Abstract
We demonstrate that the assembly of an amphiphilic polyamine on the interfaces of micrometer-sized droplets of a thermotropic liquid crystal (LC) dispersed in aqueous solutions can be used to facilitate the immobilization of LC droplets on chemically functionalized surfaces. Polymer 1 was designed to contain both hydrophobic (alkyl-functionalized) and hydrophilic (primary and tertiary amine-functionalized) side chain functionality. The assembly of this polymer at the interfaces of aqueous dispersions of LC droplets was achieved by spontaneous adsorption of polymer from aqueous solution. Polymer adsorption triggered transitions in the orientational ordering of the LCs, as observed by polarized light and bright-field microscopy. We demonstrate that the presence of polymer 1 on the interfaces of these droplets can be exploited to immobilize LC droplets on planar solid surfaces through covalent bond formation (e.g., for surfaces coated with polymer multilayers containing reactive azlactone functionality) or through electrostatic interactions (e.g., for surfaces coated with multilayers containing hydrolyzed azlactone functionality). Characterization of immobilized LC droplets by polarized, fluorescence, and laser scanning confocal microscopy revealed the general spherical shape of the polymer-coated LC droplets to be maintained after immobilization, and that immobilization led to additional ordering transitions within the droplets that was dependent on the nature of the surfaces with which they were in contact. Polymer 1-functionalized LC droplets were not immobilized on polymer multilayers treated with poly(ethylene imine) (PEI). We demonstrate that the ability to design surfaces that promote or prevent the immobilization of polymer-functionalized LC droplets can exploited to pattern the immobilization of LC droplets on surfaces. The results of this investigation provide the basis of an approach that could be used to tailor the properties of dispersed LC emulsions and to immobilize these droplets on functional surfaces of interest in a broad range of fundamental and applied contexts.
Introduction
The orientational ordering of liquid crystals (LCs) is exceptionally sensitive to the structures and properties of surfaces or interfaces with which they are in contact.1,2 Because changes in the orientation of LCs can propagate over large distances (e.g., up to tens of micrometers) through the bulk,3 changes in the properties of LC interfaces that lead to small perturbations in orientation can be amplified and observed readily using polarized-light. This aspect of LC-based systems has been exploited to develop new sensing platforms that can report on the presence and/or organization of chemical or biological agents.4-17 For example, several recent studies have demonstrated that ordering transitions in LCs can be triggered by the adsorption of phospholipids,4,18-20 surfactants,21-25 polymers,14,26,27 proteins,4,9-11,28,29 viruses,8,13,17 and bacteria17 at interfaces created between LCs and solid substrates5-10,12,13,29 or LCs and aqueous phases.4,11,14-28 The results of these past studies have suggested new principles and approaches for the design of LC-based systems that have relevance in a broad range of fundamental and applied contexts (e.g., sensing).
Past studies on the behavior of aqueous/LC interfaces have focused, in large measure, on the design and investigation of interfaces that are approximately planar (for example, interfaces formed by creating a thin film of a thermotropic LC between a solid substrate and a bulk aqueous phase).21,30,31 In these experimental systems, planar solid substrates are used to provide a physical support for thin films of LC and as a means to define or control the orientation of the LC at one boundary. An alternative geometry that has recently been explored involves the use of LC droplets dispersed in a continuous aqueous phase.32-37 This approach has the advantage that surface treatment of solid substrates is not required to define the orientation of the LC.
Several past studies have demonstrated that the interfaces of LC emulsion droplets can be decorated by the spontaneous adsorption of surfactants or phospholipids, and that these interfaces can be tailored to drive ordering transitions involving topological defects that are induced by the spherical geometries.32,33,37 In addition to eliminating the need for solid substrates, as noted above, the confinement of the LC into spherical geometries offers new approaches to manipulate the ordering of the LCs.33,38 For example, the ordering of LCs confined within droplets is sensitive to the size of the droplets, thereby providing additional means to tune the response of the LC (e.g., ordering transitions) to interfacial events by controlling droplet size.33,34 Another key characteristic of LC dispersions is the ability of the droplets to move freely within the surrounding aqueous medium. While a high level of droplet mobility can be desirable in certain contexts, it can also create substantial challenges with respect to the characterization of individual LC droplets. The work reported here was motivated, in part, by challenges associated with characterizing the structures and properties of micrometer-scale droplets of the thermotropic LC 4-cyano-4′-pentylbiphenyl (5CB) decorated with amphiphilic polymers.
We have reported in several past publications that amphiphilic polymers can assemble at planar aqueous/LC interfaces in ways that trigger ordering transitions in the LCs.26,27,39 These past studies have also demonstrated that it is possible to design polymers and polymer-decorated LC interfaces that respond to external stimuli (e.g., changes in the pH of the aqueous phase or the presence of oppositely-charged polyelectrolytes).26,27,39 The work reported here sought to characterize the adsorption of amphiphilic polymers to the interfaces of micrometer-scale droplets of aqueous/LC emulsions and investigate the influence of adsorbed polymer on the orientation of the LC and defect structures of the LC droplets. We note in this context that past studies have described methods to encapsulate LC droplets dispersed in aqueous media based on the assembly of water-soluble polymers at the interfaces of the droplets.32-34,40,41 However, the polymers used in these past studies [e.g., poly(styrene sulfonate), poly(allylamine hydrochloride), or poly(vinyl alcohol)] did not contain long aliphatic side chains and were not designed to trigger changes in the ordering of the LC. It also occurred to us that the presence of polymer on the interfaces of these droplets could provide opportunities to engineer the interactions of these droplets with other objects (e.g., biomolecules in solution, other LC droplets, or solid surfaces). Thus, a second important goal of this study was to characterize the interactions of polymer-functionalized LC droplets with a variety of chemically functionalized substrates.
The following study is organized into three parts. First, we describe the design of an amphiphilic polymer containing primary, secondary, and tertiary amine functionality, and demonstrate that this polymer can adsorb to the interfaces of micrometer-scale droplets of 5CB in aqueous/LC emulsions. Evidence of adsorption is provided by the observation of changes in the ordering and defect structures of the LCs and by the results of experiments using fluorescently labeled polymer. In the second part, we investigate the nature of interactions between polymer-decorated LC droplets and surfaces coated with several different chemically functionalized polymer multilayers. Our results demonstrate that polymer-functionalized droplets can be effectively immobilized on these surfaces. In the case of surfaces presenting amine-reactive azlactone functionality, our results suggest that immobilization occurs through the formation of covalent bonds between accessible primary amines on the interfaces of the polymer-coated droplets and the azlactone functionality at the surface of the substrates. In the case of carboxylic-acid presenting surfaces, our results suggest that these polymer-coated droplets can also be immobilized through electrostatic interactions. Characterization of individual immobilized droplets by polarized light microscopy reveals that contact with these surfaces leads to distinct changes in the ordering of the LC that are dependent on the physicochemical properties of the surfaces. In the final part of this report, we demonstrate that it is possible to spatially pattern polymer-functionalized droplets of LC on the surfaces of chemically patterned substrates. Our results suggest approaches to manipulate and characterize the behavior of dispersed polymer-functionalized LC droplets of interest in a range of fundamental and applied contexts.
Materials and Methods
Materials
Linear poly(ethylene imine) (LPEI) was synthesized by hydrolysis of the side chains of poly(2-ethyloxazoline) [MW = 50,000; obtained from Polysciences, Inc., Warington, PA] and purified prior to use in analogy to procedures described previously.42 4-(2-Hydroxyethyl-1-piperazineethanesulfonic acid) (HEPES), sodium chloride, methanol, ethanol, dimethyl sulfoxide (DMSO), ethyl acetate, chloroform, dichloromethane, hexanes, trifluoroacetic acid, and glass cover slips were purchased from Fisher Scientific (Pittsburgh, PA). Acryloyl chloride, n-decylamine, 3-(dimethylamino)-1-propylamine, 1,3-diaminopropane, di-tert-butyl dicarbonate, branched poly(ethylene imine) [BPEI, MW = 25,000], fluorescein isothiocyanate labeled dextran [FITC-dextran, MW = 2,000,000], and 2,2'-azobisisobutyronitrile were purchased from Sigma-Aldrich (St. Louis, MO). 2-Vinyl-4,4-dimethylazlactone (VDMA) was a generous gift from Dr. Steven Heilmann (3M Corporation, St. Paul, MN). N-Decylacrylamide, N-[3-(dimethylamino)propyl]acrylamide, N-[3-(tert-butoxycarbonylamino)propyl]acrylamide, and poly(VDMA) (PVDMA) were synthesized in analogy to previously described procedures.43-45 Carboxytetramethylrhodamine succinimidyl ester (TMR-NHS) was purchased from Molecular Probes (Carlsbad, CA). The LC 4-cyano-4′-pentylbiphenyl (5CB) was obtained from EMD Chemicals (Hawthorne, NY). Glass beads (diameter = 3-10 μm) were purchased from Polysciences, Inc. Deionization of a distilled water source was performed using a Milli-Q system (Millipore, Bedford, MA) yielding water with a resistivity of 18.2 MΩ. All materials were used as received and without additional purification unless otherwise noted.
General Considerations
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AC+ 300 spectrometer (300.135 MHz for proton; Billerica, MA). Chemical shift values are given in ppm and are referenced with respect to residual protons from solvent. Polarized light, bright-field, and fluorescence microscopy images of 5CB droplets were acquired using an Olympus IX-71 inverted microscope equipped with a 100 W mercury lamp and filter cube with a 560 nm excitation filter and a 645 nm emission filter. Images were captured using a Hamamatsu 1394 ORCA-ER-CCD camera controlled with SimplePCI software (Hamamatsu Inc., Sewickly, PA) Laser scanning confocal microscopy (LSCM) was performed using a Bio-Rad Radiance 2100 MP Rainbow laser scanning confocal microscope. Tetramethylrhodamine and fluorescein were excited sequentially using laser lines at 543 nm and 488 nm, respectively, and fluorescence emission was collected individually from the red and green channels. Silicon substrates used for reflective infared spectroscopy experiments were prepared by depositing thin layers of titanium (10 nm) and gold (200 nm) sequentially onto silicon wafers (Si-Tech, Inc., Topsfield, MA) using an electron-beam evaporator (Tek-Vac Industries, Brentwood, NY). Characterization of multilayered films by polarization-modulation infrared reflectance-absorbance spectroscopy (PM-IRRAS) was conducted in analogy to previously reported methods.46 All experiments involving the use of 5CB were performed at ambient room temperature (~25 °C), well below the nematic/isotropic transition temperature of 5CB (33.5 °C)21 unless otherwise noted.
Synthesis of Polymer 1
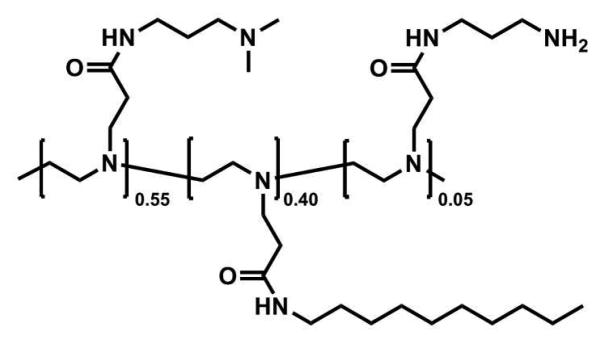
The synthesis of polymer 1 was performed by the conjugate addition of LPEI to acrylamide compounds in analogy to previously published methods.44,45 N-[3-(tert-Butoxycarbonylamino)propyl]acrylamide (13.1 mg, 0.058 mmol) was added to a solution of LPEI (50 mg, 1.16 mmol) in methanol in a screw-capped vial equipped with a magnetic stir bar. The reaction solution was stirred at 50 °C for 7 days at which time N-decylacrylamide (98.2 mg, 0.46 mmol) was added. The reaction mixture was stirred for an additional 7 days at which time N-[3-(dimethylamino)propyl]acrylamide (218 mg, 1.39 mmol) was added and the reaction mixture was allowed to stir for an additional 7 days. The resulting reaction product was isolated by repeated precipitation into a mixture of hexanes and diethyl ether (9:1, v/v) to yield the Boc-protected polymer. Removal of the Boc protecting group was achieved by dissolving the polymer in a mixture of dichloromethane and trifluoroacetic acid (1:1, v/v) in a screw-capped vial equipped with a magnetic stir bar. The reaction mixture was stirred for two hours at room temperature. The resulting reaction product was isolated by precipitation into a mixture of hexanes and diethyl ether (5:1, v/v) to yield polymer 1 as a tacky, viscous oil. Mn = 29,500; PDI=3.7. 1H NMR (CDCl3) δ (ppm) 0.877 (t, -NHCH2CH2(CH2)7CH3), 1.17 (br, -NHCH2CH2(CH2)7CH3), 1.38 (m, -NHCH2CH2(CH2)7CH3), 1.80-1.85 (b, -NHCH2CH2CH2N(CH3)2, -NHCH2CH2CH2N2), 2.81 (s, -NHCH2CH2CH2N(CH3)2), 2.87-3.27 (br m), 3.36-3.42 (b, m, -NHCH2CH2(CH2)7CH3, -NHCH2CH2CH2N(CH3)2, -NHCH2CH2CH2NH2).
Synthesis of Fluorescently Labeled Polymer 1
A fluorescently-labeled analog of polymer 1 (Polymer 1TMR) was synthesized using the following procedure. Polymer 1 (16.9 mg, 30.7 μmol) was weighed into a screw-capped vial equipped with a magnetic stir bar. DMSO (0.93 mL) and TEA (3.0 μL) were added to the vial and the solution was stirred for four hours at room temperature. TMR-NHS (7.4 mg, 14 μmol) was dissolved in 74 μL of DMSO and added to this solution. The reaction solution was stirred at room temperature for 50 h. The DMSO was removed by maintaining the vial under low pressure at 50 °C for two days. The polymer was isolated by dialysis against DI water (MW cutoff = 3500) for three weeks and lyophilized to produce a pink solid that was used without further purification.
Preparation of LC Emulsions
A dispersion of LC droplets was formed by alternately sonicating a mixture of 5CB in an aqueous buffer (1 vol%; 10 mM HEPES, pH 7.0) for 10 seconds followed by agitation with a vortex mixer for 10 seconds. This process was repeated a minimum of five times. A volume of the LC emulsion (150 μL) was added to a solution of polymer 1 (1:2 v/v, respectively) dissolved in HEPES buffer. The LC emulsion was rotated gently end-over-end using a laboratory rotator for up to 25 hours. Excess polymer solution was removed from the bulk aqueous phase by using the following washing procedure. A dispersion of LC droplets (200 μL) was combined with HEPES buffer (1 mL) in a microcentrifuge tube. The sample was centrifuged for 10 minutes at 500 g. The supernatant was removed and the droplets were resuspended in HEPES buffer (200 μL). Microscopy images of the droplets were collected by placing the dispersion of LC droplets (10 μL) on a glass coverslip (either untreated or modified with multilayered polymer films, see text). For experiments designed to investigate the immobilization of droplets on multilayered films, droplets were allowed to settle to the surface of these substrates for a period of 10 minutes. Freely suspended droplets were then removed from the solution by flowing buffer over the surface using a micropipette at a rate of approximately 20 μL/s.
Layer-by-Layer Fabrication of Polymer Thin Films
Multilayer films composed of BPEI and PVDMA were fabricated in analogy to previously reported methods.46,47 Briefly, solutions of BPEI and PVDMA were prepared in acetone (20 mM with respect to the molecular weight of the repeat unit). Glass and silicon substrates were cleaned with deionized water, methanol, ethanol, and acetone and dried under a stream of filtered, compressed air prior to the fabrication of multilayered films. Films were deposited layer-by-layer on glass or silicon manually according to the following general procedure: 1) Substrates were submerged in a solution of BPEI for 30 seconds, 2) substrates were removed and immersed in an initial acetone bath for 30 seconds followed by a second acetone bath for 30 seconds, 3) substrates were submerged in a solution of PVDMA for 30 seconds, and 4) substrates were rinsed in the manner described above. This cycle was repeated until four layer pairs (or ‘bilayers’) of BPEI/PVDMA were deposited to yield thin films (approximately 30 nm thick) terminated with a final layer of PVDMA. Films were dried under a stream of filtered, compressed air and were either used immediately or stored in a vacuum desiccator prior to use. Additional modification of these azlactone-functionalized reactive films to design amine-functionalized or carboxylate-functionalized surfaces was performed by either (i) submerging the substrates into a solution of BPEI for 30 seconds to terminate the film with a final layer of BPEI, followed by rinsing as described above, or (ii) hydrolyzing residual azlactone functionality in the films by placing the substrate in a closed vessel under complete saturation of water vapor at 37 °C for 48 hours. Complete hydrolysis of the residual azlactone functionality was confirmed by PM-IRRAS. To fabricate surfaces with spatially-defined chemical patterns, BPEI/PVDMA films terminated with a final layer of BPEI were treated with a small drop of a PVDMA solution (20 mM in DMSO) for two minutes. Films were then rinsed with cold acetone (approximately −50 °C) and dried under filtered air.
Results and Discussion
Design and Synthesis of Amphiphilic Polymer 1
We have demonstrated in past studies that water-soluble and water-insoluble amphiphilic polymers having long aliphatic side chains can assemble at planar aqueous/LC interfaces and influence the ordering of the LC.26,27,39 The general structure of the polymer used in this current study (polymer 1) was selected, in part, on the basis of these past studies for several reasons: (i) it can be synthesized readily, and in a modular manner, by the conjugate addition of linear poly(ethylene imine) (LPEI) to functionalized acrylamide compounds (e.g., hydrophobic N-decylacrylamide and hydrophilic N-[3-(dimethylamino)propyl] acrylamide), (ii) this polymer is water-soluble, and can thus be adsorbed directly onto the interfaces of LCs in contact with aqueous solutions, and (iii) our past studies demonstrate that the adsorption of polymers having this general structure to aqueous/LC interfaces leads to functional interfaces that can respond reversibly to changes in pH.26 In this study, we designed polymer 1 to include 5 mol% of a primary amine-functionalized side chain to permit conjugation of a fluorescent label and provide a reactive handle for the immobilization of polymer-functionalized LC droplets on amine-reactive surfaces. The ratio of hydrophobic, tertiary amine-functionalized, and primary amine-functionalized side chains in the polymer 1 used in this study was 55:40:5, respectively (see Materials and Methods for additional details related to polymer synthesis and characterization).
Polymer 1.
Adsorption of Polymer 1 to LC Emulsion Droplets
We performed a series of experiments to determine whether polymer 1 could adsorb from bulk aqueous solutions onto the interfaces of dispersed droplets of LC. For these experiments, we sonicated a mixture of the thermotropic LC 5CB and HEPES buffer (1% v/v; pH = 7.0) to produce a dispersion of spherical LC droplets. Characterization of this dispersion by optical microscopy revealed approximately 95% of the droplets to be between 1 μm and 8 μm in diameter. The dispersion was then added to a solution of polymer 1 and incubated with continuous and gentle agitation. Samples of this dispersion were removed at various times and characterized by bright-field and polarized light microscopy. We note again that the method used to prepare these LC dispersions resulted in a distribution of droplet sizes, and that past studies have demonstrated that droplet size can have a strong influence on the ordering of the LC.33,34 With this in mind, we restricted all analyses described here to the characterization of droplets having sizes in the range of 3 μm to 6 μm in diameter. In this part of our study, images of LC droplets were acquired by adjusting the focal plane of the microscope to a position far above the surface of the glass microscope slide to characterize freely moving droplets and minimize the potential influence of contact with the glass slide on the ordering of the LC within the droplets.
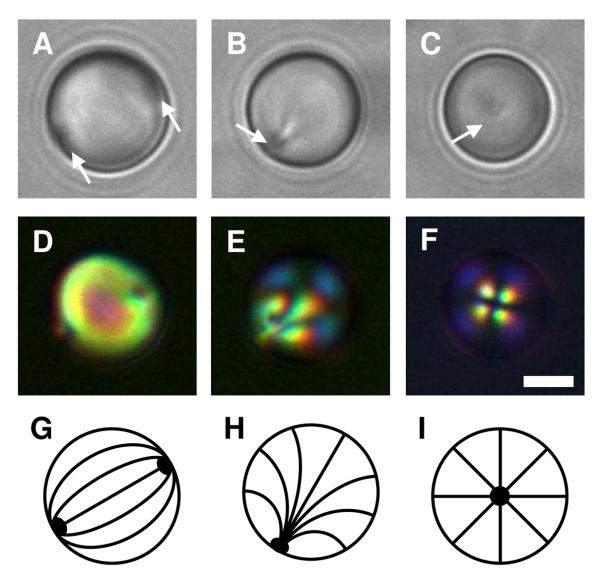
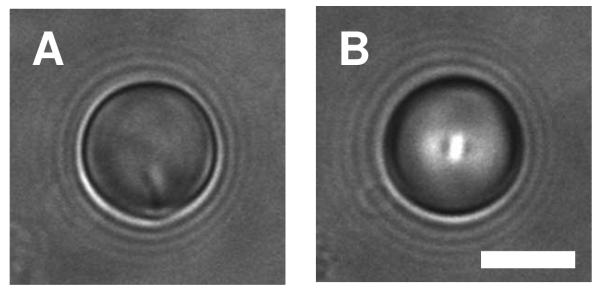
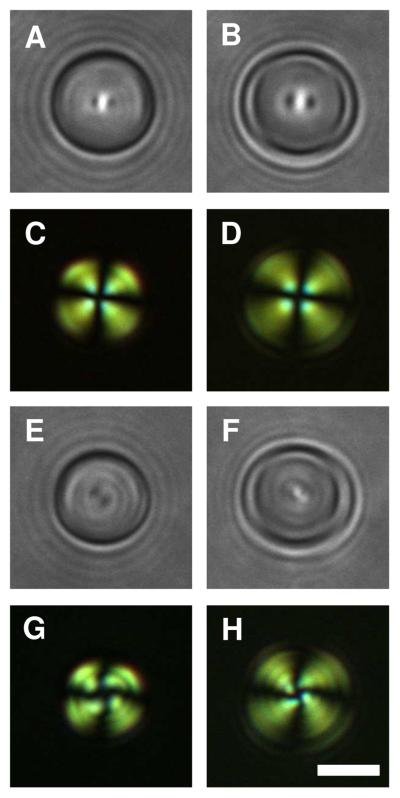
Figures 1A and 1D show representative bright-field and polarized light micrographs, respectively, of LC droplets in HEPES buffer (i.e., in the absence of polymer). These LC droplets exhibit a so-called bipolar configuration in which the director of the LC is oriented parallel to the surface of the droplets and connects two point defects located at opposite poles of the droplet.38,48 Figure 1G presents a schematic illustration of this director profile. These point defects scatter light, and can thus be observed by bright-field microscopy (e.g., indicated by the white arrows in Figure 1A). The observation of a bipolar configuration for these droplets is consistent with the behavior of droplets of LC dispersed in aqueous phases reported in other past studies.32,37
Figure 1.
A-C) Bright-field and D-F) polarized light micrographs of dispersed 5CB droplets incubated in A, D) a buffer solution (10 mM HEPES, pH 7); B, E) a solution of polymer 1 at 0.1 mg/mL; and C, F) a solution of polymer 1 at 1.0 mg/mL. The 5CB emulsions were under continuous agitation for 2 h. G-I) Schematic illustrations of the director profiles for G) bipolar, H) preradial, and I) radial configurations. Point defects in the 5CB droplets shown in bright-field images are indicated by the white arrows. Scale bar = 3 μm.
We observed large changes in the ordering of LC within droplets incubated in the presence of polymer 1. These changes are exemplified by the bright-field and polarized light micrographs shown in Figures 1B, 1C, 1E, and 1F. The images in Figures 1B and 1E correspond to droplets incubated in a solution of polymer 1 (at a concentration of 0.1 mg/mL) for two hours. In contrast to the images shown in Figures 1A and 1D, these images reveal the presence of a single point defect located at the surface of the droplet (e.g., indicated by the white arrow in Figure 1B). The presence of this single point defect is consistent with a shift of the orientation of the LC from a bipolar configuration to a so-called preradial configuration, in which the director radiates outward from the single point defect (see Figure 1H for a schematic illustration).38,48 Because the director profile for the preradial configuration is not spherically symmetric (that is, the point defect is not located in the center of the sphere), the apparent location of the defect changes as the droplet rotates in solution. This characteristic allows this type of defect to be identified easily and distinguished from the so-called radial configuration in which the defect is located in the geometric center of the LC droplet (and, thus, does not move as the droplet rotates; see Figure 1I for a schematic illustration of this configuration).38,48 We observed radial configurations in bright-field images of LC droplets incubated in the presence of higher concentrations of polymer 1 (e.g., 1.0 mg/mL) as shown in Figure 1C. This radial configuration leads to a characteristic cross-like pattern when viewed under polarized light (Figure 1F). We note again that the preradial and radial configurations shown in Figures 1B and 1C, respectively correspond to droplets incubated in solutions of polymer 1 for two hours. Additional discussion related to time-dependent changes in the configuration of the LC during incubation in solutions of polymer 1 for longer times can be found in the Supporting Information.
The change from a bipolar configuration to preradial or radial configurations upon exposure of the droplets to polymer 1 is consistent with the adsorption of polymer 1 to the interfaces of the droplets. Past studies using small molecule surfactants with long aliphatic tails have demonstrated that the ordering of LC droplets generally passes through a progression from bipolar, preradial, and radial configurations as the concentration of surfactant in the aqueous phase is increased.37 The polymer-induced ordering transitions shown in Figure 1 are consistent with these past results. These results are also generally consistent with the results of our past studies on the characterization of the adsorption of an analog of polymer 1 at planar aqueous/5CB interfaces, which demonstrated that amphiphilic polymers with long aliphatic side chains can adsorb to and drive transitions in the LC to homeotropic orientations due to the coupling between the LCs and aliphatic side chains.26
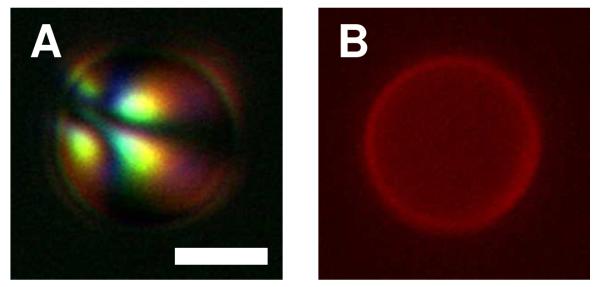
To provide additional evidence of the adsorption of polymer 1 to the interfaces of LC droplets, we performed experiments using a fluorescently labeled analog of polymer 1 (polymer 1TMR) synthesized by the conjugation of carboxytetramethylrhodamine succinimidyl ester (TMR-NHS) to the primary amine side chains of polymer 1. Figure 2 shows bright-field and fluorescence microscopy images of a droplet in the preradial configuration incubated in the presence of a polymer 1/polymer 1TMR mixture (0.1 mg/mL, 4:1 mass ratio) for two hours. This image shows bright red fluorescence distributed over the droplet interface and confirms the presence of polymer on the interface of the droplet. The results of additional characterization of LC droplets coated using polymer 1TMR are discussed later in this paper.
Figure 2.
A) Polarized light and B) fluorescence micrographs of a 5CB droplet dispersed in a mixture of polymer 1 and polymer 1TMR (4:1, 0.1 mg/mL) for 2 h. Excess polymer was removed from the bulk aqueous solution prior to imaging. Scale bar = 3 μm.
Immobilization of LC Droplets on Functional Surfaces
The results of the experiments described above demonstrate that dispersed 5CB droplets can be decorated with polymers containing amine functionality. Our next experiments sought to determine if we could exploit the primary amine functionality of polymer 1 to immobilize LC droplets on amine-reactive surfaces. The specific approach we investigated here is based on the reactive layer-by-layer assembly of thin films fabricated using poly(2-vinyl-4,4′-dimethylazlactone) (PVDMA) and branched poly(ethylene imine) (BPEI).46,47,49 Past reports have demonstrated that the azlactone functionality of PVDMA undergoes rapid ring-opening reactions with the primary amine functionality of BPEI in a manner that mediates the growth of covalently crosslinked polymer multilayers that can be modified post-fabrication using a range of primary amine functionalized molecules.46,47,49 We hypothesized that these amine-reactive multilayered films could be used to immobilize LC droplets decorated with polymer 1.
To test the feasibility of this approach, we fabricated BPEI/PVDMA thin films four bilayers thick (a ‘bilayer’ refers to one BPEI/PVDMA layer pair) on the surfaces of glass cover slips. These films were fabricated to present PVDMA as the final, topmost layer of the film and on average, were approximately 30 nm thick (as determined by ellipsometry). These azlactone-functionalized surfaces are referred to hereafter as surface 1 (shown in the schematic illustration in Figure 3A, see Materials and Methods for additional details related to film fabrication). Droplets of 5CB were decorated with polymer 1 by dispersing the droplets in a solution of polymer 1 at 0.1 mg/mL for 2 hours, as described above, after which the droplets were separated from the bulk aqueous phase by centrifugation and resuspended in fresh buffer. The polymer-laden 5CB droplets were dispensed onto surface 1 and allowed to sediment onto the surface for approximately 10 minutes before characterization by bright-field and polarized light microscopy. We observed the motion (lateral and rotational) of 5CB droplets to fall qualitatively into three different categories: i) droplets moving rapidly (at rates greater than approximately 1 μm/s), ii) droplets moving slowly (at rates less than approximately 0.1 μm/s), and iii) droplets that were completely immobile. The movement of droplets located far from the surface fell into the first category (i.e., relatively rapid motion) and was driven by a combination of fluid convection and Brownian motion. As the 5CB droplets approached the surface of the multilayers, the movement of the droplets slowed considerably (category two) due to hydrodynamic and other long range interactions until they came into contact with the surface and motion ceased altogether (category three; the ordering of LC within the droplets after contact with the surface will be discussed below). The observation that the motion of polymer-decorated LC droplets ceased when in contact with surface 1 is consistent with the hypothesis that polymer 1-decorated LC droplets can be immobilized on these amine-reactive films.
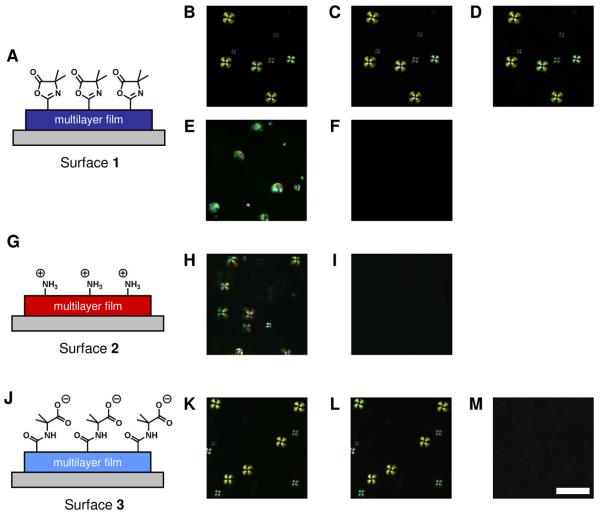
Figure 3.
Schematic illustrations representing multilayered films A) presenting azlactone functionality (surface 1), G) terminated with a layer of BPEI (surface 2) and, J) treated to hydrolyze the azlactone functionality (surface 3). Polymer 1-coated LC droplets were dispersed onto the surface of these multilayered films: (B-D) surface 1, (H, I) surface 2, and (K-M) surface 3. Images in E and F were collected using uncoated LC droplets placed on surface 1. The droplets were given time to sediment to the surface of these films (B, E, H, and K) and then rinsed with fresh buffer (C, F, I, L, see text). In an additional step, droplets were rinsed with buffer containing 1.5 M NaCl (D, M). Scale bar = 15 μm.
We characterized qualitatively the strength of the interactions between polymer 1-decorated LC droplets and surface 1 (e.g., whether droplets were bound weakly or strongly to these surfaces) by attempting to remove the droplets from the surfaces through rinsing. Figure 3 shows polarized light micrographs captured from the same field of view before (Figure 3B) and after (Figure 3C) rinsing immobilized LC droplets with solutions of fresh buffer at a rate of approximately 20 μL/s. These images reveal that the rinsing process did not change the number or location of the droplets immobilized on the multilayers and suggest that the interactions between the droplets and surface 1 are sufficiently strong to hold the droplets at surface 1 when exposed to flowing buffer.
To gain additional insight into the nature of the interactions between polymer 1-coated LC droplets and the azlactone groups of surface 1, we designed two additional surfaces displaying different chemical functionality. The first of these two additional surfaces, surface 2, was prepared by depositing one additional layer of BPEI onto the 4-bilayer films of BPEI and PVDMA (as described above). The addition of a final layer of BPEI was designed to mask the azlactone groups and increase the amount of amine functionality present at the surface of the film (schematic illustration shown in Figure 3G). The second of these additional surfaces, surface 3, was prepared by treating 4-bilayer BPEI/PVDMA films with saturated water vapor (48 hours at 37 °C) to hydrolyze residual azlactone functionality remaining within the film after fabrication of the multilayers and generate carboxylic acid functionality (see the schematic illustration in Figure 3J). Complete hydrolysis of the residual azlactone groups in these films was confirmed by reflective infrared spectroscopy (see Supporting Information, Figure S2A).
In analogy to the experiments described above, dispersions of polymer 1-coated droplets of 5CB were dispensed onto surfaces 2 and 3, the droplets were allowed to sediment onto the surfaces, and the samples were vigorously rinsed with solutions of fresh buffer. When polymer-coated droplets were placed on surface 2, the droplets continued to move freely during sedimentation and were completely displaced from the surface upon rinsing (Figures 3H and 3I), suggesting that the additional layer of BPEI was sufficient to prevent immobilization of the droplets on azlactone-containing multilayers. In contrast, polymer-coated LC droplets became immobile after coming into contact with surface 3 and were not removed by the rinsing process (Figures 3K and 3L). These results suggest that non-covalent associations (e.g., electrostatic interactions) between polymer 1-coated droplets and hydrolyzed PVDMA multilayers were sufficiently strong to immobilize the LC droplets on these surfaces.
To investigate further the nature of the immobilization of polymer 1-coated LC droplets on surface 1 and surface 3, immobilized droplets were rinsed with a buffer solution of high ionic strength. If the interactions between the surfaces and the droplets were primarily electrostatic in nature (e.g., between carboxylates at the surfaces and protonated amines of polymer 1), a high concentration of salt would screen these interactions and potentially result in the release of the droplets from the surfaces. The images shown Figure 3D and 3M were captured immediately after rinsing polymer-coated LC droplets immobilized on surface 1 or surface 3, respectively, with buffer solutions (10 mM HEPES, pH 7.0) containing 1.5 M NaCl (at a rate of approximately 20 μL/s). LC droplets on surface 1 were undisturbed by the additional rinse (Figure 3D) whereas LC droplets on surface 3 were completely rinsed from the film by the high ionic strength solution (Figure 3M). These results suggest that electrostatic forces are likely responsible for the immobilization of the droplets to the surfaces of hydrolyzed PVDMA multilayers (surface 3).
The observation that LC droplets on surface 1 were not dislodged after treatment with high salt concentrations is consistent with the view that the polymer-decorated LC droplets are covalently immobilized on surface 1 as a result of ring-opening reactions between the primary amine functionality of polymer 1 and the azlactone groups in these films. We note that it is possible that the azlactone functionality presented on surface 1 could hydrolyze during contact with the aqueous phase of the LC emulsions during these experiments, thereby eliminating or reducing available azlactone functionality. However, Heilmann, et al. have reported that reactions between primary amines and 2-alkenyl azlactones can be carried out in aqueous solutions without significant hydrolysis.50 In addition, experiments in which BPEI/PVDMA multilayers were immersed in HEPES buffer (pH = 7.0) demonstrated that residual azlactone functionality in these films did not hydrolyzed significantly within the time frame of the experiments performed in this study (e.g., approximately 10 minutes), as characterized by reflective infrared spectroscopy (see Supporting Information, Figure S2B). Although reaction between the primary amines of polymer 1 adsorbed to LC droplets and the azlactone functionality presented on surface 1 could not be confirmed spectroscopically because of the physical constraints of this system, the results shown in Figures 3B-D suggest that the interactions between polymer 1-coated droplets and surface 1 are covalent in nature.
Finally, to establish that the immobilization of the droplets on surface 1 was a result of the presence of polymer 1 on the interfaces of the droplets and not a consequence of interactions between the surface and 5CB alone, the experiments described above were repeated using a dispersion of uncoated LC droplets. Uncoated droplets were dispensed onto surface 1 and allowed to sediment onto the film surface (Figure 3E). The droplets were completely flushed from the films after rinsing with buffer (10 mM HEPES, pH 7.0) as shown in Figures 3F. This result demonstrates that polymer 1 is necessary to immobilize the LC droplets on azlactone-containing multilayers. On the basis of the results described above and illustrated in Figure 3, we conclude that reactive multilayered films terminated with a layer of PVDMA can be used to immobilize LC droplets coated with amphiphilic polyamines such as polymer 1.
Ordering of LC Droplets Immobilized on Surfaces
As discussed above, polymer 1-laden 5CB droplets prepared and suspended in bulk aqueous solution ([polymer 1] = 0.1 mg/mL, 2 hours of incubation) exhibited a preradial configuration (Figures 1B, 1E, and 1H). In the experiments using multilayered films described above, we observed that when polymer 1-laden 5CB droplets came into contact with surface 1, a change in the ordering of the LC occurred. Figure 4A shows a bright-field image of a polymer-laden 5CB droplet freely moving in the bulk prior to coming into direct contact with surface 1. A single characteristic point defect resides on the outer edge of the droplet, consistent with a preradial configuration. Figure 4B shows an image of the same droplet after contacting surface 1. The point defect immediately (in less than 1 second) migrated to the center of the droplet (as viewed from above) upon surface contact. The location of this defect did not change measurably when these surfaces were rinsed to remove unbound LC droplets. To determine the out-of-plane location of the point defect, we adjusted the focal plane of the microscope to focus on different horizontal sections of the droplets. The micrographs in Figures 5A-D correspond to bright-field (5A and 5B) and polarized light (5C and 5D) images of a polymer-laden droplet on surface 1 with the focal plane adjusted at approximately the midpoint of the droplet (Figures 5A and 5C) to approximately at the apex of the droplet (Figures 5B and 5D). Inspection of these images reveals that the point defect is in the same approximate focal plane as the widest point of the outer droplet edge and suggests that the point defect is located near the midpoint of the droplet. This defect structure is consistent with the radial ordering configuration.
Figure 4.
A) Bright-field micrograph of a polymer 1-laden 5CB droplet freely moving above a multilayer film presenting azlactone functionality (surface 1). B) Bright-field micrograph of the same 5CB droplet after contacting the surface of the film (immobile). Scale bar = 5 μm.
Figure 5.
(A, B, E, F) Bright-field and (C, D, G, H) polarized light micrographs of polymer 1-laden 5CB droplets immobilized on (A-D) surface 1 and (E-H) surface 3. The images were captured with the focal plane of the microscope positioned at the middle of the droplets (A, C, E, G) or positioned at the apex of the droplets (B, D, F, H; see text). Scale bar = 3 μm.
When mobile polymer 1-laden droplets contacted surface 3, the point defect was also observed to migrate from the edge to the center of the droplet (as viewed from above), similar to the result shown in Figure 4 for droplets contacting surface 1. However, adjustment of the focal plane of the microscope from the midpoint of the droplet (Figure 5E and 5G) to the apex of the droplet (Figure 5F and 5H) demonstrated that the defect was located near the apex. This defect structure, distinct from droplets immobilized on surface 1, is consistent with the preradial ordering configuration similar to the ordering of freely-moving droplets before contact with surface 3. We emphasize that the ordering of these droplets immobilized on surface 3 differs however, from freely-moving droplets in that the location of the point defect remained as the apex of the droplet. As discussed above, the apparent location of the point defect of mobile droplets changes as the droplet rotates in solution. In addition, close inspection of Figures 5G and 5H revealed the appearance of a twist distortion within the defect structure of the droplet immobilized on surface 3 that was not observed for droplets on immobilized surface 1 (Figure 5C). Previous studies have reported twisted director configurations in LC droplets in cases with perpendicular alignment at the droplet surface.23,51,52 Theoretical consideration of the twisted configurations observed in these past studies suggested that they arise from a combination of the drop size and the Frank elastic constants for different modes of elastic deformation (the elastic constant for twist is less than splay and bend deformations).52
The ordering of LCs in contact with surfaces can be influenced by a number of different factors, including the chemistry or topography of the surface.1-3 Additional characterization of the physicochemical properties of the multilayered film surfaces used here as well as the director profiles of the LC alignment within the immobilized droplets will be required to understand more completely the origin of the behavior shown in Figure 5. In the context of this current study, however, we conclude that immobilization of polymer 1-decorated 5CB droplets on polymeric films triggers ordering transitions in the LCs that are dependent on the nature of the chemical functionality and post-fabrication modification of the multilayered films.
Characterization of the Shapes of Droplets Immobilized on Surfaces
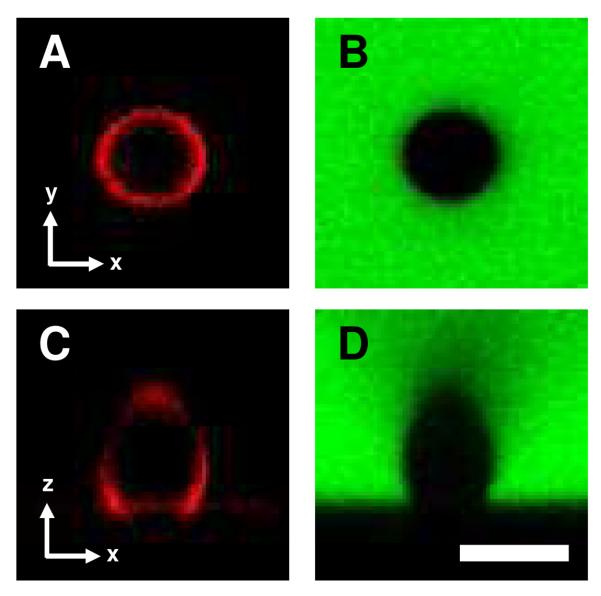
We considered the possibility that the changes in ordering of the polymer-laden LC droplets shown in Figure 5 may be driven by changes or differences in the shapes of the droplets as they contact surface 1 or surface 3. Our next experiments sought to characterize the shapes of immobilized 5CB droplets and determine if, and to what extent, their shapes change after contact with BPEI/PVDMA multilayers using laser scanning confocal microscopy (LSCM). For these experiments, droplets of 5CB were coated with polymer 1TMR (using a procedure analogous to the coating of droplets with polymer 1 described above) and allowed to sediment onto surface 1. After the polymer-laden droplets sedimented to the surface of the films, the aqueous phase was exchanged with a solution of FITC-labeled dextran (10 mg/mL in HEPES buffer). The images shown in Figure 6 are representative LSCM images of a single LC droplet captured in the x-y plane (i.e., bottom-up view of droplet; Figures 6A and 6B) or in the x-z plane (i.e., a cross-sectional side view of the droplet; Figures 6C and 6D). Figures 6A and 6C are the result of collecting fluorescence from the red channel and reveal a ring of bright red intensity corresponding to polymer 1TMR adsorbed to interface of the droplet. Independent collection of fluorescence from the green channel (Figures 6B and 6D) provided an additional view of the droplet. In these images, the regions of bright green intensity correspond to the FITC-labeled dextran dispersed throughout the aqueous solution.
Figure 6.
Confocal fluorescence micrographs of a polymer-laden LC droplet immobilized on surface 1 captured in the (A, B) x-y plane (bottom-up view) or the (C, D) x-z plane (side-on view). Images were captured by collecting the red channel (A, C; polymer 1TMR adsorbed to the surface of the LC droplets) or the green channel (B, D; FITC-dextran dispersed in the aqueous solution). Scale bar = 5 μm.
A comparison of the series of images shown in Figure 6 reveals that both approaches to characterizing the immobilized droplet (imaging polymer 1TMR on the droplet interface or a fluorescence dye distributed throughout the aqueous solution) lead to similar conclusions regarding the droplet shapes. Further inspection of Figures 6C and 6D indicates that the droplets exhibited contact angles of at least 90° on the surface. Additional control experiments using rigid glass beads revealed that the elongated distortion in the top half of these images arises from optical effects associated with imaging (see Supporting Information, Figure S3), and thus the droplets should not be interpreted to be egg-like in shape. Therefore, we limit our conclusions from these experiments to observations made of the bottom half of these images. For this reason, we also do not draw conclusions regarding differences in the distribution of polymer 1TMR on the interface of the 5CB droplets. In an analogous set of experiments, LSCM was used to characterize the shape of polymer 1TMR-coated 5CB droplets immobilized on surface 3. No significant difference between the general shape of the droplets on surface 1 (as shown in Figure 6) and surface 3 (see Supporting Information, Figure S4) was observed. In summary, these results demonstrate that the surface-dependent LC ordering transitions shown in Figures 4 and 5 were not a consequence of large differences in the shapes of the droplets immobilized on surface 1 or surface 3.
Patterning of LC droplets on Azlactone-Functionalized Surfaces
The experiments described above demonstrate that 5CB droplets decorated with polymer 1 can be immobilized on multilayers terminated with PVDMA and that films terminated in BPEI resist the adhesion or immobilization of the droplets. In a final set of experiments, we sought to determine whether our multilayered films could be chemically patterned in a manner that would provide spatial control over the immobilization of LC droplets. Past studies have demonstrated that BPEI/PVDMA multilayers can be patterned with a variety of chemical functionalities by either spotting micro-droplets of a solution on the surface of the films or by using conventional soft lithography techniques.46,47 For the experiments described here, a BPEI-terminated film (3.5 bilayers thick) was treated with a small drop (0.5 μL) of a solution of PVDMA dissolved in DMSO (20 mM with respect to repeat unit) for 2 minutes, followed by rinsing with acetone to remove excess unreacted PVDMA and the DMSO from the surface. A dispersion of polymer 1-coated LC droplets was then dispensed onto the films and the droplets were allowed to sediment to the surface before rinsing with buffer (10 mM HEPES, pH 7.0) to remove any nonimmobilized droplets.
Figure 7A shows a polarized light micrograph of the entire patterned region of the film and Figures 7B and 7C are images collected at higher magnification of the boundary between the treated and untreated regions. We note that the broader distribution of droplet sizes produced by the methods used to form the emulsion droplets (as discussed above) can be observed in these images. Inspection of Figure 7 reveals that the droplets are immobilized on the film surface primarily within the circular area that was treated with PVDMA and that the droplets are largely excluded from the surrounding BPEI terminated film. Although this patterned surface was fabricated using a slightly different approach than the approaches used to fabricate surface 1 and surface 3, the results shown in Figure 7 are consistent with the results of the experiments illustrated in Figure 3. These results demonstrate that it is possible to immobilize LC droplets within spatially-defined regions and provide a basis for the patterning of LC droplets on surfaces using other surface-patterning techniques.
Figure 7.
Polarized light micrograph of polymer 1-coated 5CB droplets on a multilayer film terminated with a layer of BPEI (i.e., surface 2) and patterned with circular region of PVDMA. The dashed boxes in A) and B) correspond to magnified regions shown in B) and C), respectively. See text for additional details. Scale bars = A) 400 μm, B) 100 μm, and C) 20 μm.
Summary and Conclusions
We have reported the design of an amphiphilic polyamine that can adsorb to the interface of LC droplets dispersed in aqueous solutions. A change in the ordering of the LC (i.e., a transition from a bipolar configuration to a preradial or radial configuration) and the observation of fluorescence around the perimeter of the droplets, when a fluorescently labeled analog of polymer 1 was used, provided evidence that the polymer adsorbed to the interface of the LC droplets. We also demonstrated that polymer 1-decorated 5CB droplets could be immobilized on surfaces coated with chemically tailored polymer multilayers. Our results suggest that the polymer-coated LC droplets can be immobilized on surfaces coated with these multilayered films by either i) covalent bonds (e.g., formed between azlactone functionality presented on surface 1 and primary amine functionality on polymer 1), or ii) electrostatic interactions (e. g., between carboxylate groups on surface 3 and the polyamines adsorped to the droplets). Immobilization of the LC droplets triggered changes in the ordering of the LCs that were dependent on the chemical functionality presented on the surface of the multilayers. We further demonstrated that polymer-coated LC droplets could be selectively immobilized within spatially defined patterns. The ability to engineer the properties of LC droplets with amphiphilic polymers may enable a range of fundamental studies on LCs in confined geometries and provide new methods to report on the presence of chemical or biological agents based on immobilized droplets of LCs (for example, through the design of arrays of surface-immobilized LC droplets that undergo analyte-induced changes in defect structure or other properties).
Supplementary Material
Acknowledgments
This research was supported by the NSF (DMR-0520527) through a grant to the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin and, in part, by a grant from the Alfred P. Sloan Foundation (to D.M.L.). We thank Dr. Steven M. Heilmann and Dr. Jerald K. Rasmussen (3M) for providing samples of 2-vinyl-4,4-dimethylazlactone and for many helpful discussions. We thank I-Hsin Lin for helpful discussions and assistance with preparing and characterizing the LC emulsions. M. E. B. was funded in part by an NIH Chemistry-Biology Interface Training Grant (NIGMS T32 GM008505).
Footnotes
Supporting Information Available. Additional figures and other data showing the ordering configurations of LC droplets incubated in solutions of polymer 1 as a function of time, characterization of BPEI/PVDMA films by reflectance infrared spectroscopy, LSCM images of glass beads on multilayer films, and LSCM images of polymer 1-coated 5CB droplets immobilized on surface 3. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jerome B. Rep. Prog. Phys. 1991;54:391. [Google Scholar]
- 2.Cognard J. Mol. Cryst. Liq. Cryst. 1982:1. [Google Scholar]
- 3.de Gennes PG, Prost J. The Physics of Liquid Crystals. Oxford University Press; London: 1994. [Google Scholar]
- 4.Brake JM, Daschner MK, Luk YY, Abbott NL. Science. 2003;302:2094. doi: 10.1126/science.1091749. [DOI] [PubMed] [Google Scholar]
- 5.Shah RR, Abbott NL. Science. 2001;293:1296. doi: 10.1126/science.1062293. [DOI] [PubMed] [Google Scholar]
- 6.Cadwell KD, Lockwood NA, Nellis BA, Alf ME, Willis CR, Abbott NL. Sens. Actuators, B. 2007;128:91. [Google Scholar]
- 7.Luk YY, Tingey ML, Hall DJ, Israel BA, Murphy CJ, Bertics PJ, Abbott NL. Langmuir. 2003;19:1671. [Google Scholar]
- 8.Jang CH, Cheng LL, Olsen CW, Abbott NL. Nano Lett. 2006;6:1053. doi: 10.1021/nl060625g. [DOI] [PubMed] [Google Scholar]
- 9.Gupta VK, Skaife JJ, Dubrovsky TB, Abbott NL. Science. 1998;279:2077. doi: 10.1126/science.279.5359.2077. [DOI] [PubMed] [Google Scholar]
- 10.Skaife JJ, Abbott NL. Langmuir. 2001;17:5595. [Google Scholar]
- 11.Birchall LS, Ulijn RV, Webb SJ. Chem. Commun. 2008:2861. doi: 10.1039/b805321a. [DOI] [PubMed] [Google Scholar]
- 12.Fang JY, Ma W, Selinger JV, Shashidhar R. Langmuir. 2003;19:2865. [Google Scholar]
- 13.Tercero Espinoza LA, Schumann KR, Luk YY, Israel BA, Abbott NL. Langmuir. 2004;20:2375. doi: 10.1021/la035774i. [DOI] [PubMed] [Google Scholar]
- 14.Price AD, Schwartz DK. J. Am. Chem. Soc. 2008;130:8188. doi: 10.1021/ja0774055. [DOI] [PubMed] [Google Scholar]
- 15.Hoogboom J, Velonia K, Rasing T, Rowan AE, Nolte RJM. Chem. Commun. 2006:434. doi: 10.1039/b514048j. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood NA, Mohr JC, Ji L, Murphy CJ, Palecek SR, de Pablo JJ, Abbott NL. Adv. Funct. Mater. 2006;16:618. [Google Scholar]
- 17.Sivakumar S, Wark KL, Gupta JK, Abbott NL, Caruso F. Adv. Funct. Mater. 2009;19:2260. [Google Scholar]
- 18.Meli MV, Lin IH, Abbott NL. J. Am. Chem. Soc. 2008;130:4326. doi: 10.1021/ja077379a. [DOI] [PubMed] [Google Scholar]
- 19.Hartono D, Bi XY, Yang KL, Yung LYL. Adv. Funct. Mater. 2008;18:2938. [Google Scholar]
- 20.Brake JM, Abbott NL. Langmuir. 2007;23:8497. doi: 10.1021/la0634286. [DOI] [PubMed] [Google Scholar]
- 21.Brake JM, Abbott NL. Langmuir. 2002;18:6101. [Google Scholar]
- 22.Brake JM, Mezera AD, Abbott NL. Langmuir. 2003;19:6436. [Google Scholar]
- 23.Poulin P, Stark H, Lubensky TC, Weitz DA. Science. 1997;275:1770. doi: 10.1126/science.275.5307.1770. [DOI] [PubMed] [Google Scholar]
- 24.Bahr C. Phys. Rev. E. 2006:73. [Google Scholar]
- 25.Price AD, Schwartz DK. J. Phys. Chem. B. 2007;111:1007. doi: 10.1021/jp066228b. [DOI] [PubMed] [Google Scholar]
- 26.Kinsinger MI, Sun B, Abbott NL, Lynn DM. Adv. Mater. 2007;19:4208. [Google Scholar]
- 27.Kinsinger MI, Buck ME, Campos F, Lynn DM, Abbott NL. Langmuir. 2008;24:13231. doi: 10.1021/la803376u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JS, Abbott NL. Adv. Mater. 2008;20:1185. [Google Scholar]
- 29.Chen CH, Yang KL. Langmuir. 2010 In press, DOI: 10.1021/la9033468. [Google Scholar]
- 30.Lockwood NA, Abbott NL. Curr. Opin. Colloid Interface Sci. 2005;10:111. [Google Scholar]
- 31.Lockwood NA, Gupta JK, Abbott NL. Surf. Sci. Rep. 2008;63:255. [Google Scholar]
- 32.Tjipto E, Cadwell KD, Quinn JF, Johnston AP, Abbott NL, Caruso F. Nano Lett. 2006;6:2243. doi: 10.1021/nl061604p. [DOI] [PubMed] [Google Scholar]
- 33.Gupta JK, Sivakumar S, Caruso F, Abbott NL. Angew. Chem., Int. Ed. 2009;48:1652. doi: 10.1002/anie.200804500. [DOI] [PubMed] [Google Scholar]
- 34.Zou J, Fang J. Langmuir. 2010 In press, DOI: 10.1021/la904257j. [Google Scholar]
- 35.Umbanhowar PB, Prasad V, Weitz DA. Langmuir. 2000;16:347. [Google Scholar]
- 36.Tixier T, Heppenstall-Butler M, Terentjev EM. Langmuir. 2006;22:2365. doi: 10.1021/la0531953. [DOI] [PubMed] [Google Scholar]
- 37.Gupta JK, Zimmerman JS, de Pablo JJ, Caruso F, Abbott NL. Langmuir. 2009;25:9016. doi: 10.1021/la900786b. [DOI] [PubMed] [Google Scholar]
- 38.Drzaic PS. Liquid Crystal Dispersions. World Scientific Publishing Co.; Singapore: 1995. [Google Scholar]
- 39.Kinsinger MI, Buck ME, Meli MV, Abbott NL, Lynn DM. J. Colloid Interface Sci. 2010;341:124. doi: 10.1016/j.jcis.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivakumar S, Gupta JK, Abbott NL, Caruso F. Chem. Mater. 2008;20:2063. [Google Scholar]
- 41.Rudhardt D, Fernandez-Nieves A, Link DR, Weitz DA. Appl. Phys. Lett. 2003;82:2610. doi: 10.1103/PhysRevLett.92.105503. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka R, Ueoka I, Takaki Y, Kataoka K, Saito S. Macromolecules. 1983;16:849. [Google Scholar]
- 43.Guichard B, Noel C, Reyx D, Thomas M, Chevalier S, Senet JP. Macromol. Chem. Phys. 1998;199:1657. [Google Scholar]
- 44.Kuroda K, DeGrado WF. J. Am. Chem. Soc. 2005;127:4128. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 45.Liu XH, Yang JW, Miller AD, Nack EA, Lynn DM. Macromolecules. 2005;38:7907. [Google Scholar]
- 46.Buck ME, Zhang J, Lynn DM. Adv. Mater. 2007;19:3951. [Google Scholar]
- 47.Buck ME, Breitbach AS, Belgrade SK, Blackwell HE, Lynn DM. Biomacromolecules. 2009;10:1564. doi: 10.1021/bm9001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavrentovich OD. Liq. Cryst. 1998;24:117. [Google Scholar]
- 49.Buck ME, Lynn DM. Adv. Mater. 2010 doi: 10.1002/adma.200903054. In press, DOI: 10.1002/adma.200903054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heilmann SM, Rasmussen JK, Krepski LR. J. Polym. Sci. A1. 2001;39:3655. [Google Scholar]
- 51.Candau S, Leroy P, Debeauva F. Mol. Cryst. Liq. Cryst. 1973;23:283. [Google Scholar]
- 52.Rudinger A, Stark H. Liq. Cryst. 1999;26:753. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.