Abstract
Recently, microRNAs (miRNAs) have received increasing attention in the field of cancer research. miRNAs play important roles in many normal biological processes; however, the aberrant miRNA expression and its correlation with the development and progression of cancers is an emerging field. Therefore, miRNAs could be used as biomarkers for diagnosis of cancer and prediction of prognosis. Importantly, some miRNAs could regulate the formation of cancer stem cells and the acquisition of epithelial-mesenchymal transition, which are critically associated with drug resistance. Moreover, some miRNAs could target genes related to drug-sensitivity, resulting in the altered sensitivity of cancer cells to anti-cancer drugs. Emerging evidences have also shown that knock-down or re-expression of specific miRNAs by synthetic antisense oligonucleotides or pre-miRNAs could induce drug sensitivity, leading to increased inhibition of cancer cell growth, invasion, and metastasis. More importantly, recent studies have shown that natural agents including isoflavone, 3,3′-diindolylmethane, and (−)-epigallocatechin-3-gallate altered miRNA expression profiles, leading to an increased sensitivity of cancer cells to conventional therapeutics. These emerging results suggest that specific targeting of miRNAs by different approaches could open new avenues for cancer treatment through overcoming drug resistance and thereby improve the outcome of cancer therapy.
Keywords: microRNA, drug resistance, cancer therapy, isoflavone, DIM, I3C, curcumin, EGCG
1. Introduction
The microRNAs (miRNAs) are a group of small RNAs, which are single-stranded and consist of 19–25 nucleotides (~22 nt). They do not code for any protein or peptide; however, they regulate gene expression by multiple mechanisms. In 1993, the first miRNA, lin-4, was discovered when authors were conducting a genetic analysis in Caenorhabditis elegans (Lee et al., 1993; Wightman et al., 1993). Lee et al identified two lin-4 transcripts of approximately 22 and 61 nt, which contain sequences complementary to a repeated sequence element in the 3′-untranslated region (UTR) of lin-14 mRNA, suggesting that miRNA lin-4 could regulate lin-14 mRNA translation via an antisense RNA-RNA interaction (Lee et al., 1993). Several years later, another important miRNA, let-7, was discovered also in Caenorhabditis elegans (Reinhart et al., 2000). Reinhart et al found that let-7 encodes a temporally regulated 21 nt RNA that is complementary to elements in the 3′-UTR of the heterochronic genes including lin-14, lin-28, lin-41, lin-42 and daf-12 (Reinhart et al., 2000), suggesting that the expression of these genes could be inhibited by let-7. It has been reported that miRNA lin-4 and let-7 could regulate developmental timing by binding and inhibiting the heterochronic genes in Caenorhabditis elegans (Reinhart et al., 2000), suggesting the importance of miRNAs in the process of development. The homologs of the let-7 gene and let-7 miRNA were soon identified in human and other animals (Basyuk et al., 2003; Pasquinelli et al., 2000). In recent years, more miRNAs have been discovered and their biological functions have been investigated (Ambros, 2001; Iorio and Croce, 2009; Vandenboom Ii et al., 2008). It has been found that miRNAs play important roles in the regulation of many physiological and pathological processes in human and animals (Ambros, 2001; Iorio and Croce, 2009; Vandenboom Ii et al., 2008). The basic mechanism of miRNA action is that miRNA could imperfectly bind to the 3′-UTR of target mRNAs, resulting in translational repression or target mRNA cleavage. However, other mechanisms may also exist.
Since miRNAs are involved in the control of the processes of development and differentiation, it is not surprising that miRNAs critically influence the development and progression of cancer. More importantly, recent studies have demonstrated that miRNAs regulate the formation of cancer stem cells (CSCs) (Peter, 2010; Shimono et al., 2009; Wellner et al., 2009) and the acquisition of the epithelial-mesenchymal transition (EMT) phenotype (Adam et al., 2009; Li et al., 2009c), which are critically associated with drug resistance. Experimental evidence also revealed that miRNAs regulate anticancer drug resistance (Zheng et al., 2010), suggesting that targeting specific miRNAs could be a novel therapeutic approach for the treatment of cancers by increasing the drug sensitivity of cancer cells or by eliminating CSCs or EMT-type cells that are typically drug resistant. Recently, it has been reported that miRNA regulation can be influenced by natural chemopreventive agents, leading to increased drug sensitivity and inhibition of tumor cell proliferation (Li et al., 2009c; Zhang et al., 2008). Therefore, targeting miRNA by natural agents could be a novel strategy for cancer therapy, in particular by combining conventional therapeutics with natural chemopreventive agents that are known to be non-toxic to humans.
2. miRNA biogenesis and gene expression
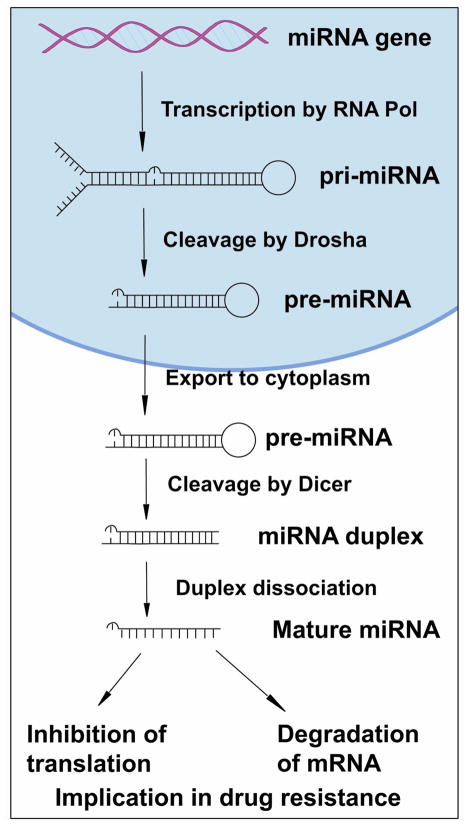
Most miRNA genes are located in intergenic regions more than one kilobase away from annotated genes. miRNA biogenesis (Fig. 1) begins with transcription by either RNA polymerase II or RNA polymerase III, yielding a primary miRNA (pri-miRNA) which is hundreds to thousands of nucleotides in length (Lee et al., 2004; Winter et al., 2009). Human pri-miRNAs usually contain a hairpin stem of 33 base-pairs, a terminal loop, and two single-stranded flanking regions upstream and downstream of the hairpin. In the next step of miRNA biogenesis, pri-miRNA undergoes several cleavages and modifications. First, the pri-miRNA is cleaved by the nuclear microprocessor complex formed by the RNase III (Drosha) and the DGCR8 (DiGeorge critical region 8) protein, yielding the precursor miRNA (pre-miRNA) which is about 70 nucleotides in length (Denli et al., 2004; Gregory et al., 2004; Han et al., 2004; Landthaler et al., 2004; Lee et al., 2002; Lee et al., 2003). Then, the pre-miRNA is transported to the cytoplasm by the nuclear export factor Exportin 5 which binds the pre-miRNA (Lund et al., 2004; Yi et al., 2003). In the cytoplasm, another RNase III (Dicer) in complex with the double-stranded RNA-binding protein TRBP cleaves the pre-miRNA hairpin and yields an RNA duplex about 22 nucleotides in length (Winter et al., 2009). Then, under the mediation of Ago2, the RNA duplex is dissociated to two single strands. One strand is the functional guide strand which is mature miRNA and complementary to the target mRNA (Hammond et al., 2000; Rand et al., 2005; Winter et al., 2009). The other strand is the passenger strand which is degraded. The mature miRNA is then incorporated into the RNA-induced silencing complex (RISC) and guides RISC to target mRNA (Hammond et al., 2000; Rand et al., 2005; Winter et al., 2009). It is commonly accepted that mature miRNAs regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of target mRNA, causing degradation of mRNA or inhibition of their translation to functional proteins (Fig. 1) (Saxena et al., 2003; Winter et al., 2009). However, how the RISC-miRNA complex actually triggers the silencing of mRNA gene expression requires further detailed investigation. In addition, miRNAs also might bind to protein factors required for translation or alter mRNA secondary structure, inhibiting protein translation (Ambros, 2001).
Figure 1.
Biogenesis of miRNA and its effect on gene expression.
3. miRNAs in cancer development and progression
miRNAs play important roles in normal biological processes including cell proliferation, differentiation, apoptotic cell death, stress resistance and physiological metabolism (Ambros, 2001; Ambros, 2003). Experimental and clinical studies have revealed that miRNAs are critically involved in the development and progression of cancers and that aberrant expression of miRNAs has been associated with the stage, progression and metastasis of cancers (Iorio and Croce, 2009; Nicoloso et al., 2009; Vandenboom Ii et al., 2008). During the process of cancer development and progression, miRNAs may function as tumor suppressor genes or oncogenes.
3.1. miRNAs function as oncogenes or tumor suppressors
Some miRNAs have been found to be down-regulated in cancer, suggesting that they could function as tumor suppressor genes (Iorio and Croce, 2009). These miRNAs include let-7, miR-15, miR-16, miR-17-5p, miR-29, miR-34, miR-124a, miR-127, miR-143, miR-145 and miR-181 (Table 1). Among these the let-7 family is the most studied miRNA group with tumor suppressor activity. The expression of let-7 is down-regulated in various cancers and let-7 might be used as biological markers for cancer detection and prognosis (Bussing et al., 2008; Johnson et al., 2007; Lee and Dutta, 2007; Takamizawa et al., 2004). It has been found that miR-15 and miR-16 also have tumor suppressor activity. Transfection with synthetic miR-16 into 22Rv1, Du145, PPC-1 and PC-3M-luc prostate cancer cells significantly reduced proliferation of prostate cancer cells (Takeshita et al., 2009). Also miR-16 inhibits prostate cancer growth by down-regulation of CDK1 and CDK2, which are associated with cell cycle control and cell proliferation (Takeshita et al., 2009).
Table 1.
The list of miRNAs which have oncogenic or tumor suppressor activity.
| miRNA | Tested in tumors | Alteration | Targeted genes | References |
|---|---|---|---|---|
| Oncogenic activity | ||||
| miR-21 | Colon, breast, pancreatic, lung, prostate, liver, and gastric cancers | Increase | PTEN, TPM1, Pdcd4, maspin | (Chan et al., 2005; Iorio et al., 2005; Li et al., 2009a; Ribas et al., 2009; Si et al., 2007; Yao et al., 2009) |
| miR-155 | Lung and breast cancers, CLL*, AML* | Increase | AT1R, TP53INP1 | (Gironella et al., 2007; Greither et al., 2009; Habbe et al., 2009; Iorio et al., 2005) |
| miR-17–92 | Lung, breast, colon, gastric, pancreatic cancers, lymphomas | Increase | Tsp1, CTGF, E2F1, AIB1, TGFBR2 | (Diosdado et al., 2009; He et al., 2005; Manni et al., 2009; Northcott et al., 2009; Uziel et al., 2009) |
| miR-106a | Colon and gastric cancers | Increase | RB-1 | (Diaz et al., 2008; Xiao et al., 2009) |
| miR-373 | Testicular tumor, Gastric cancer | Increase | LATS2 | (Lee et al., 2009) |
| miR-197 | Lung cancer | Increase | ACVR1, TSPAN3, FUS1 | (Du et al., 2009) |
| miR-221 | Breast and liver cancers, CLL | Increase | KIT, p27(Kip1), p57, PTEN | (Park et al., 2009a) |
| miR-222 | Breast and liver cancers, CLL | Increase | KIT, p27(Kip1), p57, PTEN | (Greither et al., 2009) |
| miR-372 | Testicular tumor | Increase | LATS2 | (Voorhoeve et al., 2007) |
| Tumor suppressor activity | ||||
| let-7 | Lung, ovarian cancer, breast, and colorectal cancers | Decrease | RAS, PRDM1, HMGA2, c-Myc, E2F, cyclin D2 | (Johnson et al., 2007; Johnson et al., 2005; Lee and Dutta, 2007; Takamizawa et al., 2004) |
| miR-15 miR-16 |
Gastric and lung cancers | Decrease | Bcl-2, Wt-1 | (Calin et al., 2008; Cimmino et al., 2005; Takeshita et al., 2009) |
| miR-34 | Breast, pancreatic, colon cancers | Decrease | E2F3, Notch1, CDK4, CDK5 | (Chang et al., 2007; Corney et al., 2007; Ji et al., 2009b) |
| miR-17-5p | Breast cancer, CLL* | Decrease | AIB1, E2F1, p21, BIM | (Mraz et al., 2009; Robertus et al., 2009) |
| miR-29 | Lung and breast cancers, CLL*, AML* | Decrease | MCL-1, TCL-1, DNMT3s | (Mraz et al., 2009; Park et al., 2009b) |
| miR-124a | ALL*, medulloblastoma | Decrease | CDK6 | (Agirre et al., 2009; Pierson et al., 2008) |
| miR-127 | Breast cancer, lymphoma | Decrease | Bcl-6 | (Robertus et al., 2009) |
| miR-143 | Gastric and prostate cancers | Decrease | Ras, ERK5 | (Chen et al., 2009b; Clape et al., 2009; Takagi et al., 2009) |
| miR-145 | Breast and gastric cancers | Decrease | Mucin1, ERG | (Sachdeva and Mo, 2010; Spizzo et al., 2010; Takagi et al., 2009) |
| miR-181 | Colorectal cancer | Decrease | TCL-1, E2F5, eIF5A | (Pekarsky et al., 2006) |
| miR – 146a | Pancreatic cancer | Decrease | IRAK-1, EGFR | (Li et al., 2010) |
CLL, chronic lymphoblastic leukemia; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia.
miR-34 is another important miRNA with tumor suppressor activity. miR-34a can be directly transactivated by p53 (Chang et al., 2007) and its upregulation resulted in increased apoptosis and altered the expression of genes related to cell cycle progression, apoptosis and angiogenesis (Chang et al., 2007).
Other miRNAs have been found to be upregulated in tumor cells, suggesting that they might possess oncogenic activity. The known oncogenic miRNAs include miR-21, miR-155, miR-221, miR-222 and miR-17–92 (Table 1). Knock-down of miR-21 (Chan et al., 2005; Ribas et al., 2009; Seike et al., 2009; Si et al., 2007) in breast cancer cells led to the inhibition of cell proliferation, down-regulation of Bcl-2 and induction of apoptosis in vitro and in vivo (Si et al., 2007). The miR-17–92 cluster consisting of miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92-1 also showed oncogenic activity in various cancers (Diosdado et al., 2009; He et al., 2005; Manni et al., 2009). Animal studies have demonstrated that forced expression of the miR-17–92 cluster and c-myc could accelerate tumor development in a mouse B-cell lymphoma model (He et al., 2005). The expression of miR-155 was also increased in various cancers (Greither et al., 2009; Habbe et al., 2009). A significant correlation between elevated miR-155 expression and low overall survival (p = 0.005) in pancreatic cancers was observed (Greither et al., 2009). Moreover, patients with elevated miR-155 expression level in tumor tissue had a 6.2-fold increased risk of tumor-related death compared to patients with lower expression of miR-155 (Greither et al., 2009).
3.2. miRNAs and EMT
miRNAs also control embryonic stem cell differentiation (Dirks, 2009; Lin et al., 2009; Wang et al., 2009b) and recently, miRNAs have been found to be involved in the acquisition and maintenance of CSCs and EMT-type cells, which may play important roles in drug resistance and metastasis (Adam et al., 2009; Garzia et al., 2009; Gibbons et al., 2009; Gregory et al., 2008b; Gregory et al., 2008a; Ji et al., 2009a; Ji et al., 2009b; Kong et al., 2009; Sabbah et al., 2008; Shimono et al., 2009). Recent studies suggested that glioma stem cells caused drug resistance and that miR-125b was critical for the suppression of human U251 glioma stem cell proliferation (Shi et al., 2010); This suggests that up-regulation of miR-125b may increase drug sensitivity by inhibiting glioma stem cell proliferation. miR-34 is activated by p53 and thus functioning as a tumor suppressor. miR-34 targets Notch, HMGA2 and Bcl-2, which are involved in the self-renewal process and survival of CSCs. Transfection of human gastric cancer Kato III cells with miR-34 could reduce the expression of Bcl-2 and chemosensitize Kato III cells (Ji et al., 2008). Re-expression of miR-34 also inhibited tumor sphere formation and growth (Ji et al., 2008), suggesting inhibitory effects on the self-renewal of CSCs.
Recently, we have investigated the expression levels of miR-200 and let-7 in EMT phenotypic pancreatic cancer cells (Li et al., 2009c). We found that miR-200b, miR-200c, and miR-200a were down-regulated in gemcitabine-resistant cells with an EMT phenotype. Many members of the let-7 family were also down-regulated in EMT-type gemcitabine-resistant cells. Moreover, we found that the re-expression of miR-200 family in gemcitabine-resistant cells resulted in the up-regulation of the epithelial marker E-cadherin and down-regulation of mesenchymal markers including ZEB1 and vimentin both at the mRNA and protein levels (Li et al., 2009c). After 14 days of transfection, the morphology of miR-200 transfected gemcitabine-resistant cells was partially changed from elongated fibroblastoid to epithelial cobblestone-like appearance (Li et al., 2009c). These results suggested that the loss of the miR-200 family is critical for the acquisition of EMT characteristics. Moreover, we have also found that re-expression of miR-200b in platelet-derived growth factor (PDGF)-D overexpressing EMT-type cells led to the reversal of EMT with the down-regulation of ZEB1, ZEB2 and Slug expression and the inhibition of cell invasion (Kong et al., 2009). Other investigators also reported that the expression of miR-200 was tightly associated with the epithelial phenotype and sensitivity to EGFR inhibitors-induced growth inhibition in bladder carcinoma cell lines (Adam et al., 2009).
4. The role miRNAs as diagnostic and prognostic markers in human cancers
In vitro and in vivo studies have suggested that miRNAs might be useful as diagnostic and prognostic markers. Recent data suggest that miRNA profiling can be used for tumor typing. Blenkiron, et al. examined miRNA expression in 93 primary human breast tumors using a flow cytometric miRNA expression profiling method (Blenkiron et al., 2007). They classified the breast tumors as luminal A, luminal B, basal-like, HER2+ and normal-like based on the miRNA expression profiling. Further analysis showed that miRNAs were differentially expressed between these tumor-subtypes, and that some of the miRNAs were associated with clinicopathological features. The expression of miRNAs could also be used to classify basal versus luminal tumor-subtypes (Blenkiron et al., 2007), providing additional information for pathological diagnosis. Porkka, et al. reported miRNA expression profiling for different types of prostate tumor and prostate cancer cell lines. They found differential expression of 51 miRNAs between benign tumors and carcinomas. Importantly, they were able to successfully distinguish prostate carcinoma from benign prostatic hyperplasia by miRNA expression clustering analysis (Porkka et al., 2007). Other investigators also found differential miRNA expression profiles between normal tissues and cancers (Lee et al., 2007; Mattie et al., 2006; Raponi et al., 2009; Yang et al., 2008a).
Recent evidence suggests that alterations in miRNA expression might be used as markers for prediction of cancer prognosis (Raponi et al., 2009; Wang et al., 2009a). In lung squamous cell carcinoma, several miRNAs including miR-155, let-7 and miR-146a have been found to have prognostic value (Raponi et al., 2009). Among them, miR-146b alone was found to have the strongest prediction accuracy for stratifying prognostic groups. The miRNA signatures were also used in predicting overall survival. In lung adenocarcinomas, high miR-155 and low let-7a expression correlated with poor survival (Yanaihara et al., 2006). In breast cancer, nine miRNAs including miR-21, miR-365, miR-181b, let-7f, miR-155, miR-29b, miR-181d, miR-98 and miR-29c were up-regulated whereas miR-497, miR-31, miR-355, miR-320, miR-127 and miR-30a-3p were down-regulated compared to normal adjacent tissue (Yan et al., 2008). The most significantly up-regulated miRNA was miR-21, which correlated with advanced tumor stage, lymph node metastasis and poor survival of the patients, suggesting that miR-21 could serve as a molecular prognostic marker for breast cancer aggressiveness (Yan et al., 2008). In pancreatic cancer, up-regulation of miR-21 was strongly associated with high Ki67 proliferation index and liver metastasis (Roldo et al., 2006), whereas miR-196a-2 showed predictive value in the survival of patients diagnosed with pancreatic cancer (Bloomston et al., 2007). Although these data support the idea that miRNA expression signatures could be used as prognostic markers for human cancers, none have been used as yet for prediction of the therapeutic response in patients.
It is interesting to note that miRNAs are maintained in a protected state in serum and plasma. The miRNAs in plasma were found to be packaged inside exosomes that are secreted from cells; therefore, they are protected from endogenous RNase activity (Mitchell et al., 2008). This property of miRNAs allows the detection of miRNA expression directly from human serum, increasing the value of miRNA expression profiling in diagnosis of cancer and prediction of prognosis.
5. The role of miRNAs in drug resistance
Chemotherapy is an important therapeutic strategy for cancer treatment. However, chemotherapy fails to eliminate all tumor cells because of intrinsic or acquired drug resistance, which is the most common cause of tumor recurrence (Broxterman et al., 2009; Fojo, 2007). Recent studies have suggested altered expression of specific miRNAs in drug resistant tumor cells.
5.1. miRNAs and chemotherapy resistance
miRNAs play important roles in the regulation of drug sensitivity (Table 2). The expression of three miRNAs (miR-200b, miR-194 and miR-212) was significantly down-regulated while the expression of three other miRNAs (miR-192, miR-424 and miR-98) were significantly up-regulated in docetaxel resistant SPC-A1 non-small cell lung cancer (NSCLC) cells (Rui et al., 2009), suggesting differential miRNA expression patterns between docetaxel resistant and sensitive lung cancers. The expression of miR-140 was associated with chemosensitivity to methotrexate and 5-fluorouracil (5-FU) in osteosarcoma tumor xenografts (Song et al., 2009). To confirm this, an in vitro study was conducted. Tumor cells transfected with miR-140 proved to be more resistant to methotrexate and 5-FU, whereas blocking endogenous miR-140 partially sensitized resistant colon CSC-like cells to 5-FU treatment, suggesting that miR-140 could be a candidate target to develop novel therapeutic strategy to overcome drug resistance (Song et al., 2009). Another study determined that 5-FU and oxaliplatin (L-OHP) down-regulated the expression of miR-197, miR-191, miR-92a, miR-93, miR-222 and miR-1826 in HCT-8 and HCT-116 colon cancer cells (Zhou et al., 2010). A recent study showed that miR-181a and miR-630 regulated cisplatin-induced cancer cell death in NSCLC cells (Galluzzi et al., 2010). In a clinical study, in which 57 patients with ovarian cancer were received surgical therapy and platinum-based chemotherapy (Eitan et al., 2009), miRNA expression profiles of tumor samples were assessed. Seven miRNAs were found to be significantly, differentially expressed in tumors from platinum-sensitive vs. platinum-resistant patients. These seven miRNAs included miR-27a, miR-23a, miR-30c, let-7g, miR-199a-3p, miR-378 and miR-625, which were overexpressed in platinum-resistant patients (Eitan et al., 2009).
Table 2.
The list of miRNAs which regulate drug sensitivity.
| miRNA | Status, biological effect, or regulation | Target genes | Cell or tissue | References |
|---|---|---|---|---|
| miR-125b | Suppression of human glioma stem cell through cell cycle arrest at the G1/S | CDK6, CDC25A | Glioma stem cells | (Shi et al., 2010) |
| miR-192, miR-424, miR-98 | Up-regulated in resistant cells | Docetaxel-resistant human NSCLC cells | (Rui et al., 2009) | |
| miR-26a, miR-181a | 17β-estradiol repressed miR-26a and miR-181a | Breast carcinomas | (Maillot et al., 2009) | |
| miR-128b, miR-221 | Down-regulated in resistant cells. Reexpression of these miRNAs sensitized cancer cells to glucocorticoids. | MLL, AF4, MLL-AF4, AF4-MLL, CDKN1B | Acute lymphocytic leukemia cells | (Kotani et al., 2009) |
| miR-140 | Up-regulated in colon cancer stem-like chemoresistance cells. Blocking miR-140 sensitized resistant colon cancer stem-like cells to 5-FU. |
HDAC4 | Osteosarcoma tumor xenografts | (Song et al., 2009) |
| miR-200 | miR-200 controled the EMT process and sensitivity to EGFR therapy | ERRFI-1 | bladder cancer cells | (Adam et al., 2009) |
| miR-200c | miR-200c restored chemosensitivity through inhibition of TUBB3. | ZEB1, ZEB2, TUBB3 | endometrial, breast, and ovarian cancer cells | (Cochrane et al., 2009) |
| miR-200b, miR-200c, let-7b, let-7c, let-7d, and let-7e | Reexpression of miR-200 resulted in the down- regulation of ZEB1, slug, and vimentin, and increased cell sensitivity to gemcitabine. | ZEB1 | pancreatic cancer | (Li et al., 2009c) |
| miR-200b, miR-194, miR-212 | Down-regulated in resistant cells | Docetaxel-resistant human NSCLC cells | (Rui et al., 2009) | |
| miR-21 | miR-21 inhibited tumor suppressor protein PDCD4. | PDCD4 | Breast cancer | (Bourguignon et al., 2009) |
| miR-21 | miR-21 mediated chemoresistance to VM-26 in glioblastoma cells.. | LRRFIP1 | Glioblastoma cells. | (Li et al., 2009b) |
| miR-21, miR-342, miR-489 | Down-regulated in the resistant OHT(R) cells | MCF-7 sensitive and resistant cells | (Miller et al., 2008) | |
| miR-125b | miRNA-125b had the ability of rendering LNCaP cells resistant to androgen withdrawal. | Prostate cancer | (Vere White et al., 2009) | |
| miR-205 | miR-205 increased responsiveness to Gefitinib and Lapatinib. | Breast cancer cells | (Iorio et al., 2009) | |
| miR-328 | miR-328 influenced drug disposition in human breast cancer cells | ABCG2 | Breast cancer cells | (Pan et al., 2009) |
| let-7i | Reduced let-7i expression increased the resistance of ovarian and breast cancer cells to the chemotherapy. | Ovarian and breast cancer cells | (Yang et al., 2008b) | |
| let-7i, miR-16, miR-21 | Cellular levels of let-7i, miR-16, and miR-21 affected the potencies of anticancer agents. | NCI-60 human cancer cell lines | (Blower et al., 2008) | |
| miR-34a | Ectopic miR-34a expression resulted in attenuated chemoresistance to camptothecin | SIRT1. | Prostate cancer | (Fujita et al., 2008) |
| miR-34 | miR-34 mediated suppression of self-renewal. | Bcl-2, Notch, HMGA2 | Gastric cancer | (Ji et al., 2008) |
| miR-34a, miR-148a | Down-regulated in MCF-7/AdrVp drug resistant cells | MCF-7/AdrVp cells | (Chen et al., 2009a) | |
| miR-30c, miR-130a, miR-335 | Downregulated in all the resistant cell lines. | M-CSF | paclitaxel- and cisplatin-resistant ovarian cells | (Sorrentino et al., 2008) |
| miR-221, miR-222 | Knockdown of miR-221 and/or miR-222 sensitized cancer cells to tamoxifen. | ERα | MCF-7 and T47D breast cancer cells | (Zhao et al., 2008) |
| miR-221, miR-222 | Transfection with anti-miR-221 and -222 rendered resistant cells sensitive to TRAIL. | Kit, p27kip1 | non-small cell lung cancer cells | (Garofalo et al., 2008) |
| miR-221, miR-222, miR-181 | Up-regulated in the resistant OHT(R) cells | p27Kip1 | MCF-7 sensitive and resistant cells | (Miller et al., 2008) |
| miR-521 | miR-521 mimic sensitized prostate cancer cells to radiation treatment. | CSA | Prostate cancer cells | (Josson et al., 2008) |
| miR-451 | Transfection with miR-451 resulted in the increased sensitivity of cells to DOX, | MDR1 | MCF-7/DOX- resistant cells | (Kovalchuk et al., 2008) |
| miR-451, miR-27a | Up-regulated in multidrug resistant cancer cell lines A2780DX5 and KB-V1 | MDR | MDR resistant cells | (Zhu et al., 2008) |
| miR-17-5p | Antagomir-17-5p abolished the growth of therapy-resistant neuroblastoma, | p21, BIM | Therapy-resistant neuroblastoma | (Fontana et al., 2008) |
| miR-15b, miR-16 | Overexpression of miR-15b or miR-16 sensitized cancer cells to anticancer drugs. | Bcl-2 | Multidrug-resistant gastric cancer cells | (Xia et al., 2008) |
| miR-214 | miR-214 induced cisplatin resistance through targeting PTEN. | PTEN | ovarian cancer cells | (Yang et al., 2008a) |
| miR-181a, miR-630 | miR-181a enhanced and miR-630 decreased cisplatin-induced apoptosis | A549 NSCLC cells | (Galluzzi et al., 2010) |
5.2. miRNA-21 and drug resistance
As indicated previously miR-21 is a miRNA with oncogenic activity. In breast cancer, it was recently found that miR-21 was overexpressed while the tumor suppressor protein PDCD4 was down-regulated (Bourguignon et al., 2009). These alterations caused up-regulation of inhibitors of apoptosis proteins (IAPs) and multidrug-resistant protein 1 (MDR1), leading to anti-apoptosis and chemotherapy resistance. Interestingly, transfection of MCF-7 cells with a specific anti-miR-21 sensitized the cells to undergo apoptotic cell death, suggesting that this strategy could overcome chemotherapy resistance in breast cancer cells (Bourguignon et al., 2009). Over-expression of miR-21 was also found in glioblastoma, which contributed to drug resistance since suppression of miR-21 by specific antisense oligonucleotides led to increased cytotoxic effect of a semisynthetic podophyllotoxin derivative (VM-26) against U373 MG glioblastoma cells (Li et al., 2009b). Moreover, the miRNA expression profiles of MCF-7/AdrVp (doxorubicin and verapamil resistance) and MCF-7 parent cells were compared and it was found that the levels of miR-21, let-7i and miR-141 were significantly upregulated and that miR-34a and miR-148a were down-regulated in MCF-7/AdrVp cells (Chen et al., 2009a). These results suggest that miR-21 is a critical factor involved in drug resistance and that down-regulation of this miRNA might be useful to overcome drug resistance.
5.3. miR-221 and miR-222 in anti-estrogen and in TRAIL resistance
MiR-221 and miR-222 are oncogenic miRNAs and might also contribute to drug resistance. When miRNA and mRNA expression patterns were compared between the anti-estrogen fulvestrant resistant MCF7-FR cells and the drug-sensitive parent MCF7 cells, it was found that two miRNA (miR-221 and miR-222) were upregulated, whereas 14 miRNAs (including let-7i, miR-181a, miR-638, miR-204, miR-191, miR-346, miR-212, miR-328, miR-211 and miR-424) were down-regulated in MCF7-FR cells, suggesting a role of these miRNAs in anti-estrogen resistance (Xin et al., 2009). miRNA-221 and miR-222 also appeared to negatively regulate the estrogen receptor alpha (ERα) expression by direct binding to 3′-UTR of ERα and this regulation was associated with tamoxifen resistance in breast cancer (Zhao et al., 2008). In addition, miR-221 and/or miR-222 transfected MCF-7 and T47D cells became resistant to tamoxifen and knock-down of miR-221 and/or miR-222 was found to sensitize MDA-MB-468 cells to tamoxifen-induced cell growth arrest and apoptosis (Zhao et al., 2008).
Another study showed that miR-221, miR-222 and miR-181 were upregulated while miR-21, miR-342, and miR-489 were down-regulated in tamoxifen-resistant MCF-7 cells (Miller et al., 2008). Ectopic expression of miR-221 or miR-222 rendered the parent MCF-7 cells resistant to tamoxifen through inhibiting their target p27Kip1, which was reduced by 50% in resistant cells (Miller et al., 2008). In addition to breast cancer, an increase of miR-221 and miR-222 also correlated with TRAIL resistance in NSCLC cells (Garofalo et al., 2008). Transfection with anti-miR-221 or anti-miR-222 rendered CALU-1-resistant cells sensitive to TRAIL and TRAIL-sensitive H460 cells treated with miR-221 and miR-222 pre-miRNAs became resistant to TRAIL. It is known that miR-221 and miR-222 target the 3′-UTR of both Kit and p27kip1 mRNAs; however, these miRNAs mainly targeted p27kip1 to regulate TRAIL signaling (Garofalo et al., 2008). In addition, controversial results have also been reported showing that the expression of miR-221 and miR-128b was down-regulated in MLL-AF4 ALL (Kotani et al., 2009) and re-expression of miR-128b and miR-221 cooperatively sensitized MLL-AF4 ALL cells to glucocorticoids (Kotani et al., 2009), suggesting that more investigations on miR-221 in drug resistance are needed.
5.4. miRNA let-7 family and drug resistance
A study in ovarian cancer showed that let-7i expression was significantly reduced in chemotherapy-resistant patients (n = 69, P = 0.003) and the decreased let-7i expression was significantly associated with shorter progression-free survival of patients diagnosed with late-stage ovarian cancer (Yang et al., 2008b). Furthermore, in vitro reduction of let-7i expression was associated with resistance of ovarian and breast cancer cells to cisplatin, suggesting that let-7i could be useful as a therapeutic target to modulate platinum-based chemotherapy and perhaps as a biomarker to predict chemotherapy response and survival of ovarian cancer patients (Yang et al., 2008b). However, a contradictory role of let-7 in drug resistance has been reported. In the NCI-60 human cancer cell lines (Blower et al., 2008) a change of the cellular levels of let-7i (and miR-16 and miR-21 as well) by pre-miRNA or miRNA inhibitor transfection affected the potencies of a number of the anticancer agents by up to 4-fold. Let-7i inhibition increased the sensitivity of A549 cells to NSC 670550. Similarly, let-7e expression was up-regulated in one resistant ovarian cancer cell line while it was down-regulated in another resistant cell line (Sorrentino et al., 2008).
5.5. miRNAs and therapy resistance in prostate cancer
The miRNA expression profiles between androgen responsive and castrate resistant prostate cancer cell lines were compared and found to be different. There were 17 differentially expressed miRNAs with 10 up-regulated and 7 down-regulated. Among these, miRNA-125b was found to have the ability of rendering LNCaP cells resistant to androgen withdrawal (Vere White et al., 2009).
In addition, miRNAs are also known to play important roles in the response to cellular stress caused by radiotherapy. For instance, the miR-521 mimetic significantly sensitized prostate cancer cells to radiation treatment, while radiation treatment down-regulated the levels of miR-521 and up-regulated cockayne syndrome protein A (CSA) protein (Josson et al., 2008), which plays an important role in the radio-sensitivity of prostate cancer cell lines. Therefore, regulating miRNAs could be a novel strategy for enhancing the effect of radiation treatment on prostate cancer cells.
5.6. Other miRNAs involved in drug resistance
miR-34a is down-regulated in drug resistant prostate cancer cells and ectopic miR-34a expression resulted in cell cycle arrest and growth inhibition and attenuated chemoresistance to the anticancer drug camptothecin (Fujita et al., 2008). miR-205, which targets the HER3 receptor was down-regulated in breast tumors compared with normal breast tissue. The reintroduction of miR-205 in SKBR-3 breast cancer cells inhibited their clonogenic potential and increased the responsiveness to the tyrosine kinase inhibitors of EGFR, gefitinib, and of EGFR/HER2, lapatinib, abrogating HER3-mediated resistance (Iorio et al., 2009).
Another miRNA, namely miR-328 targets the 3′-UTR of the drug transporter gene ABCG2, since transfection of miR-328 into drug resistant MCF-7/MX100 cells downregulated ABCG2 3′-UTR-luciferase activity and ABCG2 expression, resulting in increased mitoxantrone sensitivity (Pan et al., 2009). Additionally, the expression of miR-15 and miR-16 was down-regulated in the multidrug-resistant gastric cancer cell line SGC7901/VCR and in vitro it was demonstrated that forced over-expression of miR-15b or miR-16 sensitized SGC7901/VCR cells again to anticancer drugs by reducing Bcl-2 protein in SGC7901/VCR cells, suggesting that Bcl-2 is a direct target of miR-15b and miR-16 (Xia et al., 2008). Also, miR-451 was found to down-regulate the expression of the MDR1 (P-glycoprotein) gene and transfection of the MCF-7/DOX-resistant cells with miR-451 resulted in increased sensitivity of cells to doxorubicin (Kovalchuk et al., 2008).
It was reported that miR-200c levels were high in well-differentiated endometrial, breast and ovarian cancer cell lines, but extremely low in poorly differentiated cancer cells (Cochrane et al., 2009) and that high expression of TUBB3, one of the miR-200c target genes, was a common mechanism of resistance to microtubule-binding agents in many solid tumors. Importantly, re-expression of miR-200c inhibited TUBB3 expression and increased the sensitivity to microtubule-targeting agents by 85% (Cochrane et al., 2009). It was found that miR-214 induced cell survival and cisplatin resistance through targeting the 3′-UTR of the PTEN, leading to down-regulation of PTEN protein, activation of the Akt pathway and drug resistance in human ovarian cancer (Yang et al., 2008a). Collectively, these results suggest that miR-34a, miR-205, miR-200, miR-328, miR-15b, miR-16, miR-214 and miR-451 might play important roles in sensitization of tumor cells to different classes of anti-cancer drugs.
6. Targeting miRNAs to increase drug sensitivity
Targeting miRNAs for cancer therapy is an emerging field for treatment optimization aiming to enhance inhibition of cancer cell proliferation and/or to increase the sensitivity to conventional chemotherapy. Strategies to regulate miRNA expression in cancers include inactivation of oncogenic miRNAs, activation of tumor suppressor miRNAs, and targeting specific miRNAs to restore drug sensitivity. Experimental studies have shown that anti-sense oligonucleotides can block the function of miRNAs (Hutvagner et al., 2004; Meister et al., 2004; Orom et al., 2006). Importantly, in vivo studies have shown that intravenous administration of anti-sense oligonucleotides against miR-16, miR-122, miR-192 and miR-194 caused a significant reduction in the expression of the corresponding miRNA levels in different tissues (Krutzfeldt et al., 2005), demonstrating the possibility of delivery of anti-sense oligonucleotides in vivo. These results suggest that silencing of specific oncogenic miRNAs in vivo could be a novel therapeutic strategy for cancer treatment. In addition, re-expression of tumor suppressor miRNAs likewise could be another important strategy for cancer treatment. Up-regulation of let-7 tumor suppressor miRNA by pre-let-7 transfection led to the inhibition of proliferation of lung and liver tumor cells in vitro (Johnson et al., 2007), suggesting the value of restoring tumor suppressor miRNAs in cancer treatment. However, major limitations for optimal use of synthetic oligonucleotides have to be overcome, because they are easily degraded as well as due to the lack of appropriate in vivo delivery systems.
In order to overcome such limitations, recent studies have shown that natural agents including isoflavone, 3,3′-diinodolylmethane (DIM), indole-3-carbinol (I3C), curcumin, (−)-epigallocatechin-3-gallate (EGCG) and others could alter the expression of specific miRNAs (Li et al., 2009c; Melkamu et al., 2009; Sun et al., 2008; Tsang and Kwok, 2009; Li et al., 2010). Considering the relatively non-toxic characteristics of natural agents, targeting of miRNAs by these natural agents combined with conventional chemotherapy could be a novel and safer approach for achieving better treatment outcome.
Recently, we have focused our attention on designing better treatment strategies for pancreatic cancer. It is well-known that the aggressiveness of pancreatic cancer is in part due to its drug resistance, which is partly associated with pancreatic CSCs and the acquisition of an EMT phenotype. Therefore, we investigated the effects of isoflavone and DIM on miRNAs in pancreatic cancer cells that are gemcitabine-resistant and have an EMT phenotype. We found that re-expression of miR-200 by pre-miR-200 transfection or treatment of gemcitabine-resistant cells with isoflavone or DIM resulted in the up-regulation of miR-200 and the down-regulation of ZEB1, slug and vimentin, which was consistent with a morphologic reversal of the EMT phenotype leading to an epithelial cobblestone-like morphology (Li et al., 2009c). Isoflavone and DIM also induced the expression of let-7 (Li et al., 2009c), which could be mechanistically linked to the treatment effects. Importantly, we found that miR-200 re-expression or isoflavone and DIM treatment increased sensitivity of gemcitabine-resistant pancreatic cells to gemcitabine. The resistant cells transfected with miR-200b showed 20.8% – 38.2% more growth inhibition by gemcitabine treatment. The resistant cells pre-treated with DIM showed 14.8% – 17.4% more inhibition while the cells pre-treated with isoflavone showed 15.4% – 17.1% more inhibition (Li et al., 2009c). Therefore, conventional chemotherapy combined with isoflavone or DIM could be a novel strategy for more optimal treatment of pancreatic cancer.
Interestingly, Sun et al. reported that another chemopreventive agent, curcumin, also altered miRNA expression profiles in pancreatic tumor cells (Sun et al., 2008). They found that up-regulation of miR-22 expression by curcumin or by transfection with pre-miR-22s suppressed the expression of its target genes SP1 transcription factor and estrogen receptor 1 (Sun et al., 2008). Another natural agent, I3C, down-regulated miR-21 and up-regulated miR-21 target genes PTEN, PDCD4, and RECK (Melkamu et al., 2009), thus, targeting miR-21 by I3C could be a novel strategy to increase drug sensitivity. Tsang, et al. recently reported the effects of EGCG on the expression of miRNAs in human tumor cells (Tsang and Kwok, 2009). They found that miR-16 was up-regulated and Bcl-2 was down-regulated by EGCG. Since miR-16 could sensitize cancer cells to anti-cancer drugs, EGCG could increase drug sensitivity through up-regulation of miR-16.
So far, there is no report on clinical trials which utilize the miRNA approach to regulate drug sensitivity for cancer therapy. However, several phase II clinical trials are being or will be conducted to investigate differential miRNA expression profiles after cancer patients receive different chemotherapy and radiotherapy regimens (www.ClinicalTrials.gov). The anticancer drugs used in those clinical trials include cisplatin, paclitaxel, carboplatin, erlotinib, docetaxel and cetuximab for NSCLC, azacytidine and bortezomib for relapsed or refractory acute myeloid leukemia, and melphalan, prednisone, thalidomide, lenalidomide, and dexamethasone for myeloma (www.ClinicalTrials.gov). By comparison of miRNA profiles, the specific miRNAs that are critically involved in drug resistance might be identified opening new avenues for the design of novel and targeted therapeutic strategies for improving the treatment outcome of patients and we optimistically believe that such strategies are within reach.
7. Conclusions and perspectives
In conclusion, recent evidence demonstrates that miRNAs may play important roles in the regulation of anticancer drug sensitivity and resistance. Aberrant miRNA expression can reduce the response of cancer cells to anti-cancer agents such as gemcitabine, docetaxel, methotrexate, 5-fluorouracil and tamoxifen. Thus, targeting specific miRNAs is an emerging strategy to increase the sensitivity of cancer cells to anti-cancer drugs.
Interestingly, natural agents such as isoflavone, DIM, I3C, curcumin, EGCG or other unexplored “natural agents” might be very useful for the targeting of miRNAs. Therefore, targeting miRNAs by natural agents could open new avenues toward more successful treatment of cancer by eliminating CSCs or EMT-type cells or increasing the drug sensitivity in general. Strikingly, in addition to the specific miRNAs which alter drug sensitivity, several studies have implicated other non-coding RNAs, the vault RNAs, as a regulator of drug resistance (Persson et al., 2009). Vault particles are conserved organelles that in certain tumor cells correlate with drug resistance development via unknown mechanisms (Broxterman et al., 1999). Human vault RNAs (vRNAs) produce several small RNAs (svRNAs) that may regulate gene expression similar to miRNAs. It has been found that svRNAb down-regulates CYP3A4 expression, a key enzyme in drug metabolism, to alter drug resistance (Persson et al., 2009), suggesting that this newly discovered small molecule may be responsible for part of the in vivo drug resistance similar to miRNAs. Therefore much remains to be discovered on the regulation of anti-cancer drug resistance and exciting possibilities to target these small RNAs for the development of new therapeutics are coming forward.
Acknowledgments
The authors’ work cited in this review article was funded by grants from the National Cancer Institute, NIH (5R01CA083695, 2R01CA108535, 5R01CA131151, 3R01CA131151-02S109, and 1R01CA132794 awarded to FHS), and a sub-contract award to FHS from the University of Texas MD Anderson Cancer Center through SPORE grant (5P20-CA101936, 3P20CA101936-05S109) on pancreatic cancer awarded to James Abbruzzese. We also thank Puschelberg and Guido foundations for their generous contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, Bar-Eli M, Dinney C. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, Martin-Subero JI, Cordeu L, Garate L, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res. 2009;69:4443–4453. doi: 10.1158/0008-5472.CAN-08-4025. [DOI] [PubMed] [Google Scholar]
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Basyuk E, Suavet F, Doglio A, Bordonne R, Bertrand E. Human let-7 stem-loop precursors harbor features of RNase III cleavage products. Nucleic Acids Res. 2003;31:6593–6597. doi: 10.1093/nar/gkg855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavare S, Caldas C, Miska EA. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W. MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther. 2008;7:1–9. doi: 10.1158/1535-7163.MCT-07-0573. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman HJ, Gotink KJ, Verheul HM. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Updat. 2009;12:114–126. doi: 10.1016/j.drup.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Broxterman HJ, Sonneveld P, Pieters R, Lankelma J, Eekman CA, Loonen AH, Schoester M, Ossenkoppele GJ, Lowenberg B, Pinedo HM, Schuurhuis GJ. Do P-glycoprotein and major vault protein (MVP/LRP) expression correlate with in vitro daunorubicin resistance in acute myeloid leukemia? Leukemia. 1999;13:258–265. doi: 10.1038/sj.leu.2401331. [DOI] [PubMed] [Google Scholar]
- Bussing I, Slack FJ, Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400–409. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Zhao ZW, Zhou HY, Liu YJ, Yang HJ. Systematic analysis of microRNA involved in resistance of the MCF-7 human breast cancer cell to doxorubicin. Med Oncol. 2009a doi: 10.1007/s12032-009-9225-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009b;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clape C, Fritz V, Henriquet C, Apparailly F, Fernandez PL, Iborra F, Avances C, Villalba M, Culine S, Fajas L. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS One. 2009;4:e7542. doi: 10.1371/journal.pone.0007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Diaz R, Silva J, Garcia JM, Lorenzo Y, Garcia V, Pena C, Rodriguez R, Munoz C, Garcia F, Bonilla F, Dominguez G. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
- Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, Mongera S, Postma C, Meijerink WJ, Carvalho B, Meijer GA. MiR-17–92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks PB. MicroRNAs and parallel stem cell lives. Cell. 2009;138:423–424. doi: 10.1016/j.cell.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, Pertsemlidis A. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7:1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M, Hod M, Sabah G, Rosenwald S, Levavi H. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Fojo T. Multiple paths to a drug resistance phenotype: mutations, translocations, deletions and amplification of coding genes or promoter regions, epigenetic changes and microRNAs. Drug Resist Updat. 2007;10:59–67. doi: 10.1016/j.drup.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, Peschle C, Fruci D. Antagomir-17–5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, et al. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- Garofalo M, Quintavalle C, Di LG, Zanca C, Romano G, Taccioli C, Liu CG, Croce CM, Condorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- Garzia L, Andolfo I, Cusanelli E, Marino N, Petrosino G, De MD, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008a;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008b;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Greither T, Grochola LF, Udelnow A, Lautenschlager C, Wurl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2009;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:E98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Casalini P, Piovan C, Di LG, Merlo A, Triulzi T, Menard S, Croce CM, Tagliabue E. microRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69:2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009a;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009b;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Josson S, Sung SY, Lao K, Chung LW, Johnstone PA. Radiation modulation of microRNA in prostate cancer cell lines. Prostate. 2008;68:1599–1606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani A, Ha D, Hsieh J, Rao PK, Schotte D, den Boer ML, Armstrong SA, Lodish HF. miR-128b is a potent glucocorticoid sensitizer in MLL-AF4 acute lymphocytic leukemia cells and exerts cooperative effects with miR-221. Blood. 2009;114:4169–4178. doi: 10.1182/blood-2008-12-191619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–2167. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Goan YG, Hsiao M, Lee CH, Jian SH, Lin JT, Chen YL, Lu PJ. MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res. 2009;315:2529–2538. doi: 10.1016/j.yexcr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009a;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- Li Y, Li W, Yang Y, Lu Y, He C, Hu G, Liu H, Chen J, He J, Yu H. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009b;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009c;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, VandenBoom TG, II, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J. 2009;28:3157–3170. doi: 10.1038/emboj.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, Vagner S. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- Manni I, Artuso S, Careccia S, Rizzo MG, Baserga R, Piaggio G, Sacchi A. The microRNA miR-92 increases proliferation of myeloid cells and by targeting p63 modulates the abundance of its isoforms. FASEB J. 2009;23:3957–3966. doi: 10.1096/fj.09-131847. [DOI] [PubMed] [Google Scholar]
- Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkamu T, Zhang X, Tan J, Zeng Y, Kassie F. Alteration of microRNA expression in vinyl-carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2009;31:252–258. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M, Malinova K, Kotaskova J, Pavlova S, Tichy B, Malcikova J, Stano KK, Smardova J, Brychtova Y, Doubek M, Trbusek M, Mayer J, Pospisilova S. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia. 2009;23:1159–1163. doi: 10.1038/leu.2008.377. [DOI] [PubMed] [Google Scholar]
- Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Fernandez L, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van MT, Rutka JT, Croce CM, Kenney AM, Taylor MD. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orom UA, Kauppinen S, Lund AH. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene. 2006;372:137–141. doi: 10.1016/j.gene.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense Inhibition of microRNA-21 or -221 Arrests Cell Cycle, Induces Apoptosis, and Sensitizes the Effects of Gemcitabine in Pancreatic Adenocarcinoma. Pancreas. 2009a;38:e190–199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009b;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- Persson H, Kvist A, Vallon-Christersson J, Medstrand P, Borg A, Rovira C. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat Cell Biol. 2009;11:1268–1271. doi: 10.1038/ncb1972. [DOI] [PubMed] [Google Scholar]
- Peter ME. Regulating cancer stem cells the miR way. Cell Stem Cell. 2010;6:4–6. doi: 10.1016/j.stem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Pierson J, Hostager B, Fan R, Vibhakar R. Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma. J Neurooncol. 2008;90:1–7. doi: 10.1007/s11060-008-9624-3. [DOI] [PubMed] [Google Scholar]
- Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Raponi M, Dossey L, Jatkoe T, Wu X, Chen G, Fan H, Beer DG. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009;69:5776–5783. doi: 10.1158/0008-5472.CAN-09-0587. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69:7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus JL, Harms G, Blokzijl T, Booman M, de JD, van IG, Rosati S, Schuuring E, Kluin P, van den BA. Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol. 2009;22:547–555. doi: 10.1038/modpathol.2009.10. [DOI] [PubMed] [Google Scholar]
- Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- Rui W, Bing F, Hai-Zhu S, Wei D, Long-Bang C. Identification of microRNA profiles in docetaxel-resistant human non-small cell lung carcinoma cells (SPC-A1) J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, Ouelaa R, Bracke M, De WO, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Mo YY. MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J Biol Chem. 2003;278:44312–44319. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A. 2009;106:12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang J, Pan T, Zhou J, Gong W, Liu N, Fu Z, You Y. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–126. doi: 10.1016/j.brainres.2009.11.056. [DOI] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, Fodstad O, Ju J. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478–486. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Spizzo R, Nicoloso MS, Lupini L, Lu Y, Fogarty J, Rossi S, et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010;17:246–254. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, Kawamata M, Kelnar K, Bader AG, Brown D, Ochiya T. Systemic Delivery of Synthetic MicroRNA-16 Inhibits the Growth of Metastatic Prostate Tumors via Downregulation of Multiple Cell-cycle Genes. Mol Ther. 2009;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2009;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Uziel T, Karginov FV, Xie S, Parker JS, Wang YD, Gajjar A, He L, Ellison D, Gilbertson RJ, Hannon G, Roussel MF. The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci U S A. 2009;106:2812–2817. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenboom TG, II, Li Y, Philip PA, Sarkar FH. MicroRNA and Cancer: Tiny Molecules with Major Implications. Curr Genomics. 2008;9:97–109. doi: 10.2174/138920208784139555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere White RW, Vinall RL, Tepper CG, Shi XB. MicroRNAs and their potential for translation in prostate cancer. Urol Oncol. 2009;27:307–311. doi: 10.1016/j.urolonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhoeve PM, le SC, Schrier M, Gillis AJ, Stoop H, Nagel R, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv Exp Med Biol. 2007;604:17–46. doi: 10.1007/978-0-387-69116-9_2. [DOI] [PubMed] [Google Scholar]
- Wang QZ, Xu W, Habib N, Xu R. Potential uses of microRNA in lung cancer diagnosis, prognosis, and therapy. Curr Cancer Drug Targets. 2009a;9:572–594. doi: 10.2174/156800909788486731. [DOI] [PubMed] [Google Scholar]
- Wang Y, Russell I, Chen C. MicroRNA and stem cell regulation. Curr Opin Mol Ther. 2009b;11:292–298. [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- Xiao B, Guo J, Miao Y, Jiang Z, Huan R, Zhang Y, Li D, Zhong J. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta. 2009;400:97–102. doi: 10.1016/j.cca.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Xin F, Li M, Balch C, Thomson M, Fan M, Liu Y, Hammond SM, Kim S, Nephew KP. Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics. 2009;25:430–434. doi: 10.1093/bioinformatics/btn646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008a;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, et al. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008b;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Li L, Wu DP, Fan JH, Li X, Wu KJ, Wang XY, He DL. A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol Sin. 2008;29:1060–1068. doi: 10.1111/j.1745-7254.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer. 2010;126:2–10. doi: 10.1002/ijc.24782. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhou Y, Yin B, Hao W, Zhao L, Ju W, Bai C. 5-Fluorouracil and oxaliplatin modify the expression profiles of microRNAs in human colon cancer cells in vitro. Oncol Rep. 2010;23:121–128. [PubMed] [Google Scholar]
- Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]