Abstract
Plasmodium vivax is the second leading cause of malaria worldwide. Invasion of human erythrocytes by P. vivax merozoites is dependent upon the interaction between the parasite Duffy binding protein (PvDBP) and the erythrocyte Duffy antigen receptor. Therefore, disruption of this vital interaction is an attractive target for therapeutic intervention. Although Aotus nancymaae is a commonly used primate model for human P. vivax infections, it has not been confirmed that the interaction between Ao. nancymaae erythrocytes and P. vivax is Duffy antigen dependent. Our results indicate that normal Ao. nancymaae erythrocytes readily bind to PvDBPII and that this binding is completely abolished with chymotrypsin treatment of the erythrocytes. Furthermore, the results of our inhibition assays show a dose-dependent decrease in binding with increasing amounts of anti-PvDBPII polyclonal rabbit sera or anti-Fy6 monoclonal antibody. These data indicate that the interaction between Ao. nancymaae erythrocytes and P. vivax DBPII is Duffy antigen dependent, validating this model system for in vivo studies of anti-PvDBP inhibition.
Plasmodium vivax results in an estimated 70-80 million cases of malaria annually, with an estimated 2.6 billion people at risk for infection (Mendis et al., 2001; Guerra et al., 2006). Plasmodium vivax is seasonally transmitted and produces little sustained immunity, resulting in repeated infections throughout childhood and adulthood. These recurrent infections are rarely lethal, but produce a significant physical, economic and educational burden for affected individuals (Mendis et al., 2001; Fernando et al., 2003). Reports of emerging drug resistance along with reported cases of severe clinical complications due to P. vivax infection also point to P. vivax as an important target of vaccine development (Baird, 2007; Barcus et al., 2007; Kochar et al., 2009).
Invasion of host erythrocytes by Plasmodium merozoites is a complex, multi-step process involving specific interactions between parasite ligands and host cell receptors (Dvorak et al., 1975; Bannister and Dluzewski, 1990; Barnwell and Galinski, 1995; Chitnis and Blackman, 2000; Galinski, 2005). Plasmodium vivax invasion of host erythrocytes is dependent on the interaction between the microneme Duffy binding protein (specifically region II, PvDBPII) and the host Duffy antigen receptor (Miller et al., 1976; Haynes et al., 1988; Barnwell et al., 1989; Wertheimer and Barnwell, 1989; Adams et al., 1990; Adams et al., 1992; Chitnis and Miller, 1994). Individuals who do not express the Duffy antigen on their erythrocytes appear completely protected from blood-stage infection by P. vivax (Miller et al., 1976). The parasites’ reliance on this interaction makes it an attractive target for therapeutic intervention.
Vaccine testing necessitates the use of appropriate animal models. Plasmodium parasites infect a wide variety of hosts in a species-specific manner, including rodents, chickens and primates, among others. Plasmodium vivax is known to infect a number of primates, including Aotus nancymaae, a commonly used model for Plasmodium vaccine testing (Collins et al., 2005; Darko et al., 2005; Williams et al., 2005; Collins et al., 2006; Makobongo et al., 2006; Singh et al., 2006; Rojas Caraballo et al., 2007). However, invasion of Ao. nancymaae erythrocytes has not been shown to be Duffy antigen dependent. Confirmation of this interaction is pertinent because Saimiri boliviensis monkeys, which are also used as a model of human Plasmodium infection, particularly for P. vivax, are infected by P. vivax in a non-Duffy antigen dependent fashion (Barnwell et al., 1989; Wertheimer and Barnwell, 1989). The DBP ligand does not bind to Saimiri sp. erythrocytes. In order to validate the Aotus system for P. vivax vaccine testing, we characterized the binding interaction between intact Ao. nancymaae erythrocytes and recombinant PvDBPII protein expressed on the surface of COS7 cells. Our results indicate that the interaction between Ao. nancymaae erythrocytes and PvDBPII is Duffy antigen dependent, validating this model for in vivo studies of anti-PvDBP inhibition.
Salvador I strain PvDBPII DNA was cloned into the pEGFP-N1 plasmid with flanking signal sequences from the herpes simplex virus glycoprotein D1 allowing expression of a green fluorescent protein (GFP) fusion protein on the surface of transiently transfected COS7 cells (Michon et al., 2000). A negative control plasmid, pEGFP-PkDBPβII, was cloned in a similar fashion. Recombinant plasmid DNA was purified using an endotoxin-free plasmid DNA purification system (Qiagen, Valencia, California).
COS7 cells were plated in 24-well plates at a density of 40,000 cells per well and grown for 3 hrs at 37°C, 5% CO2. The COS7 cells were then transiently transfected with endotoxin-free pEGFP-PvDBPII DNA (37.5ng/well) using Lipofectamine (Invitrogen, Carlsbad, California) in incomplete Dulbecco’s modified Eagle medium (DMEM; Sigma, St. Louis, Missouri). Sixteen hours post-transfection, the medium was replaced with complete DMEM (10% fetal bovine serum (FBS)). Forty-two hours post-transfection, the transfected COS7 cells were incubated with erythrocytes for 2 hrs at room temperature (1% final suspension, previously washed 3 times with incomplete DMEM). Wells were washed 3 times with PBS to remove nonadherent erythrocytes and binding was scored by counting the number of rosettes per 30 fields of view at 200x magnification. Inhibition assays were carried out in the same manner except that transfected COS7 cells were preincubated with antisera for 1 hr at 37°C, 5% CO2 prior to addition of the erythrocyte suspension.
Aotus nancymaae erythrocytes were collected into ACD Solution A. Immediately prior to incubation with transfected COS7 cells the erythrocytes were washed 3 times with incomplete DMEM and added to a final suspension of 1%. Chymotrypsin treatment of erythrocytes was carried out by first washing 3 times with RPMI-1640 and then adding chymotrypsin to a final concentration of 1mg/mL, 50% hematocrit. This suspension was incubated at 37°C for 1 hr with rocking and then rinsed with RPMI-1640. Soybean trypsin inhibitor was added to a final concentration of 0.5mg/mL, 50% hematocrit and incubated at room temperature for 10 min with rocking. Erythrocytes were then washed 2 times with RPMI-1640 before addition to transfected COS7 cells.
Antiserum against recombinant PvDBPII was produced as previously described (Grimberg et al., 2007) and kindly provided by Dr. Christopher King of Case Western Reserve University. Anti-Fy6 monoclonal antibody (mAb) was produced as previously described (Nichols et al., 1987). Anti-Fy6 mAb was precipitated from ascites with addition of saturated ammonium sulfate to 45% (v/v) and then dissolved in and dialyzed against PBS. This was performed 2 times resulting in a final concentration of 0.45mg/mL. Statistical analyses were performed using the Prism 4 program (GraphPad Software, La Jolla, California). Results were analyzed using 1-way analysis of variance (ANOVA) and Tukey’s post-test. Results from inhibition assays were first normalized to the control as percentages.
In order to determine if Ao. nancymaae erythrocytes interact with the parasite produced PvDBP, we initially examined the binding of Ao. nancymaae erythrocytes to recombinant PvDBPII as compared to recombinant PkDBPßII. PkDBPß is a PvDBP paralogue found in P. knowlesi, having more than 70% sequence identity (Adams et al., 1992). PkDBPß does not bind to human erythrocytes, although it readily binds Rhesus erythrocytes (Barnwell et al., 1989). Binding assays were carried out by transiently transfecting adherent COS7 cells with either pEGFP-PvDBPII or pEGFP-PkDBPßII, incubating with Ao. nancymaae erythrocytes and then determining the binding phenotype by counting the number of erythrocyte rosettes. Aotus nancymaae erythrocytes showed the same specificity of binding as human erythrocytes, readily binding to PvDBPII, but not at all to PkDBPßII (Fig. 1). Chymotrypsin treatment removes the Duffy antigen receptor from erythrocytes (Barnwell et al., 1989). In order to determine if Ao. nancymaae erythrocytes interact with PvDBP via the Duffy antigen receptor, we performed binding assays in which we compared untreated erythrocytes with chymotrypsin-treated erythrocytes. Chymotrypsin treatment of Ao. nancymaae erythrocytes completely abolished binding to PvDBPII (Fig. 2).
FIGURE 1.
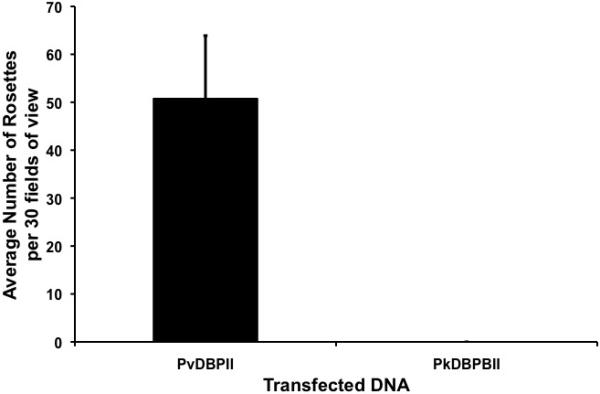
Aotus nancymaae erythrocytes bind specifically to PvDBPII. Results are shown for four experiments performed in triplicate.
FIGURE 2.
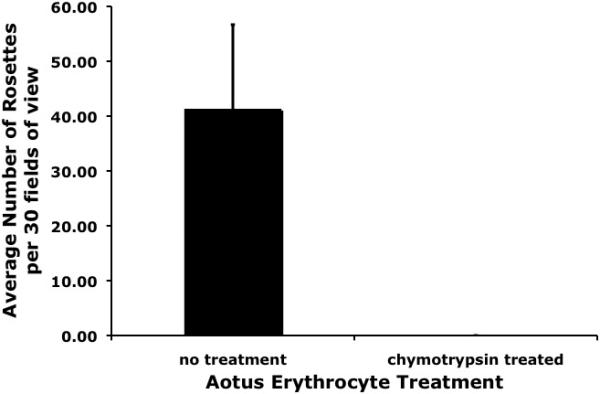
Chymotrypsin removal of the Duffy antigen receptor abolishes binding of Aotus nancymaae erythrocytes to PvDBPII. Results are shown for two experiments performed in triplicate.
To further show that Ao. nancymaae erythrocytes interact specifically with PvDBP, we performed inhibition assays using polyclonal rabbit sera raised against recombinant PvDBPII protein (Grimberg et al., 2007). Aotus nancymaae binding to PvDBPII is inhibited by anti-PvDBPII sera in a dose-dependent fashion (Fig. 3). We also performed inhibition assays using anti-Fy6 mAb, which specifically binds to the Duffy antigen receptor. Blocking of the Duffy antigen receptor using anti-Fy6 mAb disrupts binding of Ao. nancymaae erythrocytes in a dose-dependent manner (Fig. 4).
FIGURE 3.
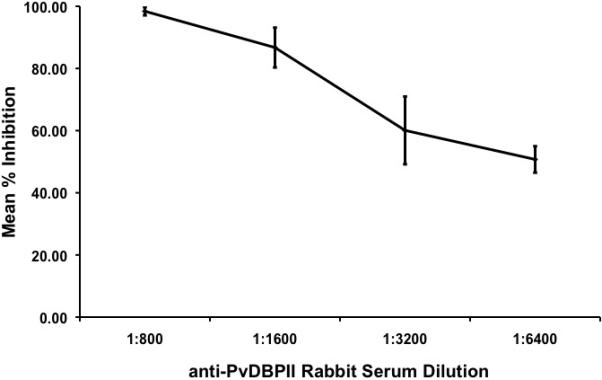
Blocking of PvDBPII with polyclonal anti-PvDBPII rabbit sera inhibits binding of Aotus nancymaae erythrocytes to PvDBPII in a dose-dependent manner. Inhibition is shown as a percentage of the control (sera from a naïve rabbit). Results are shown for four experiments performed in triplicate.
FIGURE 4.
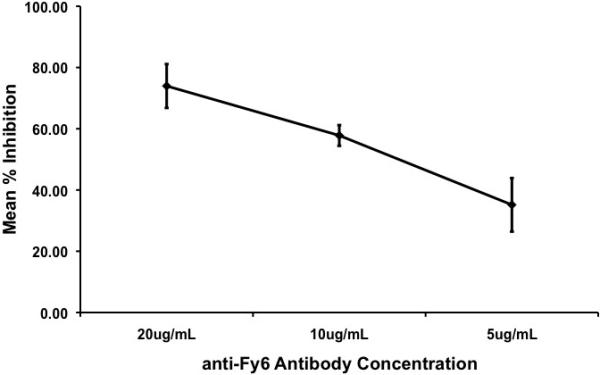
Blocking of the Duffy antigen receptor with anti-Fy6 mAb inhibits binding of Aotus nancymaae erythrocytes to PvDBPII in a dose-dependent manner. Inhibition of binding is shown as a percentage of the control (no sera). Results are shown for four experiments performed in triplicate.
Reports of drug resistance in P. vivax strains along with reports of severe clinical malaria cases due to P. vivax, call for an increased focus on P. vivax vaccine development (Baird, 2007; Barcus et al., 2007; Kochar et al., 2009). One of the main targets of P. vivax vaccine research is the Duffy binding protein, a parasite ligand required for erythrocyte invasion (Miller et al., 1976; Haynes et al., 1988; Wertheimer and Barnwell, 1989; Adams et al., 1990; Adams et al., 1992; Chitnis and Miller, 1994). Testing of an anti-PvDBP vaccine will require an appropriate animal model. This model will need to have a similar course of disease as human P. vivax infections as well as interact with P. vivax parasites in a Duffy dependent fashion. Plasmodium vivax is capable of infecting a variety of primate erythrocytes, however not all of these infections are dependent on the interaction between PvDBP and the Duffy antigen (i.e. P. vivax infection of Saimiri monkeys (Barnwell et al., 1989)). Because of this possibility, it is important to confirm that the primate model used for testing anti-PvDBP vaccines is dependent on the PvDBP-Duffy antigen interaction.
We performed a variety of experiments to confirm that Ao. nancymaae erythrocytes interact with PvDBP in a Duffy-dependent manner. We found that Ao. nancymaae erythrocytes readily bind to PvDBPII expressed on the surface of COS7 cells and chymotrypsin treatment, which removes the Duffy antigen, completely abolishes binding (Figs. 1 & 2). We also determined that specifically blocking either the PvDBPII (with anti-PvDBPII sera) or the Duffy antigen (with anti-Fy6 mAb) inhibits binding of Ao. nancymaae erythrocytes in a dose-dependent fashion (Figs. 3 & 4). These results indicate that the interaction of Ao. nancymaae erythrocytes with PvDBP is via the Duffy antigen receptor, validating this model for use in testing anti-PvDBP vaccines.
Acknowledgments
We would like to thank Christopher L. King of Case Western Reserve University for providing the anti-PvDBPII rabbit sera. This work was funded, in part, by a National Institutes of Health grant (R01 AI33656) to J.H.A. and an Arthur J. Schmitt Presidential Fellowship to A.M.M.
LITERATURE CITED
- Adams JH, Hudson DE, Torii M, Ward GE, Wellems TE, Aikawa M, Miller LH. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–53. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A. 1992;89:7085–9. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–9. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Bannister LH, Dluzewski AR. The ultrastructure of red cell invasion in malaria infections: a review. Blood Cells. 1990;16:257–92. discussion 293-7. [PubMed] [Google Scholar]
- Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti, Elyazar I, Bangs MJ, Maguire JD, Baird JK. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern indonesian papua. Am J Trop Med Hyg. 2007;77:984–91. [PubMed] [Google Scholar]
- Barnwell JW, Galinski MR. Plasmodium vivax: a glimpse into the unique and shared biology of the merozoite. Ann Trop Med Parasitol. 1995;89:113–20. doi: 10.1080/00034983.1995.11812941. [DOI] [PubMed] [Google Scholar]
- Barnwell JW, Nichols ME, Rubinstein P. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J Exp Med. 1989;169:1795–802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis CE, Blackman MJ. Host cell invasion by malaria parasites. Parasitol Today. 2000;16:411–5. doi: 10.1016/s0169-4758(00)01756-7. [DOI] [PubMed] [Google Scholar]
- Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE, Galland GG, Barnwell JW, Udhayakumar V, Sullivan JS, Nace D, Tongren JE, Williams T, Roberts J, Shi YP, Lal AA. Preliminary observations on the efficacy of a recombinant multistage Plasmodium falciparum vaccine in Aotus nancymai monkeys. Am J Trop Med Hyg. 2005;73:686–93. [PubMed] [Google Scholar]
- Collins WE, Sullivan JS, Williams A, Nace D, Williams T, Galland GG, Barnwell JW. Aotus nancymaae as a potential model for the testing of anti-sporozoite and liver stage vaccines against Plasmodium falciparum. Am J Trop Med Hyg. 2006;74:422–4. [PubMed] [Google Scholar]
- Darko CA, Angov E, Collins WE, Bergmann-Leitner ES, Girouard AS, Hitt SL, McBride JS, Diggs CL, Holder AA, Long CA, Barnwell JW, Lyon JA. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect Immun. 2005;73:287–97. doi: 10.1128/IAI.73.1.287-297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975;187:748–50. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- Fernando SD, Gunawardena DM, Bandara MR, De Silva D, Carter R, Mendis KN, Wickremasinghe AR. The impact of repeated malaria attacks on the school performance of children. Am J Trop Med Hyg. 2003;69:582–8. [PubMed] [Google Scholar]
- Galinski MR, Dluzewski AR, Barnwell JW. A Mechanistic Approach to Merozoite Invasion of Red Blood Cells: Merozoite Biogenesis, Rupture, and Invasion of Erythrocytes. In: Sherman IW, editor. Molecular Approaches to Malaria. ASM Press; New York City: 2005. pp. 113–168. [Google Scholar]
- Grimberg BT, Udomsangpetch R, Xainli J, McHenry A, Panichakul T, Sattabongkot J, Cui L, Bockarie M, Chitnis C, Adams J, Zimmerman PA, King CL. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med. 2007;4:e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–8. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Dalton JP, Klotz FW, McGinniss MH, Hadley TJ, Hudson DE, Miller LH. Receptor-like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med. 1988;167:1873–81. doi: 10.1084/jem.167.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8. [PubMed] [Google Scholar]
- Makobongo MO, Keegan B, Long CA, Miller LH. Immunization of Aotus monkeys with recombinant cysteine-rich interdomain region 1 alpha protects against severe disease during Plasmodium falciparum reinfection. J Infect Dis. 2006;193:731–40. doi: 10.1086/500150. [DOI] [PubMed] [Google Scholar]
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Michon P, Fraser T, Adams JH. Naturally acquired and vaccine-elicited antibodies block erythrocyte cytoadherence of the Plasmodium vivax Duffy binding protein. Infect Immun. 2000;68:3164–71. doi: 10.1128/iai.68.6.3164-3171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–4. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Nichols ME, Rubinstein P, Barnwell J, Rodriguez de Cordoba S, Rosenfield RE. A new human Duffy blood group specificity defined by a murine monoclonal antibody. Immunogenetics and association with susceptibility to Plasmodium vivax. J Exp Med. 1987;166:776–85. doi: 10.1084/jem.166.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas Caraballo J, Delgado G, Rodriguez R, Patarroyo MA. The antigenicity of a Plasmodium vivax reticulocyte binding protein-1 (PvRBP1) recombinant fragment in humans and its immunogenicity and protection studies in Aotus monkeys. Vaccine. 2007;25:3713–21. doi: 10.1016/j.vaccine.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, Martin LB, Saul AJ, Miller LH, Long CA. Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun. 2006;74:4573–80. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer SP, Barnwell JW. Plasmodium vivax interaction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol. 1989;69:340–50. doi: 10.1016/0014-4894(89)90083-0. [DOI] [PubMed] [Google Scholar]
- Williams AM, Barefield SJ, Carter ER, Collins WE, Sullivan JS, Tate MK. Adaptation of Plasmodium vivax to growth in owl monkeys (Aotus nancymai) Comp Med. 2005;55:528–32. [PubMed] [Google Scholar]


