Abstract
Importance of the field
Immunotherapy of cancer has not improved disease-free or overall patient survival. The lack of concordance between immunologic and clinical responses in cancer immunotherapy trials is thought to result from the pervasive presence of tumor-driven immune suppression that allows tumor to escape and that has not been adequately targeted by current therapies.
Areas covered in this review
Because multiple mechanisms of tumor induced suppression have now been identified and shown to contribute to tumor escape, the opportunity arises to interfere with these mechanisms. A range of known tumor-derived inhibitors (enzymes, receptors, ligands, microvesicles and soluble factors) can now be blocked or neutralized by biologic or metabolic agents. Used alone or in combination with each other or with conventional cancer therapies, these agents offer novel therapeutic strategies for the control of tumor escape.
What the reader will gain
This review deals with currently available inhibitors for counteracting tumor immune escape. The restoration of effective anti-tumor immunity in patients with cancer will require new approaches aiming at: (a) protection of immune cells from adverse effects of myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg) or inhibitory factors thus enhancing effector functions, and (b) prolong survival of central memory T cells thus ensuring long-term protection.
Take home message
Inhibitors of mechanisms responsible for tumor escape could restore anti-tumor immune responses in patients with cancer.
Keywords: adenosine, immune suppression, inhibitory cytokines, MDSC, Treg, tumor escape
Introduction
Recent evidence supports the concept that the host immune system interacts with the developing tumor and plays a role in the arrest of tumor growth in experimental animals and in man [{Whiteside, 2010 #211}]. These interactions between the host and the tumor have been referred to as “immune surveillance,” and they conjure an image of a vigilant immune system poised to eliminate malignant cells. Indeed, antibodies (Abs) specific for tumor-associated antigens (TAA) are commonly found in patients with cancer [2, 3]. T lymphocytes specifically able to recognize these antigens are detectable as well [3], and non-specific but activated immune effector cells able to eliminate tumor cells, e.g., natural killer (NK) cells, macrophages or NKT cells, are present in the circulation of patients with cancer and at the tumor site [4]. The presence of these various elements of the host immune system in cancer-bearing hosts implies that immune cells and their products, i.e., antibodies, cytokines, enzymes or other soluble factors are potentially capable of mediating tumor rejection. Further, because some of the TAA-specific T cells present in the circulation have the memory phenotype, it has been suggested that long-lasting immunologic memory could prevent or delay tumor recurrence following a successful oncologic therapy [5]. Nevertheless, the fact remains that tumors progress in immunologically competent hosts in the face of existing and measurable anti-tumor immune responses and despite various oncologic therapies administered to cancer patients. Thus, although the components necessary for mounting an effective anti-tumor immune response are present in patients with cancer, the host usually fails to arrest tumor progression.
Numerous molecular and cellular mechanisms have been invoked in the past to explain this counterintuitive situation, largely focusing on the “immune escape” of tumors and suggesting that tumors develop the capability to avoid tumor-specific immune responses generated by the host or altogether disable the host anti-tumor immunity. This process referred to as “tumor-induced immune suppression” has been recognized in recent years and is being intensively investigated [6, 7]. It appears that tumors can interfere with all components of the immune system, affecting all stages of the anti-tumor immune response [4]. Tumor-induced immune suppression is not a classical immunodeficiency, however, as it represents a highly selective functional inhibition of those immune cells that are responsible for anti-tumor immune responses, leaving anti-bacterial or antiviral immunity unimpaired in patients with cancer.
Human tumors engineer an immune escape via a wide-range of mechanisms. Many of these mechanisms have been only recently identified [4, 6, 8]. By and large, these mechanisms either interfere with the generation of anti-tumor immune cells, thus reducing their numbers, or subvert their functions and survival [4]. Tumor-induced immunosuppression has been recognized as a serious and pervasive problem that has interfered with available cancer therapies and with the development of drugs able to control tumor progression.
Immunotherapies, including anti-tumor vaccines, adoptive T-cell transfers or delivery of exogenous cytokines are designed to activate, mobilize and otherwise up-regulate the host innate and adaptive immune responses directed at the tumor. In effect, they are expected to eliminate or reverse tumor-induced immunosuppression. This has proven to be a difficult objective to achieve. The lack of convincing and reproducible associations between immunologic and clinical responses in cancer patients treated with immunotherapy has cast doubts on the utility and effectiveness of biologic therapies in overcoming tumor-induced immune suppression [9]. Given the multiplicity of known mechanisms involved in this process and the prevailing practice of treating advanced metastatic disease with immune therapies, this lack of association between immune and clinical responses to therapy is not surprising.
Recent advances in tumor immunology have allowed for a better understanding of the mechanisms tumors use for immune escape and of the relationship each tumor establishes with the host immune system. Current investigations are overwhelmingly directed at the discovery of biomarkers that could be used to establish an “immune signature” of the tumor [10, 11]. The goal is to define the genetic, molecular and functional profiles of immune cells present in the tumor microenvironment. It is expected that definition of the immunologic profile, which is likely to be unique for every tumor, might be the key to personalized selection of immunotherapy that will ultimately result in a successful control of the tumor progression. As the molecular mechanisms responsible for tumor escape are defined and various lesions in anti-tumor functions of immune cells are identified, the selection of the best therapeutic options for the cancer patient becomes a reality. This new “personalized strategy” uses current insights into mechanisms of tumor-induced immunosuppression for the selection of therapy that fits best to the tumor immune signature and thus has the best chance for eliminating suppression and restoring normal immune cell functions.
Today, a number of biologic and non-biologic agents are available that have been pre-tested in animal models of cancer, target a known immunosuppressive mechanism induced by tumors and show an excellent therapeutic potential for cancer therapy. The objective of this review is to feature those compounds that are currently in the lead, illustrating their molecular and cellular targets as well as the mechanisms responsible for their therapeutic activity in cancer. However, it is first necessary to briefly describe the most common immunosuppressive mechanisms used by human tumors and to illustrate their role in tumor progression. With this background in mind, it will then be possible to discuss strategies available for the elimination of tumor-induced immune suppression using specific examples in the context of the tumor-specific “immune signature.”
How tumors escape from the host immune system
Molecular alterations that occur in tumor cells as the tumor progresses from the pre-malignant to metastatic phenotype are a result of genetic instability, now recognized as a principal characteristic of all tumors. Genetic changes are already detectable during early stages of tumorgenesis, become more pronounced as the tumor progresses, are the greatest in metastatic cells and are responsible for tumor heterogeneity and alterations in the antigenic epitope profile of tumor cells [12]. It has been suggested that the immune system might drive these antigenic changes via “immune editing” by eliminating those malignant cells that are sensitive to immune intervention and allowing for the selection and survival of immunoresistant variants [13]. The net result of immune editing is the tumor escape from the host immune system. Several of the mechanisms commonly used by the tumor to execute an immune escape are listed below:
1. Loss of surface antigens and selection of epitope-loss tumor variants
Due to genetic instability characterizing human tumors, molecules present on the surface of tumor cells constantly change. If anti-tumor cytotoxic T lymphocytes (CTL) are present in the microenvironment, the tumor executes a hiding strategy changing its antigenic profile. Over time, the immune system of the host selects for “epitope-loss” tumors [14] by eliminating the tumor cells expressing the epitope and leaving behind those that are epitope-negative. The result is the tumor, which is “invisible” and highly resistant to T cells responsible for tumor elimination.
2. HLA class I antigen loss
Low or absent HLA class I antigen expression on the surface of tumor cells in culture and in tissue sections has been documented for most types of human solid tumors, using monoclonal antibodies (mAbs) to monomorphic determinants of HLA class I antigens and immunohistochemical (IHC) staining [15, 16]. The frequency of HLA class I antigen loss or down-regulation ranges from 15% in primary melanoma lesions to 50% in primary prostate carcinoma [15]. In addition, selective losses in single alleles of HLA class I molecules are often seen in tumors [15, 16].
3. Down-regulation of antigen processing machinery (APM) components
Using reagents newly developed by Dr. S. Ferrone, (U. of Pittsburgh) it has been possible to demonstrate multiple defects in components of the APM in tumor cells [17]. These alterations are predicated to affect the repertoire of peptides presented by HLA class I molecules to T cells and may be responsible for the observed increased resistance of tumors to lysis mediated by CTL. The absence or diminished display of HLA class I/peptide complexes limits the recognition of tumor cells by CTL. This could be of major clinical significance, since defects in expression of HLA class I molecules by a patient’s tumor appears to be associated with a short disease-free interval and with poor survival in breast carcinoma and melanoma [18].
4. The “self” nature of TAA
The fact that most known TAA are “self”, i.e., contain non-mutated sequences, explains in part the poor immunogenicity of many tumors and the resistance of tumors to low-avidity T cells that have persisted avoiding post-central and/or peripheral tolerance selection [19]. Indeed, in the context of low avidity T-cell recognition typically requiring that high densities of specific HLA class I/tumor peptide complexes be presented, the HLA/APM system might only require subtle down-modulation to prevent tumor recognition and elimination by CTL [20].
5. Low levels of co-stimulation
IHC studies of tumor cell lines and tumor tissues have shown that human tumors often lack or express low levels of co-stimulatory molecules [21]. Although tumor cells are not “professional” APC, insufficient expression of co-stimulatory molecules on tumor cells or down-regulation of co-stimulatory molecules on APC may result in death or anergy of TAA-specific CTL. Also, dendritic cells (DC) conditioned by the tumor may be locked in an immature state, expressing reduced levels of co-stimulatory molecules and showing a diminished capacity to fully activate T cells in productive immune responses. Such interactions may result in the development of tolerance instead of anti-tumor immunity.
6. Generation of inhibitory signals
In the tumor, activating signals necessary for sustaining T-cell functions may be substituted by inhibitory signals, such as those mediated by CTLA-4, which counteracts stimulatory effects of CD28 ligation [22]. The balance of CD28 and CTLA-4-derived signals is critical to maintaining the balance between T-cell activation vs. tolerance [22, 23]. In addition, tumor cells may express inhibitory ligands such as PDL1, for example, that lead to functional skewing of effector T cells to less productive phenotypes or to the enhanced sensitivity of T cells to activation-induced cell death [4, 7].
7. Release of tumor-derived microvesicles (TMV)
Tumor cells are a source of membraneous vesicles (50–100nM in size), which are present in sera and body fluids of patients with cancer. These TMV express the membrane-form of FasL as well as HLA class I molecules, induce apoptosis of activated T cells, and promote proliferation and suppressor activity of regulatory T cells (Treg) [24]. TMV are also involved in elimination of immune cells in the peripheral circulation of patients with cancer.
8. Regulatory T cells (Treg)
CD4+CD25highFOXP3+ Treg accumulate in human tumors and the peripheral circulation of patients with cancer [25]. It is unclear whether these cells migrate to tumors or expand in situ. Because TAA are self, it is possible that Treg accumulation is a response to enforce immune tolerance. Treg contribute to down-regulation of immune activity of effector T cells by a variety of mechanisms including IL-10 and TGF-β1 production [26], enzymatic degradation of ATP to immunosuppressive adenosine [{Mandapathil, 2010 #106}, or engagement of the Fas/FasL and granzyme/perforin pathways [28]. Tumors benefit from immunosuppressive effects mediated by Treg.
9. Myeloid-derived suppressor cells (MDSC)
These bone marrow-derived immature myeloid cells (CD34+CD33+CD13+CD15−) are increased in frequency in the peripheral circulation and tumors of nearly all cancer patients [29]. They are recruited by tumor-derived soluble factors such as TGF-β1, IL-10, VEGF, GM-CSF, IL-6, PGE2. They favor tumor growth by suppressing T-cell responses via several mechanisms, including production of an enzyme involved in L-arginine metabolism, arginase 1, as well as activation of iNOS [30]. They also control production by the tumor of indoleamine-2,2-dioxygenase (IDO) involved in the catabolism of tryptophan, an amino acid essential for T-cell differentiation [31].
10. Apoptosis of activated T lymphocytes
Tumor-induced “counterattack” on immune cells results in apoptosis of substantial proportions of circulating CD8+ effector T cells in patients with cancer [{Whiteside, 2010 #211}. The responsible mechanism has been identified as overexpression of Fas (CD95) on the surface of activated T cells and cross-linking of this receptor by FasL expressed on human tumor cells [{Whiteside, 2010 #211}. More recent data suggest that tumor-mediated down-regulation of the common cytokine receptor γ chain, a subunit of receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 which binds Jak3 enabling it to phosphorylate STAT5, is responsible for defective signaling of T cells [7]. Tumor-derived factors such as PGE2, for example, can down-modulate Jak3 expression, thus interfering with the IL-2R signaling and inducing down-regulated expression of the pro-survival members of the Bcl-2 family [32]. This defective signaling in T cells represents a molecular basis for their demise in patients with cancer.
11. Tumor stem cells
The well known phenomenon of tumor “dormancy” has been recently linked to the presence in tumors of cancer initiating or cancer stem cells [33]. Characterized by the ability of self-renewal and resistance to immune as well as other signals, these tumor calls are responsible for tumor survival during oncologic therapies and for tumor recurrence.
12. Tumor associated chronic inflammation
Tissue trauma normally engenders infiltration into tissues of inflammatory cells and production of a variety of growth factors suppressing or promoting cellular proliferation. Human tumors are usually well infiltrated by mononuclear cells. Sustained inflammation at tumor sites leads to release of pro-inflammatory cytokines and reactive oxygen species (ROS). If inflammation becomes persistent, involving cell death and necrosis, the resulting cytokine cascades either up-regulate or suppress local immune responses, depending on the cellular makeup of the microenvironment [34]. Recently performed studies in cytokine KO mice show that pro-inflammatory cytokines, such as TNF-α, play a key role in inducing carcinogenesis, probably acting via the NFκB pathway [35]. Thus, chronic inflammation might promote tumor growth and favor tumor escape by establishing an immunosuppressive microenvironment in tissues [36].
The above selected examples of the effects human tumors exercise with respect to immune cells represent a spectrum of mechanisms and illustrate the diversity of immune escape strategies. Table 1 presents a selected list of molecularly-defined immunosuppressive factors produced by human tumors. It is important to note that each of these factors targets a distinct aspect of the anti-tumor immune response. The tumor co-opts different functions of immune cells and uses them to support its own growth. At the same time, the tumor develops the ability to hide from the immune attack either by mounting a “counterattack” or developing resistance to immune intervention. Thus, the escape from the host immune system is driven by the ever-evolving ability of human tumors to actively subvert local and systemic innate as well as adaptive anti-tumor immune responses. Tumors differ in their abilities to subvert immune cells by engaging one or more of the above mechanisms, and tumor aggressiveness may depend on how successfully such subversion is established and accomplished. Therefore, the immune signature of every tumor might be unique, resulting from an interaction between the tumor and the host and representing a considerable challenge to therapeutic interventions.
Table 1.
Tumor-derived factors with immunoinhibitory activities.a
| 1. Small Molecules | |
| Prostaglandin E2 (PGE2) |
|
| Epinephrine | |
| Adenosine | |
| ROS (reactive oxygen species) | Inhibits leukocyte functions via superoxide generation |
| 2. Cytokines | |
| TGF-β | Inhibits lymphocyte proliferation and perforin and granzyme mRNA expression; promotes Treg expansion |
| IL-10 | Inhibits cytokine production, including that of IL-12; promotes Treg expansion |
| GMCSF | Promotes expansion of immunosuppressive tumor-associated macrophages; recruits MDSC |
| Proinflammatory cytokines (IL-6, IL-1β, TNF-α) | Promote chronic inflammation favoring tumor growth and the immunosuppressive microenvironment |
| 3. Enzymes | |
| Indoleamine-2,3-dioxygenase (IDO) | Inhibits T-cell activation by depleting tryptophan, an essential amino acid |
| Arginase I | Metabolizes L-arginine, another essential amino acid for T cells |
| iNOS | Produces immunosuppressive nitric oxide |
| COX2 | Produces immunosuppressive PGE2 |
| Ectonucleotidases | Catalyze breakdown of ATP to immunosuppressive adenosine |
| 4. Death-receptor Ligands | Induce apoptosis via the corresponding receptors expressed on immune cells |
| FASL\FAS | |
| TRAIL\TRAIL-R | |
| TNF\TNF-R1 | |
| 5. Immune regulatory ligands | |
| B7-H1 (PD-L1) | Binds to PD1 and inhibits lymphocyte and DC functions |
| MICA/B | Block cytolytic activity of NK cells and T cells |
| 6. Tumor-derived microvesicles (MV) | Induce apoptosis of activated CD8+ T cells; promote expression/function of Treg and MDSC |
| 7. Viral-related products | |
| p15E (CKS-17, synthetic peptide) | Inhibits production of type I cytokines, upregulates IL-10 synthesis |
| EBI-3 (homologue of IL-12 p40) | Inhibits IL-12 production |
| 8. Tumor-associated gangliosides | Inhibit IL-2-dependent lymphocyte proliferation; induce T-cell apoptosis; suppress NFκB activation; interfere with DC generation |
This is a partial listing of tumor-derived immunoinhibitory factors. Human tumors have evolved a wide range of mechanisms which effectively incapacitate the host immune system.
Strategies for counteracting tumor escape
The problem presented by cancer-induced immune suppression has been acknowledged by the medical community relatively recently and since then, immunotherapy of cancer, which is aimed at the restoration of the anti-tumor host response, has been gaining acceptance. Most oncologic therapies are directed at the elimination of tumor cells and decreasing tumor load. In general, little attention has been paid to interactions between the tumor and the host immune system in selecting therapies, and until recently, no consideration has been given to the possibility that immunotherapy as well as conventional radio-chemotherapy might alter these interactions in unpredictable and surprising ways. Unfortunately, mechanisms evolved by tumors for disarming host defenses and for escape emerge as the major obstacles to successful immunotherapy. The problem of how to reduce or eliminate tumor-induced immunosuppression is complicated not only by a large number of responsible mechanisms, as described above, but also by the fact that each tumor creates a unique microenvironment and has a unique immune signature. Hence, the rational strategy for cancer immunotherapy consists of the initial definition of this signature followed by the informed selection of therapies that would target the predominant suppressive mechanisms at hand and test the safety and effectiveness of these therapies in appropriately designed clinical trials. Table 2 and Figure 1 list potential strategies that are being advanced for a design of more effective therapies aiming at the elimination of immune suppression in the tumor microenvironment, with a concomitant activation of anti-tumor immune responses.
Table 2.
Potential strategies for the design of therapies aimed at counteracting tumor-induced immunosuppression.
| Immunostimulation to induce and sustain activity and survival of anti-tumor effector cells: |
| Antibody-based therapies |
| Anti-tumor vaccines |
| Adoptive T-cell transfers |
| Delivery of “survival” cytokines |
| Use of potent adjuvants to switch Th2 to Th1 responses |
| Elimination of existing immune suppression: |
| Inhibit production of tumor-derived suppressive factors |
| Inhibit activity of tumor-derived suppressive factors |
| Inhibit generation/function of Treg and/or MDSC |
| Alteration of the tumor microenvironment in favor of immune but not tumor cells |
| Combination of immunotherapies with chemotherapy |
| Treatment of early disease or in an adjuvant setting before immunosuppression overwhelms anti-tumor immune responses |
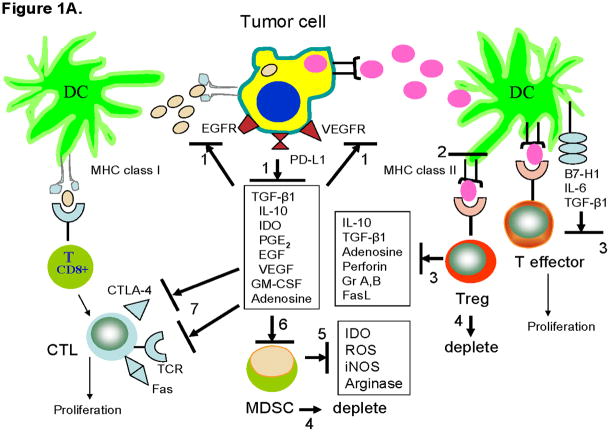
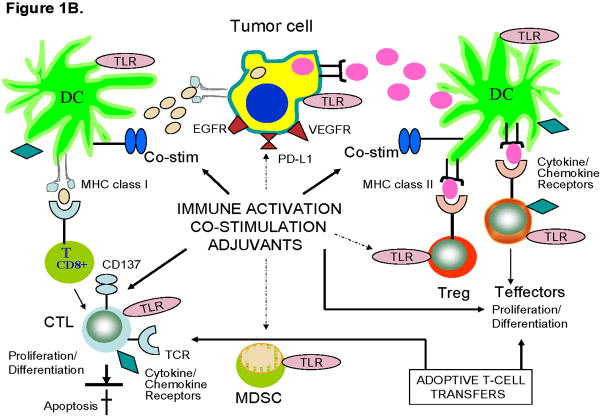
Figure 1. Strategies for countering tumor-induced immune suppression.
A. Inhibition of tumor-induced inhibitors. The tumor presents antigens to DC using the MHC class I or class II molecules. In the suppressive tumor environment, anti-tumor immune responses are inhibited by a variety of mechanisms. To restore anti-tumor immune responses and to counter tumor-induced inhibitory effects, it is now possible to block these mechanisms using biologic or non-biologic agents. Blocking of inhibitory pathways or signals is indicated by solid black lines. The numbers refer to various available inhibitors as follows: 1) mAbs specific for growth factor receptors on tumor cells or for inhibitory receptors such as PD-L1; 2) interference with Treg induction by altering the DC program; 3) neutralization of Treg-derived or DC-derived factors/molecules with mAbs or inhibitors; 4) depletion of Treg and/or MDSC with antibodies, immunotoxins or drugs such as Sunitinib; 5) blocking activity of MDSC-derived immunoinhibitory factors with metabolic inhibitors; 6) inhibition of MDSC recruitment from the bone marrow or their activity with mAbs or inhibitors specific for tumor-derived factors; 7) T-cell checkpoint blockade with mAbs such as anti-CTLA-4 or others targeting receptors that can bind tumor-derived inhibitory ligands. Metabolic antagonists are available to block activities of IDO, PGE2 and adenosine (Table 3).
B. Activation of immune cells in the tumor microenvironment, their replacement via adoptive transfers or cytokine-mediated extension of their survival. Solid arrows indicate activating signals targeting TLRs or other receptors and co-stimulatory molecules, e.g., APC-associated CD40, expressed on immune cells. Dashed arrows indicate the possibility that tumor cells, MDSC and Treg, all of which express TLRs, may be also activated with adjuvants targeting these receptors. The adoptively transferred T cells with anti-tumor activity re-populate the lymphocyte pool. Chemokines and cytokines such as IL-7 or IL-15 promote T-cell growth, protect them from apoptosis and prolong their survival. In A and B, thin arrows indicate that CTL and CD4+ effector T cells proliferate/differentiate into competent anti-tumor effector T cells and memory T cells as a result of immune activation or inhibition of tumor-derived factors.
The currently available therapies that target tumor-driven immunosuppressive mechanisms are listed in Table 3 and fall into several distinct categories: 1. use of mAb specific for TAA in order to eliminate TAA-expressing tumor cells, promote formation of strongly immunogenic Ag-Ab complexes, and enhance the development of anti-tumor humoral and cellular responses (mAb therapy); 2. cytokine-mediated protection of activated immune T cells from apoptosis, re-modeling of pro-inflammatory tumor microenvironment and broadly-based up-regulation of immune cell functions (cytokine therapy); 3. delivery of immune adjuvants generally in combination with therapeutic anti-tumor vaccines to patients with cancer, aiming at the activation of anti-tumor responses and the development of long-lived immunologic memory (adjuvant/vaccination therapy); 4. stimulation of T cells alone or in conjunction with adoptive transfers of in vitro engineered T cells in order to increase anti-tumor effector functions and in vivo survival of these cells (cellular therapy); 5. elimination of Treg and/or MDSC and blocking of the soluble factors produced by these cells (cell depletion/neutralization therapy); 6. use of small molecules to block suppressive signaling (molecular therapy); 7. identification and elimination of cancer stem cells (cancer stem cell therapy).
Table 3.
Immunotherapy drugs aimed at counteracting tumor-induced immunosuppression.a
| Therapy | Targeted pathway | References |
|---|---|---|
| Monoclonal antibodies approved by FDAb | ||
| Rituximab (anti-CD20) | Inhibits B-cell proliferation/survival | [110] |
| Trastuzumab (anti-HER2/neu) | Inhibits receptor signaling | [111] |
| Cetuximab (anti-EGFR) | Inhibits receptor signaling | [112] |
| Bevacizumab (anti-VEGF) | Blocks blood vessel growth | [113] |
| Monoclonal antibodies in developmentc | ||
| anti-CTLA-4 (Ipilimumab, Tremelimumab) | T-cell checkpoint blockade | [42] |
| anti -PD1 | T-cell checkpoint blockade | [44] |
| anti-CD137 (anti-4-1BB) | T-cell activator | [114] |
| anti- IL-10 | Inhibitor of suppression | [52] |
| anti-CD40 (CP870,893) | APC stimulator | [55] |
| anti-TNF-α | Blocks pro-tumor activity | [79] |
| Cytokines | ||
| IL-15 | T-cell growth factor | [65] |
| IL-7 | T-cell growth factor | [67] |
| IFN-α2 | Immune cell activator | [72] |
| IL-12 | DC activator, vaccine adjuvant | [70] |
| GM-CSF | DC polarization, vaccine adjuvant | [73] |
| Immune adjuvants | ||
| TLR ligands and agonists: | Vaccine adjuvants | [80] |
| MPL | Vaccine adjuvant | [81] |
| Resiquimod | Vaccine adjuvant | [115] |
| CpG | Vaccine adjuvant | [82] |
| Poly I:C and Poly ICLC | Vaccine adjuvant | [83] |
| Small molecule inhibitors | ||
| Indomethacin, Diclofenac, Colecoxib | Block COX-2/PGE2 activity | [88] |
| Metalloproteinase inhibitors | Block MICA/MICB cleavage | [116] |
| Aminobisphosphonates (zoledronate) | γδ T-cell agonist | [87] |
| Circumventing activity of Treg or MDSC | ||
| Daclizumab (anti-CD25) | T reg depletion | [60] |
| Ontak™ (denileukin diftitox) | Treg-depleting immunotoxin | [58] |
| Anti-CD25 + toxins | Treg-depleting immunotoxin | [117] |
| Ectonuclotidase inhibitors | Inhibit ATP hydrolysis by Treg | [94] |
| Adenosine receptor antagonists | Block adenosine signaling | [94] |
| 1-MT(1-methyl tryptophan) | IDO inhibitor | [31] |
| N-hydroxy-nor-L-arginine | Arginase I inhibitor | [93] |
| Sunitinib; other drugs | Eliminates MDSC/Treg | [63] |
| Phosphodiesterase-5 (PDE5) inhibitors | Block MDSC activity | [118] |
| Adoptively transferred T cells | ||
| After lymphodepletion | Re-population with TAA-specific T cells | [99] |
| Engineered T cells | Improve delivery to tumor cells | [101] |
| Targeting of cancer stem cells | Elimination by Abs and/or T cells | [33] |
The drugs/biologic agents listed are selected from a larger portfolio of available drugs based on recommendations of the 2007 NCI Workshop [37] and current use in clinical trials for patients with cancer. Other promising drugs are listed in the summary of the Workshop [37].
Not listed are agents countering oxidative stress or hypoxia usually present in the tumor microenvironment. The four mAbs listed represent a group of nine currently approved by FDA for therapy of cancer.
These mAbs represent other mAbs now in development based on their exceptional pre-clinical therapeutic potential for inhibition of tumor-induced suppression or activation of immune cells in the tumor microenvironment.
At the time human tumors are diagnosed and treated, they are well established and have immunosuppressive mechanisms in place. The balance between immunogenic and tolerogenic signals delivered to immune cells in the tumor microenvironment is strongly skewed toward tolerance. Therefore, to tip the balance in favor of immunostimulation and away from immunosuppression, immune therapies should be administered in the minimal residual disease setting, i.e., following surgery or other adjuvant therapy, when the tumor is at a disadvantage and the immune system is at least partially relieved from suppression.
Immunotherapy drugs targeting cancer-induced immune suppression
The recent workshop organized by NCI (July 12, 2007) has developed a ranked list of immunotherapy agents with a high potential to serve as effective anti-tumor drugs [37]. It names 12 different agents, all of which have a proven ability to either augment T-cell responses or reverse immune inhibition. Further, the list arranges the drugs in order of priorities established by the workshop participants and based on the future potential of the drugs to exert a significant impact on therapy of cancer. It is important to note that antibodies (Abs) to inhibitory cytokines and to molecules that mediate downregulatory signals occupy a prominent place among these agents. It has been acknowledged by the participants that blocking of tumor-induced immunosuppression is a major unsolved problem in oncology, and that the development of approaches to eliminate or reduce debilitating effects of tumor-derived factors on the host immune system is a priority. Based largely but not entirely on the criteria established during the workshop, Table 3 lists the immunotherapy drugs designed for counteracting immune suppression that are either already in the clinic or are being developed for the near-future clinical use.
Antibodies (Abs). Tumor-targeting Abs have been in the clinical use for many years. Today, they belong to the category of “molecular targeted therapy” of cancer and are considered as promising anti-cancer “drugs.” Monoclonal anti-cancer Abs (mAbs) of yesterday have been humanized and re-designed for safer and more effective delivery to cancer patients. However, only a handful of these Abs is currently approved by Food and Drugs Administration (FDA) for cancer therapy (Table 3), including Rituximab (Rituxan) targeting CD20 and Trastuzumab (Herceptin), the two Abs widely used for therapy of B-cell lymphoma [38] or HER-2-positive breast cancer, respectively [39]. These and other FDA-approved Abs (currently, nine Abs targeting six TAA are approved) were tested alone or in combination with chemotherapy in phase III clinical trials and were shown to be clinically effective for specific indications. Many of these Abs target tumor components or tumor vessels aiming at malignant cell elimination or suppression of tumor growth. Other Abs target immunoinhibitory factors known to be produced by human tumors. In view of the promising clinical results obtained with the approved Abs, albeit often in a subset of cancer patients only, large-scale efforts are ongoing to produce new Abs reactive with targets expressed on human tumors or to identify new targets for already approved Abs. Anti-cancer Abs are considered to be promising anti-cancer drugs, because they have a favorable toxicity profile and simultaneously engage several distinct host effector mechanisms [40]. In addition to directly interfering with tumor cell progression and/or survival, Abs activate cell-mediated cytotoxicity (ADCC) by ligating activating Fc receptors (FcγRI, FcγRIIA, FcγRIIC and FcγRIII) expressed on various immune cells and form immunogenic Ag-Ab complexes, which are avidly processed by APC, favoring the development of Th1-type immune responses. Binding of immune complexes to FcRs on APC results in their phagocytosis and presentation of peptides on MHC class I and class II molecules. This form of cross-presentation elicits especially effective peptide-specific T-cell responses [41]. Abs neutralizing immunosuppressive cytokines such as IL-10 or TGF-β1, Abs inhibiting signals induced by tumor-associated inhibitory ligands or Abs designed to remove suppressor cells, all target immuno-suppressive elements produced or expressed by human tumors (Table 3). More recent data suggest that Abs also promote the development of tumor-specific CTL, perhaps via the mobilization of several anti-tumor mechanisms, which leads to release of TAA in the biologically-active form thus skewing APC toward the Th1-type response. Table 3 lists several of the especially promising Abs that are already being tested in ongoing clinical trials.
Abs that are not yet FDA approved but are being widely evaluated in clinical trials include anti-CTLA-4 Ab, which recognizes cytotoxic T lymphocyte antigen-4, an inhibitory molecule expressed on activated T cells as well as on Treg [22, 42]. CTLA-4 is a negative immunoregulatory receptor. It competes for the same ligands (B7.1 and B7.2 expressed on APC or tumor cells) as CD28 but with substantially higher affinity and avidity [42]. Thus, it out-competes CD28, and plays a key role in down-modulating immune responses initiated by interaction of APC with T cells. In cancer, where activation of tumor-reactive T cells is desirable, negative signaling via CTLA-4 inhibits T-cell effector functions and limits responses to TAA. Anti-CTLA-4 Abs inhibit negative signaling in T cells, restore their responsiveness to TAA and up-regulate anti-tumor activities. For these reasons, the CTLA-4 blockade with Abs is being explored as a novel and potentially effective therapy for melanoma and other solid tumors [43].
Programmed death-1 (PD-1) is another negative receptor that regulates T-cell functions. [44]. PD-1 engagement down-regulates T cell activation [44]. Its ligand, the programmed death -1 ligand (PD-L1), is expressed on tumor cells and plays a role in tumor escape. It enables the tumor to trigger receptor-mediated apoptosis in activated T or NK cells, which are positive for PD-1. Blocking of the PD-1/PD-L1 pathway can increase anti-tumor T-cell immunity and has led to tumor rejection in experimental animals [45]. Recently, it has been reported that the PD1 blockade also reversed suppression of melanoma antigen-specific CTL by reducing Treg activity [46]. Anti- PD1 Abs and/or anti-.PD-L1 (anti-B7-H1) Abs with a potential to interfere at this important T-cell checkpoint are in the development stage.
Neutralizing Abs to immunoinhibitory cytokines, IL-10 and TGF-β1, are among the agents prominently considered at the NCI-sponsored workshop [37], because of their potential to successfully reverse tumor-induced suppression. These cytokines are products of tumors, tumor-infiltrating immune cells and/or tissue cells in the tumor microenvironment [47]. For example, the abilities of TGF-β1 to suppress immune cells functions is well documented as are its contributions to the re-modeling of the tumor microenvironment, recruitment of Treg and MDSC to the tumor and enhancing Treg survival and function by inducing expression of the transcription factor, FOXP3 [47, 48]. A number of Abs have been raised against TGF-β, and some are in early clinical trials for therapy of human solid tumors, with preliminary data indicating little toxicity [49]. IL-10 can be produced by tumor cells, TAMs, TILs or PBMC in cancer patients and has pleiotropic activities [50]. Its significance in cancer progression or down-regulation of anti-tumor immunity is controversial [50]. Tumor-derived IL-10 down-regulates expression of co-stimulatory CD40 in APC, thus impairing their stimulatory functions and interfering with anti-tumor responses [51]. Abs to IL-10 or its receptors are in human clinical trials for indications other than cancer [52], and it is expected that in the near future, they will be applied as promising signaling inhibitors for the treatment of cancer.
Agonistic Abs to CD40 and/or CD40L belong to the class of drugs that up-regulate stimulatory activities of APC [53]. CD40, a member of the tumor necrosis factor (TNF) receptor super family, is a co-stimulatory protein expressed on APC and it is the target of the agonistic Ab. Signaling via this receptor enhances HLA expression and IL-12 production by APC and leads to T-cell activation [54]. Other potentially therapeutic effects of these Abs include direct tumor inhibition, interference with angiogenesis and activation of NK cells, B cells and macrophages. Agonistic anti-CD40 mAbs are currently in phase I clinical trials in patients with cancer [54], and preliminary results are encouraging. Pre-clinical studies indicate that CD40 agonists also have a great therapeutic potential when used in combination with other oncologic therapies.
Circumventing Treg accumulation
Depletion of CD4+CD25high Treg, which accumulate in response to cancer progression and suppress functions of effector T cells, by using anti-CD25 Abs has been successfully performed in tumor-bearing animals, human PBMC in vitro and in clinical trials [55, 56]. Early clinical trials utilized the immunotoxin, denileukin diftitox (Ontak™ Ligand Pharmaceuticals), approved for therapy of cutaneous T cell lymphoma [57]. More recently, denileukin diftitox has been also used in therapies of patients with other tumors in combination with anti-tumor vaccines [58]. This immunotoxin depletes Treg due to its ability to bind to CD25. Another anti-CD25 MAb, daclizumab [Hoffman-La Roche] has been used for treatment of T cell leukemia [59] and more recently in conjunction with a multipeptide vaccine in patients with metastatic breast cancer [60]. Engineered IL-2 antagonists which comprise mutants with signal-deficient β or γ subunits have been developed and represent a novel approach to CD25-mediated inhibition of Treg [60]. However, because anti-CD25 Abs deplete not only Treg but also activated (CD25+) effector T cells, and because they have only transient effects on Treg depletion, other Abs with specificity for human Treg, e.g., anti-glucocorticoid-induced TNF receptor (GITR) Abs, would be a potentially more acceptable alternative. In view of the lack of a definitive surface marker for human Treg, the development of suitable Treg depleting Abs has been delayed, and Treg-depleting, low doses of cyclophosphamide are currently used for this purpose [61].
Circumventing accumulation of MDSC
Sunitinib is a tyrosine kinase inhibitor which has proved to be effective in reducing tumor-induced immune suppression [62]. Approved for therapy of patients with renal cell carcinoma (RCC), Sunitinib has a reported response rate of 48% as a front line drug [62]. The mechanisms responsible for this remarkable response involve not only direct anti-tumor effects of the drug but also its ability to deplete MDSC that accumulate at the tumor site as well the peripheral circulation of patients with cancer. As shown by Finke et al, Sunitinib selectively induces apoptosis in MDSC, effectively decreasing their numbers and restoring T-cell ability to secrete IFN-γ [63]. As such, Sunitinib is one of the most promising drugs for eradicating tumor-induced immune suppression. Pre-clinical studies suggest that other chemotherapeutic agents, notably gemcitabine and 5-fluorouracil also target and eliminate MDSC.
Cytokines for counteracting immune suppression
In addition to above-discussed IL-10 and TGF-β, Table 3 lists several other cytokines available as recombinant proteins that show exceptional promise for reducing in various ways tumor-induced immune suppression. One category of these cytokines is represented by T-cell growth factors, IL-15 and IL-7. IL-15 inhibits antigen-induced cell death of T cells, reverses T-cell anergy induced by tumor-derived factors [64] promotes in vitro differentiation of DC, enhances NK cell activity it is necessary for maintenance and survival of CD8+ T cells [65] and, unlike IL-2, it does not support activity of Treg [66]. IL-7, is another survival cytokine for T cells, primarily naïve T cells, and thus is essential for T-cell development [67]. Administered to patients, it induces dramatic increases in numbers of peripheral CD4+ and CD8+ T cells without apparent toxicity [68]. These two cytokines currently viewed as promising immunorestorative drugs, are likely to be increasingly often used in combination with other immunotherapies or conventional cancer therapies as adjuvants and stimulators of anti-tumor activities of immune cells.
IL-12 is a potent immune adjuvant which promotes IFN-γ release from immune cells expressing IL-12R [69]. It is capable of inducing Th1 polarization and proliferation of effector T cells producing IFN-γ. Used as a single agent in early anti-cancer clinical trials, it showed only modest anti-tumor efficacy, but more recent studies confirm its value as a potent vaccine adjuvant and a polarization cytokine for Th1 responses [70].
IFN-α2 given alone or in combination has been explored for therapy of malignant melanoma in multiple clinical trials [71, 72]. Current biologic evidence indicates that this cytokine has a potent impact on the modification of the tumor microenvironment, STAT signaling in tumor cells as well as immune cells and polarization of immune responses in favor of enhanced anti-tumor reactivity [72].
GM-CSF is approved by FDA as a hematopoietic growth factor, and thus is widely available for investigating its effects as immune cell stimulator in pre-clinical experiments and in clinical trials [73]. It is primarily used as an adjuvant in anti-tumor vaccines designed to restore immune responses blunted by the tumor [74]. More recent data suggest, however, that GM-CSF may not be an effective adjuvant; instead it may blunt vaccine-induced anti-tumor responses [75].
TNF-α is an inflammatory cytokine which plays a major role in tumor-induced inflammation [76]. Its pro-tumor activity [77] has suggested the use of TNF antagonists for therapy of cancer, which have been approved and widely used for treatment of rheumatoid arthritis and other inflammatory diseases [78]. Tumors are known to constitutively produce TNF-α, and this tumor-derived cytokine has been shown to enhance tumor proliferation in experimental animals [77]. This being the case, neutralization of TNF with anti-TNF Abs or other TNF antagonists was recently introduced as a potential therapy for patients with advanced malignancies [79].
Immune adjuvants for anti-tumor effector cells or vaccines
To be successful, therapy for overcoming tumor-induced immune suppression depends on the use of potent immune adjuvant. This is because in patients with cancer, especially those with an established or advanced disease, immune suppression is not only extensive, affecting all stages of the immune response, but also driven by a variety of mechanisms, as discussed above. To this end, a list of the available adjuvants is extensive, although many are approved for uses other than anti-tumor vaccination. Adjuvants can also be administered in combination with Abs or with other adjuvants. Among those with especially favorable potential for up-regulating immune functions are toll-like receptor (TLR) agonists [80]. Examples are monophosphoryl lipid A (MPL) specific for TLR 4 [81]; CpG, a potent TLR9 agonist [82]; poly I:C or double-stranded polyinosinic:polycytidylic acid and poly ICLC (poly I:C stabilized with poly-L-lysine and carboxymethylcellulose) which are TLR3 agonists [83] and imiquimod representing a class of drugs which are TLR7/8 agonists [84]. Imiquimod and BCG, are the two immune adjuvants currently approved for cancer therapy; however, they are not suitable for systemic delivery, and other TLR agonists, including the synthetic agonists of TLR9 such as PF-3512676, are being tested in the clinic. All these TLR agonists activate human DC, induce production of cytokine cascades from immune cells, improve antigen presentation, enhance Th1 polarization, and drive anti-tumor CTL and NK cell responses. They are being widely used as components of peptide-based anti-tumor vaccines as well as DC-based vaccines, as monotherapies for activation of innate or adaptive immune responses and in combination with other categories of adjuvants or adjuvant cytokines for therapy of cancer or viral infections. Nevertheless, it is best to remember that TLR are expressed not only by immune cells but also by tumor cells and in the presence of the respective ligands signal to promote tumor growth and increase tumor cell resistance to drugs and immune intervention [85]. Thus, the use of adjuvants in cancer may be a proverbial double-edge sword, unless the adjuvant is carefully dosed and its effects on cellular networks are understood. In addition to adjuvants broadly stimulating immune cells, factors that selectively activate one cell subset are available. Flt3 ligand (Flt3L) is a hematopoietic growth factor able to induce differentiation of DC from their progenitors [86]. Flt3L has been used as systemic therapy to increase numbers of DC after bone marrow transplantation and as a component of peptide vaccines. However, its anti-tumor effects when administered as a single agent have been limited, and its future applications will likely be in combination with vaccines to enhance DC functions.
T-cell stimulators
Agonists of cell T-cell functions, including cytokines, Abs and adjuvants are many, and some have already been described above. The best known T-cell activating cytokine is IL-2. High dose systemic administration of IL-2 has been approved for therapy of melanoma and RCC, and substantial progress has been made in making this therapy less toxic and safe for patients with cancer. More recently, a combination of zoledronate (an aminobiphosphonate widely used to treat osteoporosis or multiple myeloma) and low-dose IL-2 has been used in a phase I clinical trial to treat patients with metastatic hormone-refractory prostate cancer [87]. The objective was to activate peripheral blood γδ T cells previously reported to be responsive to zeledronate [87]. The mechanisms responsible for therapeutic effects observed in this trial are being linked to a long-term shift of γδ T cells to an activated effector-memory phenotype and to production of IFN-γ and perforin by these cells. This combination of zeledronate with IL-2 represents a novel approach to inducing immunologic and clinical responses in patients with cancer.
Metabolic inhibitors of tumor-derived factors
This category of drugs includes a large number of inhibitors used to attenuate activities of immunoinhibitory enzymes, cytokines antagonists and small molecules modulating functions of immune cells in the tumor microenvironment. Arachidonic acid degradation by COX and prostaglandin E synthase activity in tumors yield immunoinhibitory prostaglandins and thromboxanes [88]. PGE2 is a well known promoter of tumor growth, angiogenesis and tumor-cell migration. It also mediates profoundly inhibitory effects on immune cells, down-regulating production of inflammatory chemokines and Th1-type cytokines and inhibiting DC differentiation, T cell proliferation and anti-tumor functions of effector T cells [89]. Therefore, inhibition of COX-2 and of PGE2 production in the tumor microenvironment represents a desirable strategy. Selective COX-2 inhibitors, such as celecoxib or rofecoxib, have been shown to suppress growth of human tumors and to up-regulate immune reactivity in vivo [90] and to abrogate expansion of Treg in vitro [91]. However, cardiovascular risks associated with these drugs might outweigh their benefits, and the development of newer, less toxic inhibitors of this pathway will likely be undertaken in the future.
Another enzyme playing important immunosuppressive role in the tumor microenvironment is a tryptophan-catabolizing enzyme indoleamine 2, 3-deoxygenase (IDO). It is expressed in many tumor cells and catalyzes tryptophan degradation, leading to deficiency on tryptophan, an amino acid essential for lymphocyte proliferation [31]. It is apparently also involved in accumulation of Treg [92]. 1-Methyl tryptophan (1MT) is a small molecule inhibitor which effectively blocks activity of IDO and reverses immunosuppression [31]. Another enzyme present in the tumor microenvironment, arginase I, also inhibits T-cell receptor expression and antigen-specific T-cell responses by catabolizing L-arginine, an essential amino acid necessary for T lymphocyte function [93]. MDSC accumulating in cancer are responsible for arginase I production [93]. Its activity can be inhibited by N-hydroxy-nor-L-arginine also known as Nor-NOHA. Still another immunosuppressive factor often present in abundance in the tumor microenvironment is adenosine, a purine nucleoside [94]. The synthesis of adenosine involves catabolism of adenine nucleotides (ATP, ADP and AMP) by the action of extracellular ectonucleotidases, CD39 and CD73. It can be a product of tumor cells and it is also produced by Treg, which have been shown to express CD39 and CD73 [{Mandapathil, 2010 #106}. Once adenosine is released, it binds to adenosine receptors, various forms of which (i.e., A1, A2a, A2b and A3) are expressed on multiple types of immune effector cells. Adenosine can modulate immune responses mediated by these cells via up-regulation of intracellular cAMP levels depending on conditions and other signals generated in the microenvironment [94]. Adenosine is a major immunosuppressive factor produced by Treg, and a large number of small molecular weight inhibitors and adenosine receptor antagonists, some already in the clinic for other indications, are available for blocking immune suppression mediated by adenosine [95].
Adoptively-transferred T cells
This form of experimental immunotherapy has been in use for over 25 years, has been shown to induce long-term clinical responses in a small subset of patients and is currently gaining in acceptance for treatment of solid malignancies [96, 97]. It relies on the in vitro manipulation and expansion of autologous cancer-reactive T cells, which can be obtained from peripheral blood and body fluids such as malignant ascites or tissues such lymph nodes or tumors. Following the in vitro expansion, these T cells are re-infused to patients with cancer in expectation that they will eliminate tumor cells on the one hand and generate anti-tumor immunity and memory on the other [98]. Tumor-infiltrating lymphocytes (TIL), considered to be enriched in tumor-reactive T cells, have been successfully expanded in culture and delivered to patients with metastatic disease, especially melanoma. Adoptive transfer of T cells is enhanced in patients lymphodepleted with chemotherapy, perhaps because elimination of endogenous lymphocytes “creates room” for re-population with transferred and more functional T cells and/or because it eliminates competition for endogenous cytokine pools necessary for T-cell differentiation and survival [99]. In the context of adoptive transfers of ex vivo cultured or genetically-engineered T cells performed following lymphodepletion, recombinant IL-2 has been usually employed to provide transferred T cells with a factor able to support their survival and functions [100]. More recently, the “survival” cytokines, IL-15 and IL-7, have emerged as new and better additives that appear to significantly increase treatment efficacy.
Recently, adoptive T-cell therapy with modified or engineered T cells has offered a greater therapeutic potential by supplying to patients T cells with the improved capability to recognize tumor antigens and home to tumors. Two strategies for T-cell engineering have been developed: (a) genetic transfers of T cell receptors (TCR) selected for recognition of tumor antigens of choice and (b) genetic modifications of T cells to express “chimeric” or fusion receptors, which combine antigen-binding domains of the B-cell receptor with the signaling components (the ζchain complex) of the TCR [101]. The so called “T-bodies” can directly bind to antigens on tumor cells, leading to T cell activation. Both strategies are being tested in clinical trials with promising initial results that are, however, tempered by unexpected toxicities due to the target antigen expression on vital organs such as liver. The choice of a target antigen is the critical issue in adoptive therapy with engineered T cells because of their potential for inducing damage of normal tissues [102].
Cancer stem cell elimination
The existence of cancer stem cells (CSC) and their role in promoting tumor progression have been controversial. Nevertheless, current studies suggest that cells characterized by multi-drug resistance and the ability to self-renew, proliferate and form tumors upon transfer into experimental animals can be identified and isolated from human tumors. Sometimes referred to as “cancer-initiating cells,” they were considered to be rare components of human tumors [33]. While CSC may not directly mediate tumor-induced suppression, they provide tumors with a way to acquire resistance to therapy and to survive. Hence, their detection and elimination is of considerable importance. However, recent evidence suggests that CSC now being identified by ever expanding set of biomarkers may not be as rare and as resistant to immune intervention as previously thought. In our hands, for example, aldehyde dehydrogenase 1 family member A1 (ALDHA1)bright cells present in human head and neck cancers (HNC) have all of the properties of CSC, and yet are highly sensitive to ALDHA1-specific CD8+ T effector cells in vitro [103]. These effector cells can also in vivo eradicate ALDHA1bright cells in xenografts growing in immunodeficient mice [A. DeLeo, unpublished data]. Furthermore, our preliminary data suggest that tumor-derived ALDHA1bright cells are also sensitive to the mAb targeting a surface marker chondroitin sulfate glycoprotein 4 (CSGP4) expressed on these cells [S. Ferrone, unpublished data]. These experiments open a possibility for the future concomitant targeting of CSC with Mabs and specific T cells, alone or in combination with chemotherapy, providing yet another therapeutic strategy for overcoming tumor escape and disease recurrence.
Conclusions
Immunotherapy targeting human solid tumors remains an important and desirable goal, particularly since conventional therapies have only limited effects on cancer patients’ long-term survival, while introducing considerable toxicities and adverse reactions. Among the most important strategies that are currently being developed for cancer therapy are those designed to eliminate or reduce tumor-induced immune suppression as also discussed by others [104, 105]. Many agents have already been developed and tested in animal tumor models that specifically target mechanisms used by tumors to evade the immune response mounted by the host. Some are actively being tested in clinical trials. Among the latter, therapies using mAbs specific for immunosuppressive cytokines, eliminating Treg or MDSC, blocking tolerizing signals or otherwise restoring functions of immune cell subsets appear to be most promising. In addition, however, numerous other strategies are now available for translation to clinical trials with the expectation that tumor-derived inhibitory effects can be effectively abolished and anti-tumor immune responses restored. This review considers many, but by no means all, of these strategies. At this time, no strategy of choice can be recommended, although a recent NCI Workshop has identified several agents that have a strong potential for inducing immunologic and clinical responses in patients with cancer [37]. The complexity of tumor-immune cell interactions also reflects the fact that most TAA are self, and thus therapeutic activation of TAA-specific responses is invariably accompanied by a wave of tolerance to self mediated by the immune system. Therefore, achieving the correct balance between therapy-driven immune activation, obligatory contraction of up-regulated immune responses and tumor-induced immune suppression represents a challenge that faces basic scientists as well as clinicians. Because the tumor tends to dominate the microenvironment, which invariably is hostile to immune effector cells, elimination of effects mediated by Treg, MDSC and inhibitory cytokines seems to be of primary importance for immunotherapy of cancer. Fortunately, a continually improving understanding of molecular mechanisms involved in cellular interactions taking place in the tumor environment has provided us with well defined targets, a solid rationale for clinically addressing these targets and a score of novel biologics and metabolites which can be translated to cancer clinical trials in expectation of a future therapeutic success.
Expert opinion
Tumors can use a variety of mechanisms to create an immunosuppressive environment which favors suppression of anti-tumor immune responses. In patients with advanced malignancies, tumor-induced tolerance becomes predominant and, consequently, the host is unable to mount an immune attack against the tumor. Instead, the tumor counterattacks the immune system of the host, often using immune cells to create an immunosuppressive network which promotes tumor growth, protects the tumor from immune attack and attenuates the efficacy of immune therapies. Among the mechanisms that tumors can utilize to suppress the host immune system are the secretion of inhibitory factors, alterations in APC functions, interference with co-stimulatory signals, enrichment in numbers/functions of Treg and many others [105, 106]. The variety, complexity and multiplicity of immunoinhibitory mechanisms operative in the tumor microenvironment have now been recognized. Considerable efforts are being made to achieve a better understanding of those tumor-immune cell interactions that lead to the development of immunosuppressive cellular and molecular networks in tumor-bearing hosts. This has made it possible to begin thinking of novel therapeutic strategies that could undermine or subvert tumor-induced tolerance and optimize anti-tumor therapies. Indeed, current emphasis on the successful elimination of tumor-induced suppression has led to the development of a novel category of cancer drugs designed to restore or preserve immune responses in cancer, and increasing numbers of these therapeutic agents targeting tumor-induced immune suppression are already in the clinic. This trend is likely to continue in the near future.
A partial and often long-lasting restoration of anti-tumor immune responses has been reported to occur in cancer patients enrolled in various immunotherapy trials. Frequently, however, these newly-augmented immune responses do not correlate with the clinical evidence of tumor control, i.e., relapse-free and overall survival or with the development of immunologic memory in these patients [107, 108]. Further, detrimental clinical effects have been reported as a result of some anti-cancer vaccines [9, 75]. Presumably, immune activation in this setting accompanied by the induction of anti-tumor effector cells leads to the activation and induction of Treg [75, 109]. This paradigm obviously calls for strategies that could inhibit the inhibitor, thus introducing the requirement for combined therapies, if we are to achieve tumor control. The problem is further complicated by the existence of the unique tumor immune signatures, suggesting that each tumor creates its own unique immunosuppressive network. This introduces an additional requirement for the identification of the relevant components of a given network and for inhibitors that specifically target these components. Because increasing numbers of biologic and conventional drugs with activities potentially able to overcome tumor-induced suppression and to restore anti-tumor immune competence are becoming available, future immune interventions in cancer will focus on selection and combination of drugs that synergize in their abilities to restore anti-tumor immunity. In fact, there is much to be learned from clinical applications of drugs such as TNF-α antagonists or metabolic inhibitors of immune suppressive pathways that are already in clinical trials for indications other than cancer, e.g., in autoimmune diseases. However, based on clinical experience to date, it appears unlikely that any one of these agents used singly would achieve significant clinical benefits in patients with advanced cancer. Combinatorial therapies of the immune system-stimulating agents with selected inhibitors targeting immune networks at critical points to block the inhibitors, up-regulate the development and sustain the activity of anti-tumor effector cells are emerging as a future approach to cancer therapies. Whether in combination with immune therapies or conventional chemotherapy and/or radiation, selective inhibition of tumor-induced immunoinhibitory networks is a necessary next step toward achieving improved therapeutic results in cancer.
Article Highlights
Tumor escape from the host immune system has been a major problem in immunotherapy of human cancer.
“Immune escape” of tumors is a common event.
To escape from the host immune system tumors utilize a variety of mechanisms.
Various strategies that can be used for counteracting tumor escape are discussed.
Drugs and biologic targeting cancer-induced immune suppression are currently in clinical trials.
-
Categories of drugs with a high potential to inhibit tumor-induced immunosuppression includes:
- Antibodies
- Cytokines
- Immune adjuvants
- T-cell stimulators
- Metabolic inhibitors
To subvert tumor-induced tolerance it will be necessary to selectively block inhibitors induced by tumors.
Combinational therapies including agents blocking the inhibitors are emerging as a future approach to treatment of cancer.
Footnotes
Declaration of interest
This paper has been sponsored by NIH Grants PO1-CA109688 to TLW and RO1-CA112643 to D. Shin (TLW co-investigator).
References
- 1.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–83. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. Immune effector cells in the tumor microenvironment: their role in regulation of tumor progression. In: Yefenof E, editor. Innate and Adaptive Immunity Responses in the Tumor Microenvironment. Springer Science; The Netherlands: 2008. pp. 1–34. [Google Scholar]
- 5.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–76. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faries MB, Hsueh EC, Ye X, et al. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–7035. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–34. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadun RE, Sachsman SM, Chen X, et al. Immune signatures of murine and human cancers reveal unique mechanisms of tumor escape and new targets for cancer immunotherapy. Clin Cancer Res. 2007;13:4016–25. doi: 10.1158/1078-0432.CCR-07-0016. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda H. Gene and chromosomal alterations in sporadic breast cancer: correlation with histopathological features and implications for genesis and progression. Breast Cancer. 2009;16:186–201. doi: 10.1007/s12282-009-0124-x. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 14.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–90. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;4:A40–5. doi: 10.1016/s0264-410x(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 16.Aptsiauri NT, Cabrera R, Mendez A, et al. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–31. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside TL, Stanson J, Shurin MR, Ferrone S. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173:1526–34. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 18.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–74. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114:468–471. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–95. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 21.Lang S, Atarashi Y, Nishioka Y, et al. B7.1 on human carcinomas: costimulation of T cells and enhanced tumor-induced T-cell death. Cell Immunol. 2000;201:132–43. doi: 10.1006/cimm.2000.1651. [DOI] [PubMed] [Google Scholar]
- 22.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–18. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 23.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 24.Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–30. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–49. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 27.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells(TREG) J Biol Chem. 2009;285:7176–86. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182:1469–80. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaraj S, Schrum AG, Cho HI, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–16. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7:31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 32.Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–66. [PubMed] [Google Scholar]
- 33.Zhou BB, Zhang H, Damelin M, et al. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 34.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol. 2006;16:80–8. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–66. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 36.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 37.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–68. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 38.Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert Opin Drug Saf. 2009;8:223–35. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 39.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpressed HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 40.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 41.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–59. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–61. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 43.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer. 2009;15:169–73. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 44.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–97. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Lau R, Yu D, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–04. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shevach EM, Davidson TS, Huter EN, et al. Role of TGF-Beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol. 2008;28:640–46. doi: 10.1007/s10875-008-9240-1. [DOI] [PubMed] [Google Scholar]
- 49.Terabe M, Ambrosino E, Takaku S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–69. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vicari AP, Trinchieri G. Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev. 2004;202:223–36. doi: 10.1111/j.0105-2896.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 51.Shurin MR, Yurkovetsky ZR, Tourkova IL, et al. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61–8. doi: 10.1002/ijc.10576. [DOI] [PubMed] [Google Scholar]
- 52.Llorente L, Richaud-Patin Y, Garcia-Padilla C, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2:61–5. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–88. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 55.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann NY Acad Sci. 2009;1174:99–06. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 56.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–33. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 57.Kaminetzky D, Hymes KB. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics. 2008;2:717–24. doi: 10.2147/btt.s3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol. 2007;27:1–18. doi: 10.1007/s10875-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 60.Liu DV, Maier LM, Hafler DA, Wittrup KD. Engineered interleukin-2 antagonists for the inhibition of regulatory T cells. J Immunother. 2009;32:887–94. doi: 10.1097/CJI.0b013e3181b528da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–48. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biswas S, Eisen T. Immunotherapeutic strategies in kidney cancer--when TKIs are not enough. Nat Rev Clin Oncol. 2009;6:478–87. doi: 10.1038/nrclinonc.2009.91. [DOI] [PubMed] [Google Scholar]
- 63.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 64.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teague RM, Sather BD, Sacks JA, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–41. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 66.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–17. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caserta S, Alessi P, Basso V, Mondino A. IL-7 is superior to IL-2 for ex vivo expansion of tumour-specific CD4(+) T cells. Eur J Immunol. 2010;40:470–79. doi: 10.1002/eji.200939801. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–19. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 70.Cocco C, Pistoia V, Airoldi I. New perspectives for melanoma immunotherapy: role of IL-12. Curr Mol Med. 2009;9:459–69. doi: 10.2174/156652409788167140. [DOI] [PubMed] [Google Scholar]
- 71.Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. J Transl Med. 2008;6:62. doi: 10.1186/1479-5876-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–55. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 73.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–67. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 74.Zhou X, Jun DY, Thomas AM, et al. Diverse CD8+ T-cell responses to renal cell carcinoma antigens in patients treated with an autologous granulocyte-macrophage colony-stimulating factor gene-transduced renal tumor cell vaccine. Cancer Res. 2005;65:1079–88. [PubMed] [Google Scholar]
- 75.Eggermont AM. Immunostimulation versus immunosuppression after multiple vaccinations: the woes of therapeutic vaccine development. Clin Cancer Res. 2009;15:6745–47. doi: 10.1158/1078-0432.CCR-09-2377. [DOI] [PubMed] [Google Scholar]
- 76.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 77.Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baldwin HM, Ito-Ihara T, Isaacs JD, Hilkens CM. TNF{alpha} blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.110502. In press. [DOI] [PubMed] [Google Scholar]
- 79.Brown ER, Charles KA, Hoare SA, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19:1340–46. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- 80.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 81.Mata-Haro V, Cekic C, Martin M, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 82.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baldridge JR, McGowan P, Evans JT, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–38. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 84.Smits EL, Cools N, Lion E, et al. The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells. Cancer Immunol Immunother. 2010;59:35–46. doi: 10.1007/s00262-009-0721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–13. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis ID, Chen Q, Morris L, et al. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 87.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–57. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sarkar FH, Adsule S, Li Y, Padhye S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev Med Chem. 2007;7:599–08. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]
- 89.Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 90.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–70. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 91.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 92.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, andmyeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 94.Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009;193:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- 96.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–05. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 98.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romo de Vivar Chavez A, de Vera ME, Liang X, Lotze MT. The biology of interleukin-2 efficacy in the treatment of patients with renal cell carcinoma. Med Oncol. 2009;26 (Suppl 1):3–12. doi: 10.1007/s12032-008-9162-z. [DOI] [PubMed] [Google Scholar]
- 101.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–29. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Offringa R. Antigen choice in adoptive T-cell therapy of cancer. Curr Opin Immunol. 2009;21:190–99. doi: 10.1016/j.coi.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Visus CD, Ito DA, Amoscato A, et al. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–45. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 104.Morse MA, Hall JR, Plate JM. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer. Expert Opin Biol Ther. 2009;9:331–39. doi: 10.1517/14712590802715756. [DOI] [PubMed] [Google Scholar]
- 105.Pandolfi F, Cianci R, Lolli S, et al. Strategies to overcome obstacles to successful immunotherapy of melanoma. Int J Immunopathol Pharmacol. 2008;21:493–500. doi: 10.1177/039463200802100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–18. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lens M. The role of vaccine therapy in the treatment of melanoma. Expert Opin Biol Ther. 2008;8:315–23. doi: 10.1517/14712598.8.3.315. [DOI] [PubMed] [Google Scholar]
- 108.Engell-Noerregaard L, Hansen TH, Andersen MH, et al. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lacelle MG, Jensen SM, Fox BA. Partial CD4 depletion reduces regulatory T cells induced by multiple vaccinations and restores therapeutic efficacy. Clin Cancer Res. 2009;15:6881–90. doi: 10.1158/1078-0432.CCR-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stolz C, Schuler M. Molecular mechanisms of resistance to Rituximab and pharmacologic strategies for its circumvention. Leuk Lymphoma. 2009;50:873–85. doi: 10.1080/10428190902878471. [DOI] [PubMed] [Google Scholar]
- 111.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–84. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 112.Martinelli E, De Palma R, Orditura M, et al. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs. 2009;23:289–04. doi: 10.2165/11317600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 114.Houot R, Goldstein MJ, Kohrt HE, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–38. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu JJ, Huang DB, Tyring SK. Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses. Antiviral Res. 2004;64:79–83. doi: 10.1016/j.antiviral.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Fridman JS, Caulder E, Hansbury M, et al. Selective inhibition of ADAM metalloproteases as a novel approach for modulating ErbB pathways in cancer. Clin Cancer Res. 2007;13:1892–02. doi: 10.1158/1078-0432.CCR-06-2116. [DOI] [PubMed] [Google Scholar]
- 117.Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J. 2006;8:E532–51. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]