Abstract
Anti-amyloid immunotherapy has been proposed as an appropriate therapeutic approach for Alzheimer’s disease (AD). Significant efforts have been made towards the generation and assessment of antibody-based reagents capable of preventing and clearing amyloid aggregates as well as preventing their synaptotoxic effects. In this study, we selected a novel set of human anti-amyloid-beta peptide 1-42 (Aβ1-42) recombinant monoclonal antibodies in a single chain fragment variable (scFv) and a single domain (VH) formats. We demonstrated that these antibody fragments recognize in a specific manner amyloid beta deposits in APP/Tg mouse brains, inhibit toxicity of oligomeric Aβ1-42 in neuroblastoma cell cultures in a concentration-dependently manner and reduced amyloid deposits in APP/Tg2576 mice after intracranial administration. These antibody fragments recognize epitopes in the middle/C-terminus region of Aβ, which makes them strong therapeutic candidates due to the fact that most of the Aβ species found in the brains of AD patients display extensive N-terminus truncations/modifications.
Keywords: Alzheimer’s disease, recombinant antibody fragment, domain antibody
1. INTRODUCTION
The accumulation of extracellular and intracellular amyloid-beta (Aβ) peptide aggregates in the human brain has been hypothesized to play a central role in the neuropathology of Alzheimer’s Disease (AD) (Haass and Selkoe, 2007; LaFerla et al, 2007; Walsh and Selkoe, 2007). Anti-Aβ immunotherapy has been shown to disrupt Aβ aggregates, block aggregation, attenuate toxicity, as well as promote the clearance of the peptide in the central nervous system (CNS) in animal models. Immunotherapy approaches, both active immunization with Aβ peptide and passive transfer of anti-Aβ antibodies, have shown therapeutic efficacy in several amyloid precursor protein transgenic (APP/Tg) mouse models, which develop AD-like amyloid plaque pathology (Schenk et al., 1999; Bard et al., 2000; Janus et al., 2000; Hartman et al., 2005; Morgan, 2006; Brody et al., 2008), as well as in a canine and a primate models of amyloidosis (Lemere et al., 2004, Head et al., 2008). However, active immunization in human clinical trials resulted in the development of brain inflammation in some patients (Munch et al., 2002; Gilman et al., 2005; Holmes et al., 2008).
The hypothesis that passive antibody immunotherapy could be more appropriate as a therapeutic approach for AD promoted efforts towards the generation and application of antibody-based reagents capable of preventing and clearing amyloid deposits as well as preventing their synaptotoxic effects and increasing structural plasticity of dendritic spines (Bard et al., 2000; Kotilinek et al., 2002; Lombardo et al., 2003; Fukuchi et al., 2006; Levites et al., 2006; Wilcock et al., 2008; Wisniewski et al., 2008, Spires-Jones et al., 2009). A Phase IIa study of passive immunization in patients with mild to moderate AD using an antibody binding to a central region of Aβ1-42 peptide and named LY2062430 started in May 2006 and Eli Lilly announced that they initiated a U.S. phase 3 clinical trial in 2009. Another monoclonal antibody AAB-001 directed against an amino-terminal part of Aβ1-42 peptide is being used for passive immunotherapy by Elan/Wyeth in a phase 3 clinical trial. However, there are reports that passive immunization also may show adverse side effects, such as, vasogenic edema, microhemorrhages and increased vascular amyloid angiopathy observed after administration of mouse monoclonal anti-Aβ antibodies in transgenic mice (Pfeifer et al., 2002; Wilcock et al., 2004, Lee et al., 2005). Efforts have been made to generate antibodies recognizing other linear or conformational epitopes within Aβ1-42 that may be biologically functional without being associated with the development of adverse events. This could be achieved, for example, by using phage-displayed recombinant antibody (Ab) fragment libraries that results in the generation of a panel of specific antibodies quickly, easily and cost-effectively in vitro. The antibody fragments, such as single chain Fv (scFv), Fab and domain or single-domain Abs (dAbs or sdAbs) are now considered, along with conventional intact Abs, as powerful therapeutic and diagnostic agents, particularly for targeting cancer, inflammatory, autoimmune and infectious diseases (Holliger and Hudson, 2005). Several Ab fragments are currently in clinical trials including late stages of clinical development (Holt et al.,2003; Demarest et al., 2008; Weisser and Hall, 2009; Wilcock et a., 2009). However, despite the general acceptance of the utility of Abs targeting Aβ as promising therapeutics for AD and a number of reports on Aβ-specific recombinant antibody fragments (scFvs and Fabs), these molecules are not progressing currently to late-phase clinical trials (Frenkel et al., 2000; Tammer et al., 2002, Manoutcharian et al., 2003; Manoutcharian et al., 2004; Levites, 2006; Lichtlen and Mohajeri, 2008; Zameer et al., 2008).
The dAbs, bearing Ig heavy (VH) or light chain (VL) variable domains, are Ab-derived recognition units of 11–15 kDa and have been shown to possess unique and superior properties for a range of diagnostic and therapeutic applications (Holliger and Hudson, 2005). Interest to these types of molecules was recently revived after the discovery that camelids and cartilaginous fish naturally produce heavy chains Abs (with VH domains called VHH and V-NAR, respectively) that are devoid of light chains. Several VH, VHH and VL dAbs against various antigen targets were isolated from camel, shark, mouse and human phage display antibody libraries and evaluated in vivo (Holt et al., 2003; Holliger and Hudson, 2005; De Bernardis et al., 2007; Harmsen and De Haard, 2007). Recently, a conformational antibody domain that prevented mature amyloid fibril formation by stabilizing Aβ protofibrils was selected from a fully synthetic library of camelid VHH-domains (Habicht et al., 2007). Also, two VHH antibodies, binding to low-molecular weight Aβ oligomers were obtained from an immunized alpaca phage display library (Lafaye et al., 2008).
In the present study, we developed a novel strategy for the generation of dAbs consisting in expression of Ig VH repertoire, fused to M13 bacteriophage cpVIII, at high copy numbers on the phage surface. We used this approach to construct a phage display VH domain library from mice immunized with Aβ1-42. This immune mouse library as well as a non-immune human scFv antibody library were used for selection of recombinant antibody fragments recognizing Aβ1-42 peptide. Five Aβ-specific antibody fragments were selected and shown to neutralize the toxicity of oligomeric Aβ42 tested in vitro in differentiated SH-SY5Y and IMR-32 cell cultures. In addition, these antibodies bound specifically to amyloid-beta deposits present in transgenic mouse brain. Finally, we showed that one of the tested VH antibody fragments reduced amyloid load after intracranial delivery into the Tg2576 mouse. These antibody fragments may be considered as potential therapeutic candidates for passive AD immunotherapy.
2. MATERIALS AND METHODS
2.1. Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Synthetic human Aβ1-42, Aβ1-16, Aβ8-42, Aβ12-28, Aβ17-42 and Aβ35-25, as well as N-pyroglutamate modified peptides AβN3(pE) and AβN11(pE), were purchased from Ana Spec (San Jose, CA, USA). A non-related peptide used as a negative control (NRP; amino acid sequence: AALSPGSSAYPSATVLA) was synthesized in our laboratory. 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), Thioflavin T, all-trans retinoic acid and dibutyryl cAMP were from Sigma. HRP-conjugated anti-mouse IgG, IgG1 and IgG2b and HRP-conjugated goat anti-rabbit IgG were from Zymed (San Francisco, CA, USA). Super Signal West Dura Extended Duration Substrate kit was from Pierce, Rockford, IL, USA. Cell culture media (DMEM/F12, 1:1) were from GIBCO (Grand Island, NY, USA).
2.2. Construction of phage displayed VH library from mouse immunized with Aβ1-42
Construction of VH library was carried out essentially as described in our previous study (Manoutcharian et al., 2003). All molecular biology procedures were carried out using standard protocols or as recommended by manufacturers. Restriction enzymes, DNA isolation/purification kits, mRNA extraction and cDNA synthesis kits, DNA polymerase, T4 DNA ligase and helper phage were obtained from Amersham Biosciences (Piscataway, NJ, USA), Invitrogen (Carlsbad, CA, USA) or New England Biolabs (MA, USA). The oligonucleotides were synthesized at Invitrogen. The phagemid vector pG8SAET allowing the expression of foreign polypeptides as fusions with the major coat protein (cpVIII) on M13 phage and described previously in our studies was used (Manoutcharian et al., 2005). To allow the cloning of cDNAs coding for VH domains, new restriction sites Xho I, Hind III and Not I were introduced by cloning a DNA fragment into the pG8SAET vector at Nco I and Bam HI sites. This DNA was generated by combining a pair of complementary oligonucleotides 5MP: CATGCCATGGTCTCGAGAAGCTTGCGGCCGCTGGTGCGCCGGTGCCGTA TCCGGACCCACTGGAACCGCGTGCCTAGG and 3ANMP: GGTACCAGAGCTCTTCGAACGCCGGCGACCACGCGGCCACGGCATAGGC CTGGGTGACCTTGGCGCACGGATCCCTAG in an annealing reaction creating Nco I and Bam HI restriction sites at 5′ and 3′ ends of the DNA fragment, respectively. About 1 μg of this DNA was ligated using T4 DNA ligase to approximately 0.5 μg of Nco I/Bam HI digested and gel-purified pG8SAET vector DNA. The ligation mixture was used to transform chemically competent E.coli TG1 bacteria and transformed cells were plated on LB-Amp plates. The correct cloning was confirmed by DNA sequencing of several clones. The plasmid DNA of modified pG8SAET vector was isolated and used for the cloning of VH library. The cDNA fragments coding for Ig VH domains were generated as described previously (Manoutcharian et al., 2003). Briefly, the mRNA was extracted from the splenocytes of mice immunized with Aβ peptide using QuickPrep mRNA Purification Kit (Amersham) and first strand cDNA was synthesized from mRNA using random pd(N)6 primers according to RPAS Mouse ScFv Module (Amersham). The VH domain genes were amplified by PCR using specific primers from the same kit and the obtained DNA, after gel purification, using Concert Rapid Gel Extraction System (Marligen Biosciences, MD, USA), was used as template in a second PCR. Two primers carrying restriction sites Nco I and Hind III (underlined) were used for PCR reamplification of VH genes, 5PCANT: ATATTGCATGCTCGAGACGCGTATCCATGGTAGTTGTTCCTTTCTATGCGG CCCAGCCGGCC and 3LINK: TTCTTAGATCGTCGAC AAGCTTCGATCCGCCACCGCCAGAGCCACCTCCGCCT. The PCR products were gel purified, digested with Nco I and Hind III, column purified and ligated with similarly digested DNA of modified pG8SAET vector as described above. The ligated DNA was column purified and introduced into Escherichia coli TG1 cells by electroporation using Gene Pulser II System (Bio-Rad Laboratories). Ten electroporations were performed, and the transformed TG1 cells were plated on LB-Amp plates to determine the diversity of the library. Ten individual bacterial colonies were used to analyze the quality of the library by PCR. The resultant phagemid library was rescued/amplified using M13KO7 helper phage (Invitrogen), purified by double PEG/NaCl (20% w/v polyethylene glycol-8000; 2.5 M NaCl) precipitation and resuspended in Tris-buffered saline (TBS). The typical phage yields were 1011–1012 colony-forming units (cfu) per milliliter of culture medium.
2.3. Peptide preparation and WB
Aβ1-42 was dissolved in HFIP to allow a conversion to the monomer and, after evaporation of solvent, was stored in aliquots at −20°C. Oligomeric Aβ1-42, AβN3(pE) and AβN11(pE) were prepared from monomers essentially as described previously (Klein, 2002). Oligomerization was performed at 4°C or at 37°C for 72 hrs. Aβ1-16, Aβ8-42, Aβ12-28, Aβ17-42, Aβ35-25 and a non-related peptide (NRP, amino acid sequence: AALSPGSSAYPSATVLA) were dissolved in water at a concentration of 1 mg per ml. All oligomeric Aβ1-42 preparations were separated by electrophoresis on 4–12% polyacrilamide precast NuPAGE Bis-Tris gels (Invitrogen) at 100 V for 1 h 45 mins. After electrophoresis, the peptides were transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA) using a semi dry blot system (Bio-Rad) at 25 V for 50 min. Then the membranes were blocked in PBS/2% non-fat dry milk/0.2% Triton X-100 overnight at 4°C. Incubation with primary antibodies – 4G8 (1:2000), a monoclonal anti-β-amyloid antibody (Sigma) – was carried out overnight at 4°C. After washing with PBS/0.2% Tween, the membranes were incubated with HRP-conjugated anti-mouse IgG2b, 1:2500, for 2 h at RT. Immunoreactive bands were detected by chemiluminescence using SuperSignal West Dura Extended Duration Substrate kit (Pierce).
2.4. Selection of Aβ-specific VH and scFv antibodies by biopanning against Aβ1-42 peptide
Selection and amplification procedures for the immune mouse VH library and a non-immune human scFv library were carried out essentially as described (Manoutcharian et al., 2003; Manoutcharian et al., 2004). Three rounds of biopanning were performed. In each round, we rescued phagemid library using helper phage M13K07. After each round, 20 to 40 clones were isolated and their DNA inserts were analyzed by PCR. Phage clones carrying full-length DNA inserts were further evaluated in ELISA as described previously (Manoutcharian 2003, Manoutcharian et al., 2004). Expression in pSyn1 vector and purification of soluble antibody fragments were performed essentially as described in our previous reports (Riaño-Umbarila et al., 2005). All soluble amino terminal His6-tagged VHs were produced using the baculovius expression system. Briefly, the corresponding VH region (VH1.27, 2.8 or 1.28) was amplified by PCR using the primers VH1.27 (5′-atcagatctgaaatgcagcagtca-3′ and 5′-agagtcgactggagacggtgaccgtggt-3′), VH2.8 (5′-atcagatctcaggtcaaactgcaggagggag-3′ and 5′ atcgtcgactggagacggtgaccgt-3), and VH1.28 (5′-atcagatctcaagtgcaggagtctgg-3′ and 5′-atcgtcgactggagacggtgaccg-3′) containing the restrictions sites BglII and SalI (underlined). After amplification, gel purified and digested fragments were ligated into pBlueHis2B (Invitrogen, Carlsbad, CA) using the same restriction sites. The resulting plasmids were designed pBlueHis-VH1.27, pBlueHis-VH2.8 and pBlueHis-VH1.28. The recombinant baculoviruses were subsequently generated using the Bac-N-Blue system (Invitrogen, Carlsbad, CA) as previously reported and designed BacHis-VH1.27, BacHis-VH2.8 and BacHis-VH1.28, respectively. The recombinant virus selection and amplification were performed as previously described (Luz-Madrigal et al., 2007). For large-scale production of His6-tagged VHs, separately 1×108 (2×106/ml) Sf9 (Spodoptera frigiperda) cells were infected at a multiplicity of infection (MOI) of 5 and were collected after 72 h by centrifugation at 1000 × g during 10 min at 4°C. Cells were treated with insect lysis buffer [10 mM Tris.Cl, pH7.5, 130 mM NaCl, 1% (v/v) Triton X-100, 10 mM NaF, 10 mM sodium phosphate, pH7.5, 10 mM sodium pyrophosphate] containing complete protease inhibitors cocktail (Roche, biochemicals) and incubated during 45 min on ice. The lysate was clarified by centrifugation at 40, 000 × g during 30 min at 4°C. The scFvs and His6-tagged VHs were purified by Ni2+-NTA affinity chromatography (Qiagen, Hilden, Germany), and eluted with 1 mL of 250 mM imidazole.
2.5. ELISA binding of positive VH- or scFv-bearing phage clones to full-length Aβ1-42 and fragments
ELISA analysis was carried out using MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) coated overnight with a synthetic peptide at a concentration of 2 μg/ml in carbonate buffer (pH 9.6). After washing with phosphate buffer containing 0.2% Tween-20 (PBS-Tween), plates were blocked with PBS/non-fat milk (2%) for 1 h at room temperature (RT). Plates were washed, then phage previously incubated for 30 minutes at RT with PBS/non-fat milk (2%)/triton X-100 (0.2%), were added at a concentration of 1011 per ml, and after incubation for 2 hrs at RT, plates were washed with PBS-Tween. HRP/Anti-M13 monoclonal conjugate (Amersham) diluted in PBS/milk/triton was added, and plates were incubated for 1 h at RT. Monoclonal anti-β-amyloid antibodies BAM90.1 and 4G8, used as a positive control to test the peptide binding to well surface, were from Sigma. Polyclonal mouse anti-human Aβ1-42 and Aβ8-42 antibodies as well as rabbit anti-AβN3(pE) and AβN11(pE) antibodiesa, also used as positive controls, were produced previously in our laboratory (Manoutcharian et al., 2003; Acero et al., 2009). HRP-Goat anti-mouse IgG (H+L) conjugate (for mouse sera analysis), HRP-conjugated rabbit anti-mouse IgG1 (for BAM90.1) and HRP-conjugated rabbit anti-mouse IgG2b (for 4G8) diluted in PBS/milk/triton were used as recommended by manufacturer. Plates were washed and 2,2′-azino-bis- (3-ethyl-benzthiazoline-6-sulphonic acid (ABTS) single solution (Zymed) was added. The OD reading at 405 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies, Chantilly, VA, USA).
2.6. DNA sequencing
The DNA sequences of the inserts of phage clones were determined on automated ABI Prism 310 Genetic Analyzer (Applied Biosystems, CA, USA) using miniprep-purified double-stranded DNA and pG8SAET vector-based 5′ and 3′ primers. The DNA and deduced amino acid sequences were analyzed by computer search with ExPASy Molecular Biology server, BLAST and IMGT (International ImMunoGeneTics System) databases.
2.7. Inhibition of Aβ1-42 toxicity in vitro by selected VH and scFvs in SH-SY5Y and IMR-32 cell cultures
Human neuroblastoma SH-SY5Y and IMR-32 cells obtained from the American Type Culture Collection (ATCC, VA, USA) were maintained in DMEM/F12 (1:1) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO) and penicillin-streptomycin (GIBCO) and differentiated for 8–10 days in the presence of 10 μM all-trans retinoic acid (SH-SY5Y) or 1mM dibutyryl cAMP (IMR-32 cells). To test the inhibitory effects of compounds on the neuronal toxicity induced by Aβ1-42, cells were plated at a density of 1 × 104 cells/200 μl per well in 96-well tissue culture plates (Corning, NY, USA). For all assays, Aβ1-42 peptide preparations were mixed to a final concentration of 20 μmol/ml in OPTI-MEM serum free medium containing 200 u/ml penicillin and 200 μg/ml streptomycin. Synthetic Aβ35-25 was used as a negative control. Phage bearing VH antibody (c.c1.27, c.c1.28 and c.c2.8) or scFv antibody (3.20, 4.4, 4.8) and control phage diluted in TBS were mixed with Aβ1-42 peptide to a final concentration from 1010 to 1012 cfu/ml and incubated overnight at 4°C. Before treatment of cells, the old medium was removed and the peptide preparation (with or without phage) was added to each well and incubated for 24 hrs. Cell viability was assessed using an XTT cytoxicity assay (sodium 3′ {1-[phenylaminocarbonyl]-3-4 tetrazolium}-bis {4-methoxy-6-nitro} benzene sulfonic acid hydrate) (Roche, IN, USA) according to manufacturer’s instructions. 50 μl of XTT was added to each plate and incubated for 4 hrs at 37°C and 5% CO2. The OD reading at 500 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies).
2.8. Immunohistochemical analysis
Antibody fragments were also used to assay if they recognized dense core plaques on brain sections from APP Tg2576 mice (Hsiao et al., 1996). Briefly, 18 months old mice APP/Tg and wild type mice were overdosed with 100 mg/kg Nembutal, intracardially perfused with ice-cold PBS, pH 7.4, brains were collected and fixed over night in 4% paraformaldehyde. Coronal sections 40 μm thick were mounted on glass slides and air-dried. After antigen retrival in 90% formic acid for 5 min, and autofluorescence quenching by incubating in TBS containing 10% methanol and 3% H2O2 for 30 min at room temperature, scFv antibody fragments were diluted 1:100 and added to the slides. After overnight incubation at 4°C, slides were washed and mouse anti-myc antibodies (Zymed) were added, followed by goat anti-mouse Alexa-Fluor 488 (Invitrogen). Slides were then incubated with rabbit anti-Aβ1-42 antibodies (established in the Lab) at 1:1000 followed by goat anti-rabbit Alexa-Fluor 594 (Invitrogen) at 1:1000. Alter extensive washing, slides were coversliped with Fluoromunt-G (Southern Biotech, Birmingham, AL) (Petrushina et al., 2007). In the case of VH fragments, rabbit polyclonal anti-histidine (His-probe H-15, Santa Cruz Biotechnology, CA, USA) antibodies were used followed by goat anti-rabbit Alexa-Fluor 594 (Invitrogen). Then slides were incubated with Thioflavin T (0.5% in 50% ethanol), and after extensive washing, were coversliped as described above.
Confocal images (size 1024 × 1024 pixel or 800 × 800 pixel) were collected sequentially at RT on a FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan) using a 60x or 40x objective lens (UPLSAPO NA=1.35 and UPLFLN NA=1.30, respectively). FITC or Thioflavin T fluorescence was obtained after exciting the samples with a wavelength of 488 nm and reading the fluorescence at 519 nm. For the detection of rhodamine the excitation wavelength was 543 nm and emission was colleted at 578 nm. The fluorescence intensity was set optimally for control slides (scFv untreated slides) and this exposure was retained for slides treated with a negative scFv and those treated with scFv (3.20, 4.4 and 4.8) and VH (1.27, 2.8 and 1.28). Images reconstruction was achieved by performing optical slices of hippocampus of mouse brain taken 0.3–0.45 μm apart in stacks about 20–25 images. All images were obtained from a minimum of 8 slices and at least 5 different plaques. From these image stacks, three-dimensional (3D) digital models of plaques were reconstructed by image thresholding and surface rendering employing Imaris 5.0.3 software (Bitplane AG, Zurich, Switzerland) and CorelDRAW X3. Images from controls and treated cells were processed under similar conditions.
2.9. Intracranial injections
The 14-month-old Tg2576 mice (Hsiao et al., 1996) were used in this study. Mice were anesthetized with Nembutal (50 mg/kg body weight) and placed in a stereotactic apparatus (MyNeuroLab, St. Louis, MO) with a mouse adaptor as described previously (Vasilevko et al., 2007)). Briefly, experimental and control phage were diluted in PBS (1010 pfu/μl), and anti-Aβ monoclonal 6E10 antibody (Covance Research Products, Berkley, CA) at concentration of 1 mg/ml was used as a positive control. Experimental and control agents were injected into the left hippocampus through a 33 gauge injector attached to a 10μl Hamilton syringe (Hamilton, Reno, NV). The coordinates, with respect to bregma, were 2.7mm posterior, 2.5 mm lateral, and 3.0 mm ventral to the skull. Two microliters of agents were injected over the span of 5 min, after which the cannula was left in place for an additional 2 min to allow for diffusion. Animals were placed on a warming pad until they had fully recovered from anesthesia and were kept in individual cages to prevent damage to the sutures. Seven days later, mice were overdosed with 100 mg/kg Nembutal and intracardially perfused with ice-cold PBS, pH 7.2, their brains were fixed in 4% paraformaldehyde overnight. For immunostaining of Aβ deposits, sections were cut in the coronal plane at 40 μm using a vibratome, and after the antigen retrival in 70% formic acid, were stained with rabbit polyclonal anti-Aβ primary antibodies followed by goat anti-rabbit biotinylated secondary antibodies and ABC–HRP system from Vector Laboratories (Burlingame, CA). Primary antibodies were visualized with diaminobenzidine solution (Vector Laboratories). At least five sections both ancestral and posterior to the site of injection were stained for Aβ load and analyzed using ImageJ software.
2.10. Statistical Analysis
For multiple comparisons ANOVA and post-hoc Tukey test were used. SPSS Release 9.0.0 statistical software program was employed.
3. RESULTS
3.1. Construction of phage displayed VH library and selection of Aβ-specific VH and scFv antibodies by biopanning against Aβ
For construction of phage displayed VH immune library, spleen cells from mice immunized with Aβ1-42 peptide were used as a source of Ig VH genes. The cDNAs coding for VH domains were amplified by PCR and cloned into the phagemid vector allowing the expression of VH fused to major coat protein VIII (cpVIII) of M13 phage at high copy numbers. The estimated number of cpVIII fusions expressed on hybrid phage particles is 300–900 copies per phage (Willis et al., 1993). Antibody VH single-domain phage display library consisting of 8×104 independent clones was generated and, more than 90% of clones were shown to carry the inserts of correct size (data not shown).
To select recombinant antibody fragments recognizing Aβ1-42, the constructed VH mouse immune library as well as a scFv human naïve library, described in our previous studies (Manoutcharian et al., 2004), were screened against oligomeric Aβ1-42. All oligomeric preparations of Aβ1-42 contained two prominent bands corresponding to trimers and tetramers as well as a monomer as demonstrated (Fig. 1). Higher molecular weight bands corresponding to 12- and 24-mers were also observed (Fig. 1). All bands were identified by 4G8 antibody known to bind to an internal epitope of Aβ (aa17–24).
Fig. 1.
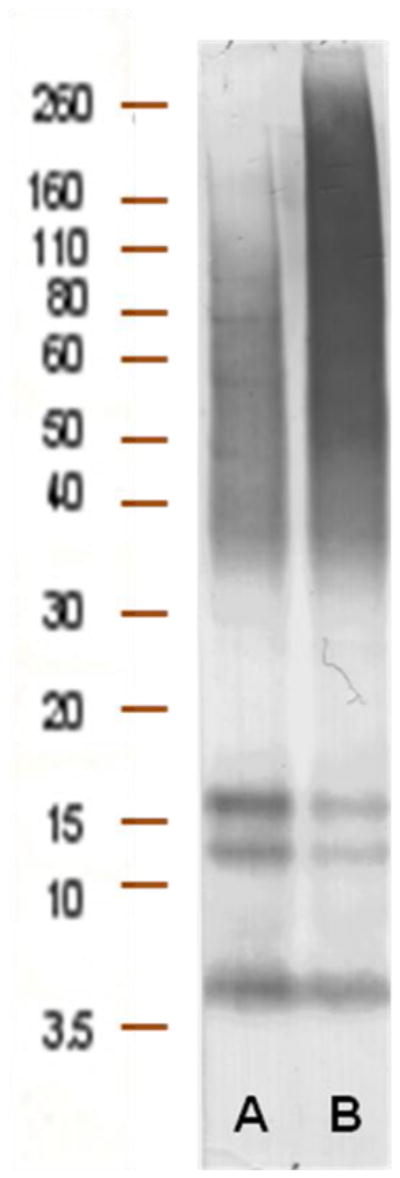
Biopanning of Ab fragments was done against preferentially oligomeric Aβ forms. Oligomers were prepared and analyzed as described in Materials and Methods section. Aβ1-42 was incubated at 4°C (A) and at 37°C (B). Migration of the molecular mass standards is indicated.
After the third round of panning against β-amyloid peptide, three VH domain-expressing phage clones (C 1.27, C 1.28 and C 2.8) and two scFv-expressing phage clones (scFv 4.8 and scFv 3.20), with the highest ELISA values, were selected for further studies. DNA sequencing of positive VH domain-expressing phage clones revealed three unique sequences which were aligned according to IMGT numbering for V-DOMAIN in the IMGT/DNA PLOT directory (Table 1) (Lefranc et al., 2003). As shown in Table 1, these VH-domains were originated from different germline genes/segments and carried multiple mutations at both nucleotide and amino acid level. No sequence homology was found between HCDR3 regions of the clones, and only VH framework region 3 (FR3)-derived sequences had high homology between the clones.
Table 1.
Germline genes/segments and CDR sequences of VH domain antibodies isolated from immune library selected on β-amyloid peptidea
| Clone | VH germline | DH germline | JH germline | HCDR1 | HCDR2 | HCDR3 |
|---|---|---|---|---|---|---|
| C 1.27 | IGHV3-6*02(6)b | IGHD2-14*01 | IGHJ4*01 (11)b | GYSITSGYY | ISYDGSN | CARDYRYDGMDYC |
| C 2.8 | IGHV3-1*02(14)b | IGHD3-3*01 | IGHJ1*01 (11)b | GYSITSGYS | MQYSGTT | CARGDGAYW |
| C 1.28 | IGHV3-2*02(4)b | IGHD3-2*01 | IGHJ2*01 (5)b | GYSITSDYA | ISYSGST | CARRGNYLDYW |
Mouse germline VH, DH and JH segments have been assigned as detailed in the International ImMunoGeneTics Information System (IMGT). The amino acid sequences of CDRs (according to IMGT nomenclature) are shown.
Differences in nucleotides from VH and JH germline sequences.
3.2. VH- or scFv-bearing phage clones do not bind to an epitope within the immunodominant N-terminal region of Aβ1-42
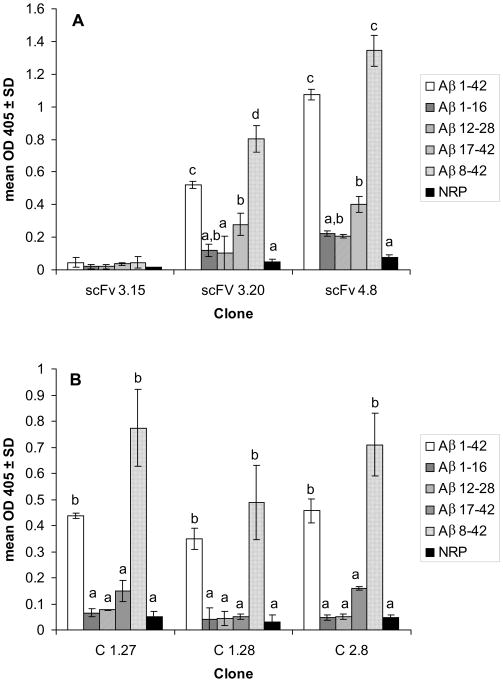
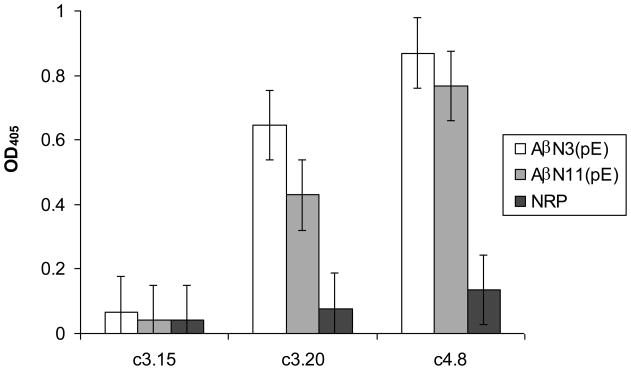
To define more precisely the binding epitope of selected antibody fragments, ELISAs were performed using full-length Aβ1-42 and four short fragments (Fig. 2). All recombinant antibodies bound to full-length Aβ1-42 and to N-truncated Aβ8-42 as well as to N-pyroglutamate modified peptides AβN3(pE) and AβN11(pE) (Fig. 3). Also, four of five antibodies bound moderately to Aβ17-42 (Fig. 2). These results indicate that epitope recognized by selected recombinant antibody fragments is located in the central/C-terminal part of the full-length Aβ1-42. In all experiments monoclonal anti-β-amyloid antibodies BAM90.1 and 4G8, as well as polyclonal mouse anti-human Aβ1-42 and Aβ8-42 antibodies produced previously in our laboratory (Manoutcharian et al., 2003), were used as positive controls to test the peptide binding to well surface.
Fig. 2.
Selected Ab fragments recognize epitopes in middle and/or C-terminus of Aβ1-42. ELISA analysis of binding of selected scFv- and VH-bearing phage clones to synthetic Aβ peptides. NRP - a non-related control peptide. Data are means ± SD of 2 independent experiments. Means denoted with different letters are statistically different (P<0.05). In the same assay BAM90.1 bound to Aβ 12-28 (OD=1.074 ± 0.08), 4G8 bound to Aβ12-28 (OD=1.53 ±0.02) and Aβ17-42 (OD=1.125 ±0.03). Mouse anti-human Aβ1-42 polyclonal antibodies bound to Aβ1-42 and Aβ1-16, OD=1.98 ± 0.04 and OD=1.99 ± 0.05, respectively. Mouse anti-adjuvant serum was used as a negative control and showed OD405 < 0.1.
Fig. 3.
Selected Ab fragments bound to N-truncated/pyroglutamate modified peptides AβN3(pE) and AβN11(pE). ELISA analysis of binding of selected clones to synthetic Aβ 1-42, AβN3(pE) and AβN11(pE). Data are means±SD of 2 independent experiments.
3.3. Inhibition of Aβ1-42 toxicity in vitro by selected VHs and scFvs in differentiated neuroblastoma cells
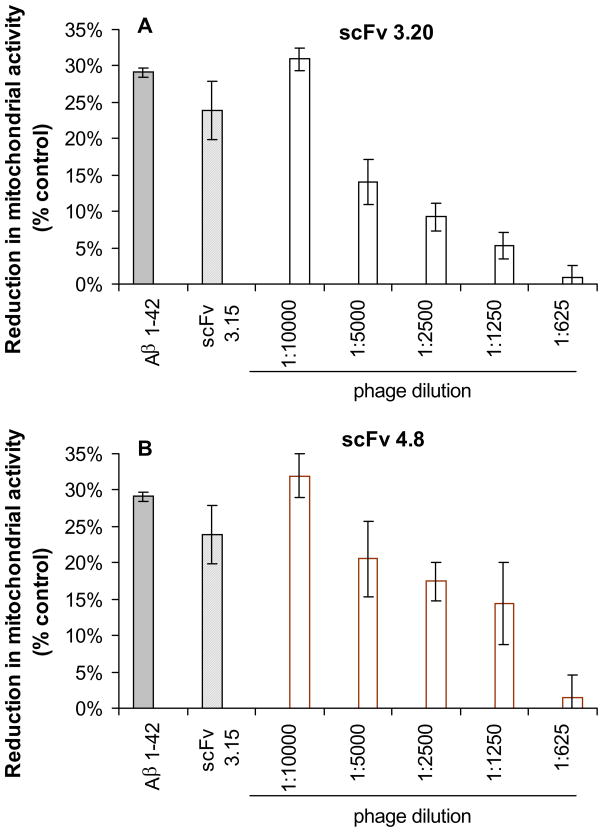
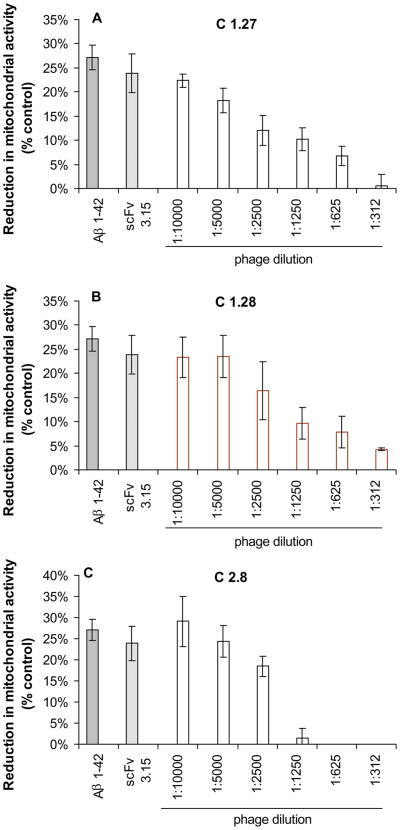
We assessed the ability of selected VHs and scFvs to inhibit Aβ1-42-induced cytotoxicity in SH-SY5Y and IMR-32 differentiated neuroblastoma cells using XTT assay. Overnight preincubation of Aβ1-42 with VHs and scFvs prior to addition to cells resulted in a dose-dependent inhibition of the reduction in mitochondrial activity compared with a control phage (Fig. 4 and Fig. 5). A control phage expressing a non-related antibody fragment did not show any inhibition activity.
Fig. 4.
Selected scFv-bearing phage clones from human phage library inhibit Aβ42 induced neurotoxicity in human neuroblastoma SH-SY5Y cell cultures. Cell viability was assessed using an XTT toxicity assay. Data presented are means ±SE of three independent experiments.
Fig. 5.
Selected VH-bearing phage clones from immune mouse phage display library inhibit Aβ42-induced neurotoxicity in human neuroblastoma SH-SY5Y cell cultures. Cell viability was assessed using an XTT toxicity assay. Data presented are means±SE of 3 independent experiments.
3.4. Selected VHs and scFvs bind to amyloid deposits in Tg mouse brain sections
In order to further characterize the binding properties of selected antibody fragments, we tested whether soluble scFvs may detect naturally occurring Aβ deposits in Tg2576 mouse brain tissue. All recombinant antibody fragments showed a specific binding on transgenic mouse brain sections (Fig. 6 and Fig. 7). No binding to control mouse brain was observed. Control non-related scFv or Vh did not bind to amyloid aggregates on transgenic mouse brain sections.
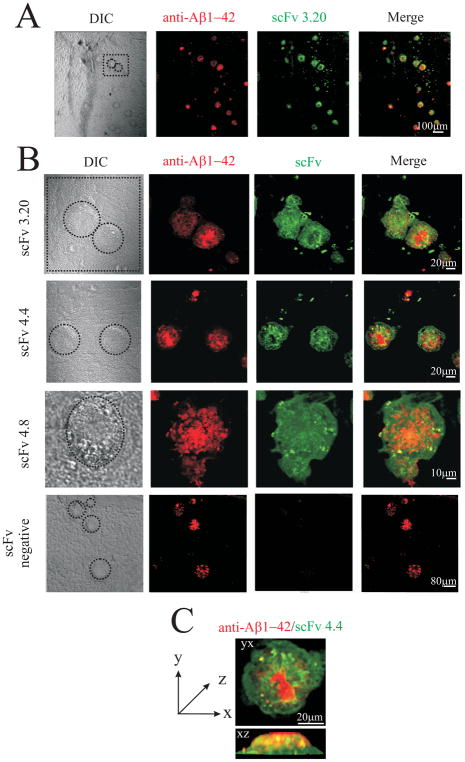
Fig. 6.
Purified soluble scFv antibodies from human non-immune phage display library specifically recognize Aβ deposits in the hippocampus of transgenic mice (Tg2576). Each panel shows, from the left: the reactivity of a rabbit anti-Aβ1-42 antibody (shown in red); the reactivity of soluble scFv fragments (shown in green); the merge between red and green channels. (A) Low magnification provides an overview of the hippocampus in which amyloid deposits are recognized by the soluble scFv fragment (scFv3.20). (B) High magnification provides a detailed view of binding of scFv 3.20, scFv 4.4 and scFv 4.8 to amyloid deposits. Merged images illustrate clear colocalization of red and green fluorescence (in yellow) as well as only-green and only-red areas. Only a background staining is observed with a control scFv antibody. (C) Three-dimensional reconstruction of sequential confocal sections (z-stacks, taken 0.3–0.45 μm apart in stacks) of an amyloid deposit in yx and xz planes.
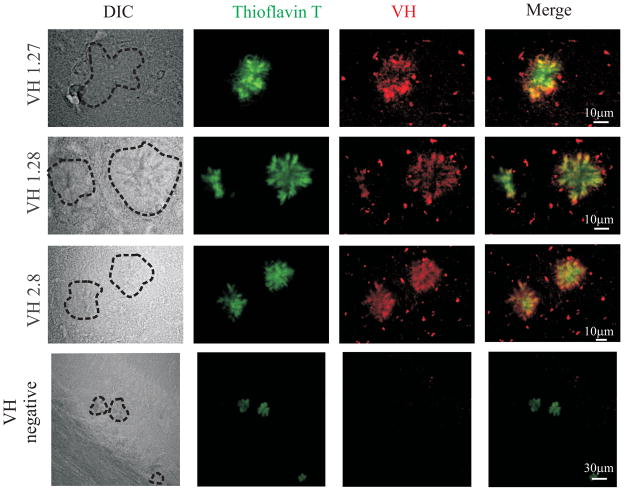
Fig. 7.
Purified soluble VH antibodies from mouse immune phage display library specifically recognize Aβ deposits in the hippocampus of transgenic mice (Tg2576). Each panel shows, from the left: Thioflavin T staining (shown in green); the reactivity of soluble VH fragments (shown in red); the merge between green and red channels. Merged images illustrate clear colocalization of red and green fluorescence (in yellow).
3.5. Aβ-specific VH recombinant antibody fragment clears amyloid deposits after intracranial delivery
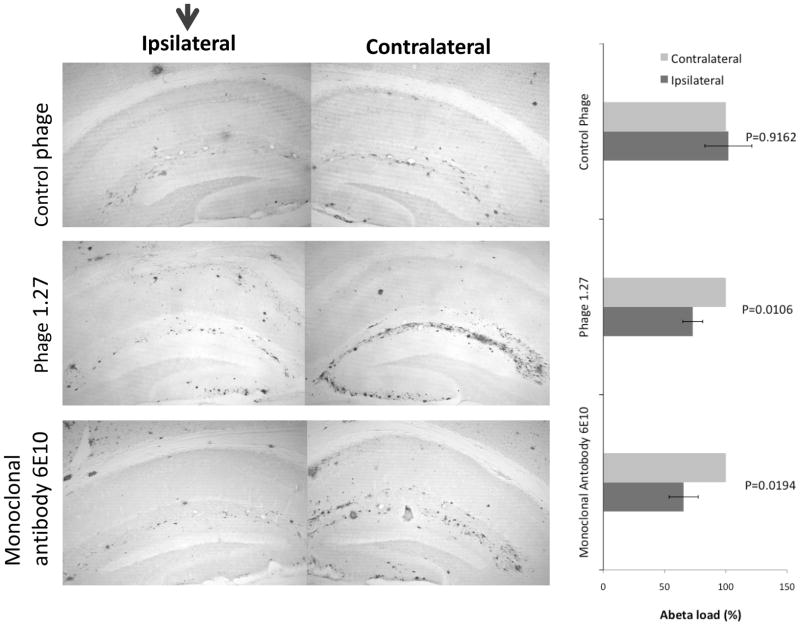
To demonstrate therapeutic efficacy of the discovered antibody fragments, we injected phage C1.27 bearing Aβ-specific VH antibody fragment into the left hemisphere of 14 month old APP/Tg 2576 mouse. As controls we used negatively selected phage 3.17 or Aβ-specific mouse monoclonal antibody 6E10, which was effective in our previous studies. Seven days later mice were sacrificed and brain sections were stained for amyloid deposits analysis (Fig. 8). Right hemisphere of the mouse brain was used for the comparison of amyloid load. In these experiments, phage 1.27 was almost as effective as a control Aβ-specific monoclonal antibody (6E10) in amyloid clearance, while negative control phage did not reduce amyloid load (Fig. 8).
Fig. 8.
Purified phage bearing anti-Aβ antibody fragment efficiently clears amyloid deposits after intracranial delivery. Purified phage C1.27 bearing anti-Aβ antibody fragment, as well as negative control phage or monoclonal 6E10 antibody, were intracranially injected into the left hippocampus of 14-month-old Tg2576 mice. Seven days later, brain sections were immunostained for Aβ with polyclonal rabbit anti-Aβ antibody. Original magnification was at 40x. Aβ load from the contralateral side was considered as 100%. Data are presented as mean±SE.
4. DISCUSSION
In this study, we selected a novel set of human anti-Aβ1-42 recombinant monoclonal antibodies in a single chain fragment variable (scFv) and a single domain (VH) formats from a human and mouse phage display antibody libraries, respectively. We also evaluated their binding characteristics as well as their capacity to inhibit Aβ1-42 toxicity in vitro, using SH-SY5Y and IMR-32 cell cultures. In addition, we demonstrated that these antibodies bound to Aβ1-42 present in transgenic mouse brains and clear amyloid deposits after intracranial administration in APP/Tg mice.
Immunotherapy approaches, both active immunization with Aβ peptide, or passive transfer of anti-Aβ antibodies, have shown therapeutic efficacy in several APP/Tg mouse models, which develop AD-like amyloid plaque pathology (Brody et al., 2008), as well as in a canine and a primate models of amyloidosis (Lemere et al., 2004, Head et al., 2008). However, in some cases inflammatory complications and deleterious side effects such as increased hemorrhages were observed. Rapid microglial response around amyloid pathology and interaction of microglia via Fc receptor with full-length antibody-opsonized amyloid were shown to result in increased inflammation (Lue et al., 2002; Koenigsknecht-Talboo et al., 2008). However, in an interesting study by Morgan and collaborators, minimal vascular side effects were observed after passive administration of deglycosylated compared with intact anti-Aβ antibodies (Wilcock et al., 2006). Deglycosylation of IgG molecule results in defective binding to Fc receptor and complement C1q leading to decreased activation of microglia. Moreover, it has been demonstrated that the reduction in Aβ accumulation after Aβ1-42 immunization of FcR-γ chain knock-out Tg2576 mice was equivalent to the reduction in Aβ deposits seen in immunized age-matched FcR sufficient Tg2576 mice, suggesting that the Fc portion of the anti-Aβ antibody required for interaction with FcR may not be necessary for Aβ immunotherapy to be effective (Das et al., 2003). Using antibody fragments lacking the functional Fc portion of the whole Ig molecule may represent an alternative approach in passive immunotherapy treatment strategies against AD (Bacskai et al., 2002).
Interestingly, antibody fragments selected in this study did not bind to amino-terminal part of Aβ1-42. These binding characteristics of the selected antibody fragments may be important considering that an increase in both the incidence and severity of cerebral amyloid angiopathy-associated microhemorrhages after passive immunization with anti-amino terminal antibody (3D6) was observed in previous studies (Pfeifer et al., 2002; Racke et al., 2005). In addition, anti-amino terminal antibodies may interact also with APP at the cell surface, potentially interfering with its function and processing.
Analyses of samples extracted from the brain of subjects with AD revealed the presence of low molecular weight oligomers - dimers, trimers and tetramers (McLean et al., 1999; Cleary et al., 2005, Shankar et al., 2008). Importantly, these low-n aggregates were shown to exist in both intracellular and extracellular compartments, impair synaptic plasticity and disrupt cognitive function (Walsh et al., 2002; Cleary et al., 2005; Klyubin et al., 2005; Townsend et al., 2006; Shankar et al., 2008). Aβ1-42 preparations used in our study contained mainly low-n oligomers, and, importantly, antibody fragments selected in this study neutralized the toxic effects of these species.
Another important fact to consider for immunotherapeutics preparation for AD is the presence of N-truncated and N-modified forms of Aβ peptide in diffuse and dense core plaques in AD and Down’s syndrome patients as well as in transgenic mouse models of AD. However, the majority of antibodies used in previous studies recognized the N-terminal immunodominant epitope (EFRH) of the full length Aβ, which is absent in N-amino truncated peptides. Recombinant antibody fragments described in our study were shown to bind to N-truncated Aβ peptides and, thus, would target a broad range of toxic Aβ species.
The field of recombinant antibody fragments has rapidly progressed during the last several years because of the interest in their therapeutic use. The discovery that camelids have functional antibodies devoid of light chain let researchers to turn to single-domain antibody fragments (VHHs or nanobodies) and isolate VH domain antibodies binding to a number of antigens of interest in cancer studies and a number of infectious, inflammatory and other diseases (Harmsen and De Haard, 2007). Among advantages of VH domain antibodies are their high stability and solubility, rapid tissue penetration and recognition of hidden antigenic sites as well as cost-effective production in microorganisms. Three Aβ-specific VH domain antibodies selected in this study may be of potential interest for antibody-based AD therapy.
An additional interesting application of selected VHs and scFvs, will be their expression as intracellular Abs (intrabodies) to allow the targeting of intracellular pool of Aβ (Gouras et al., 2005; LaFerla et al., 2007). A number of intracellular recombinant antibodies, binding to the microtubule associated protein tau, intracellular aggregates of huntingtin and a pro-apoptotic protein Bax, were isolated and characterized from both synthetic and naïve antibody libraries (Lecerf et al., 2001; Visintin et al., 2002; Gueorguieva et al, 2006). Importantly, these recombinant Ab fragments were stably expressed in mammalian cells as intrabodies and were shown to be nontoxic to the cells. In addition, the effectiveness in improving learning and memory deficits in Tg2576 mice of intracellular expression of anti-Aβ scFv via adeno-associated virus was demonstrated recently by Fukuchi and collaborators (Fukuchi et al., 2006). More recently, a panel of anti-Aβ scFvs, targeting intracellular Aβ oligomers was selected (Mali et al., 2009). Thus, the selection of anti-Aβ recombinant intracellular antibodies may represent a new interesting approach for the development of therapeutic strategies for AD.
Finally, all soluble VHs and scFv antibodies selected in this study showed a specific reactivity on transgenic mouse brain sections. Interestingly, contrary to results obtained with a rabbit anti-Aβ1-42 antibodies, the immunoreactivity patterns with our antibody fragments showed no plaque core staining. Recently, the similar staining pattern in brain of Tg2576 mice was observed by O’Callaghan and collaborators with a mouse monoclonal anti-Aβ1-42 antibody (6E10) (O’Callaghan et al., 2008). The binding of selected antibody fragments to naturally occurring Aβ aggregates is important for considering them as promising molecules targeting amyloid deposits in human brain. Indeed, we showed that one of the tested antibody fragments cleared amyloid deposits after intracranial delivery into the Tg2576 mouse.
In conclusion, in the present study we described five recombinant antibody fragments isolated from an anti-Aβ mouse immune VH domain antibody library and a naïve human scFv library. Considering the great potential of antibodies or their recombinant fragments for AD immunotherapy, VHs and scFvs may be of interest as components of future antibody-based therapeutics for AD as well as other neurodegenerative diseases.
Acknowledgments
Authors thank Gonzalo Acero, Carmen Calixto, Alicia Sampieri and Gabriel Orozco Hoyuela for technical assistance and Patricia De La Torre for DNA sequencing. This work was supported by grants from the NIH to GG (NIA-AG023534) and DHC (NIA-AG20241 & NIA-AG00538, NINDS-NS50895); by grants from DGAPA-UNAM, Mexico (IN200907 to GG and IN203706 to KM) and by a grant from CONACyT (58081) to G.G. M.M. was a recipient of a doctorate fellowship from CONACyT, and Posgrado en Ciencias Biologicas, UNAM, Mexico.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acero G, Manoutcharian K, Vasilevko V, Munguia ME, Govezensky T, as G, Luz-madrigal A, Cribbs D, Gevorkian G. Immunodominant epitope and properties of pyroglutamate-modified Aβ-specific antibodies produced in rabbits. J Neuroimmunol. 2009;213:39–46. doi: 10.1016/j.jneuroim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games H, Grajeda T, Guido K, Hu J, Huang K, Johnson-Wood K, Khan D, Kholodenko M, Lee I, Lieberburg R, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch K, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid-β peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer’s disease. Nature Medicine. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-b immunization effectively reduces amyoid deposition in FcRγ−/− knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBernardis F, Liu H, O’Mahony R, La Valle R, Bartollino S, Sandini S, Grant S, Brewis N, Tomlinson I, Basset RC, Holton J, Roitt IM, Cassone A. Human domain antibodies against virulence traits of candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- Demarest SJ, Glaser SM. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr Opin Drug Discov Devel. 2008;11:675–687. [PubMed] [Google Scholar]
- Frenkel D, Solomon B, Benhar I. Modulation of Alzheimer’s β-amyloid neurotoxicity by site-directed single-chain antibody. J Neuroimmunol. 2000;106:23–31. doi: 10.1016/s0165-5728(99)00232-5. [DOI] [PubMed] [Google Scholar]
- Fukuchi KI, Tahara K, Kim HD, Maxwell JA, Lewis TL, Accavitti-Loper MA, Kim H, Ponnazhagan S, Lalonde R. Anti-Aβ single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer’s disease. Neurobiol Dis. 2006;23:502–511. doi: 10.1016/j.nbd.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, AN 1792 (QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Habicht G, Haupt C, Friedrich RP, Hortschansky P, Sachse C, Meinhardt J, Wieligmann K, Gellermann GP, Brodhun M, Gotz J, Halbhuber KJ, Rocken C, Horn U, Fandrich M. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc Natl Acad Sci USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-β antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Pop V, Vasilevko V, Hill M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jines RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JAR. Long-term effects of Aβ42 immunization in Alzheimer’s disease: follow-up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM. Domain antibodies: proteins for therapy. Trends Biotechnol. 2003;21:484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mattews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Neslin D, French J, Mount NTJ, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. Ab peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Mayer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzmann DM. Rapid microglial response around amyloid pathology after systemic anti-Ab antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ferla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s Disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lafaye P, Achour I, England P, Duyckaerts C, Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol Immunol. 2008;46:695–704. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Lecerf JM, Shirley TL, Zhu Q, Kazantsev A, Amersdorfer P, Housman DE, Messer A, Huston JS. Human single-chain Fv intrabodies counteract in situ huntingtin aggregationin cellular models of Huntington’s disease. Proc Natl Acad Sci USA. 2001;98:4764–4769. doi: 10.1073/pnas.071058398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Lee VMY, Trojanowski JQ. Meningoencephalitis associated with passive immunization of a transgenic murine model of Alzheimer’s amyloidosis. FEBS Lett. 2005;579:2564–2568. doi: 10.1016/j.febslet.2005.03.070. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease Aβ vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levites Y, Das P, Price DW, Rochette MJ, Kostura LA, McGowan EM, Murphy MP, Golde TE. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtlen P, Mohajeri MH. Antibody-based approaches in Alzheimer’s research. Safety, pharmacokinetics, metabolism, and analytical tools. J Neurochem. 2008;104:859–874. doi: 10.1111/j.1471-4159.2007.05064.x. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG. Modeling Alzheimer’s disease immune therapy mechanisms: interactions of human postmortem microglia with antibody-opsonized amyloid beta peptide. J Neurosci Res. 2002;70:599–610. doi: 10.1002/jnr.10422. [DOI] [PubMed] [Google Scholar]
- Luz-Madrigal A, Clapp C, Aranda J, Vaca L. In vivo transcriptional targeting into the retinal vasculature using recombinant baculovirus carrying the human flt-1 promoter. Virol J. 2007;4:88. doi: 10.1186/1743-422X-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoutcharian K, Acero G, Munguia ME, Montero JA, Govezensky T, Cao C, Ugen K, Gevorkian G. Amyloid-beta peptide specific single Chain Fv antibodies isolated from an immune phage display library. J Neuroimmunol. 2003;145:12–17. doi: 10.1016/j.jneuroim.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Manoutcharian K, Acero G, Munguia ME, Becerril B, Massieu L, Govezensky T, Ortiz E, Marks JD, Cao C, Ugen K, Gevorkian G. Human single chain Fv antibodies and a complementarity determining region-derived peptide binding to amyloid beta 1-42. Neurobiol Dis. 2004;17:114–121. doi: 10.1016/j.nbd.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Meli G, Visintin M, Cannistraci I, Cattaneo A. Direct in vivo intracellular selection of conformation-sensitive antibody domains targeting Alzheimer’s amyloid-β oligomers. J Mol Biol. 2009;387:584–606. doi: 10.1016/j.jmb.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Munch G, Robinson SR. Potential neurotoxic inflammatory responses to Aβ vaccination in humans. J Neural Transm. 2002;109:1081–1087. doi: 10.1007/s007020200091. [DOI] [PubMed] [Google Scholar]
- Morgan D. Immunotherapy for Alzheimer’s disease. J Alzheimers Dis. 2006;9:425–432. doi: 10.3233/jad-2006-9s348. [DOI] [PubMed] [Google Scholar]
- O’Callaghan P, Sandwall E, Li J-P, Ravid R, Guan Z-Z, van Kuppevelt TH, Nilsson LNG, Ingelsson M, Hyman BT, Kalimo H, Lindahl U, Lannfelt L, Zhang X. Heparan sulfate accumulation with Ab deposits in Alzheimer’s Disiease and Tg2576 mice is contributed by glial cells. Brain Pathol. 2008;18:548–561. doi: 10.1111/j.1750-3639.2008.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrushina I, Ghochikyan A, Mkrtchyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadanian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaño-Umbarila L, Juarez-Gonzalez VR, Olamendi-Portugal T, Ortiz-Leon M, Possani LD, Becerril B. A strategy for the generation of specific human antibodies by direct evolution and phage display. An example of a single-chain antibody fragment that neutralizes a major component of scorpion venom. The FEBS J. 2005;272:2591–2601. doi: 10.1111/j.1742-4658.2005.04687.x. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia Munoz A, Shepardson NE, Smith I, Brett FM, Farell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Mielke ML, Rozkalne A, Mayer-Luehmann M, de Calignon A, Bacskai BJ, Schenk D, Hyman BT. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol Dis. 2009;33:213–220. doi: 10.1016/j.nbd.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammer AH, Coia G, Cappai R, Fuller S, Masters CL, Hudson P, Underwood JR. Generation of a recombinant Fab antibody reactive with the Alzheimer’s. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein in hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572 (Pt2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin M, Settanni G, Maritan A, Graziosi S, marks JD, Cattaneo A. The intracellular antibody capture technology (IACT): towards a consensus sequence for intracellular antibodies. J Mol Biol. 2002;317:73–83. doi: 10.1006/jmbi.2002.5392. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers – a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Weisser NE, Hall JC. Applications of single-chain variable fragment antibodies in therapeutics and diagnostics. Biotechnol Adv. 2009;27:502–520. doi: 10.1016/j.biotechadv.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Colton CA. Anti-amyloid beta immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimer’s Dis. 2008;15:555–569. doi: 10.3233/jad-2008-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosental A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. Deglycosylated anti-amyloid-β antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Gharkholonarehe N, Van Nostrand W, davis J, Vitek MP, Colton CA. Amyloid reduction by amyloid-β vaccination also reduces mouse tau pathology and protects from neuron loss in two mouse models of Alzheimer’s disease. J Neurosci. 2009;29:7957–7965. doi: 10.1523/JNEUROSCI.1339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer’s disease. Lancet Neurol. 2008;7:805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zameer A, Kasturirangan S, Emadi S, Nimmagadda SV, Sierks MR. Anti-oligomeric Abeta single-chain variable domain antibody blocks Abeta-induced toxicity against human neuroblastoma cells. J Mol Biol. 2008;384:917–928. doi: 10.1016/j.jmb.2008.09.068. [DOI] [PubMed] [Google Scholar]