diet TD 05381, Harlan-Teklad, Madison, WI) for 11 days. Group 2 (0.3% I3C; n=6) Cyp1a1-Ren2 rats were fed a normal diet containing 0.3% I3C for 11 days to induce ANG II-dependent malignant hypertension. The rats were then placed a normal non-I3C containing diet (TD 99414, Harlan-Teklad) for 3 weeks. Group 3 (0.3% I3C+Lis; n=6) rats were fed 0.3% I3C and treated chronically with the angiotensin-converting enzyme (ACE) inhibitor, lisinopril (Lis; Sigma Chemical, St. Louis, MO) for 11 days. Lisinopril was added to the drinking water at a concentration of 50 mg/L.
Measurement of systolic blood pressure was obtained in conscious rats using tail-cuff plethysmography (Model 6R22931, IITC Life Science; Woodland Hills, CA). All rats were trained for two weeks prior to the beginning of the experiment in order to habituate them to this procedure. Blood pressures were measured every 1–3 days throughout the duration of the study. Body weight was measured every day throughout the course of the study. At the completion of the experimental protocol, renal clearance experiments were performed on all rats. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and placed on a thermostatically controlled surgical table to maintain body temperature at 37°C. A tracheostomy was performed, and the animals were allowed to breathe air enriched with oxygen, which has been shown to improve the stability of arterial blood pressure of pentobarbital-anesthetized rats.12,18,19 The left femoral artery was cannulated to allow continuous monitoring of arterial blood pressure. Blood pressure was monitored with a Statham pressure transducer (model P23 DC) and recorded using a computerized data acquisition system (MP100 system; BIOPAC Systems, Santa Barbara, CA) with the Acqknowledge software package (version 3.2.4, BIOPAC). The left external jugular vein was cannulated to allow intravenous infusion of solutions and additional anesthetic. The rats were infused intravenously, at a rate of 1.2 ml/hr, with isotonic saline containing 6% albumin (bovine; Calbiochem, San Diego, CA) during the surgery and thereafter with isotonic saline containing 1% albumin, 7.5% polyfructosan (Inutest, Lentz, Austria), and 1.5% para-aminohippuric acid (PAH, Merck & Co., INC., Whitehouse Station, NJ). A suprapubic incision was made and the bladder was exposed by blunt dissection through the abdominal wall. The bladder was catheterized to allow timed urine collections to be made. Following the surgery the rats were allowed to stabilize for one hour.
The experimental protocol consisted of an initial 60-min control period after which the rats received a single intravenous injection of the AT1 receptor antagonist, losartan (Merck & Co.; 10 mg/kg, iv). After a 30-min equilibration period, a 60-min experimental period was initiated. The effectiveness of the blockade of AT1 receptors was assessed by determining the magnitude of the pressor response to intravenous bolus injections of 50 ng of ANG II (Sigma; 50 ml in volume) during control conditions and at the end of the experimental period (some 90 min after losartan administration). During both control and experimental periods, timed urine collections and arterial blood samples (each blood sample being ~300 μl in volume) were obtained to determine whole kidney hemodynamic function.
At the end of each experiment both kidneys were removed, decapsulated, blotted dry, and weighed. Urine volume was determined gravimetrically. Inulin and PAH concentrations in both urine and plasma were measured by standard spectrophotometry. Glomerular filtration rate (GFR) and renal plasma flow (RPF) were estimated from the clearance of inulin and PAH, respectively. Values presented for RPF were not corrected for the renal extraction of PAH. Statistical analyses were performed using one-way and two-way ANOVA, one-way repeated measures ANOVA, and two-way repeated measures ANOVA followed by Student-Newman-Keuls test where appropriate. All statistical analyses were performed using SigmaPlot for Windows (version 11, Systat Software Inc., San Jose, CA). Statistical significance was defined as P < 0.05. All data are expressed as means ± SE.
Results
The effects of dietary administration of I3C on conscious systolic blood pressures (SBP) and body weights of Cyp1a1-Ren2 transgenic rats are summarized in Figs. 1 and 2. Chronic administration of 0.3% I3C for 11 days resulted in the development of severe hypertension compared with noninduced controls (229±11 vs. 132±3 mmHg, P<0.001) (Fig. 1). The development of hypertension was associated with a marked decrease in body weight (303±4 to 222±2 g, P<0.001) (Fig. 2). As shown in Fig. 1, SBP remained unaltered in non-induced Fischer 344 rats and Cyp1a1-Ren2 rats induced with I3C and treated chronically with the ACE inhibitor, lisinopril, confirming that the development of malignant hypertension is dependent on generation of ANG II secondary to the expression of the mouse Ren2 renin gene. Body weight increased from 348±3 to 356±4 grams (P<0.001) in the non-induced Fischer 344 rats, and from 327±5 to 331±5 grams (P<0.05) in Cyp1a1-Ren2 rats induced with I3C and treated chronically with the ACE inhibitor, lisinopril (Fig. 2). When I3C administration was terminated, body weight increased to normal levels within 12 days and continued to increase during the subsequent 9 days to a value 13% higher than the pre-I3C value (343±4 grams, P<0.001). However, as shown in Fig. 1, SBP remained significantly elevated (172±1 mmHg, P<0.01) for up to 3 weeks following cessation of dietary I3C administration. Body weight remained unaltered in non-induced Fischer 344 rats and Cyp1a1-Ren2 rats induced with I3C and treated chronically with the ACE inhibitor, lisinopril (Fig. 2).
FIG. 1.
Systolic blood pressures of transgene-negative Fischer 344 rats fed a normal diet containing 0.3% I3C (non-induced; closed squares); Cyp1a1-Ren2 rats induced with 0.3% I3C (0.3% I3C; closed circles) for 11 days; Cyp1a1-Ren2 rats induced with 0.3% I3C and treated chronically with the ACE inhibitor, lisinopril (50 mg/L in drinking water) (0.3% I3C+Lis; closed triangles). * P<0.01 vs. day 0.
Fig. 2.
Body weights of transgene-negative Fischer 344 rats fed a normal diet containing 0.3% I3C (non-induced; closed squares); Cyp1a1-Ren2 rats induced with 0.3% I3C (0.3% I3C; closed circles) for 11 days; Cyp1a1-Ren2 rats induced with 0.3% I3C and treated chronically with the ACE inhibitor, lisinopril (50 mg/L in drinking water) (0.3% I3C+Lis; closed triangles). * P<0.01 vs. day 0.
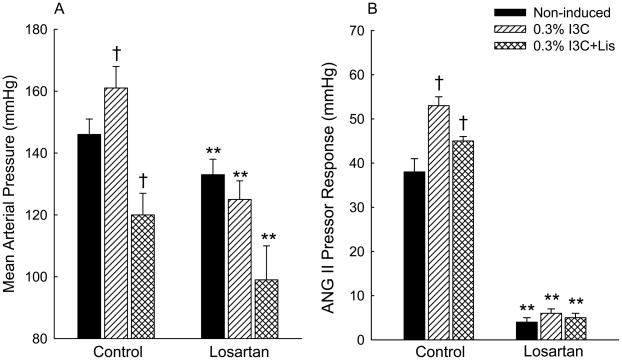
The effects of acute intravenous administration of losartan on mean arterial pressure (MAP) and on the magnitude of the pressor response to bolus injections of 50 ng of ANG II are shown in Fig. 3. Administration of losartan decreased MAP from 146±5 to 133±5 mmHg (P<0.01) in the non-induced rats, and from 120±7 to 99±11 mmHg (P<0.01) in Cyp1a1-Ren2 rats induced with I3C and treated chronically with lisinopril (Fig. 3A). Losartan administration elicited a substantially greater decrease (P<0.05) in MAP in Cyp1a1-Ren2 rats induced with I3C (from 161±7 to 125±6 mmHg, P<0.01) than in the other two groups (Fig 2A). As shown in Fig. 3B, the magnitude of the blood pressure response to intravenous bolus administration of 50 ng of ANG II three weeks following cessation of dietary I3C (53±2 mmHg) was substantially greater (P<0.05) than that observed in non-induced normotensive control rats (38±3 mmHg) and in rats induced with I3C and treated chronically with lisinopril (45±1 mmHg). Acute losartan administration markedly decreased the magnitude of the pressor response to 50 ng of ANG II in noninduced rats (4±1 mmHg, P<0.01), in rats induced with I3C and treated chronically with lisinopril (5±1 mmHg, p<0.01), and in Cyp1a1-Ren2 rats three weeks following cessation of dietary I3C administration (6±1 mmHg, P<0.01) (Fig. 3B).
Fig. 3.
Effects of acute administration of losartan (10 mg/kg, iv) on mean arterial blood pressures (A) and the pressor responses to intravenous administration of 50 ng of ANG II (B) in transgene-negative Fischer 344 rats fed a normal diet containing 0.3% I3C (non-induced; filled bars); Cyp1a1-Ren2 rats induced with 0.3% I3C (0.3% I3C; hatched bars) for 11 days; Cyp1a1-Ren2 rats induced with 0.3% I3C and treated chronically with the ACE inhibitor, lisinopril (50 mg/L in drinking water) (0.3% I3C+Lis; cross-hatched bars). **P<0.01 vs. corresponding control value; † P<0.05 vs. non-induced.
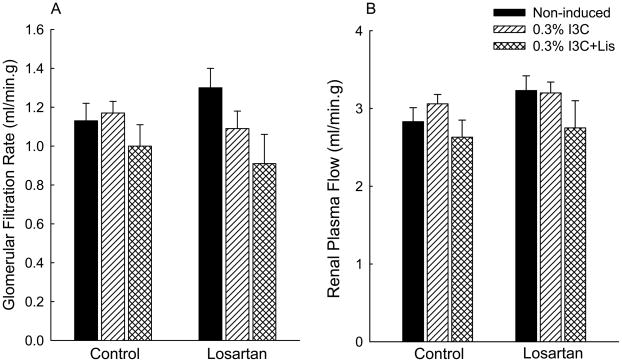
As shown in Fig. 4, despite the markedly elevated blood pressure, the control values for glomerular filtration rate (GFR) and renal plasma flow (RPF) in the hypertensive rats (i.e., rats induced with I3C alone) averaged 1.17±0.06 and 3.06±0.12 ml/min.g, respectively, values similar to those in the non-induced rats (1.13±0.09 and 2.83±0.18, respectively) and in the rats induced with I3C and treated chronically with lisinopril (1.00±0.11 and 2.63±0.22 ml/min.g, respectively). Administration of losartan did not alter GFR or RPF in any of the three groups of rats (Fig. 4).
Fig. 4.
Effects of acute administration of losartan (10 mg/kg, iv) on glomerular filtration rate (A) and renal plasma flow (B) in transgene-negative Fischer 344 rats fed a normal diet containing 0.3% I3C (non-induced; filled bars); Cyp1a1-Ren2 rats induced with 0.3% I3C (0.3% I3C; hatched bars) for 11 days; Cyp1a1-Ren2 rats induced with 0.3% I3C and treated chronically with the ACE inhibitor, lisinopril (50 mg/L in drinking water) (0.3% I3C+Lis; cross-hatched bars).
Discussion
Cyp1a1-Ren2 transgenic rats with inducible expression of the mouse Ren2 renin gene allow induction of various degrees of ANG II-dependent hypertension.1,13 Dietary administration of I3C at a dose of 0.15% induces a slowly developing form of ANG II-dependent hypertension whereas dietary administration of a higher dose (0.3%) of I3C results in the development of ANG II-dependent malignant hypertension.13 Cessation of administration of 0.15% I3C results in the normalization of blood pressure, indicating the reversibility of hypertension induced by this dose of I3C.10 The present study was performed to determine if ANG II-dependent malignant hypertension is similarly reversible following cessation of dietary administration of 0.3% I3C. Malignant hypertension is a form of severe hypertension characterized by fibrinoid necrosis of arterioles and vascular damage in many tissues, including the kidney.1,16,20–23 This transgenic line was generated by inserting a mouse Ren2 renin gene into the genome of the Fischer 344 rat.1 Extrarenal Ren2 renin gene expression is induced by the administration of the aryl hydrocarbon, I3C, resulting in the development of ANG II-dependent malignant hypertension.1,11–17 Such induction of Ren2 renin gene expression using a benign and naturally occurring dietary supplement leads to the development of ANG II-dependent hypertension as a result of increased renin gene expression and plasma renin levels, which are not subject to normal physiological feedback control mechanisms.
In the present study, induction of the Ren2 renin gene by dietary administration of 0.3% I3C for 11 days resulted in the development of severe hypertension. As described previously11–17, the hypertension was associated with a marked decrease in body weight, and the rats exhibited extreme lethargy, assumption of a hunched posture, and piloerection, which are clinical manifestations of malignant hypertension in the rat.1,13,20–23 When administration of 0.3% I3C was terminated, SBP decreased from 229±11 to 167±4 mmHg and body weight increased to normal levels (310±2 g, P<001) within 12 days. However, SBP remained significantly elevated (172±1 mmHg) for up to 3 weeks following termination of dietary administration of 0.3% I3C. Thus, in contrast to the previous observation that cessation of dietary administration of 0.15% I3C resulted in the normalization of blood pressure, indicating that the slowly developing hypertension induced by this dose of I3C is completely reversible, the present findings indicate that transient induction of ANG II-dependent malignant hypertension results in prolonged elevations of arterial blood pressure in Cyp1a1-Ren2 transgenic rats. This indicates that transient induction of malignant hypertension induces a prolonged inability of the kidneys to maintain arterial blood pressure within the normotensive range. The observation that acute losartan administration normalized the elevated arterial pressures of Cyp1a1-Ren2 rats transiently induced with I3C indicates that the prolonged elevation of arterial blood pressure observed 3 weeks following cessation of dietary I3C administration was ANG II-dependent.
The present study demonstrates that the magnitude of the blood pressure response to intravenous bolus administration of ANG II three weeks following cessation of dietary I3C was substantially greater than that observed in non-induced normotensive control rats and in rats induced with I3C and treated chronically with lisinopril. This finding indicates that the hypertensive Cyp1a1-Ren2 rats exhibited augmented peripheral vasoconstrictor responses to ANG II. Such enhanced peripheral vascular responsiveness to ANG II would likely enable the elevated circulating ANG II levels13 to exert greater peripheral vasoconstrictor effects and thereby contribute to an increased peripheral vascular resistance in hypertensive Cyp1a1-Ren2 rats. Thus, the augmented pressor responsiveness to ANG II might contribute in part to the prolonged elevation of blood pressure that occurs following transient induction of ANG II-dependent malignant hypertension in Cyp1a1-Ren2 transgenic rats.
The increased sensitivity to ANG II, in addition to the effects of losartan to normalize blood pressure, suggests a fundamental role for ANG II in maintaining the hypertension following cessation of dietary I3C administration. In this regard, we have previously demonstrated that induction of malignant hypertension by 0.3% I3C is associated with increased plasma and intrarenal ANG II levels.13 In addition, we have recently shown that AT1 receptor mRNA and protein expression in kidney cortex of Cyp1a1-Ren2 rats with malignant hypertension are not different from those in non-induced normotensive control rats.24 Thus, it is possible that the plasma and intrarenal ANG Ievels remain inappropriately elevated three weeks following cessation of dietary I3C administration. Such inappropriately elevated circulating and intrarenal ANG II levels together with maintained AT1 receptor expression in the kidney cortex would likely contribute importantly to the sustained elevation of blood pressure following transient induction of ANG II-dependent malignant hypertension.
It should also be recognized that induction of malignant hypertension may have caused renal injury which in turn could have contributed to the maintenance of hypertension following cessation of dietary I3C administration. In this regard, we have demonstrated that the kidneys of Cyp1a1-Ren2 rats with malignant hypertension exhibit myointimal hyperplasia and tubular dilation, glomerulosclerosis, and tubulointerstitial inflammation and proliferation, particularly in the perivascular areas.16 These findings demonstrate that the renal pathological changes that occur in Cyp1a1-Ren2 rats with malignant hypertension consist of inflammation and cellular proliferation in cortical vessels and tubulointerstitium.16 It is likely that such renal injury would similarly be present in the kidneys of Cyp1a1-Ren2 rats three weeks following cessation of dietary I3C administration. To the extent that this is the case, one would predict that such morphological changes, together with increased ANG II generation by the inflammatory tubulointerstitial infiltrate which has been observed in the post- ANG II salt-sensitive hypertension model25,26, would contribute to the sustained elevation of blood pressure following transient induction of malignant hypertension in Cyp1a1-Ren2 transgenic rats.
In the present study, neither GFR nor RPF was altered in the hypertensive rats compared with corresponding values observed in non-induced normotensive control rats and in rats induced with I3C and treated chronically with lisinopril. In addition, GFR and RPF remained unaltered in all 3 groups of rats following acute administration of losartan. These findings indicate that ANG II-dependent reductions in renal hemodynamics were not primarily responsible for maintaining the prolonged elevation of blood pressure that occurs following transient induction of ANG II-dependent malignant hypertension in Cyp1a1-Ren2 transgenic rats. Rather, the present findings that neither GFR nor RPF was altered in the hypertensive rats suggests that enhanced tubular reabsorption of salt and water contributes importantly to the prolonged hypertension that occurs following transient induction of ANG II-dependent malignant hypertension in Cyp1a1-Ren2 transgenic rats. Additional studies are required to address this issue.
In summary, the present findings demonstrate that transient induction of ANG II-dependent malignant hypertension results in prolonged elevations of arterial blood pressure in Cyp1a1-Ren2 transgenic rats. This indicates that transient induction of malignant hypertension induces a prolonged inability of the kidneys to maintain arterial blood pressure within the normotensive range. The observation that acute losartan administration normalized the elevated arterial pressures of Cyp1a1-Ren2 rats transiently induced with I3C indicates that the prolonged elevation of arterial blood pressure observed 3 weeks following cessation of dietary I3C administration was ANG II-dependent. The data also demonstrate that transient induction of ANG II-dependent malignant hypertension results in a marked augmentation of the pressor response to ANG II in Cyp1a1-Ren2 transgenic rats. Such augmented pressor responsiveness to ANG II might contribute in part to the prolonged elevation of blood pressure that occurs following transient induction of ANG II-dependent malignant hypertension in Cyp1a1-Ren2 transgenic rats.
Acknowledgments
The authors would like to thank Porcha D. Davis for excellent technical assistance. We also thank Dr. L. Gabriel Navar for helpful comments and suggestions, and Dr. Barb Mickelson, Harlan-Teklad, for help with the design and production of the I3C-containing rat diet. This study was supported by The Tulane COBRE in Hypertension and Renal Biology (NCRR 2P20RR017659-06), and NHLBI grant HL26371.
References
- 1.Kantachuvesiri S, Fleming S, Peters J, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SJ, Carlotti F, Hall PA, et al. Regulation of the CYP1A1 promoter in transgenic mice: an exquisitely sensitive on-off system for cell specific gene regulation. J Cell Sci. 1996;109:2619–2625. doi: 10.1242/jcs.109.11.2619. [DOI] [PubMed] [Google Scholar]
- 3.Forrester LM, Henderson CJ, Glancey MJ, et al. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281:359–368. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JD, Wong E, Ginsberg M. Cytochrome P450 1A1 promoter as a genetic switch for the regulatable and physiological expression of a plasma protein in transgenic mice. Proc Natl Acad Sci USA. 1995;92:1926–11930. doi: 10.1073/pnas.92.25.11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 6.Jellinck PH, Forkert PG, Riddick DS, et al. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 7.Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975;54:985–988. [PubMed] [Google Scholar]
- 8.Pelkonen O, Nebert DW. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- 9.Fujii-Kuriyama Y, Masatsuga E, Junsei M, et al. Polymorphic forms of the Ah receptor and induction of the CYP1A1 gene. Pharmacogenetics. 1995;5:149–153. doi: 10.1097/00008571-199512001-00018. [DOI] [PubMed] [Google Scholar]
- 10.Howard LL, Patterson ME, Mullins JJ, et al. Salt-sensitive hypertension develops after transient induction of ANG II-dependent hypertension in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol. 2005;288:F810–F815. doi: 10.1152/ajprenal.00148.2004. [DOI] [PubMed] [Google Scholar]
- 11.Patterson ME, Mouton CR, Mullins JJ, et al. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2005;289:F754–F759. doi: 10.1152/ajprenal.00419.2004. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell KD, Mullins JJ. Enhanced tubuloglomerular feedback in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2005;289:F1210–F1216. doi: 10.1152/ajprenal.00461.2004. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell KD, Bagatell SJ, Miller CS, et al. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. Journal of the Renin Angiotensin Aldosterone System. 2006;7(2):74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 14.Opay AL, Mouton CR, Mullins JJ, et al. Cyclooxygenase-2 inhibition normalizes arterial blood pressure in CYP1A1-REN2 transgenic rats with inducible ANG-dependent malignant hypertension. Am J Physiol Renal Physiol. 2006;291:F612–F618. doi: 10.1152/ajprenal.00032.2006. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz RM, Graciano ML, Mullins JJ, et al. Aldosterone receptor antagonism alleviates proteinuria, but not malignant hypertension in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol. 2007;293:F1584–F1591. doi: 10.1152/ajprenal.00124.2007. [DOI] [PubMed] [Google Scholar]
- 16.Graciano ML, Mouton CR, Patterson ME, et al. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2007;292:F1858–F1866. doi: 10.1152/ajprenal.00469.2006. [DOI] [PubMed] [Google Scholar]
- 17.Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2008;294:F205–F211. doi: 10.1152/ajprenal.00150.2007. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell KD, Navar LG. Modulation of tubuloglomerular feedback responsiveness by extracellular ATP. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F458–F466. doi: 10.1152/ajprenal.1993.264.3.F458. [DOI] [PubMed] [Google Scholar]
- 20.Kantachuvesiri S, Haley CS, Fleming S, et al. Genetic mapping of modifier loci affecting malignant hypertension in TGRmRen2 rats. Kidney Int. 1999;56:414–420. doi: 10.1046/j.1523-1755.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Whitworth CE, Fleming S, Cumming AD, et al. Spontaneous development of malignant phase hypertension in transgenic ren-2 rats. Kidney Int. 1994;46:1528–1532. doi: 10.1038/ki.1994.437. [DOI] [PubMed] [Google Scholar]
- 22.Mann SJ, Atlas SA. Hypertensive Emergencies. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Maintenance. 2. New York, NY: Raven; 1995. pp. 3009–3022. [Google Scholar]
- 23.Whitworth CE, Fleming S, Kotelevtsev Y, et al. A genetic model of malignant phase hypertension in rats. Kidney Int. 1995;47:529–535. doi: 10.1038/ki.1995.66. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Villalobos RA, Davis PD, Kim C, et al. Blockade of AT1 receptors attenuates the increase in proteinuria and elevations of kidney tissue ANG II levels in Cyp1a1-Ren2 transgenic rats fed a high salt diet. Hypertension. 2009;54(4):e65. [Google Scholar]
- 25.Franco M, Martinez F, Rodriguez-Iturbe, et al. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006;291:F1281–1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 26.Franco M, Martinez F, Quiroz Y, et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertensions. Am J Physiol Renal Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]