Summary
Investigation of how specialized olfactory cues, such as pheromones, are detected has primarily focused on the function of receptor neurons within a subsystem of the nasal cavity, the vomeronasal organ (VNO). Behavioral analyses have long indicated that additional, non-VNO olfactory neurons are similarly necessary for pheromone detection; however the identity of these neurons has been a mystery. Recent molecular, behavioral, and genomic approaches have led to the identification of multiple atypical sensory circuits that display characteristics suggestive of a specialized function. This review focuses on these non-VNO receptors and neurons, and evaluates their potential for mediating stereotyped olfactory behavior in mammals.
Introduction
Olfaction powerfully instructs behavior, primarily through experiential association. For example, the smell of a previously unremarkable odor in a stressful environment is likely to initiate stress upon subsequent detection. In contrast, exceptional subsets of odorants have the unique capacity to generate behavior that is largely stereotyped both between individuals and upon repeated exposure (Table 1). While the stimuli that trigger most mammalian sensory systems are well defined, pheromones and those odors that instruct stereotyped behavior are largely unknown. This is due, in part, to the immense number of candidate odorants as well as technical limitations that have prohibited the use of traditional methods to de-orphanize mammalian odorant receptors [1]. Odorant receptors are located in the nasal cavity which is separated into distinct subsystems (Fig 1) that include the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). MOE neurons project their axons to particular neuropils (glomeruli) in the main olfactory bulb (MOB) with subsequent circuits primarily projecting to the olfactory cortex. In contrast, VNO neurons project to the accessory olfactory bulb (AOB) whose output neurons innervate the limbic system [2]. The clear anatomical segregation of these olfactory subsystems, and their expression of molecularly distinct populations of sensory neurons, led to the theory that they may each be specialized for differing function. Indeed, the MOE is established to sense odorants while the VNO has been found to detect pheromones [3]. Therefore, until recently, the search for chemical cues and receptors of specialized function, such as mediating species-specific behaviors, was primarily focused on the VNO. Interestingly however, genetic and behavioral studies have consistently indicated that VNO activity is not necessary for the display of some olfactory-mediated instinctual behavior [4] indicating the existence of uncharacterized, functionally specialized, behaviorally relevant olfactory sensory circuits outside the VNO. While the investigation of VNO circuits that influence behavior remains of great interest (see Box 1), recent studies have now identified neurons in the MOE and additional (non-VNO) olfactory sub-systems that display characteristics consistent with the detection of specialized odorants and the ability to mediate stereotyped behavior. Molecular and functional identification of these neurons has the potential to determine the rationale of multiple olfactory subsystems, the range of olfactory coding strategies, and provide molecular tools to determine if olfactory specializations function in humans.
Table 1. Classification of olfactory cues by behavioral significance.
| Pheromone | Instinctive cue | Odorant | |
|---|---|---|---|
| Definition | Social cues emitted by one member of a species that provoke a specific behavioral or physiological response when detected by another member of the same species [49]. | Chemical cues that initiate a conserved, stereotyped behavior on first and subsequent exposures. | Chemicals that encode a contextually dependent perceptual quality and can trigger an associative or learned behavior with appropriate training. |
| Example | Major urinary proteins (Mups) found in the urine of adult male mice initiate aggressive behavior in rival males [50]. | Spoiled food odors elicit avoidance in rodents [41••]. | Mice rapidly learn odors that are paired with a reward, and will prefer that odor on subsequent exposure [15•,41••]. |
Figure 1. Olfactory sub-systems implicated in mediating innate behavior.
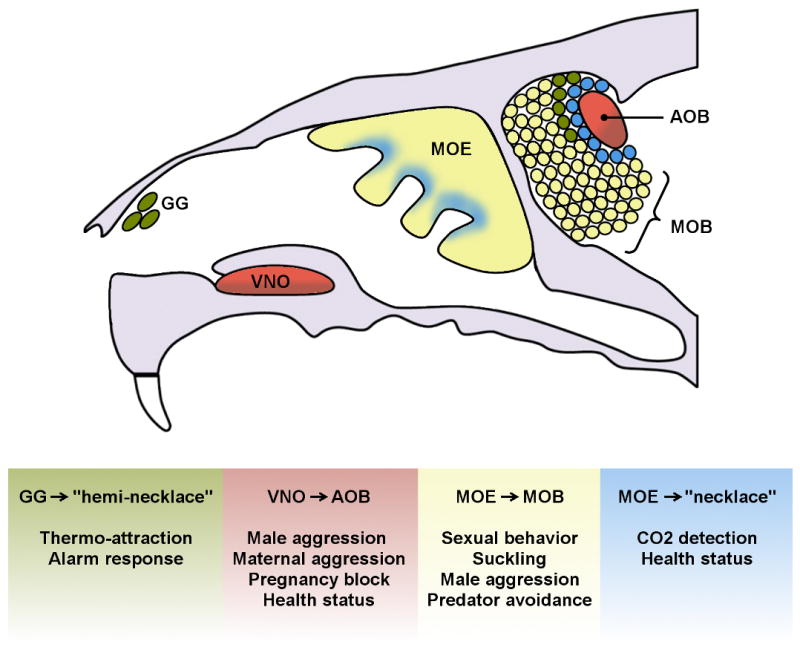
Upper: Organization of olfactory subsystems in the mouse; vomeronasal organ neurons (VNO) innervate the accessory olfactory bulb (AOB), canonical main olfactory epithelium (MOE) neurons project axons to the main olfactory bulb (MOB, yellow), Gruenberg ganglion (GG) neurons project to the MOB hemi-necklace (green), and molecularly specialized MOE neurons project to the MOB necklace (blue). Lower: Innate, pheromone, or specialized olfactory responses reported to be mediated through each sub-system.
Box 1. VNO ligands and receptors.
Recently there have been exciting advances towards identifying the molecules and mechanisms that underpin VNO function. Functional screening has revealed over 50 new ligands with pheromone characteristics. Two large clusters of genes, the Major urinary proteins (Mups) and exocrine-gland-secreting peptides (ESPs), have been shown to encode secreted ligands [51-52]. Variants of both show sexually-dimorphic expression, activate VNO sensory neurons of the mouse [50,53] and, in the case of Mups, elicit stereotyped male aggressive behavior [50]. Additionally, a new class of metabolite, sulfated steroids, was found to be sexually dimorphic, expressed in female mouse urine, and to robustly activate VNO neurons [54]. Strikingly, 80% of the VNO neural response to female urine is lost by sulfatase treatment supporting their importance as olfactory ligands [54]. Sulfated steroids have been shown to function as a migratory pheromone in lamprey [55]. Their function in mice is unknown but they are hypothesized to provide information about gender and physiology, such as recent stress [54].
Parallel discoveries have advanced our understanding of VNO sensory detection. A third receptor family, the formyl peptide receptor-like (FPR) proteins, was discovered in the VNO [56•-57•]. Though their function has not been established, they are activated by modified peptides characteristically produced by bacteria [57•]. Therefore it has been proposed that FPR-expressing neurons may detect disease state in other animals. Finally, stimulation with major histocompatibility complex (MHC) peptides provided insight into mechanistic logic of VNO neuron activation. Remarkably, only a subset of neurons expressing a labeled VNO receptor (V1Rb2) responded to a purified MHC stimulus, suggesting that this receptor may not be sufficient to determine ligand response [58].
Pheromone detection, beyond the VNO?
Classic studies showing the attractive response of the sow to androstenone (naturally released by boars) as well as the robust suckling behavior of the European Hare [5-6] have directly implicated the MOE in mediating pheromone-evoked responses. Functional experiments indicate that in both instances behavioral responses are VNO independent [5,7-8]. More recently, deletions of CNGA2 and ACIII, essential components of the canonical MOE signaling pathway, as well as complementary deletions in TrpC2, the primary sensory channel of the VNO, revealed that neurons outside the VNO are sufficient to display suckling and mating behavior and necessary to generate male-male aggression [9-13]. Whether these findings are due to the specific deletion of specialized signaling circuits or an indirect result of catastrophic olfactory loss is unknown. It has been suggested that anosmic mice either reduce sniffing which inhibits VNO function, or enter a severely depressed motivational state thereby secondarily impairing all social behavior [9,14]. This was addressed recently by engineering a mouse with over 95% of the olfactory sensory neurons expressing the same odorant receptor (M71) [15•]. These mice do display impaired olfactory discrimination but can still detect a surprising range of odorants, presumably utilizing the remaining 5% of OSNs that express a natural variety of receptors. This residual olfactory capacity relieves concerns about secondary motivational defects or general VNO function. Interestingly, these mice do not display appropriate stereotyped mating behavior, which further strengthens the hypothesis that, like VNO neurons, MOE neurons similarly mediate social behaviors. In a system that has over 1200 distinct sensory neuron types how can the relevant specialized subset be identified? Investigation has focused on neurons whose cell bodies or axonal projections are anatomically segregated from canonical MOE neurons as well as those that express novel sensory transduction molecules (Fig. 2).
Figure 2. Mechanisms encoding innate behavior: beyond the vomeronasal organ.
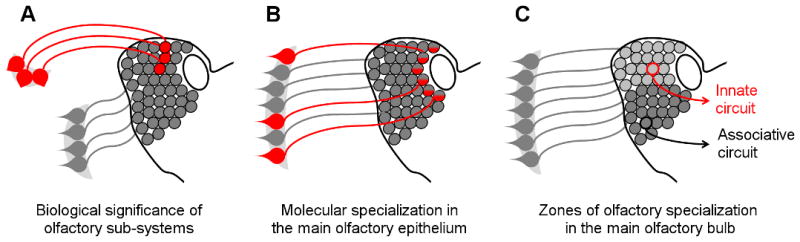
Multiple mechanisms have been postulated to encode innate olfactory information. Three current hypotheses: A) Anatomical segregation of sensory neurons distinguishes specialized processing (red) from general odor detection (grey). The VNO and GG (represented) are being investigated to support this mechanism. B) Molecular specialization of MOE neurons may enable pheromone detection (red). GC-D (represented) or TAAR expressing neurons may utilize this mechanism. C) The MOB glomeruli may be organized in functional zones. Analysis of mice lacking the dorsal zone (shaded light) suggests its necessity for innate coding.
Specialized sub-systems, specialized functions?
Recently a group of morphologically distinct neurons, whose cell bodies reside outside of the MOE, have been implicated in pheromone-mediated behavior. The Grueneberg ganglion (GG) was first thought to represent a branch of the terminal nerve until its rediscovery as an olfactory sub-system in 2005 [16-18]. GG neurons are present at birth, found in many mammalian species, and project to a “hemi-necklace” of glomeruli at the very caudal MOB (Fig. 1, 2A) [18] a region thought to be activated during nursing [19-20], collectively leading to hypotheses that they detect suckling pheromones [17]. However, lesioning the GG axon tract has no effect on nursing behavior [21••]. Instead, two distinct functions have recently been described. Mamasuew et al found that a subset of GG neurons of neonatal mice induce cFos in response to cool ambient temperatures, thereby acting as a thermosensor [22]. Interestingly, this may achieve the goal of a maternal pheromone, attracting a pup to the warmth of its mother, using an alternative sensory modality.
Independently, Brechbühl et al monitored intracellular calcium concentrations in individual GG neurons when exposed to urine, milk, mammary secretions, CO2 or temperature change, but observed no responses. However they did find that critically stressed mice release a signal that activates over 95% of the assayed GG neurons [21••]. The mechanism of this robust response is unclear considering that the molecular profile of GG neurons is not homogeneous. Indeed the GG has been shown to express components common with both VNO neurons (receptor V2R83b, Gαi2, Gαo) and canonical MOE neurons (olfactory marker protein, ACIII, Gαolf, and odorant receptors) [23]. Some neurons express the membrane receptor guanylyl cyclase sub-type, GC-G [24] while others alternately express GC-A [25], neither of which are found in the MOE or VNO. Importantly, behavioral analysis revealed strong freezing response to the stress-emitted signal, which is abolished upon neonatal GG lesion. The authors conclude that the GG mediates an innate response to an alarm pheromone emitted from the stressed mice [21••]. Identification of this stress signal and its sensory receptor(s) will provide the tools to confirm this function. If the GG is validated to detect pheromones it reinforces conclusions drawn from VNO analysis: that the organization of the nasal cavity into distinct subsystems may serve to generate specialized olfactory function (Fig 2A).
Necklace MOE neurons defy conventional mechanisms
Classical anatomical studies have identified another morphologically distinct set of glomeruli embedded in the MOB. These specialized “necklace” glomeruli, named because they encircle the caudal olfactory bulb like beads on a string [26], appear to be adjacent to, but different from the “hemi-necklace” of the GG neurons (Fig. 1)[18]. The necklace glomeruli are innervated by molecularly atypical neurons that do not express the canonical cAMP signaling proteins that define MOE neurons and instead express components of a cGMP signaling cascade [27-28]. No GPCRs have been identified in these neurons; however a membrane receptor guanylyl cyclase (GC-D) has been shown to be necessary for function [29•].
Early experiments associated the necklace region with suckling [19-20], but a series of recent independent studies came to alternate, unexpected conclusions. Utilizing electrophysiological techniques, one group found that GC-D neurons detect two natriuretic peptides, uroguanylin and guanylin [29•], at least one of which is present in mouse urine (a rich source of known VNO-mediated pheromones). Heterologous expression found GC-D to be activated by uroguanylin, but not guanylin [30]. Neurons from GC-D mutant mice fail to respond to natriuretic peptides indicating a primary role for GC-D in signaling. It has been reported that sub-sets of GC-D expressing neurons differentially detect guanylin, uroguanylin, or both [29•], however the mechanism underlying peptide specificity is unclear given that no other membrane receptor has been identified in these neurons. Both peptides are involved in regulating electrolyte balance suggesting the possibility that necklace neurons monitor metabolic status. Another elegant series of studies reported that the same neurons express a carbonic anhydrase that converts carbon dioxide to bicarbonate [31•]; this directly activates the cyclase domain of GC-D and triggers neural activation [32•-33]. Near atmospheric concentrations of CO2 delivered to anesthetized mice specifically induced activity from mitral cells that innervate necklace glomeruli, establishing the beginnings of a functional circuit [31•].
The importance of CO2 and uroguanylin in olfaction is unclear as both are emitted from other animals as well as endogenously present in the nasal cavity. It has been observed that naris occlusion diminishes tyrosine hydroxylase activity throughout the MOB, including the necklace, suggesting either a role in detecting external stimuli or the requirement of active respiration [34]. It remains of great interest to determine if the molecular and anatomical distinction of necklace neurons generates a specialized behavior, as no deficits were identified in GC-D knockout mice [29•]. Interestingly, while all other studied MOB glomeruli are exclusively innervated by neurons expressing the same GPCR [35], necklace glomeruli defy this convention. Instead they contain mixed sensory input both from GC-D neurons as well as canonical MOE neurons [36]. It is tempting to speculate that this unusual heterogeneous composition of necklace glomeruli may provide a mechanism for signals from odorants to be integrated with stimuli that encode specialized functions.
Olfactory receptors revisited
The hypothesis that olfactory functional specialization may be achieved through molecular specification prompted the search for additional atypical classes of MOE neurons. Liberles and Buck reasoned that pheromone sensing neurons of the MOE may express a GPCR of a different sub-family from those expressed by canonical neurons. Through genomics and expression analysis they identified a small population of MOE neurons that express trace amine-associated receptors (TAARs) [37••]. Importantly, each individual neuron expresses only one TAAR and displays no co-incidental expression with canonical olfactory receptors, suggesting the TAAR molecularly defines a specific sensory circuit. Moreover, the approximately 90:1 ratio of canonical receptors to TAARs suggests that they may indeed serve a highly specialized function in the MOE. Heterologous expression of TAARs enabled the functional screening of odorants to find that they selectively bind volatile amines. These amines may convey social information as at least one is naturally enriched in the urine of stressed rodents and humans [37••]. At this time, the functional importance of TAAR expressing neurons has not yet been established but their characteristics are consistent with candidate pheromone detectors [38]. Whether the glomeruli innervated by TAAR neurons project to an anatomically distinct region of the MOB, similar to the necklace (Fig 2B), remains to be determined. Interestingly, volatile amines are odorous and therefore may also be detected by ensembles of canonical olfactory receptors. If so, it will be important to determine whether amine odor activity generated from canonical MOE and TAAR neurons is differentially processed in the brain.
Spatial organization, zones of innate behavior?
An alternate mechanism to specialize olfactory function may not depend on molecular or anatomical characteristics of sensory neurons, but instead may occur through synaptic distinctions (Fig 2C). Both the Dulac and the Buck labs have shown by independent methods that subsets of MOE neurons establish primary circuits to GnRH-expressing neurons in the hypothalamus, confirming the occurrence of such post-synaptic specialization [39-40]. Recently Sakano and colleagues created genetic tools to selectively ablate large ensembles of either dorsal or ventral glomeruli, generating a platform to test the biological significance of each MOB domain [41••]. They analyzed the ability of these mutants to detect and behaviorally respond to a series of innately aversive odorants including 2-methylbutyric acid, the odor of spoiled food, or trimethyl-thiazoline, a pungent odor secreted by foxes. Interestingly, mice missing dorsal glomeruli lack both innate aversion and a characteristic stress response to both odors. Strikingly, the mutant mice could still detect these odors, discriminate them from other odorants and be conditioned to avoid them through the remaining ventral glomerui. The authors conclude that a number of glomeruli in the dorsal zone are necessary for the innate aversive response, while ventral glomeruli are sufficient for olfactory conditioning (Fig 2C) [41••]. Further screening of these mutants with a larger panel of stereotypically aversive odors will help determine if a general feature of the dorsal MOB is to generate innate behavior. If so, it will be of interest to determine if behavior is mediated by canonical neurons or by molecularly specialized neurons within the zone.
Molecular evaluation of human pheromone detection
Human pheromomes are the subject of intense popular fascination, but have yet to be definitively identified. Their study is particularly challenging, not least because experimentally controlling the identification of learned associative responses from instinctive responses is nearly impossible using human subjects. A functional VNO is likely absent in humans and key VNO-specific genes have been demonstrated to be pseudogenized in our lineage [42]. Like the VNO, the GG has been identified only in human embryos and likely regresses during fetal development [16,21]. GC-D is also pseudogenized in primates, and human necklace glomeruli have not been described [43]. In contrast, TAAR receptors persist in the genome of many diverse vertebrate species, including humans, and are widely expressed in the olfactory neurons of bony fish [44-45], however it is currently unknown whether TAARs are expressed in human olfactory neurons.
Perhaps the strongest candidate for human pheromone status is androstadienone, a component of male sweat that is reported to maintain cortisol levels in women and influences their attraction to men in some contexts [46-47]. The human olfactory receptor for androstadienone was recently identified as OR7D4 and found to have naturally occurring sequence variants that correlate with both in vitro activation of the receptor and the pleasantness of androstadienone perception [48••]. Interestingly this receptor is a canonical olfactory GPCR, with no obvious signs of molecular specialization for pheromone detection. Therefore if androstadienone is a human pheromone, it appears likely that the organization of its glomeruli or post-synaptic projections will be what distinguishes its sensory neurons from those responding to other odorants.
Though there have been many recent advances, the identification of cognate ensembles of ligands, receptors, circuits and behavior will ultimately be required to determine the general mechanisms that initiate stereotyped olfactory-mediate behavior in mammals.
Acknowledgments
We would like to thank K. Baldwin, F. Papes, and C. Dulac for helpful comments on the text. LS is supported by grants from the NIH-NIDCD and the Skaggs Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Those of special [•] or outstanding interest [••]
- 1.Bush CF, Hall RA. Olfactory receptor trafficking to the plasma membrane. Cell Mol Life Sci. 2008;65:2289–2295. doi: 10.1007/s00018-008-8028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 3.Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89:921–956. doi: 10.1152/physrev.00037.2008. [DOI] [PubMed] [Google Scholar]
- 4.Stowers L, Marton T. What is a pheromone? Mammalian pheromones reconsidered. Neuron. 2005;46:699–702. doi: 10.1016/j.neuron.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and Behavioral Responses to the Pheremone Androstenone are not Mediated by the Vomeronasal Organ in Domestic Pigs. Brain Behav Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- 6.Schaal B, Coureaud G, Doucet S, Delaunay-El Allam M, Moncomble AS, Montigny D, Patris B, Holley A. Mammary olfactory signalisation in females and odor processing in neonates: ways evolved by rabbits and humans. Behav Brain Res. 2009;200:346–358. doi: 10.1016/j.bbr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Hudson R, Distel H. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav. 1986;37:123–128. doi: 10.1016/0031-9384(86)90394-x. [DOI] [PubMed] [Google Scholar]
- 8.Distel H, Hudson R. The contribution of the olfactory and tactile modalities to the nipple-search behaviour of newborn rabbits. J Comp Physiol A. 1985;157:599–605. doi: 10.1007/BF01351354. [DOI] [PubMed] [Google Scholar]
- 9.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet LJ, Gold GH, Ngai J. General Anosmia Caused by a Targeted Disruption of the Mouse Olfactory Cyclic Nucleotide-Gated Cation Channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 12.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 13.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mucignat-Caretta C, Bondi M, Caretta A. Time course of alterations after olfactory bulbectomy in mice. Physiol Behav. 2006;89:637–643. doi: 10.1016/j.physbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15•.Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, et al. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study the authors attempted to create a transgenic line that expressed a single canonical olfactory receptor in every single OSN, a challenging technical feat. Their best efforts resulted in a mouse with 5% of OSNs still expressing other receptors. Surprisingly, this mouse has a remarkably mild olfactory phenotype. However it does display similar defects in innate behavior to fully anosmic mice, suggesting that within the 95% of the MOE neurons disrupted lies circuits that mediate pheromones.
- 16.Gruneberg H. A ganglion probably belonging to the N. terminalis system in the nasal mucosa of the mouse. Z Anat Entwicklungsgesch. 1973;140:39–52. [PubMed] [Google Scholar]
- 17.Koos DS, Fraser SE. The Grueneberg ganglion projects to the olfactory bulb. Neuroreport. 2005;16:1929–1932. doi: 10.1097/01.wnr.0000186597.72081.10. [DOI] [PubMed] [Google Scholar]
- 18.Fuss SH, Omura M, Mombaerts P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur J Neurosci. 2005;22:2649–2654. doi: 10.1111/j.1460-9568.2005.04468.x. [DOI] [PubMed] [Google Scholar]
- 19.Greer CA, Stewart WB, Teicher MH, Shepherd GM. Functional development of the olfactory bulb and a unique glomerular complex in the neonatal rat. J Neurosci. 1982;2:1744–1759. doi: 10.1523/JNEUROSCI.02-12-01744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teicher MH, Stewart WB, Kauer JS, Shepherd GM. Suckling pheromone stimulation of a modified glomerular region in the developing rat olfactory bulb revealed by the 2-deoxyglucose method. Brain Res. 1980;194:530–535. doi: 10.1016/0006-8993(80)91237-8. [DOI] [PubMed] [Google Scholar]
- 21••.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]; This study demonstrates that the neurons of a specialized olfactory subsystem respond to volatile stimulii emitted by stressed conspecifics, but not to a range of secretions from unstressed mice. Importantly, the authors convincingly show that alarm stimuli detection exposure elicits a freezing response in other mice, which is selectively abolished when the neurons are lesioned. This study provides strong behavioral evidence that the GG functions in pheromone detection.
- 22.Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 23.Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H. Olfactory receptors and signaling elements in the Grueneberg ganglion. J Neurochem. 2006;98:543–554. doi: 10.1111/j.1471-4159.2006.03894.x. [DOI] [PubMed] [Google Scholar]
- 24.Fleischer J, Mamasuew K, Breer H. Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem Cell Biol. 2009;131:75–88. doi: 10.1007/s00418-008-0514-8. [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Fraser SE, Koos DS. Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. J Comp Neurol. 2009;516:36–48. doi: 10.1002/cne.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinoda K, Shiotani Y, Osawa Y. “Necklace olfactory glomeruli” form unique components of the rat primary olfactory system. J Comp Neurol. 1989;284:362–373. doi: 10.1002/cne.902840304. [DOI] [PubMed] [Google Scholar]
- 27.Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study genetically targeted GC-D, the membrane guanylyl cyclase expressed in sensory neurons that project to the necklace glomeruli. Using a combination of electrophysiology and calcium imaging analysis, they identified two peptide ligands that activate these neurons. These may act as pheromones in mice, but the study was unable to confirm their ethological function, as the deficient mice had no obvious behavioral phenotype.
- 30.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 31•.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]; This study genetically labeled GC-D expressing neurons of the MOE with GFP in order to directly characterize their activation profile. They show these neurons express carbonic anhydrase and detect near-atmospheric concentrations of CO2. Their analysis of wild-type and mutant mice supports behavioral significance for CO2 detection.
- 32•.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci U S A. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the same group describe the molecular mechanism through which CO2 detection occurs. They demonstrate that the intracellular domain of GC-D is directly activated by bicarbonate, explaining how these unusual chemosensory neurons function in the absence of a GPCR.
- 33.Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockerham RE, Margolis FL, Munger SD. Afferent activity to necklace glomeruli is dependent on external stimuli. BMC Res Notes. 2009;2:31. doi: 10.1186/1756-0500-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treloar HB, Feinstein P, Mombaerts P, Greer CA. Specificity of glomerular targeting by olfactory sensory axons. J Neurosci. 2002;22:2469–2477. doi: 10.1523/JNEUROSCI.22-07-02469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cockerham RE, Puche AC, Munger SD. Heterogeneous sensory innervation and extensive intrabulbar connections of olfactory necklace glomeruli. PLoS One. 2009;4:e4657. doi: 10.1371/journal.pone.0004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]; Fifteen years after the landmark description of canonical G-protein coupled receptors underpinning the the molecular basis of olfaction, this study identified a second sub-family of olfactory receptors: the trace amine-associated receptors. These receptors are activated by a number of chemicals that display the hallmarks of pheromones, and are preserved in the genome of many vertebrates, including humans.
- 38.Liberles SD. Trace amine-associated receptors are olfactory receptors in vertebrates. Ann N Y Acad Sci. 2009;1170:168–172. doi: 10.1111/j.1749-6632.2009.04014.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 41••.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]; The authors selectively ablate glomeruli across two spatial zones, functionally dividing the MOB in half. Using odor mapping and behavioral assays they report that glomerular activation in the dorsal zone is both sufficient and necessary for innate, stereotyped “fear” responses to a number of pungent odors. However, these mice can still discriminate these odors from related compounds and be conditioned to avoid them, suggesting the existence of distinct circuits for both innate and learned odor responses.
- 42.Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci U S A. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young JM, Waters H, Dong C, Fulle HJ, Liman ER. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS One. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashiguchi Y, Nishida M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol Biol Evol. 2007;24:2099–2107. doi: 10.1093/molbev/msm140. [DOI] [PubMed] [Google Scholar]
- 45.Hussain A, Saraiva LR, Korsching SI. Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. Proc Natl Acad Sci U S A. 2009;106:4313–4318. doi: 10.1073/pnas.0803229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyart C, Webster WW, Chen JH, Wilson SR, McClary A, Khan RM, Sobel N. Smelling a single component of male sweat alters levels of cortisol in women. J Neurosci. 2007;27:1261–1265. doi: 10.1523/JNEUROSCI.4430-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saxton TK, Lyndon A, Little AC, Roberts SC. Evidence that androstadienone, a putative human chemosignal, modulates women's attributions of men's attractiveness. Horm Behav. 2008;54:597–601. doi: 10.1016/j.yhbeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 48••.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]; In this study 335 human olfactory receptors were screened for androstenone sensitivity. Only one, OR7D4, was found to be robustly and selectively activated by both androstenone and androstadienone, candidate human pheromones. The authors then studied sequence variants of OR7D4 gene that are present in the human population. They found that different alleles encoded receptors that had a range of sensitivities in vitro. Furthermore, receptor genotype strongly correlated with an altered perception of odor quality and intensity that were specific to androstenone and androstadienone.
- 49.Karlson P, Luscher M. Pheromones': a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 50.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 51.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 52.Logan DW, Marton TF, Stowers L. Species specificity in major urinary proteins by parallel evolution. PLoS ONE. 2008;3:e3280. doi: 10.1371/journal.pone.0003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol. 2007;17:1879–1884. doi: 10.1016/j.cub.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 54.Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine JM, Sorensen PW. Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J Chem Ecol. 2008;34:1259–1267. doi: 10.1007/s10886-008-9535-y. [DOI] [PubMed] [Google Scholar]
- 56•.Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci U S A. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]; In these two independent studies, the FPR proteins are identified as a third family of receptors in the VNO of mammals. They are expressed in neurons dispersed throughout the VNO epithelium in a cell-specific manner, and were found to be senstitive to peptides that are formylated by bacteria. Therefore the authors propose that they may function as detectors of pathogenic state.
- 58.Leinders-Zufall T, Ishii T, Mombaerts P, Zufall F, Boehm T. Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat Neurosci. 2009;12:1551–1558. doi: 10.1038/nn.2452. [DOI] [PubMed] [Google Scholar]


