Abstract
The parent-into-F1 model has led to important advances in our understanding of lupus. Here, we review previous work in murine lupus which elucidated the role of T cells and supports the conclusion that the parent-into-F1 model of induced lupus compares favorably with de facto ‘gold standard’ spontaneous models of lupus. The review then focuses on recent work in parent-into-F1 mice, which has yielded novel insights into unresolved controversies, such as the role of apoptosis in the pathogenesis of lupus and lupus in patients receiving TNF blockade. The article concludes with evidence that supports a potential role for CD8 T cells, both cytotoxic and memory cells, in mediating disease remission.
Parent-into-F1 model: two major disease phenotypes
The basics of the P→F1 model and its resemblance to human lupus have been outlined previously (reviewed in [1–4]. Briefly, the transfer of homozygous parental strain T cells into unirradiated semi-allogeneic F1 recipients induces a graft-vs.-host reaction resulting from donor T cell recognition of host alloantigens. As naïve donor T cells mature into effector T cells, a graft-vs.-host disease (GVHD) ensues taking either a lymphopenic (acute GVHD) or lymphoproliferative (chronic GVHD) phenotype depending on the donor T cell subsets involved. If both CD4 and CD8 donor T cells are activated following transfer into an MHC I + II disparate F1 host, mice develop acute GVHD i.e. a potentially lethal immunodeficiency in which donor CD8 CTL attack MHC I disparate cells and eliminate immunocompetent host lymphocytes with B cells among the earliest cells eliminated followed by CD4 T cells, APC and lastly CD8 T cells which exhibit greater resistance to elimination [5,6]. By contrast, chronic GVHD requires only the activation of the donor CD4 T cell subset and can be induced either by depletion of CD8 T cells in the donor inoculum or by transfer of unfractionated donor lymphocytes into a host differing only by MHC II. Chronic GVHD is characterized by expansion of host lymphocytes, particularly B cells due to allo-reactive donor CD4 T cell activation following encounter with foreign host MHC II resulting in cognate polyclonal host B cell help [7], production of characteristic IgG lupus autoantibodies and a lupus-like immune complex glomerulonephritis (ICGN) that can be lethal in some P→F1 combinations.
Acute and chronic GVHD phenotypes can be distinguished as early as two weeks after donor transfer using flow cytometric analysis of splenocytes (reviewed in [6]. Important two-week distinguishing features of acute GVHD are: a) engraftment of both donor CD4 and CD8 T cells and b) near complete elimination of host B cells (≤ 10% of normal F1values). By contrast, the key features of chronic GVHD are: a) engraftment of donor CD4 T cells only (i.e. absent or impaired donor CD8 engraftment); and b) host B cell expansion (≥120% of normal F1 values). These two-week phenotypes (Table 1) reliably the predict long term clinical phenotypes of acute and chronic GVHD and can serve as early surrogate markers for long-term disease. Two-week end points are useful for interventional studies and allow for rapid screening of candidates for therapeutic or immunomodulatory potential. Only those candidates showing promise need undergo long-term studies to confirm an effect on clinical disease phenotype (e.g. lupus-like renal disease). An emerging issue with the use of two-week surrogate marker analysis is the occurrence of intermediate phenotypes (seen with knock out donor strains or biological intervention) and the empirical determination of the attendant long term phenotypes (reviewed in [6].
Table 1.
Three common donor (parent) strains using the B6D2F1 mouse as recipient and the resulting short and long term phenotypes.
| DBA→F1 | B6→F1 | B6 (CD8)→F1 | |
|---|---|---|---|
| Donor strain | DBA | B6 | B6 |
| Lymphocytes injecteda | Unfractionated | Unfractionated | CD8 depleted |
| Host | BDF1 | BDF1 | BDF1 |
| GVHD type | Chronic | Acute | Chronic |
| Long-term phenotypeb | Lupus-like | Lethal immune deficient |
Lupus-like |
| Short term phenotype | Stimulatoryc | Cytotoxicd | Stimulatory |
| (10–14 days) |
Typical injection doses are 80–90 × 106 DBA donor cells and 50 × 106 B6 donor cells (undepleted or CD8-depleted).
Lupus-like disease in chronic GVHD mice is characterized by (i) ICGN, (ii) lupus specific autoantibodies, (iii) anti-nuclear antibodies, (iv) Ig and complement deposition in skin and (v) anti-RBC antibody. Mice with long-term acute GVHD exhibit an early mortality at 2–4 weeks, and survivors exhibit persistent immunodeficiency and a disease resembling acute GVHD in humans after a bone marrow transplant [29].
Stimulatory phenotype: significant engraftment of donor CD4 T cells only, host B-cell expansion (≥150% versus control), low level IFN-g production, low lever host B-cell Fas expression and no detectable donor anti-host CTL activity.
Cytotoxic phenotype: significant engraftment of both CD4 and CD8 donor T cells, host B-cell reduction (≤ 20% versus control), striking increase in both IFN-g production and host B-cell Fas expression, and detectable donor anti-host CTL activity.
Chronic GVHD strongly resembles human lupus
Early studies demonstrated that chronic GVHD mice resembled several human connective tissue diseases depending on the parent and F1 strains used (reviewed in [1,8]. A striking resemblance to human lupus was seen following the transfer of DBA/2 lymphocytes into BDF1 hosts (DBA→F1). It was subsequently shown that due to the lack of MHC II expression on murine T cells, a semi-allogeneic F1 recipient is not absolutely required and that transfer of CD4 T cells into MHC II disparate congenic mice such as B6 lymphocytes into the bm12 mutant (B6 →B6bm12) also results in a lupus-like chronic GVHD [4]. Human lupus is highly heterogeneous and likely represents a syndrome rather than a single disease. As a result, classification criteria were developed by the American College of Rheumatology (ACR) and revised [9] to ensure that lupus patients included in published studies actually had lupus. Taken collectively, the lupus-like features reported in various strain combinations that could be considered to meet human ACR criteria are: a) immune complex glomerulonephritis (ICGN) consisting of deposition of Ig and C’ with cellular proliferation and proteinuria [10,11]; b) lupus-like skin lesions consisting of deposition of Ig and C’ at the dermal-epidermal junction i.e. positive lupus band test [10,12]; c) anti-RBC ab and Coombs positivity [8]; 4) anti-nuclear antibodies [10,13]; and 5) characteristic lupus autoantibodies to include anti-dsDNA, anti-Sm and more recently, antibodies to poly (ADP-ribose) polymerase 1 (PARP-1) [1,4,14]. Importantly, lupus-like features vary depending on the strain combinations used with some combinations exhibiting only lupus-like autoantibodies and no renal disease [15] while other combinations such as either Balb/c or A/J→(Balb × A/J)F1 exhibited a closer resemblance to rheumatoid arthritis, Sjogren’s syndrome and undifferentiated connective tissue disease [16,17].
Three P→F1 combinations that strongly resemble human lupus have been studied extensively: a) unfractionated DBA/2 lymphocytes into BDF1 hosts (DBA→F1); b) unfractionated lymphocytes from B6 donors into bm12 hosts or vice versa (B6<→bm12); and c) CD8 depleted B6 or B10 lymphocytes into BDF1 hosts (B6 CD8 depleted→BDF1). The pathology and lupus criteria met for each model are summarized in Table 2. DBA→F1 mice exhibit the following features consistent with the ACR criteria: 1) lupus like ICGN; 2) characteristic lupus-like skin lesions with positive lupus band test; 3) anti-RBC ab; 4) anti-nuclear antibodies; and 5) lupus-specific antibodies to include anti-dsDNA, anti-histone/chromatin and anti-PARP [1,8,14]. As with human lupus, organ specific autoantibodies are not observed [8]. Moreover, ICGN in DBA→F1 mice that in females is histologically more severe and progresses to nephrotic more often than in males [18,19] making the model useful for the study of sex based differences in lupus pathogenesis Because chronic GVHD in DBA→BDF1 mice results from defective DBA CD8 maturation into CTL [20,21], this P→F1 combination is useful for screening in vivo agents with CTL promoting potential which are detected by conversion of disease phenotype to acute GVHD. A disadvantage of the DBA→F1 model is the lack of mutant strains on the DBA/2 background.
Table 2.
Lupus-like features of three common donor and host strains used to induce chronic GVHD.
| DBA→F1 | B6→bm12a | B6 (CD8)→F1 | ||
|---|---|---|---|---|
| Donor strain | DBA/2 | B6 | B6 | |
| lymphocytes injected2 | unfractionated | unfractionated | CD8 depleted | |
| Host | BDF1 | bm12 | BDF1 | |
| Lupus criteria met | ||||
| 1) ICGN | yes | yes | yes | |
| 2) Anti nuclear ab | yes | yes | yes | |
| 3) Lupus specific ab | yes | yes | yes | |
| Anti-dsDNA | + | + | + | |
| Anti-chromatin/histone | + | + | + | |
| Anti-Sm | n.r. | + | n.r. | |
| 4) Anti-RBC ab | yes | n.r. | yes | |
| 5) Skin disease | yes | n.r. | n.r. | |
| (+ lupus band test)c | ||||
The reverse transfer bm12→B6 gives similar results.
splenocytes or lymph node cells
lupus-like immune complex glomerulonephritis with Ig and complement deposition and cellular infiltration consistent with either proliferative glomerulonephritis or membranous glomerulonephritis
deposition of Ig and complement at the dermal-epidermal junction n.r., not reported
(see text for references)
Lupus-like features of B6<→bm12 mice are: 1) ICGN which is mild and without sex based differences in severity [22]; 2) anti-nuclear antibodies; and 3) characteristic lupus autoantibodies to include ant-dsDNA, anti-chromatin and anti-Sm [4]. The use of B6 mice as either donor or hosts is a significant experimental advantage as a large number of knock out and transgenic mice are available on the B6 background allowing the role of single molecules or genes in lupus pathogenesis to be probed for their role in either donor or host cells. By using B6 mice as hosts, this model is readily amenable to the study of host T and B cell responses in lupus and does not require breeding of a transgenic or knock out F1 as does the P→F1 model.
Lupus-like features of B6 CD8 depleted→F1 model are: 1) ICGN; 2) anti-nuclear ab; and 3) anti-dsDNA ab; and 4) anti-RBC ab (Coombs positivity) [23,24]. This P→F1 combination is useful for studying the role of donor T cell molecules by using B6 knock out or transgenic mice. Additionally, the BDF1 host has the potential to exhibit severe ICGN and sex based differences in renal disease although the latter have not been reported using in this combination. Although a number of injection protocols have been used, disease can be reliably induced in all three combinations with a single dose of donor cells and disease severity is directly correlated with the number of donor cells transferred [24,25] The use of Balb/c donor cells and (B6 × Balb/c) F1 hosts also results in lupus-like ICGN however disease onset requires ≥ 6 months compared to 2–3 months for the three combinations in Table 2 [26,27] making this combination less attractive for experimental study.
As reviewed by Reeves et. al. [28] NZB/W F1 mice meet three ACR criteria for lupus and Pristane-induced lupus mice meet 4 or 5 criteria depending on the strain. Thus, neither human lupus patients nor lupus mice exhibit all 11 ACR criteria for lupus, however the combinations in Table 2 strongly resemble the subset of human lupus with high tittered autoantibodies and ICGN. Because lupus nephritis is a major cause of morbidity, this a valuable end point. Moreover, it is important not only that murine models of lupus resemble aspects of human lupus but also that they have predictive value in elucidating mechanisms and identifying therapeutic targets in humans. These are discussed further below.
P→F1 GVHD vs. human GVHD
Despite the similar names, chronic GVHD in the P→F1 model has only a moderate resemblance to human chronic GVHD in allogeneic bone marrow transplant recipients due primarily to elimination of immunocompetent host B cells in human transfers, whereas host B cells are intact in the P→F1 model and are the source of lupus-like autoantibodies. Thus, chronic GVHD in P→F1 mice resembles human lupus more closely than human chronic GVHD. In contrast, acute GVHD in the P→F1 model exhibits many similarities to acute GVHD in humans following allogeneic bone marrow transplants [29] and may become of increasing relevance with the advent of non myeloablative conditioning regimens. Acute GVHD in P→F1 mice is also a useful model of normal in vivo allo-specific CTL development which has important implications about both lupus development and potential lupus treatment (see below).
A normal in vivo alloantigen specific T cell response can cause lupus
In spontaneous murine lupus, neither the exact time of disease onset nor the specificity of the disease initiating T cells are known. In contrast, both of these variables are known in the P→F 1 model. Because both donor and host cells are normal prior to transfer, the P→F1 model is a paradigm for a generic in vivo ag-driven immune response taking either a cell mediated form (acute GVHD) or an antibody (ab) mediated form (chronic GVHD). Because the ag recognized by donor CD4 and CD8 T cells is host allogeneic MHC II and MHC I respectively, the responding T cell frequency is therefore high enough to permit tracking of the cells that initiate disease (i.e. donor T cells) by flow cytometry without the use of tetramers thereby allowing separation of the ag-specific (donor) T cell response from non-specific secondarily activated (host) T cells response, the latter may be primarily involved in down regulation the GVHR [5]. Moreover, the use of normal donor splenocytes avoids skewing of the T cell repertoire as seen with T cell transgenic adoptive transfer models. As opposed to some models of spontaneous lupus where T cells may exhibit intrinsic abnormalities that result in lupus [30], disease phenotype in the P→F1 model is a consequence of a normal cell mediated or ab-mediated response that is abnormally targeted to self-MHC. Thus, normal allo-specific CD4 T cells can initiate lupus if abnormally targeted to all host B cells. Autoantigen specific T cells are not absolutely required for lupus initiation.
Conceptual advances in pathogenesis of lupus stemming from chronic GVHD: previous work
Central role of CD4 T cells in pathogenesis of lupus
A well-established concept in the pathogenesis of lupus is that pathogenic autoantibody production is T-cell driven and that CD4 T cells are necessary and sufficient for disease (summarized in Ref. [30]). This major conceptual advance in the pathogenesis of lupus was initially based on mechanistic studies in mouse models. Work in spontaneous murine lupus as early as the 1980s demonstrated that disease was improved when T-cell function, particularly CD4 T-cell function, was experimentally impaired or altered by a variety of methods [30–36]. The crucial role for CD4 T cells in the pathogenesis of lupus was also shown in the 1980s by using P→F1 chronic GVHD mice [23,37]. An important advantage of the P→F1 model is that, unlike spontaneous murine lupus models, the T cells responsible for disease initiation are of donor origin and can be identified experimentally. This operational advantage allowed the pioneering work by the Morris et al., which demonstrated that the CD4 T-cell help driving host B-cell autoantibody production is cognate rather than bystander [7] and is more effective if CD4 help is polyclonal rather than oligoclonal/monoclonal [38]. Cognate T-cell help for autoantibody production was subsequently demonstrated in lpr mice [39].
Because mechanistic studies are difficult to design in humans, the evidence supporting a central role of CD4 T cells from human studies was largely indirect i.e. pathogenic autoantibodies exhibit all the features of a normal antigen-driven response, including somatic mutation, affinity maturation and isotype switching to IgG, all of which are T-cell dependent (reviewed in Ref.[40]). Clear evidence of the central role of CD4 T cells in human lupus was revealed not from direct experimental study of human lupus T cells but rather from indirect observational studies of patients with SLE and co-existing HIV infection. Initial reports that SLE remission occurred in HIV patients exhibiting CD4 depletion [41] were followed by reports of SLE relapse in association with successful drug-induced HIV remission [42], which provided strong, albeit indirect, support for a central role of CD4 T cells in human lupus.
Thus, the importance of murine lupus models, both spontaneous and induced (i.e chronic GVHD), in advancing understanding of the pathogenesis of lupus is substantiated by the elucidation in murine lupus models of the crucial role of CD4 T cells, long before this mechanism could be indirectly corroborated in humans. Not only was the chronic GVHD model the contemporary of the spontaneous models but it also extended our understanding by elucidating cognate help as a mechanism.
T cells as therapeutic targets in SLE
The therapeutic implications of the central role of CD4 T cells in lupus support clinical targeting of T cells in humans. Given the crucial role of T cells in immune responses and the trade off of immunodeficiency versus active lupus reported in HIV patients, targeting CD4 T cells must be selective rather than global. One such approach is based on targeting T-cell activation by blocking the CD28-mediated co-stimulation pathway using a CTLA-4 fusion protein, CTLA4-Ig, that binds to CD80 and CD86. A beneficial effect of CTLA4-Ig was shown in both spontaneous murine lupus (reviewed in Ref.[43]) and in lupus-like chronic GVHD [44]. Human trials using CTLA-4Ig (abatacept) are currently underway. Although there is optimism that co-stimulatory blockade with abatacept will be useful as induction therapy in lupus patients [45], clinical translation of promising agents in mice to humans is unpredictable and further refinement of T-cell targeting might be necessary. Regardless of the eventual clinical role of abatacept in lupus, its development for human SLE was predicated on its beneficial role in murine lupus, and results in the P→F1 model were concordant with results in spontaneous models.
Secondary, induced T-cell abnormalities are early predictors of disease severity
A long-standing observation in both human and murine lupus is that T cells exhibit functional abnormalities consisting of evidence of activation in conjunction with depressed function and aberrant signaling (reviewed in Refs [46,47]). It has not been clear whether T-cell defects have a primary, causative role or have a secondary role as a consequence of immune activation. Results from the P→F1 model provide insight into this controversy and have been reviewed in detail [48]. Briefly, in DBA→F1 mice, both donor and host immune systems are normal before transfer and, importantly, neither strain is lupus prone. A mild in vitro defect in T helper (Th)-cell function (i.e. reduced IL-2 production and impaired IL-2-dependent CTL generation) is observed as early as 10 days after donor transfer. Similar in vitro defects were found in spontaneous murine lupus [49] and in humans with lupus [50]. Importantly, the clinical severity of human SLE was found to correlate with the severity of Th-cell dysfunction in vitro [51]. Thus, in vitro defects in IL-2 production in T cells are inducible secondary events that are valuable early and sensitive marker of ongoing lupuslike T-cell-B-cell collaboration. These results do not exclude the existence of primary intrinsic T-cell defects in some cases of lupus; however, such primary defects are not required for the development of a secondary defect.
Recent implications of chronic GVHD and lupus pathogenesis
Role of apoptosis
Pre-existing defects in apoptosis clearance mechanisms are associated with lupus in both humans and mice (reviewed in Ref. [52]). Moreover, apoptotic blebs contain nearly all of the autoantigens targeted by the humoral immune system in SLE [53,54], which led to the postulate that abnormalities in apoptosis predispose to lupus. However, hereditary defects in clearance or apoptosis account for only a minority of cases of SLE in humans. Thus, the exact role of altered apoptosis in the pathogenesis of lupus remains controversial.
Recent work in DBA→F1 mice provides a mechanism by which lupus autoantibodies target apoptosis-related molecules [14], particularly antigens cleaved by caspases, e.g. poly (ADP ribose) polymerase 1 (PARP-1). After 4 weeks of disease, female F1 recipients exhibited increased splenic apoptosis (TUNEL positive cells) and serum levels of anti-PARP-1 antibodies. Values for males were significantly lower but could be increased by experimentally increasing the apoptotic burden through co-administration of UVB irradiated syngeneic F1 splenocytes. BDF1 host mice have no known defects in complement or apoptotic clearance. Moreover, donor CD8 CTL development is defective in DBA→F1 mice with minimal anti-host activity prior to day 14 after which donor CD8 T cells exhibit contraction, well before the appearance at 4 weeks of increased splenic apoptosis and anti-PARP-1 ab. Thus, it is unlikely that excessive apoptotic material results from CD8 CTL killing of host cells or due genetic defects in clearance. Instead, our results support the conclusion that lymphocyte activation i.e CD4 T cell driven B cell hyperactivity and attendant cell turnover characteristic of active lupus in the DBA→F1 model is of sufficient strength and duration that otherwise normal apoptosis clearance mechanisms can be saturated, particularly in females, thereby permitting the antibody response to target apoptotic autoantigens.
Conceptual advances in pathogenesis of lupus stemming from acute GVHD: previous work
Crucial role of CD8 T cells
The crucial role of donor CD8 T cells in preventing lupus-like phenotype was shown by the demonstration that depletion of CD8 T cells but not of CD4 T cells from the donor inoculum converts acute GVHD in B6→BDF1 mice to chronic GVHD both in the long term [23] and at two weeks [20]. Further underscoring the crucial role of CD8 T cells is the demonstration that DBA→BDF1 mice exhibit a lupus phenotype rather than the expected acute GVHD as a result of a naturally occurring defect in CD8 T-cell maturation. Typically, the transfer of parental CD4 and CD8 T cells into MHC I and II disparate F1 BDF1 mice results in acute GVHD; however, the transfer of DBA CD4 and CD8 T cells into BDF1 mice (DBA→BDF1) paradoxically results in chronic GVHD and lupus-like disease in the long term. The mechanism involves both an intrinsic CD8 defect (i.e. reduced anti-F1 precursor CTL frequency) [20] and an extrinsic defect (i.e skewing of CD4 T-cell help to Th2 cytokines) [21]. Importantly, CD8 CTL defects in DBA→BDF1 mice can be circumvented by in vivo manipulations that promote CTL function, such as rIL-12 treatment resulting in acute rather than chronic GVHD [55]. Acute GVHD can also be induced in DBA→F1 mice by treatment with anti-CD80 mAb which results in impaired down regulation of CD8 CTLs [56].
Thus, the DBA→F1 model not only attests to the crucial importance of donor CD8 CTL effector function in spelling out the difference between acute or chronic GVHD but also serves as a useful model for screening of agents that promote CTLs and thereby prevent lupus-like disease.
Partial defects in donor CD8 CTL function
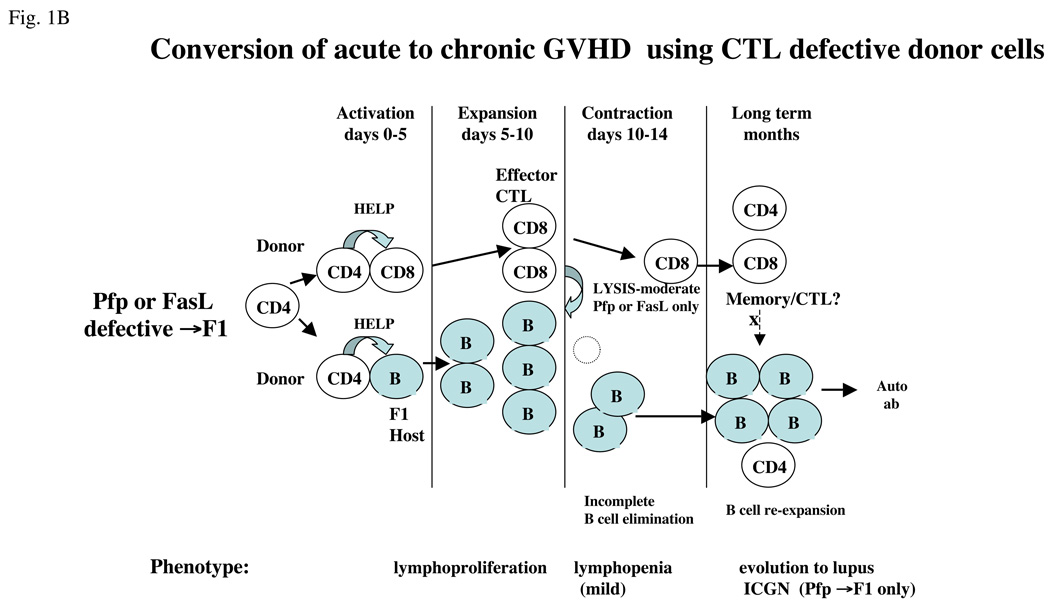
In the B6→F1 model of acute GVHD nearly all of the CTL activity in vivo can be accounted for by perforin (pfp) and Fas/FasL pathways [57]. The use of donor T cells with a partial defect in parental CD8 CTL activity (i.e. perforin knockout (pfp KO→F1) or FasL defective (gld→F1) donor T cells) results in an attenuated acute GVHD phenotype at two weeks [58,59] such that host B-cell elimination is impaired compared with WT B6→BDF1 mice because of the loss of either the FasL or pfp pathway, respectively. This finding confirms ex vivo data indicating that both pathways are important in eliminating and controlling activated B cells in vivo. It might be expected that a defect in a single killing pathway simply delays the onset of the acute GVHD phenotype; however, in pfp KO→F1 mice, the opposite occurs and mice develop lupus long term. This surprising outcome is a result of normal homeostatic contraction of activated CTL effectors from days 10–14 after donor cell transfer [5,6], a time when host B-cell elimination is typically complete for F1 mice receiving wild-type B6 donor T cells but is incomplete using pfp KO or gld donor B6 T cells. Donor CD8 T-cell contraction allows the persisting donor CD4 T cells to provide cognate help to residual host B cells resulting in their re-expansion, autoantibody production and eventually ICGN. Thus, in the presence of impaired but not completely defective CD8 CTL function, F1 mice develop an initial two week phenotype consistent with an incomplete acute GVHD but over time mice evolve into a lupus-like phenotype (reviewed in Ref. [6]).
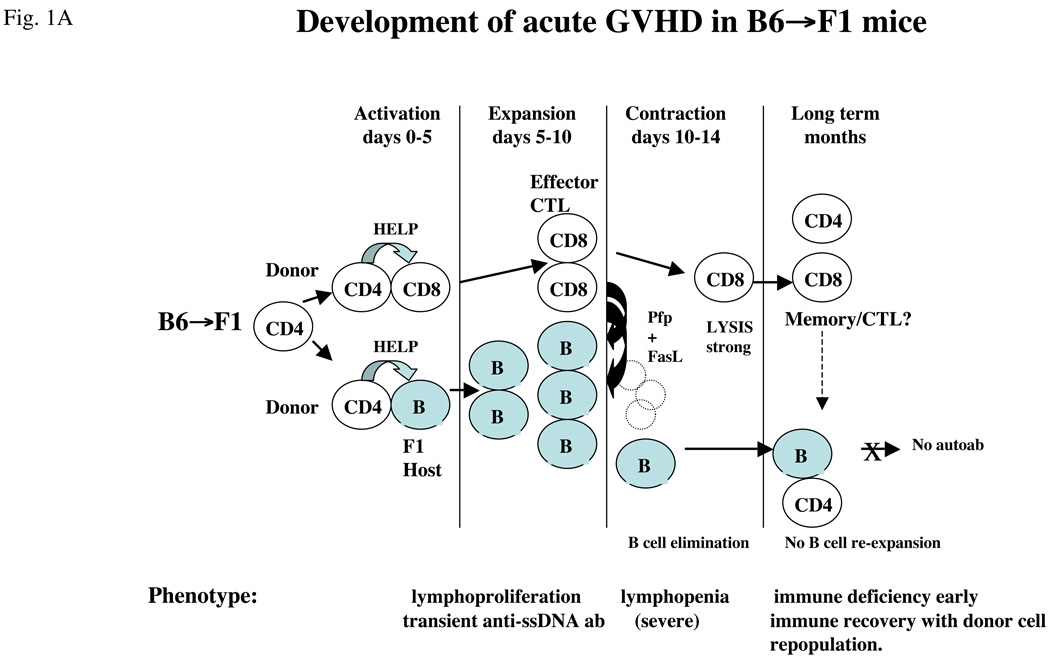
These results have important implications for the role of CTLs in lupus. Firstly, effector CTLs can contract after a finite period regardless of whether foreign antigen has been completely eliminated. In the setting of persistent foreign antigen and contracted CD8 CTL, the immune response shifts to an antibody-mediated response with the potential for lupus-like disease in the long term. Thus, not only are antigen-specific CD8 CTLs important in initially limiting lupus-like B-cell hyperactivity by eliminating activated B cells, but their failure, either complete or partial, to eliminate B cells in a timely fashion can permit the development of a lupus phenotype. The sequence of events is schematically illustrated in Figure 1 in which the acute GVHD phenotype using wild-type B6 donor cells (Figure 1a) is compared with the effect of selective perforin or FasL deficient donor cells (Figure 1b).
Figure 1.
Conversion of acute to chronic GVHD phenotype in B6→F1 mice. (a) Mechanics of acute GVHD induction. Transfer of wild-type B6 T cells into normal B6D2F1 mice results in B6 CD4 T cell activation that in turn provides help to host B cells and to donor CD8 T cells (days 0–5), leading to expansion and maturation of both host B cells (with transient anti-ssDNA ab production) and donor CD8 T cells into CTLs (days 5–10). Donor CTL effectors eliminate activated host B and T cells through both perforin (Pfp) and FasL pathways (days 10–14) and then undergo homeostatic contraction (days 12–14). Host mice exhibit a profound immunodeficiency that gradually recovers with donor lymphocyte re-population. Despite the presence of donor CD4 T cells in the long term, host B cells do not re-expand, possibly because of the presence of memory donor CD8 T cells. (b) Partial defects in CTL killing convert acute to chronic GVHD. The transfer of B6 donor cells defective in either Pfp (pfp KO) or FasL (gld) results in impaired donor CD8 CTL elimination of host B cells during days 10–14. As a result, homeostatic of donor contraction of donor CD8 CTL (days 12– 14) is associated with a significant number of residual host B cells capable of re-expansion in the presence of donor CD4 T cells. Both gld→F1 and Pfp KO→F1 exhibit an intermediate 2 week phenotype consisting of impaired elimination of host B cells. Only pfp KO→F1 mice have been shown to develop lupus long term. It is not known whether Pfp or FasL play a role in long- term inhibition by donor CD8 T cells of B-cell expansion. For all figures, donor cells are shown as clear and host cells are shaded.
TNF blockade in acute GVHD: links to human lupus
Therapeutic TNF blockade in humans has been associated with autoantibody production and, less commonly, clinical lupus (reviewed in Ref. [60]). The mechanism involved is incompletely understood; however, results of in vivo TNF blockade in mice with acute GVHD provide a possible mechanism [61] in that the expected acute GVHD phenotype is converted to a chronic lupus-like GVHD two week phenotype due to a dose-dependent block in donor anti-host CTL maturation. Importantly, TNF blockade acted not at the effector CTL level but rather blocked the activation and induction phase of CTLs (days 0–7). Moreover, TNF blockade selectively impaired production of the Th1 cytokine IFN-g, however there was no inhibition of cytokines, such as IL-6, IL-4 or IL-10, that are important in antibody production. Thus, in an ongoing primary immune response in which both cell-mediated and antibody-mediated responses are initiated, TNF blockade selectively blocks Th1 cytokines and impairs CD8 CTL maturation resulting in unopposed function of cytokines important in antibody responses, thereby skewing the immune response towards humoral immunity. Because the association of TNF blockade treatment and autoantibody production is relatively uncommon and the development of clinical lupus is even less common, other factors are probably involved, for example the nature of the underlying pathogen, its cellular tropism and the genetics of the host response. This previously unknown crucial role of TNF in a primary CD8 CTL response provides not only a mechanism by which humoral autoimmunity could occur in patients receiving TNF blockade but also raises questions about the long-term trade offs in anti-viral and anti-tumor immunity, particularly in juvenile patients who may not have a fully developed memory CD8 repertoire. Supporting this latter concern, the FDA has recently issued an alert that TNF blockade may be associated with an increased risk of leukemia and lymphoma, particularly in children [62].
Recent implications of acute GVHD and lupus pathogenesis
Memory CD8 T cells and lupus remission
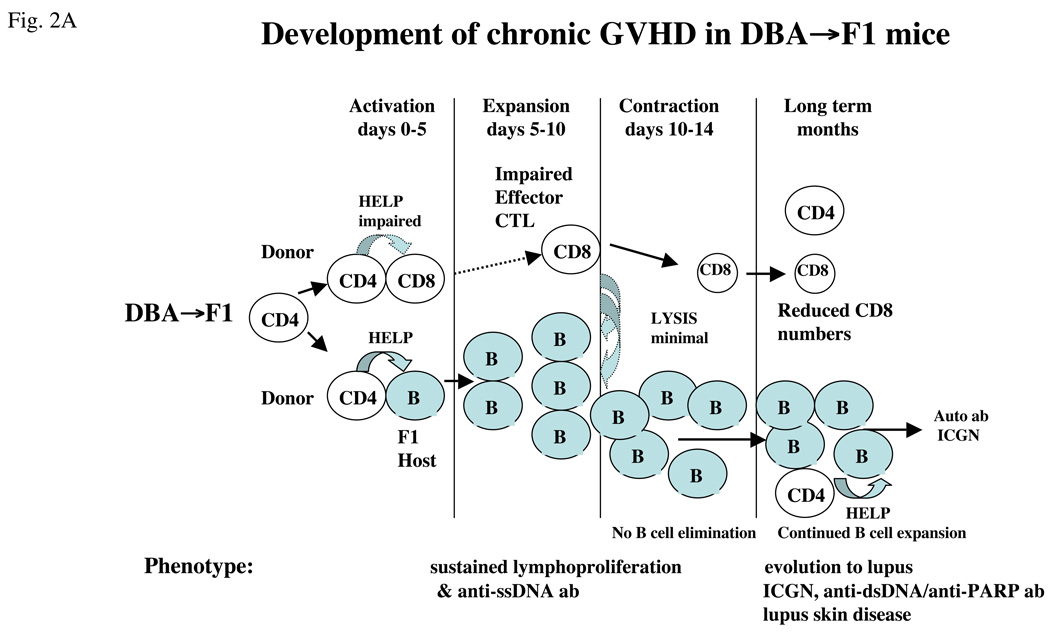
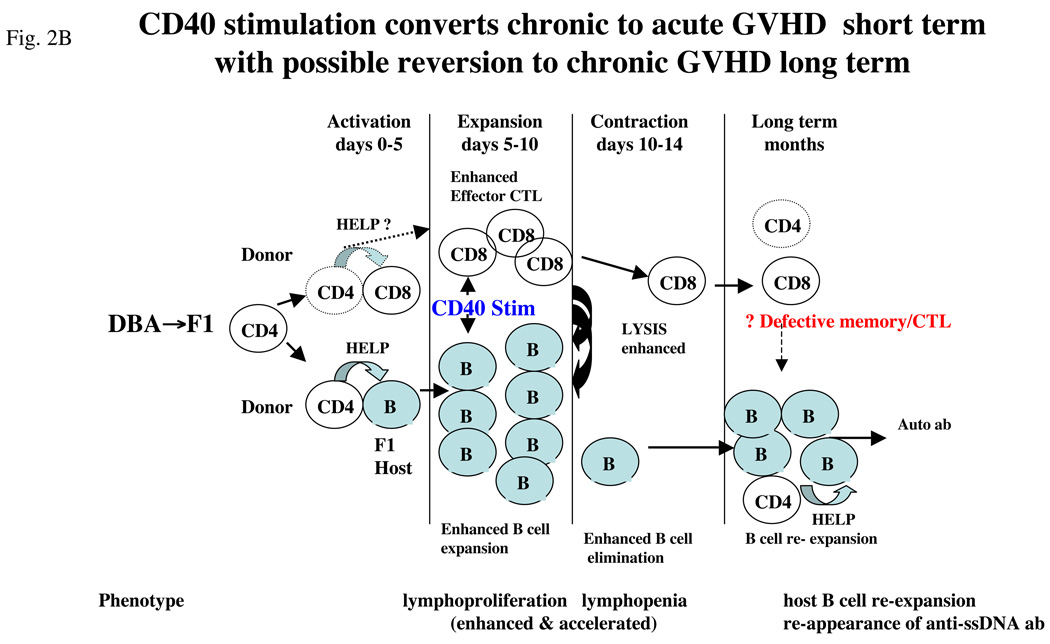
Agonist CD40 mAb has both B-cell and T-cell stimulatory properties and is beneficial against mouse tumors because of its CTL-promoting ability [63,64]. Similarly, a single dose of agonist anti-CD40 mAb in DBA→F1 mice at the time of donor cell transfer exhibited both strong B-cell stimulatory and CTL promoting effect and successfully converted the two week disease phenotype from chronic to acute GVHD [5]. Promotion of DBA CD8 CTLs was due to the ability of anti-CD40 mAb to bypass donor CD4 T-cell help and license APCs to activate donor CD8 CTLs directly [5]. So effective was anti-CD40 mAb treatment of DBA→F1 mice that early development of the acute GVHD phenotype was accelerated compared with acute GVHD in B6→F1 mice. Although lupus-like renal disease was prevented at 12 weeks in anti-CD40 treated DBA→F1 mice, all mice in this group exhibited serological relapse (i.e. rise in autoantibodies) associated with donor CD8 T-cell engraftment and host B-cell numbers that were intermediate between acute and chronic GVHD. Thus, although anti-CD40 mAb induces an accelerated form of acute GVHD in DBA→F1 mice initially, this phenotype is not sustained in the long term, and host B cells re-expand and serum autoantibodies rise in association with a waning of donor CD8 T cells.
The mechanism of relapse in anti-CD40-treated mice very likely relates to the development of memory CD8 CTLs, which are crucially dependent on CD4 T-cell help during the initial activation of naïve CD8 precursors [65]. Although memory phenotype CD8 T cells were not directly examined in this study, it is possible that the reduced numbers of donor CD8 T cells in anti-CD40 mAb treated DBA→F1 mice in the long term reflects impaired CD8 memory T cell generation that in turn is a consequence of CD40-mediated CD4 helper T-cell bypass. B6→F1 acute GVHD mice exhibit significantly greater numbers of donor CD8 T cells long term compared to anti-CD40 treated DBA→F1 mice and do not exhibit delayed expansion of host B cells or a rise in serum autoantibodies. By contrast, in DBA→F1 mice, primary donor anti-host CD8 CTL development is minimal and transient [5] which likely underpins the significantly reduced long term donor CD8 T cell engraftment vs. anti-CD40 treated DBA→F1 mice and their persistent host B cell activation and expansion.
Thus, in addition to the well-described role of donor CD8 CTLs in the initial (< day 14) prevention of autoantibody production in the P→F1 model, these results raise the novel possibility that CD8 T cells, to include memory cells, may also be required in the long term for maintaining disease remission through continued surveillance and elimination of autoantibody-producing host B cells. The sequence of events is schematically illustrated in Figure 2 in which the DBA→F1 chronic GVHD phenotype (Figure 2a) is compared with the effect of agonist anti-CD40 mAb (Figure 2b).
Figure 2.
Conversion of chronic to acute GVHD phenotype in DBA→F1 mice. (a) Mechanics of chronic GVHD induction. Transfer of DBA splenocytes into normal B6D2F1 mice results in donor CD4 T-cell activation that in turn provides help to host B cells but defective help for donor CD8 T cells which also are intrinsically defective. As a result, host B-cell expansion proceeds unchecked by mature donor CD8 CTL effectors. Early (day 7–10) B cell expansion and production of anti-ssDNA ab persists long term allowing evolution to lupus specific autoantibodies and lupus-like ICGN. (b) Conversion of chronic to acute GVHD with agonist anti-CD40 stimulation. Administration of agonist anti-CD40 mAb at the time of donor cell transfer directly stimulates host B-cell expansion and bypasses the donor CD4 T cells to stimulate donor CD8 CTL maturation by licensing of host antigen-presenting cells (APCs), resulting in an accelerated acute GVHD phenotype (i.e. host B-cell elimination) in the short term; however, in the long term, mice exhibit a rise in serum anti-ssDNA ab in conjunction with reduced numbers of donor CD8 T cells compared with WT B6→F1 mice consistent with reversion to lupus-like disease possibly as a consequence of defective donor CD8 T cell memory function.
Prolonging CD8 T-cell activation by preventing Fas-mediated down regulation
It has been proposed that the acceleration of spontaneous lupus-like autoimmunity in Fas-defective lpr mice reflects: (i) defective Fas expression on B cells allowing persistence of pathogenic autoreactive B cells [66]; and (ii) impaired Fas-mediated CD4 T-cell down regulation resulting in excessive help to pathogenic B cells [67]. Fas-defective non-autoimmune prone strains (e.g. B6.lpr mice) exhibit some lupus like autoimmune phenomena but do not exhibit lupuslike ICGN. Thus, Fas defects act not to cause lupus necessarily but rather to accelerate underlying disease. Conversely, intact Fas functioning can act to retard disease expression. By transferring Fas-defective donor cells into Fas-intact hosts, the P→F1 model allows a fine dissection of the role of Fas on antigen-specific T cells and can test whether Fas-defective CD4 T cells provide excessive or prolonged help to potentially pathogenic B cells. Moreover, the role of Fas on donor T cells in generating a CTL response in vivo (i.e. acute GVHD) can be further addressed by mixing or matching purified Fas-intact or Fas-defective donor CD4 and CD8 T cells. With this approach, it was shown that Fas expression on CD4 T cells plays a crucial role in providing help to CD8 CTL maturation but had no demonstrable role in increasing or decreasing help for CD4 driven-B cell production of IgG autoantibodies or in long term renal disease severity. [68]. Not surprisingly, Fas played a major role in the contraction of effector CD8 CTLs. Substituting normal CD4 T cells for Fas-defective CD4 T cells resulted in prolonged and enhanced effector function by Fas-defective CD8 CTLs because of impaired Fas-mediated contraction. These results support the concept shown by anti-CD80 mAb treatment [56] that in vivo CTL function can be enhanced by delaying homeostatic contraction.
These results also provide a new interpretation of the role of Fas defects in accelerating lupus in MRL/lpr mice. Both Fas and perforin are important in controlling B- cell expansion and autoimmunity in spontaneous MRL/+ mice and chronic GVHD models of lupus, because the loss of either of these pathways promotes B- cell expansion and accelerates disease [58,59,69]. A role for CD8 CTL controlling autoreactive B cells is also supported by the observation that MRL/lpr mice exhibit an age-related defect in CTL generation and IL-2 production in vitro, due in part to excessive CD4 down regulatory function, possibly mediated by regulatory T cells (Treg) [49]. Because Fas plays a more important role in CD8 CTL contraction than it does in CD4 help to B cells [68], the results from lpr→F1 mice support a role for CD8 CTL not only in chronic GVHD but also in MRL/ lupus. Specifically, it is possible that the ability of MRL CD8 CTLs to limit aberrant B-cell expansion is defective not only because of a lack of Fas expression on expanding B cells (thereby eliminating the FasL pathway of control) but also that lpr CD4 T cells provide defective help for CTL in MRL/lpr mice as shown in lpr→F1 mice [68] thereby compromising the remaining perforin pathway of autoreactive B-cell control. It is not known to what extent this compromise in CTL function is offset by a prolonged effector phase due to defective Fas-mediated homeostatic contraction.
Do CD8 T cells shape lupus disease expression?
Donor CD4 T cells are necessary and sufficient for lupus-like disease induction in P→F1 mice however donor CD8 T cells play an important role in ultimate disease expression in this model. Firstly an intact initial CD8 CTL response can prevent the onset of lupus-like disease early after donor cell transfer. Secondly, CD8 T cells (possibly memory) are important in preventing an acute GVHD from evolving into a lupus phenotype long term. Thirdly, an impaired initial CD8 CTL response results in an intermediate phenotype at two weeks that can evolve to lupus-like disease [58]. Lastly, we have recently observed that greater renal disease severity in female vs. male DBA→F1 mice is absolutely dependent on the transfer of DBA CD8 T cells [19]. Thus, despite the profound defect in initial DBA anti-host CD8 CTL response, donor CD8 T cells can still shape disease expression long term in a sex based fashion. Taken together these results support that idea that CD8 CTL may be an important mechanism in the control of autoreactive B cells and clinical lupus might in some cases result from a failure of CD8 CTL control of B cell autoreactivity.
Translating these observations regarding CD8 T cells to human lupus remains speculative. Recent work by Blanco et. al. [70] indicates that despite the well described lupus-associated in vitro defect in human CD8 CTL function [48], SLE patients with active disease can exhibit phenotypic evidence of CD8 CTL activation to include increased perforin and/or granzyme B expression. Moreover, such CD8 CTL are capable of generating high amounts of soluble nucleosomes. As with many studies in human SLE, it is not clear whether the observed phenomena are a secondary and possibly beneficial compensatory mechanism or a primary event serving to worsen disease. Results from the P→F1 model are most consistent with the former postulate i.e. a secondary beneficial or compensatory response. Specifically, CD8 CTL appearing during flare could be responsible for achieving disease remission by eliminating autoreactive B cells thereby preventing them from responding to a potentially increased nucleosome burden. Alternatively, increased CD8 CTL activity could be a primary event and further exacerbate disease by increasing chromatin/apoptotic substrates without reducing the ability of B cells to respond to these substrates. Clearly, successful unraveling the role of CD8 CTL in humans will be difficult and will likely be based on novel testable hypotheses stemming from mouse models. Regardless of the actual role of CD8 CTL in human SLE, the P→F 1 model supports the idea that targeted enhancement of CD8 CTL function resulting in selective elimination of autoreactive B cells could be beneficial in lupus.
Unresolved issues relating to human lupus
Type I interferons
Overproduction of type I IFN (IFN-1) is a characteristic feature of human lupus [71]. In murine lupus, the pristine model of induced lupus is the only model thus far to exhibit the characteristic IFN-1 signature [28]. In the P→F1 model, we have recently observed that neither male nor female DBA→F1 mice exhibit significant elevation of IFN-1 inducible genes e. g. Mx-1 at a time when significant ICGN is present [19]. The role of IFN-1 in acute and chronic GVHD in the P→F1 model is currently under investigation in our lab.
Tregs
The pathogenic role and therapeutic potential of Tregs in human lupus are the focus of active investigation [72,73]. Only a few studies have examined Tregs in the P→F1 model. Zheng et. al. demonstrated that co administration of ex vivo generated Tregs with DBA/2 splenocytes could block lupus-like features in DBA GVHD mice [74]. By contrast, lupus-like GVHD in DBA→F1 mice could be converted to acute GVHD by either: 1) depleting CD25+ DBA donor cells prior to transfer [75]; or 2) stimulation of glucocorticoid-induced TNFR-related protein with an agonist mAb [76]. With the recent description of heterogeneity among Treg subsets and more specific Treg markers e.g. Foxp3 [77], the exact implications of these studies with regard to Treg function in the P→F1 model is unclear and will require further studies.
Summary
Important advances in our understanding of lupus have been derived from murine models. Past results from the P→F1 model exhibit a striking concordance with spontaneous models. The experimental advantages of the P→F1 model are that it allows mechanistic approaches not possible in spontaneous models providing novel insights into unresolved aspects of the pathogenesis of lupus. A recent direction stemming from this model is that therapeutic enhancement of CD8 CTLs and possibly memory cells is both feasible and potentially beneficial in lupus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by National Institutes of Health grant AI047466 (CSV). The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as representing the views of the Uniformed Services University or the Department of Defense. cvia@usuhs.mil
References
- 1.Gleichmann E, et al. Graft-versus-host reactions: clues to the etiopathology of a spectrum of immunological diseases. Immuno.l Today. 1984;5:324–332. doi: 10.1016/0167-5699(84)90126-9. [DOI] [PubMed] [Google Scholar]
- 2.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol. Today. 1988;9:207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 3.Portanova JP, Kotzin BL. Lupus-like autoimmunity in murine graft-versus-host disease. Concepts Immunopatho.l. 1988;6:119–140. [PubMed] [Google Scholar]
- 4.Eisenberg R. The chronic graft-versus-host model of systemic autoimmunity. Curr. Dir. Autoimmun. 2003;6:228–244. doi: 10.1159/000066864. [DOI] [PubMed] [Google Scholar]
- 5.Puliaev R, et al. CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus. J. Immunol. 2008;181:47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puliaeva I, et al. Therapeutic potential of CD8+ cytotoxic T lymphocytes in SLE. Autoimmun. Rev. 2008 doi: 10.1016/j.autrev.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris SC, et al. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J. Exp. Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleichmann E, et al. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur. J. Immunol. 1982;12:152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Gleichmann E, Gleichmann H. Pathogenesis of graft-versus-host reactions (GVHR) and GVH-like diseases. J. Invest. Dermatol. 1985;85 doi: 10.1111/1523-1747.ep12275619. 115s–120s. [DOI] [PubMed] [Google Scholar]
- 11.Rolink AG, et al. Diseases caused by reactions of T lymphocytes to incompatible structures of the major histocompatibility complex. VII. Immune-complex glomerulonephritis. J. Immunol. 1983;130:209–215. [PubMed] [Google Scholar]
- 12.van Elven EH, et al. Diseases caused by reactions of T lymphocytes to incompatible structures of the major histocompatibility complex. II. Autoantibodies deposited along the basement membrane of skin and their relationship to immune-complex glomerulonephritis. J. Immunol. 1981;126:1684–1691. [PubMed] [Google Scholar]
- 13.Van der Veen F, et al. Diseases caused by reactions of T lymphocytes to incompatible structures of the major histocompatibility complex. IV. Autoantibodies to nuclear antigens. Clin. Exp. Immunol. 1981;46:589–596. [PMC free article] [PubMed] [Google Scholar]
- 14.Grader-Beck T, et al. Apoptotic splenocytes drive the autoimmune response to poly(ADP-ribose) polymerase 1 in a murine model of lupus. J. Immunol. 2007;178:95–102. doi: 10.4049/jimmunol.178.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Portanova JP, et al. Allogeneic MHC antigen requirements for lupus-like autoantibody production and nephritis in murine graft-vs-host disease. J. Immunol. 1988;141:3370–3376. [PubMed] [Google Scholar]
- 16.Pals ST, et al. Chronic progressive polyarthritis and other symptoms of collagen vascular disease induced by graft-vs-host reaction. J. Immunol. 1985;134:1475–1482. [PubMed] [Google Scholar]
- 17.Gelpi C, et al. Murine graft vs host disease. A model for study of mechanisms that generate autoantibodies to ribonucleoproteins. J. Immunol. 1988;140:4160–4166. [PubMed] [Google Scholar]
- 18.Lang TJ, et al. Increased severity of murine lupus in female mice is due to enhanced expansion of pathogenic T cells. J. Immunol. 2003;171:5795–5801. doi: 10.4049/jimmunol.171.11.5795. [DOI] [PubMed] [Google Scholar]
- 19.Foster A, et al. Donor CD8 T cell activation is critical for greater renal disease severity in female chronic graft-vs.-host mice and is associated with increased splenic ICOShi host CD4 T cells and IL-21 expression. Clin. Immunol. 2010 doi: 10.1016/j.clim.2010.01.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Via CS, et al. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J. Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- 21.De Wit D, et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J. Immunol. 1993;150:361–366. [PubMed] [Google Scholar]
- 22.Morris SC, et al. Experimental induction of systemic lupus erythematosus by recognition of foreign Ia. Clin. Immunol. Immunopathol. 1990;57:263–273. doi: 10.1016/0090-1229(90)90040-w. [DOI] [PubMed] [Google Scholar]
- 23.Rolink AG, Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J. Exp. Med. 1983;158:546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rus V, et al. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J. Immunol. 2007;178:3962–3972. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 25.Van Rappard-Van Der Veen FM, et al. Attempts at standardization of lupus-like graft-vs-host disease: inadvertent repopulation by DBA/2 spleen cells of H-2-different nonirradiated F1 mice. J. Immunol. 1983;130:2693–2701. [PubMed] [Google Scholar]
- 26.Gelpi C, et al. Different strains of donor parental lymphoid cells induce different models of chronic graft-versus-host disease in murine (Balb/c × A/J)F1 hybrid hosts. Clin. Immunol. Immunopathol. 1990;56:298–310. doi: 10.1016/0090-1229(90)90151-f. [DOI] [PubMed] [Google Scholar]
- 27.Tschetter JR, et al. Progression from acute to chronic disease in a murine parent-into-F1 model of graft-versus-host disease. J. Immunol. 2000;165:5987–5994. doi: 10.4049/jimmunol.165.10.5987. [DOI] [PubMed] [Google Scholar]
- 28.Reeves WH, et al. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niculescu F, et al. Both apoptosis and complement membrane attack complex deposition are major features of murine acute graft-vs.-host disease. Exp. Mol. Pathol. 2005;79:136–145. doi: 10.1016/j.yexmp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Zielinski CE, et al. Naive CD4+ T cells from lupus-prone Fas-intact MRL mice display TCR-mediated hyperproliferation due to intrinsic threshold defects in activation. J. Immunol. 2005;174:5100–5109. doi: 10.4049/jimmunol.174.8.5100. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg AD, et al. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J. Immunol. 1980;125:871–873. [PubMed] [Google Scholar]
- 32.Seaman WE, et al. Treatment of autoimmune MRL/Ipr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J. Immunol. 1983;130:1713–1718. [PubMed] [Google Scholar]
- 33.Santoro TJ, et al. The contribution of L3T4+ T cells to lymphoproliferation and autoantibody production in MRL-lpr/lpr mice. J. Exp. Med. 1988;167:1713–1718. doi: 10.1084/jem.167.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jevnikar AM, et al. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J. Exp. Med. 1994;179:1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng SL, et al. Murine lupus in the absence of alpha beta T cells. J. Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 36.Rozzo SJ, et al. Evidence for polyclonal T cell activation in murine models of systemic lupus erythematosus. J. Immunol. 1994;153:1340–1351. [PubMed] [Google Scholar]
- 37.Rolink AG, et al. Allosuppressor and allohelper T cells in acute and chronic graft-vs.-host disease. II. F1 recipients carrying mutations at H-2K and/or I-A. J. Exp. Med. 1983;157:755–771. doi: 10.1084/jem.157.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busser BW, et al. Activation of diverse repertoires of autoreactive T cells enhances the loss of anti-dsDNA B cell tolerance. J. Clin. Invest. 2003;112:1361–1371. doi: 10.1172/JCI18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobel ES, et al. T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted. J. Immunol. 1994;152:6011–6016. [PubMed] [Google Scholar]
- 40.Hoffman R, Maldanado ME. T-cells and Systemic Lupus Erythematosus. In: Tsokos GC, et al., editors. Systemic Lupus Erythematosus: A Companion to Rheumatology. Mosby: 2007. pp. 95–102. [Google Scholar]
- 41.Molina JF, et al. Coexistence of human immunodeficiency virus infection and systemic lupus erythematosus. J. Rheumatol. 1995;22:347–350. [PubMed] [Google Scholar]
- 42.Drake WP, et al. Reactivation of systemic lupus erythematosus after initiation of highly active antiretroviral therapy for acquired immunodeficiency syndrome. J. Clin. Rheumatol. 2003;9:176–180. doi: 10.1097/01.RHU.0000073591.34503.4e. [DOI] [PubMed] [Google Scholar]
- 43.Davidson A, et al. Block and tackle: CTLA4Ig takes on lupus. Lupus. 2005;14:197–203. doi: 10.1191/0961203305lu2136oa. [DOI] [PubMed] [Google Scholar]
- 44.Via CS, et al. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J. Immunol. 1996;157:4258–4267. [PubMed] [Google Scholar]
- 45.Jancin B. SLE drug pipeline: an embarrasment of riches. Rheumatology News. 2008;7:12. [Google Scholar]
- 46.Hannahs Hahn B, Tsao BP. Pathogenesis of Systemic Lupus Erythematosus. In: Firestein G, et al., editors. Firestein: Kelley's Textbook of Rheumatology. W. B. Saunders; 2008. [Google Scholar]
- 47.Crispin JC, et al. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. doi: 10.1016/j.it.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Via CS, Shearer GM. Defective in vitro IL-2 production in lupus is an early but secondary event paralleling disease activity: evidence from the murine parent-into-F1 model supports staging of IL-2 defects in human lupus. Autoimmunity. 2010;43:23–31. doi: 10.3109/08916930903374808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Via CS, Shearer GM. Functional heterogeneity of L3T4+ T cells in MRL-lpr/lpr mice. L3T4+ T cells suppress major histocompatibility complex-self-restricted L3T4+ T helper cell function in association with autoimmunity. J. Exp. Med. 1988;168:2165–2181. doi: 10.1084/jem.168.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Via CS, et al. T cell-antigen-presenting cell interactions in human systemic lupus erythematosus. Evidence for heterogeneous expression of multiple defects. J. Immunol. 1993;151:3914–3922. [PubMed] [Google Scholar]
- 51.Bermas BL, et al. T helper cell dysfunction in systemic lupus erythematosus (SLE): relation to disease activity. J. Clin. Immunol. 1994;14:169–177. doi: 10.1007/BF01533366. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan MJ. Apoptosis in systemic lupus erythematosus. Clin. Immunol. 2004;112:210–218. doi: 10.1016/j.clim.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Casciola-Rosen LA, et al. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson LS, Winchester R. Systemic Lupus Erythematosus: Pathogenesis. In: Koopman WJ, Moreland LW, editors. Arthritis & Allied Conditions. Lippincott Williams & Wilkins; 2005. pp. 1523–1559. [Google Scholar]
- 55.Via CS, et al. IL-12 stimulates the development of acute graft-versus-host disease in mice that normally would develop chronic, autoimmune graft-versus-host disease. J. Immunol. 1994;153:4040–4047. [PubMed] [Google Scholar]
- 56.Lang TJ, et al. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J. Immunol. 2002;168:3786–3792. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- 57.Shustov A, et al. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-gamma production. J. Immunol. 1998;161:2848–2855. [PubMed] [Google Scholar]
- 58.Shustov A, et al. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J. Clin Invest. 2000;106:R39–R47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Via CS, et al. A major role for the Fas pathway in acute graft-versus-host disease. J. Immunol. 1996;157:5387–5393. [PubMed] [Google Scholar]
- 60.Williams EL, et al. Anti-TNF-induced lupus. Rheumatology (Oxford) 2009;48:716–720. doi: 10.1093/rheumatology/kep080. [DOI] [PubMed] [Google Scholar]
- 61.Via CS, et al. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J. Immunol. 2001;167:6821–6826. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 62.FDA. Information for Healthcare Professionals: Tumor Necrosis Factor (TNF) Blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) 2009 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/uc m174474.htm.
- 63.Murphy WJ, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J. Immunol. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 64.Lode HN, et al. Melanoma immunotherapy by targeted IL-2 depends on CD4(+) T-cell help mediated by CD40/CD40L interaction. J. Clin. Invest. 2000;105:1623–1630. doi: 10.1172/JCI9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bourgeois C, et al. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 66.Rathmell JC, et al. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 67.Bossu P, et al. Mature CD4+ T lymphocytes from MRL/lpr mice are resistant to receptor-mediated tolerance and apoptosis. J. Immunol. 1993;151:7233–7239. [PubMed] [Google Scholar]
- 68.Puliaeva I, et al. Fas expression on antigen-specific T cells has costimulatory, helper, and down-regulatory functions in vivo for cytotoxic T cell responses but not for T cell-dependent B cell responses. J. Immunol. 2008;181:5912–5929. doi: 10.4049/jimmunol.181.9.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng SL, et al. Perforin protects against autoimmunity in lupus-prone mice. J. Immunol. 1998;160:652–660. [PubMed] [Google Scholar]
- 70.Blanco P, et al. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 71.Ronnblom L, et al. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 72.Horwitz DA, et al. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin. Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 73.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–425. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 74.Zheng SG, et al. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 75.Kim J, et al. Maintenance of CD8+ T-cell anergy by CD4+CD25+ regulatory T cells in chronic graft-versus-host disease. Exp. Mol. Med. 2006;38:494–501. doi: 10.1038/emm.2006.58. [DOI] [PubMed] [Google Scholar]
- 76.Kim J, et al. Conversion of alloantigen-specific CD8+ T cell anergy to CD8+ T cell priming through in vivo ligation of glucocorticoid-induced TNF receptor. J. Immunol. 2006;176:5223–5231. doi: 10.4049/jimmunol.176.9.5223. [DOI] [PubMed] [Google Scholar]
- 77.Matarese G, et al. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12–17. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]