Abstract
The development of new approaches to treat patients with hepatic diseases that can eliminate the need for liver transplantation is imperative. The use of cell therapy as a means of repopulating the liver has several advantages over whole organ transplantation, since it would be less invasive, less immunogenic, and would allow the use, in some instances, of autologous-derived cells. Stem/progenitor cells that would be ideal for liver repopulation would need to have characteristics such as availability and ease of isolation, the ability to be expanded in vitro, ensuring adequate numbers of cells, susceptibility to modification by viral vector transduction/genetic recombination, to correct any underlying genetic defects, and the ability of restoring liver function following transplantation. Bone marrow-derived stem cells such as Hematopoietic, Mesenchymal and Endothelial Progenitor cells possess some or most of these characteristics, making them ideal candidates for liver regenerative therapies. Here, we will summarize the ability of each of these stem cell populations to give rise to functional hepatic elements which could mediate repair in patients with liver damage/disease.
Keywords: hematopoietic stem cells, mesenchymal stem cells, hepatocytes, liver regeneration, cell therapy
INTRODUCTION
Liver failure is a potentially life-threatening condition for which organ transplantation is the only definitive therapy [1]. However, the current shortage of available livers for transplant results in the death of many patients while awaiting transplantation [2]. Thus, it is imperative that new approaches for repairing the liver are developed, so that the need for transplanting a partial or complete human liver to cure the patient can be eliminated. Presently, cell therapies represent one of the most promising alternative solutions to entire or partial liver transplant [3–12]. A logical alternative to performing liver transplantation would be to isolate and transplant human hepatocytes. Unfortunately, human livers would still be required as a source of cells and, in addition, isolation of human hepatocytes is difficult and inefficient. Furthermore, differentiated hepatocytes cannot be effectively expanded in culture, greatly limiting the number of hepatocytes that could be obtained from each liver [13, 14]. Therefore, numerous studies have concentrated on investigating the ability of a variety of stem cells that can be readily isolated using non-invasive procedures, to give rise to hepatocytes both in vitro and in vivo. In addition, many of these cell populations can be expanded significantly in vitro, making it possible to generate large numbers of cells for transplantation from a fairly small number of initial stem cells. Since some of these stem cell populations are present within the adult, and could thus be isolated from the patient to be treated, it would be possible to produce personalized, immunologically matched hepatocytes.
Here we will discuss bone marrow as a source of some of the most promising stem cell candidates for liver regeneration/repair. We will present data supporting the ability of each of the stem cell populations to give rise to functional hepatic elements which could mediate repair in patients with liver damage/disease, and a summary clinical trials using cell therapy for liver regeneration.
Hematopoietic Stem Cells for Liver Regeneration
Hematopoietic stem cells (HSC) are probably the most studied and best understood stem cell within the body [15]. Since pioneering studies in the early sixties demonstrating that HSC existed within the marrow and were able to completely repopulate the hematopoietic system of lethally irradiated murine recipients [16–18], HSC have been the center of intensive research. HSC have been proven to be invaluable in the clinic, in the treatment of numerous hematologic and non-hematologic malignancies as well as a range of both inherited and acquired diseases. In addition to the bone marrow, HSC can also be harvested from cytokine-mobilized peripheral blood and umbilical cord blood, making it possible to harvest these cells from the patient in adequate amounts, using relatively non-invasive procedures. Therefore, HSC meet the requirements of cells that would be ideally suited for cells therapies such as availability, ease of isolation, and the ability to be harvested from the patient to be treated.
In the field of liver regeneration, HSC have received a great deal of attention as a result of ground-breaking studies showing that HSC, following transplantation, had the ability to give rise to hepatocyte-like cells in vivo and completely repopulate the liver of FAH mice, correcting their disease phenotype [19, 20]. These studies were followed by others using a variety of rodent model systems to rigorously test the hepatocytic potential of HSC from various sources [19, 21–37]. Table 1 shows the sources of cells used in studies that demonstrated the hepatocytic potential of enriched populations of HSC in various murine models with a wide range of genetic lesions and injuries induced by either chemicals or physical means. Since each group used different criteria for isolating HSC, and each injury/disease model seems to have its own unique characteristics, resulting in differing outcomes, the results from these studies have been rather hard to interpret, even when the same or very similar types of HSC are transplanted. However it safe to say that umbilical cord blood HSC, appear to more consistently generate higher levels of hepatocytes following transplantation than HSC isolated from bone marrow or mobilized peripheral blood. Furthermore, it is clear from these studies that HSC give rise to higher levels of liver engraftment when they are transplanted into models in which the host’s endogenous hepatocytes are defective either as a result of genetic lesion or due to treatment with agents that prevent host hepatocyte replication. It appears that the only way to achieve high levels of donor HSC-derived hepatocytes is to somehow impart a proliferative/survival advantage to the transplanted cells. The mechanism whereby the hepatocytes are generated from the transplanted HSC has also been a controversial point among research groups. In some cases, such as the FAH model, it appears that the donor-derived hepatocytes are generated almost entirely through fusion of the transplanted HSC with the host’s endogenous hepatocytes [20, 35, 38]. However, in other models, the hepatocytes are generated almost exclusively through what seems to be true differentiation of the HSC into hepatocytes without exchange of any genetic or cellular elements between the host and the engrafted human cells [21, 25, 34, 36, 39]. It has also been shown that the phenotype and purity of the cell population transplanted also influences the production of hepatocytes and whether fusion was observed or not [8, 9, 40, 41]. Therefore, depending on the source of cells and etiology of disease, the outcome will differ significantly in terms of numbers and how hepatocytes are being generated. Using a non-injury model of transplantation in which the procedure is performed during the fetal period when the liver is rapidly proliferating and differentiating, it is possible to obtain high levels of engraftment and differentiation of the donor human cells without forcing the transplanted cells to adopt a specific fate by damaging/inducing regeneration within the recipient liver [42–45]. The lack of injury is therefore important, since in the presence of disease/damage, the host hepatic microenvironment may be far more conducive to apoptosis than engraftment and differentiation [42], making it very hard to assess the true potential/ability of the transplanted cells [43–48]. Using the fetal sheep model, we performed a detailed head-to-head comparison between HSC from bone marrow versus those of cord blood, and those from mobilized peripheral blood [49] with respect to their ability to give rise to functional hepatocytes in vivo. Our results showed that phenotypically identical HSC from three different clinically relevant sources possess marked differences in their hepatocytic potential, with cord blood HSC giving rise to the greatest numbers of hepatocytes, followed closely by bone marrow-derived HSC, all in the absence of fusion. Of particular note, HSC derived from mobilized peripheral blood exhibited substantially lower hepatocytic potential than those from either cord blood or marrow, suggesting that mobilized peripheral blood will not likely prove to be the ideal source of HSC for hepatic repair/regeneration. We also compared the hepatocytic potentials among different putative HSC phenotypes and found that adult human BM CD34+Lin−CD38− cells were able to generate more hepatocytes in vivo than any other phenotype tested, suggesting that this fraction of cells are enriched not only for HSCs but also for cells with hepatocytic potential [49]. In addition, our studies demonstrated that for each given HSC population, the hepatocytes generated from the hematopoietic graft expanded over time, gradually comprising a larger percentage of the total hepatic mass.
TABLE 1.
Studies demonstrating HSC hepatocytic potential in rodent models
| Type of Hematopoietic stem cells | |
|---|---|
| adult mouse BM KTLS | Lagasse et al., 2000 |
| adult male mouse whole BM | Theise et al., 2000 |
| adult male mouse BM HSC purified by elutriation, Lin- | Krause et al., 2001 |
| adult male Lin- BM cells from L-PK-Bcl-2 transgenic mice | Mallet et al., 2002 |
| adult mouse whole BM | Wang et al., 2002 |
| human cord blood CD34+ or CD45+ | Ishikawa et al., 2003 |
| human cord blood or mPB CD34+ | Kollet et al., 2003 |
| human cord blood mononuclear cells | Newsome et al., 2003 |
| male mouse Lin- BM cells | Vassilopoulos et al., 2003 |
| human cord blood or BM CD34+ or CD34+CD38-CD7- | Wang et al., 2003a |
| mouse BM cells | Wang et al., 2003b |
| adult male mouse BM HSC purified by elutriation, Lin- | Jang et al., 2004 |
| human cord blood mononuclear cells OR eGFP transgenic mouse BM cells | Sharma et al., 2005 |
| male GFP transgenic rat b2microglobulin(−) Thy-1(+) BM cells | Muraca et al., 2007 |
| T-depleted mouse BM cells | Eggenhofer et al., 2008 |
Mesenchymal Stem Cells for Liver Regeneration
Mesenchymal stem cells (MSC) make up part of the bone marrow stromal microenvironment that provides support to the hematopoietic stem cell and drives the process of hematopoiesis [50, 51]. Despite their important role within the bone marrow, MSC are rare cells with estimates for MSC frequency ranging from 0.001–0.01% of the total nucleated cell population present within the marrow [52]. Like HSC, MSC cannot be isolated to absolute purity, although numerous culture methods and surface markers have been characterized that enable one to enrich for MSC, with each laboratory preferring its own method of isolation. This makes the comparison of results obtained by various laboratories very difficult, since each lab is likely studying somewhat different cell populations despite the fact that all of these cells have collectively been referred to as MSC. In addition to the bone marrow, these cells have now been isolated from numerous tissues, including brain, liver, lung, fetal blood, umbilical cord blood, kidney, and even liposuction material [53–59], leading one to postulate that MSC are likely to play a critical role in organ homeostasis, perhaps providing supportive factors like in the bone marrow, and/or mediating maintenance/repair within their respective tissue.
Recent studies have shown that MSC, like HSC, have far greater differentiative abilities than previously thought. They in fact appear to be capable of giving rise to cells of all three germinal layers [60], including albumin-producing hepatocyte-like cells in vitro and in vivo [61–64]. However, in contrast to HSC, MSC can be expanded in culture for long periods of time without any seeming loss of differentiation capacity. Furthermore, since MSC are quite amenable to genetic modification/correction, MSC could be harvested from the patient’s own marrow, even if the liver disease present was the result of an underlying genetic defect, and genetically corrected autologous MSC could thus be propagated to generate sufficient numbers of cells to achieve meaningful levels of engraftment following transplantation. Similar to the studies performed with human HSC, each group of investigators using MSC defined these cells in different ways ranging from specific antigen profile to simple plastic adherence, generating data that were not always in agreement. However, it is recognized overall that MSC appear to be able to exert beneficial effects in a wide range of injuries and disease states within the liver, and that fusion does not appear to play a major role in the beneficial effects of transplanted MSC. One issue which has complicated interpretation of the data generated from these studies in liver is that it appears that the transplantation of MSC somehow stimulates the host’s liver to repair itself without the donor cells actually having to persist long-term within the recipient. Several studies clearly demonstrated that secretion of factors which stimulate the regeneration of endogenous parenchymal cells were likely to play important roles in promoting tissue recovery [42, 65–69]. These findings led to the question of whether MSC can actually generate hepatocytes or if, perhaps, all the effects they produce are simply mediated through release of soluble factors. In vitro studies have now provided definitive evidence that MSC can, under appropriate conditions be made to differentiate into cells with all of the characteristics of functional hepatocytes that can currently be assessed in culture [70–73]. Furthermore, MSC possess other equally important therapeutic effects besides contribution to the cellular pool. A variety of evidence from animal studies has now indicated that MSC have the ability to enhance fibrous matrix degradation, likely through the induction of metalloproteinases, suggesting that MSC may be ideally suited for treatment of liver diseases involving fibrosis [3, 74–80]. However, these results must be interpreted carefully since other studies have suggested that under different conditions, transplanted MSC may actually contribute to the myofibroblast pool and thus enhance the fibrotic process within the liver [3, 81–84]. To rigorously test whether these qualities could be exploited to generate significant numbers of hepatocytes in vivo, we examined the ability of clonally-derived human MSC from adult bone marrow to generate functional albumin-producing hepatocytes in vivo following transplantation into fetal sheep recipients, comparing two routes of administration, intraperitoneal and intrahepatic [61]. Our results showed that, although MSC efficiently generated significant numbers of hepatocytes by both routes of administration, the intrahepatic injection resulted in substantially more efficient generation of hepatocytes. In addition to higher levels of hepatocytes, the animals that received an intrahepatic injection also exhibited a widespread distribution of hepatocytes throughout the liver parenchyma, while those receiving an intraperitoneal injection exhibited a preferential periportal distribution of human hepatocytes that produced higher amounts of albumin [61]. These studies thus provided compelling evidence that MSC represent a valuable source of cells for liver repair and regeneration and demonstrate that, by altering the site of injection, the generation of hepatocytes occurs in different hepatic zones. In other studies, we evaluated the ability of mesenchymal cells derived from non-hematopoietic organs to form hepatic cells in vitro and in vivo [53]. After culture in specific inducing media, cells with hepatocyte-like morphology and phenotype were obtained, suggesting that metanephric-derived MSC could also serve as a source of cells with hepatic repopulating ability. Also, like their bone marrow counterparts, these cells gave rise to significant numbers of human albumin-producing hepatocyte-like cells upon in utero transplantation. Similar results were also obtained using a novel adherent MSC-like cell population isolated from umbilical cord blood, which the authors termed unrestricted somatic stem cells, or USSC [85]. This cord blood-derived MSC population was capable of giving rise to albumin-producing human parenchymal hepatic cells at levels of over 20% in the recipient liver, in the absence of any injury or genetic defect. Another key aspect to assessing the utility of stem cell therapy for regenerative medicine for the liver, and for other organs as well, is the mechanism whereby the transplanted cells replace/repopulate the recipient liver [61]. We showed that MSC could give rise directly to cells within the liver without the need for first forming hematopoietic elements[86]. In more recent studies, we have now shown that the ability to directly contribute to liver repopulation without the need for a hematopoietic intermediate enables the transplanted MSC to rapidly begin contributing to the growing liver, producing cells with hepatic markers within as little as 24 or 48 hours post-transplant [86]. The findings of these more recent studies confirmed our prior findings regarding the lack of a need for fusion, and furthered our understanding of the mechanism of hepatic repopulation by demonstrating that the generation of hepatocytes occurs independently of the transfer of either mitochondria or membrane-derived vesicles between the transplanted donor cells and the cells of the recipient liver [86]. These findings thus provide strong evidence to support genetic reprogramming and differentiation of the transplanted stem cells. The lack of fusion as a requirement for liver repopulation was in contrast to the results of numerous other studies employing injury models, raising the possibility that the efficacy and mechanism of stem cell repair will likely depend upon not only the stem cell population being transplanted, but also the nature of the injury/defect within the liver, and therefore the conditions within the hepatic microenvironment at the time of stem cell transplantation.
Endothelial Progenitor Cells (EPC) for Liver Regeneration
A population of cells that may prove valuable in the repair/regeneration of damaged/diseased liver through the promotion of supportive factors necessary to the host’s endogenous hepatocyte repair mechanisms is the so-called endothelial progenitor cells, or EPC. These cells have now been shown to engraft within the injured liver and generate new blood vessels, through the secretion of numerous growth factors such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) that assist in the regenerative process [87–91]. Therefore, EPC improved the survival of mice that were subjected to severe liver injury, probably through stimulating regeneration of the liver via its own endogenous cellular reserves [89]. Also, in a rat model of cirrhotic liver disease, transplanted EPC incorporated into the portal tracts and fibrous septa, significantly reducing liver fibrosis [87]. Furthermore, in similarity to some of the effects observed following transplantation with bone marrow-derived MSC, livers of animals receiving EPC transplantation showed significantly increased levels of matrix metalloproteinase (MMP)-2, -9 and -13, with a concomitant reduction in levels of tissue inhibitor of metalloproteinase-1. Likewise, in rats with chronic liver injury induced by intraperitoneal injection of dimethylnitrosamine, EPC stimulated liver repair and suppression of liver fibrogenesis [88]. Stimulation of this regenerative process allowed maintenance of normal liver function parameters such as transaminase, total bilirubin, total protein, and albumin despite the continued administration of DMN [88]. Thus, while only a small number of studies have been performed to date investigating the potential of EPC’s for liver regeneration/repair, these investigations have provided compelling evidence that EPC transplantation is effective both at preventing liver fibrosis, and also for promoting regeneration in chronically damaged livers in which fibrosis is already well established. Therefore, EPC’s may prove to be a crucial cell type for therapies for liver disorders in which the host’s own hepatocytes do not possess a genetic defect, and are thus capable of responding with proliferation to the array of supportive factors released by the engrafted cells repopulating the damaged regions of the liver.
Bone Marrow-Derived Stem Cells in Clinical Trials
The clinical use of bone marrow-derived cells for repair/regeneration within the liver is still in its infancy. The unwillingness to test these cells in human patients is mainly due to the uncertainty of the outcome, based on conflicting studies in animal models, and the possibility of cellular fusion as a repair mechanism that may lead to genetically unstable hepatocytes within the environment of a diseased liver [5]. Five clinical feasibility studies, in which the number of patients in each case has been small and no control arm has been included, have been reported thus far. The first of these investigated the effect of infusing autologous BM-derived CD133+, in patients that were undergoing partial hepatectomy for liver cancer, to expand a remnant segment of liver [92]. The results of this study were promising, in that patients receiving the infusion of BM cells (which likely contained both HSC and EPC) exhibited 2.5-fold higher mean proliferation rates when compared with a group of three consecutive patients who did not receive BM cells. Infusion of autologous CD34+ cells via either the portal vein or the hepatic artery was also shown to transiently improve serum bilirubin and albumin for greater than 60 days in 5 patients suffering from cirrhosis [93]. Furthermore, a long-term follow-up in these patients demonstrated that 4 of the 5 patients maintained improved clinical parameters for roughly 12 months post-infusion [94]. Two other recent studies have tested the safety and efficacy of using both CD34+ BM-derived cells [95] and BM MSC [96] to treat patients with cirrhosis. The infusion of CD34+ cells via the hepatic artery resulted in hepatorenal syndrome and death of 1 patient, with the remaining 3 patients showing no evidence of significant clinical improvement. In contrast, infusion of BM-derived MSC via a peripheral vein was found to be well tolerated and have a definite therapeutic effect for 2 of the 4 patients in the trial [96]. In the one other study that has thus far been reported, nine patients with cirrhosis were treated by portal vein infusion of autologous whole, unselected BM cells. 24 week follow-up revealed some improvement in Child-Pugh score, albumin, and biopsy evidence of an increase in hepatocyte turnover.
Although these studies provide hope that BM-derived cells may prove to be a valuable resource for cell-based therapies for liver disease, the results must be interpreted with some caution, given the limited number of patients enrolled in each trial and the lack of appropriate controls. Furthermore, since autologous cells were used in these trials, there was no way for the investigators to assess the actual engraftment, persistence, or differentiative potential of the transplanted cells, leaving the mechanism responsible for the observed clinical improvements open to speculation.
SUMMARY
The current shortage of donor organs available for transplant and the severe morbidity and mortality associated with this procedure underscores the need for alternatives to liver transplantation. The ability to repair the liver by transplanting bone marrow-derived cells such as HSC, MSC, or EPC, rather than the liver itself, has shown great promise in animal models. Unfortunately, the lack of standardization of protocols for isolating specific cell types and the use of a variety of injury/disease models has made the interpretation of these results rather difficult, and has left open questions regarding the mechanism(s) whereby these cells mediate their beneficial effects. The results with human clinical trials, although promising, are not yet definitive, but have certainly sustained a reserved optimism as to the role of cell therapies for treating liver diseases, once methodologies have become standardized and optimized.
FIGURE 1.
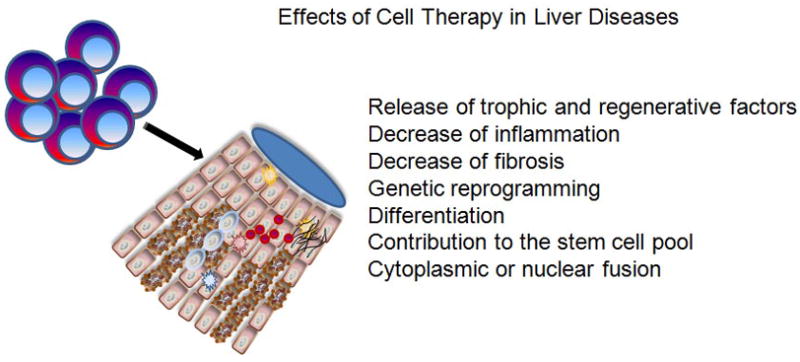
Effects of cell therapy in liver diseases.
Acknowledgments
This work was supported by: National Institutes of Health (Bethesda, MD, USA) grants HL73737, HL97623, and HL52955.
Footnotes
Conflict of interest disclosure: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology (Baltimore, Md. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WR, Kremers WK. Benefits of “the benefit model” in liver transplantation. Hepatology (Baltimore, Md. 2008;48:697–698. doi: 10.1002/hep.22497. [DOI] [PubMed] [Google Scholar]
- 3.Alison M, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. The Journal of pathology. 2008 doi: 10.1002/path.2453. [DOI] [PubMed] [Google Scholar]
- 4.Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. Journal of inherited metabolic disease. 2006;29:431–435. doi: 10.1007/s10545-006-0245-8. [DOI] [PubMed] [Google Scholar]
- 5.Enns GM, Millan MT. Cell-based therapies for metabolic liver disease. Molecular genetics and metabolism. 2008;95:3–10. doi: 10.1016/j.ymgme.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl. 2001;7:835–844. doi: 10.1053/jlts.2001.27865. [DOI] [PubMed] [Google Scholar]
- 7.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology (Baltimore, Md. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 8.Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716–724. doi: 10.1136/gut.2006.098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lysy PA, Campard D, Smets F, Najimi M, Sokal EM. Stem cells for liver tissue repair: current knowledge and perspectives. World J Gastroenterol. 2008;14:864–875. doi: 10.3748/wjg.14.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochimica et biophysica acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Seminars in liver disease. 1999;19:39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 12.Strom SC, Fisher RA, Rubinstein WS, et al. Transplantation of human hepatocytes. Transplantation proceedings. 1997;29:2103–2106. doi: 10.1016/s0041-1345(97)00252-2. [DOI] [PubMed] [Google Scholar]
- 13.Serralta A, Donato MT, Martinez A, et al. Influence of preservation solution on the isolation and culture of human hepatocytes from liver grafts. Cell transplantation. 2005;14:837–843. doi: 10.3727/000000005783982495. [DOI] [PubMed] [Google Scholar]
- 14.Serralta A, Donato MT, Orbis F, Castell JV, Mir J, Gomez-Lechon MJ. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicol In Vitro. 2003;17:769–774. doi: 10.1016/s0887-2333(03)00122-x. [DOI] [PubMed] [Google Scholar]
- 15.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. The American journal of pathology. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCulloch EA, Till JE. The sensitivity of cells from normal mouse bone marrow to gamma radiation in vitro and in vivo. Radiation research. 1962;16:822–832. [PubMed] [Google Scholar]
- 17.Siminovitch L, McCulloch EA, Till JE. The Distribution of Colony-Forming Cells among Spleen Colonies. Journal of cellular physiology. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 18.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research. 1961;14:213–222. [PubMed] [Google Scholar]
- 19.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature medicine. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 20.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 21.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nature cell biology. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 22.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. The Journal of clinical investigation. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 24.Mallet VO, Mitchell C, Mezey E, et al. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology (Baltimore, Md. 2002;35:799–804. doi: 10.1053/jhep.2002.32530. [DOI] [PubMed] [Google Scholar]
- 25.Newsome PN, Johannessen I, Boyle S, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 26.Popp FC, Piso P, Schlitt HJ, Dahlke MH. Therapeutic potential of bone marrow stem cells for liver diseases. Current stem cell research & therapy. 2006;1:411–418. doi: 10.2174/157488806778226759. [DOI] [PubMed] [Google Scholar]
- 27.Sharma AD, Cantz T, Richter R, et al. Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. The American journal of pathology. 2005;167:555–564. doi: 10.1016/S0002-9440(10)62997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theise ND, Krause DS. Bone marrow to liver: the blood of Prometheus. Seminars in cell & developmental biology. 2002;13:411–417. doi: 10.1016/s1084952102001283. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. The American journal of pathology. 2002;161:565–574. doi: 10.1016/S0002-9440(10)64212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggenhofer E, Popp FC, Renner P, et al. Allogeneic bone marrow transplantation restores liver function in Fah-knockout mice. Experimental hematology. 2008;36:1507–1513. doi: 10.1016/j.exphem.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Fox IJ, Strom SC. To be or not to be: generation of hepatocytes from cells outside the liver. Gastroenterology. 2008;134:878–881. doi: 10.1053/j.gastro.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 33.Theise ND, Krause DS, Sharkis S. Comment on “Little evidence for developmental plasticity of adult hematopoietic stem cells”. Science (New York, NY. 2003;299:1317. doi: 10.1126/science.1078412. author reply 1317. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa F, Drake CJ, Yang S, et al. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Annals of the New York Academy of Sciences. 2003;996:174–185. doi: 10.1111/j.1749-6632.2003.tb03245.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 36.Muraca M, Ferraresso C, Vilei MT, et al. Liver repopulation with bone marrow derived cells improves the metabolic disorder in the Gunn rat. Gut. 2007;56:1725–1735. doi: 10.1136/gut.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology (Baltimore, Md. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 38.Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV, et al. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology (Baltimore, Md. 2006;43:108–116. doi: 10.1002/hep.21005. [DOI] [PubMed] [Google Scholar]
- 39.Tang XP, Zhang M, Yang X, Chen LM, Zeng Y. Differentiation of human umbilical cord blood stem cells into hepatocytes in vivo and in vitro. World J Gastroenterol. 2006;12:4014–4019. doi: 10.3748/wjg.v12.i25.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. The Journal of clinical investigation. 2004;113:1266–1270. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nature medicine. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 42.Kuo TK, Hung SP, Chuang CH, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. e2111–2113. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almeida-Porada G, Porada C, Zanjani ED. Adult stem cell plasticity and methods of detection. Reviews in clinical and experimental hematology. 2001;5:26–41. doi: 10.1046/j.1468-0734.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 44.Almeida-Porada G, Porada C, Zanjani ED. Plasticity of human stem cells in the fetal sheep model of human stem cell transplantation. International journal of hematology. 2004;79:1–6. doi: 10.1007/BF02983526. [DOI] [PubMed] [Google Scholar]
- 45.Almeida-Porada G, Zanjani ED. A large animal noninjury model for study of human stem cell plasticity. Blood cells, molecules & diseases. 2004;32:77–81. doi: 10.1016/j.bcmd.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Civin CI, Almeida-Porada G, Lee MJ, Olweus J, Terstappen LW, Zanjani ED. Sustained, retransplantable, multilineage engraftment of highly purified adult human bone marrow stem cells in vivo. Blood. 1996;88:4102–4109. [PubMed] [Google Scholar]
- 47.Porada GA, Porada C, Zanjani ED. The fetal sheep: a unique model system for assessing the full differentiative potential of human stem cells. Yonsei medical journal. 2004;45(Suppl):7–14. doi: 10.3349/ymj.2004.45.Suppl.7. [DOI] [PubMed] [Google Scholar]
- 48.Zanjani ED, Almeida-Porada G, Flake AW. The human/sheep xenograft model: a large animal model of human hematopoiesis. International journal of hematology. 1996;63:179–192. doi: 10.1016/0925-5710(96)00445-8. [DOI] [PubMed] [Google Scholar]
- 49.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–2590. doi: 10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- 50.Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12:99–108. doi: 10.1097/00007890-197108000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Experimental hematology. 1999;27:1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 53.Almeida-Porada G, El Shabrawy D, Porada C, Zanjani ED. Differentiative potential of human metanephric mesenchymal cells. Experimental hematology. 2002;30:1454–1462. doi: 10.1016/s0301-472x(02)00967-0. [DOI] [PubMed] [Google Scholar]
- 54.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells, tissues, organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 55.Fan CG, Tang FW, Zhang QJ, et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell transplantation. 2005;14:311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 56.Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90:1017–1026. [PubMed] [Google Scholar]
- 57.in‘t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 58.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Experimental hematology. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 59.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 60.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Current stem cell research & therapy. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 61.Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology (Baltimore, Md. 2007;46:1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 62.Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology (Baltimore, Md. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 63.Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. The Journal of clinical investigation. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banas A, Teratani T, Yamamoto Y, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem cells (Dayton, Ohio) 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 66.Parekkadan B, van Poll D, Megeed Z, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochemical and biophysical research communications. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 69.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. Journal of cellular physiology. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. :2008. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 71.Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology (Baltimore, Md. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 72.Pan RL, Chen Y, Xiang LX, Shao JZ, Dong XJ, Zhang GR. Fetal liver-conditioned medium induces hepatic specification from mouse bone marrow mesenchymal stromal cells: a novel strategy for hepatic transdifferentiation. Cytotherapy. 2008;10:668–675. doi: 10.1080/14653240802360704. [DOI] [PubMed] [Google Scholar]
- 73.Stock P, Staege MS, Muller LP, et al. Hepatocytes derived from adult stem cells. Transplantation proceedings. 2008;40:620–623. doi: 10.1016/j.transproceed.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 74.Abdel Aziz MT, Atta HM, Mahfouz S, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clinical biochemistry. 2007;40:893–899. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 76.Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. Journal of gastroenterology. 2008;43:419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- 78.Zhao ZH, Xin SJ, Zhao JM, et al. Dynamic expression of matrix metalloproteinase-2, membrane type-matrix metalloproteinase-2 in experimental hepatic fibrosis and its reversal in rat. Zhonghua shi yan he lin chuang bing du xue za zhi = Zhonghua shiyan he linchuang bingduxue zazhi = Chinese journal of experimental and clinical virology. 2004;18:328–331. [PubMed] [Google Scholar]
- 79.Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology (Baltimore, Md. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 80.Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology (Baltimore, Md. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 81.Asawa S, Saito T, Satoh A, et al. Participation of bone marrow cells in biliary fibrosis after bile duct ligation. Journal of gastroenterology and hepatology. 2007;22:2001–2008. doi: 10.1111/j.1440-1746.2006.04708.x. [DOI] [PubMed] [Google Scholar]
- 82.Baba S, Fujii H, Hirose T, et al. Commitment of bone marrow cells to hepatic stellate cells in mouse. Journal of hepatology. 2004;40:255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 83.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. Journal of hepatology. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Russo FP, Alison MR, Bigger BW, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 85.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. The Journal of experimental medicine. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colletti E, Airey JA, Liu W, Simmons PJ, Zanjani ED, Porada CD, Almeida-Porada G. Generation of tissue-specific cells by MSC does not require fusion or donor to host mitochondrial/membrane transfer. Stem Cell Research. 2008 doi: 10.1016/j.scr.2008.08.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura T, Torimura T, Sakamoto M, et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:91–107. e101. doi: 10.1053/j.gastro.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 88.Ueno T, Nakamura T, Torimura T, Sata M. Angiogenic cell therapy for hepatic fibrosis. Medical molecular morphology. 2006;39:16–21. doi: 10.1007/s00795-006-0311-1. [DOI] [PubMed] [Google Scholar]
- 89.Taniguchi E, Kin M, Torimura T, et al. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology. 2006;130:521–531. doi: 10.1053/j.gastro.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 90.Liu F, Fei R, Rao HY, Cong X, Ha MH, Wei L. The effects of endothelial progenitor cell transplantation in carbon tetrachloride induced hepatic fibrosis rats. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2007;15:589–592. [PubMed] [Google Scholar]
- 91.Beaudry P, Hida Y, Udagawa T, et al. Endothelial progenitor cells contribute to accelerated liver regeneration. Journal of pediatric surgery. 2007;42:1190–1198. doi: 10.1016/j.jpedsurg.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 92.am Esch JS, 2nd, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem cells (Dayton, Ohio) 2005;23:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 93.Gordon MY, Levicar N, Pai M, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem cells (Dayton, Ohio) 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 94.Levicar N, Pai M, Habib NA, et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell proliferation. 2008;41(Suppl 1):115–125. doi: 10.1111/j.1365-2184.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Archives of Iranian medicine. 2007;10:459–466. [PubMed] [Google Scholar]


